Abstract
Melanoma is a cutaneous malignant neoplasm of melanocytes. Primary malignant melanoma (MM) of the lung is very rare. Although previous reports have described the radiologic features of pulmonary MM, its rarity means that many factors are unknown. Thus, radiologic diagnosis is very difficult. Furthermore, there is little information regarding diagnostic application and/or the usefulness of [18F]‐fluorine‐2‐fluoro‐2‐deoxy‐D‐glucose positron emission tomography‐computed tomography (FDG‐PET‐CT) for primary pulmonary MM. A 69‐year‐old patient with a productive cough lasting three weeks was admitted to our hospital. Chest CT showed a large single mass with a multi‐lobulated margin and homogeneous enhancement in the right upper lobe, which was subsequently diagnosed as a primary pulmonary MM with multiple metastases. On PET‐CT images, the pulmonary mass and multiple bone lesions showed very increased uptakes of FDG. Considering that pulmonary metastasis from a mucocutaneous melanoma is the main differential diagnosis of primary pulmonary MM, systemic assessment of the whole body is more important than for other types of lung malignancies. This report introduces PET‐CT as a useful diagnostic modality for pulmonary MM, especially in cases of distant multiple metastases.
Keywords: Diagnosis, lung, PET‐CT, primary malignant melanoma
Introduction
Primary malignant melanoma (MM) of the lung is quite rare, accounting for 0.01% of all malignant lung neoplasms.1 Melanoma is a malignant neoplasm of melanocytes and usually originates from skin. MMs involving non‐cutaneous organs, in particular the respiratory system, are practically metastatic.1 Hence, clinical information, such as general characteristics, pathogenesis, radiologic features, diagnostic approach, and treatment for primary MM of the lung is still to be elucidated.
In particular, the radiologic features of primary pulmonary MM have rarely been described. Little is known regarding the application of positron emission tomography‐computed tomography (PET‐CT) to detect distant metastases in the whole body and patterns of [18F]‐fluorine‐2‐fluoro‐2‐deoxy‐D‐glucose (FDG) uptake.
In this report, we demonstrate the radiologic features of a 69‐year‐old patient with primary pulmonary MM and an interesting FDG uptake pattern with images of distant metastases, found by systemic PET‐CT scan.
Case report
A 69‐year‐old man was admitted with a productive cough lasting three weeks. Vital signs were stable and no abnormal findings were observed in laboratory and physical examinations. He was a never‐smoker and had no specific medical, family, or psychosocial history. CT showed a 5 × 3.4 cm sized mass with a multi‐lobulated margin and homogeneous enhancement (28 Hounsfield units) in the right upper lobe (Fig 1a). PET‐CT revealed an intense increase in FDG uptake into the mass at the right upper lobe of the lung (standardized uptake value 11.42; Fig 1b). In addition, on chest magnetic resonance imaging (MRI), the mass was hypo‐intense on T1‐weighted images (T1WI; Fig 1c) and hyper‐intense on T2‐weighted images (T2WI; Fig 1d). Interestingly, enhanced MRI showed homogenous enhancement of the right upper lobar lobulated mass on T1WI (Fig 1e). Bronchoscopic examination revealed no definitive endobronchial lesion and bronchial washing was negative for malignancy. Bronchoalveolar lavage cultures were negative for bacteria, mycobacterium, virus, and fungi. For pathologic diagnosis, we performed percutaneous trans‐thoracic needle biopsy. On microscopic examination, we found a tumor composed of polygonal cells with large amounts of acidophilic granular cytoplasm without melanin pigments, which displace the nucleus to the periphery of the cell (Fig 2a, b). Immunohistochemically, positive reaction of the tumor cells for human melanoma black 45 (HMB‐45; Fig 2c), S‐100 protein (Fig 2d), and vimentin (Fig 2e) were seen, while epithelial membrane antigen (EMA), pan‐cytokeratin (CK), CK7, CK20, thyroid transcription factor (TTF)‐1, renal cell carcinoma (RCC) marker, prostate specific antigen (PSA), CD68, and actin were negative. On the basis of these pathologic findings, the tumor was diagnosed as malignant melanoma of the lung.
Figure 1.
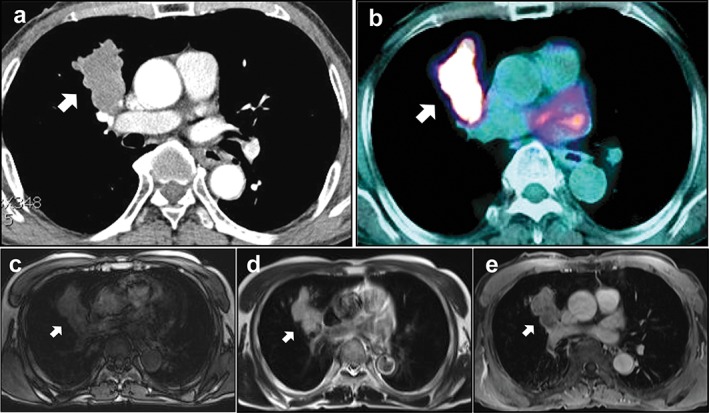
Chest image findings of malignant melanoma in the lung. (a) A rounded, well‐demarcated, multi‐lobulated mass was observed in the right upper lobe on spiral chest computed tomography. (b) This nodule showed strong [18F]‐fluorine‐2‐fluoro‐2‐deoxy‐D‐glucose (FDG) uptake on FDG‐positron emission tomography. Chest magnetic resonance imaging revealed that the mass appeared as (c) hypo‐intense on T1WI, (d) hyper‐intense on T2WI, and (e) with homogenous enhancement on enhanced T1WI. Arrows indicate the lesion.
Figure 2.
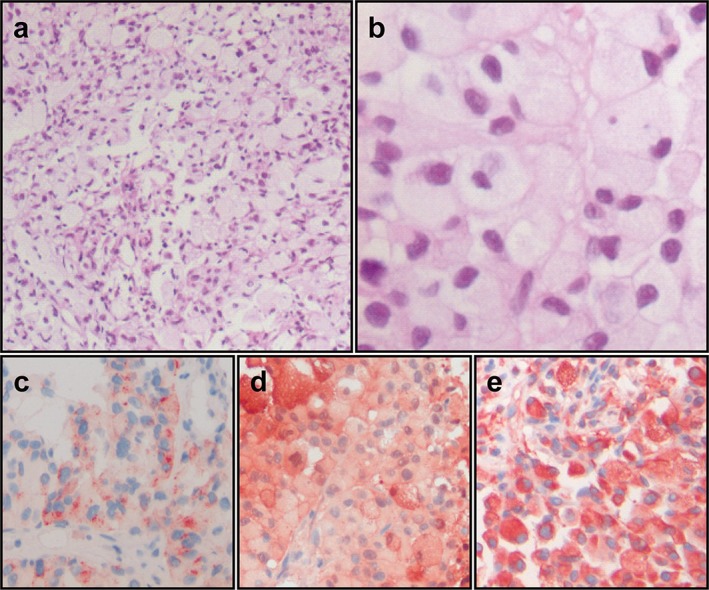
Histologic and immunohistochemical analyses of malignant melanoma of the lung. A photomicrograph of the lung tumor showed malignant cells in an alveolar pattern (a, hematoxylin & eosin × 100). The tumor was composed of polygonal cells with large amounts of acidophilic granular cytoplasm without melanin pigments, which displace the nucleus to the periphery of the cell (b, hematoxylin & eosin × 400). Immunohistochemistry showed positive reactions of the tumor cells for (c) HMB‐45, (d) S‐100 protein, and (e) vimentin.
Whole body assessment using PET‐CT revealed that increased FDG uptake was observed at the third thoracic vertebra, left ilium, and left proximal femur, suggesting hematogenous bone metastases (Fig 3a–d). Brain MRI showed two additional metastatic tumors in both frontal lobes (Fig 3e–g). They appeared as hypo‐intense on T1WI and hyper‐intense on T2WI, known as the amelanotic pattern, one of the typical MRI presentations of melanoma, with homogenous strong enhancement on T1WI. Dermatologic investigation, performed by two dermatologists in the university hospital, did not reveal any abnormal findings suggesting melanoma as the primary focus. Other organs, including the eyes, gastrointestinal tract, and oral and nasal cavities, which can be primary sites of melanoma, were thoroughly examined and no evidence of melanoma was found. However, any histologic examination of distant metastatic lesions, such as brain and bone metastases, could not be performed as a result of patient refusal.
Figure 3.
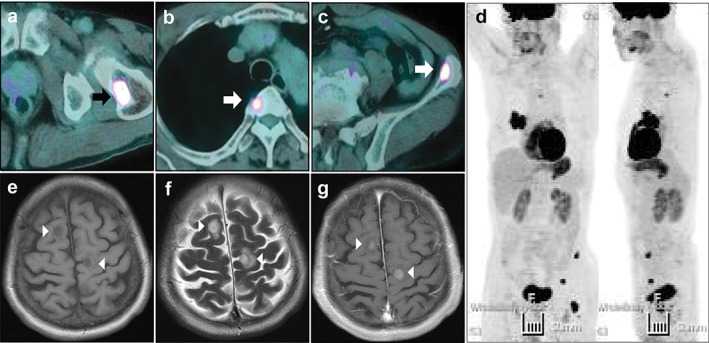
Assessment of the whole body for distant metastasis. Multiple bone metastases were observed in the (a) left proximal femur, (b) third thoracic vertebra, and (c) left ilium. (d) Primary and metastatic lesions in the maximum intensity projection view of positron emission tomography‐computed tomography. Brain magnetic resonance imaging showed two metastatic tumors in both frontal lobes in which the masses appeared as (e) hypo‐intense on T1WI, (f) hyper‐intense on T2WI, and (g) with homogenous enhancement on enhanced T1WI. Arrows and arrowheads indicate metastatic lesions.
Discussion
Melanoma is a malignant neoplasm characterized by uncontrolled proliferation of melanocytes. It usually originates from the skin but other organs, such as the eyes, nasal cavity, stomach, liver, and cervix have been reported as primary sites.1 The lung is a very unusual site for the primary involvement of MM. Because of its rarity, it is difficult to clinically suspect primary pulmonary MM when physicians encounter pulmonary masses. In addition, radiologic features are not specific for pulmonary MM. Therefore, histological assessment is still the gold standard for the diagnosis of pulmonary MM. However, it is also very difficult to pathologically distinguish primary MM from metastatic MM in the lung because of their microscopic similarities. Approximately 5–10% of patients with metastatic melanoma have a primary melanoma of unknown origin.2, 3 Therefore, systemic assessment of the whole body, including meticulous dermatologic examination, is more important than for other types of lung malignancies. In addition, radiologic findings are definitely helpful to narrow the scope of probable diagnoses.
In CT scan images, pulmonary MM presents as a well demarcated solitary nodule/mass with a round contour and lobulation, similar to the radiologic images of neuroendocrine tumors, adenosquamous tumors, lymphomas, or pseudolymphomas.4, 5 The usual appearances of malignant melanoma on MRI include melanotic and amelanotic patterns. A melanotic pattern shows hyper‐intensity on T1WI and hypo‐intensity on T2WI, while an amelanotic patterned lesion is hypo‐intense or isointense on T1WI and hyper‐intense or isointense on T2WI.6, 7 Because of these characteristics, MRI is the best diagnostic method for detecting brain metastases of MM, one of the common metastatic sites.8 Recent studies have reported that FDG‐PET‐CT can successfully detect metastatic lesions of MM, which show false negative findings with CT and may be a valuable diagnostic tool for restaging and management of recurred MM.9, 10 Specifically, a number of recent reports have described PET/CT scan images of MM in the lung from the skin as lesions with increased uptake of the radiotracer.11, 12 However, information on primary MM of the lung (not metastatic) is still limited.
Pathologically, primary pulmonary MM resembles skin or mucosa and metastatic melanoma, and exhibits morphologic variability within the tumor sample. Microscopically, the tumor is composed of epithelioid cells arranged in nests, or spindle cells arranged in fascicles, with or without melanin pigment deposition. Mitotic figures are readily apparent.13 Because of the difficulty of pathologic diagnosis, final diagnosis of a primary pulmonary MM of the lung is usually based on clinical, radiological, and pathological findings.14, 15, 16 Additionally, recent studies have indicated that immuno‐histochemical markers can be used to distinguish a primary MM from a metastatic one, as a result of the different expression patterns.17, 18 Usually, a melanoma is stained positive for S‐100, HMB‐45, and vimentin, whereas epithelial markers (pan‐CK, CK7, CK20, EMA), keratin, carcinoembryonic antigen (CEA), P63, TTF‐1, CD45, neuroendocrine markers (synaptophysin, chromographin), and muscular markers (actin and desmin) are negative.13 Metastatic melanomas tend to lose their specific typical markers, such as S‐100 and HMB‐45. Furthermore, metastatic melanoma seems to exhibit aberrant expression of epithelial‐associated markers, including EMA. CK AE1/AE3 was also noted in the metastases. B‐cell lymphoma 2, CD117, and TTF‐1 also showed a modest increase in antigenic expression at metastatic sites over the primary lesions.17, 18 Therefore, immuno‐histochemical staining analysis is a useful tool to differentially diagnose primary MM from metastatic.
As for the differential diagnosis of pulmonary MM from other types of lung malignancies, such as non‐small cell lung cancer (NSCLC) and small cell lung cancer (SCLC), radiologic examinations with enhanced CT and MRI are helpful. In fact, on enhanced CT or enhanced T1WI of MRI, pulmonary MM exhibits homogenous enhancement of the mass, while other lung malignancies usually show heterogeneity on CT scan and enhanced T1WI on MRI.7, 19 In addition, in cases of brain metastasis of MM, strong homogenous enhancement is shown on enhanced T1WI, whereas other lung malignancies usually exhibit ring enhancement.7 Histologic differential diagnosis using immune‐histochemical staining in which epithelial neoplasms, such as NSCLC and SCLC, are negative for melanoma markers and positive for epithelial markers, neuroendocrine tumors are positive for neuroendocrine markers, and neurogenic sarcoma is positive for S‐100, but negative for HMB‐45 and Melan‐A, is also useful.20
In the present report, an enhanced CT scan revealed primary pulmonary MM as a huge, well demarcated, homogenous, enhanced mass with lobulation. Chest MRI showed hypo‐intensity on T1WI and hyper‐intensity on T2WI with homogenous strong enhancement of the mass on enhanced T1WI, patterns that were also observed in the multiple metastatic lesions on brain MRI. Most importantly, we performed PET‐CT for the systemic assessment of the patient for pulmonary MM. On PET‐CT, intense FDG uptake into the pulmonary mass was observed, and multiple distant metastatic lesions with the same natured FDG uptake in bones were found. To determine whether the pulmonary tumor was a primary or metastatic lesion, we used immuno‐histochemistry to reveal the positivity of vimentin, HMB‐45, and S‐100 in tumor cells and observed negativity in various markers, such as CK, EMA, TTF‐1, and RCC.
We suggest that in cases of primary pulmonary MM, although pathologic diagnosis is confirmative, chest CT and MRI are informative and PET‐CT is a useful and convenient tool for systemic assessment of metastatic lesions in the body, supporting the final diagnosis.
In summary, we describe a primary pulmonary MM accompanying multiple bone and brain metastases, which is a very rare condition in pulmonary malignancies. Despite its rarity, this current case indicates that MM can develop in the lung and also present as a well‐enhanced lobulated mass with intense FDG uptake on PET‐CT. In addition, systemic assessment using PET‐CT seems to be a very useful diagnostic tool for multiple organ‐involved tumors.
Disclosure
No authors report any conflict of interest.
Acknowledgment
We thank Professor Mie‐Jae Im (Chonbuk National University Medical School, Jeonju, South Korea) for critical readings of the manuscript. This study was supported by the Korea Healthcare Technology R&D Project, Ministry for Health and Welfare, Republic of Korea (Grant A121931), the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT and future Planning (NRF‐2014R1A2A1A01002823), and by the Biomedical Research Institute, Chonbuk National University Hospital.
References
- 1. Wilson RW, Moran CA. Primary melanoma of the lung: A clinicopathologic and immunohistochemical study of eight cases. Am J Surg Pathol 1997; 21: 1196–1202. [DOI] [PubMed] [Google Scholar]
- 2. Serna MJ, Vázquez‐Doval J, Sola MA, Ruiz de Erenchun F, Quintanilla E. Metastatic melanoma of unknown primary tumour. Cutis 1994; 53: 305–308. [PubMed] [Google Scholar]
- 3. Chang AE, Karnell LH, Menck HR. The National Cancer Data Base report on cutaneous and noncutaneous melanoma: A summary of 84, 836 cases from the past decade. The American College of Surgeons Commission on Cancer and the American Cancer Society. Cancer 1998; 83: 1664–1678. [DOI] [PubMed] [Google Scholar]
- 4. Shin AR, Shin BK, Choi JA, Oh YW, Kim HK, Kang EY. Large cell neuroendocrine carcinoma of the lung: Radiologic and pathologic findings. J Comput Assist Tomogr 2000; 24: 567–573. [DOI] [PubMed] [Google Scholar]
- 5. Erasmus JJ, Connolly JE, McAdams HP, Roggli VL. Solitary pulmonary nodules: Part I. Morphologic evaluation for differentiation of benign and malignant lesions. Radiographics 2000; 20: 43–58. [DOI] [PubMed] [Google Scholar]
- 6. Woodruff WW Jr, Djang WT, McLendon RE, Heinz ER, Voorhees DR. Intracerebral malignant melanoma: High‐field‐strength MR imaging. Radiology 1987; 165: 209–213. [DOI] [PubMed] [Google Scholar]
- 7. Escott EJ. A variety of appearances of malignant melanoma in the head: A review. Radiographics 2001; 21: 625–639. [DOI] [PubMed] [Google Scholar]
- 8. Chiarion‐Sileni V, Murr R, Pigozzo J, Sarti S, Tomassi O. Brain metastases from malignant melanoma. Forum (Genova) 2003; 13: 170–185. [PubMed] [Google Scholar]
- 9. Pfluger T, Melzer HI, Schneider V et al PET/CT in malignant melanoma: Contrast‐enhanced CT versus plain low‐dose CT. Eur J Nucl Med Mol Imaging 2011; 38: 822–831. [DOI] [PubMed] [Google Scholar]
- 10. Stas M, Stroobants S, Dupont P et al 18‐FDG PET scan in the staging of recurrent melanoma: Additional value and therapeutic impact. Melanoma Res 2002; 12: 479–490. [DOI] [PubMed] [Google Scholar]
- 11. dos Santos CL, Fernandes LR, Meruje M, Barata F. Primary pulmonary melanoma: The unexpected tumour. BMJ Case Rep 2013; pii: bcr2013200706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chakraborty PS, Dhull VS, Karunanithi S, Verma S, Kumar R. Malignant melanoma with cavitary pulmonary metastasis: Diagnostic dilemma resolved by FDG PET/CT guided biopsy. Indian J Nucl Med 2014; 29: 196–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gong L, Liu XY, Zhang WD et al Primary pulmonary malignant melanoma: A clinicopathologic study of two cases. Diagn Pathol 2012; 7: 123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Allen MS Jr, Drash EC. Primary melanoma of the lung. Cancer 1968; 21: 154–159. [DOI] [PubMed] [Google Scholar]
- 15. Bagwell SP, Flynn SD, Cox PM, Davison JA. Primary malignant melanoma of the lung. Am Rev Respir Dis 1989; 139: 1543–1547. [DOI] [PubMed] [Google Scholar]
- 16. Alghanem AA, Mehan J, Hassan AA. Primary malignant melanoma of the lung. J Surg Oncol 1987; 34: 109–112. [DOI] [PubMed] [Google Scholar]
- 17. Plaza JA, Suster D, Perez‐Montiel D. Expression of immunohistochemical markers in primary and metastatic malignant melanoma: A comparative study in 70 patients using a tissue microarray technique. Appl Immunohistochem Mol Morphol 2007; 15: 421–425. [DOI] [PubMed] [Google Scholar]
- 18. Agaimy A, Specht K, Stoehr R et al Metastatic malignant melanoma with complete loss of differentiation markers (undifferentiated/dedifferentiated melanoma): Analysis of 14 patients emphasizing phenotypic plasticity and the value of molecular testing as surrogate diagnostic marker. Am J Surg Pathol 2016; 40: 181–191. [DOI] [PubMed] [Google Scholar]
- 19. Ganeshan B, Goh V, Mandeville HC, Ng QS, Hoskin PJ, Miles KA. Non‐small cell lung cancer: Histopathologic correlates for texture parameters at CT. Radiology 2013; 266: 326–336. [DOI] [PubMed] [Google Scholar]
- 20. Lazarou I, Purek L, Duc C, Licker MJ, Spiliopoulos A, Tschopp JM. Primary malignant achromic melanoma of the lung. Thoracic Cancer 2014; 5: 85–88. [DOI] [PMC free article] [PubMed] [Google Scholar]


