Abstract
Pulmonary sclerosing pneumocytoma (PSP) is a rare benign neoplasm that predominantly affects middle‐aged Asian women. PSP is often asymptomatic and demonstrates a solitary pulmonary nodule on radiologic examination. We report a case of PSP initially misdiagnosed as lung cancer because of strong 18F‐fluorodeoxyglucose (FDG) uptake revealed by 18F‐FDG positron emission tomography‐computed tomography scan. After surgery, pathology revealed that the tumor cells were immunopositive for epithelial membrane antigen and thyroid transcription factor‐1. The patient has been followed up without complication or recurrence.
Keywords: Positron emission tomography, pulmonary sclerosing hemangioma, solitary pulmonary nodule
Introduction
Pulmonary sclerosing pneumocytoma (PSP) is a rare benign neoplasm that was first described over 50 years ago as pulmonary sclerosing hemangioma.1 PSP predominantly affects middle‐aged adults over 50 years of age, with a female to male ratio of 5:1.2, 3 The nomenclature of this disease came from a concept that sclerosing hemangioma has an endothelial and vascular origin. However, several immunohistochemical studies have revealed that sclerosing hemangioma originates from primitive respiratory epithelium, most likely type II alveolar pneumocytes.2 The key feature of PSP is the presence of two cell types – cuboidal surface cells and stromal round cells – both of which are considered to be neoplastic.4
Pulmonary sclerosing pneumocytoma is usually a solitary, well‐circumscribed nodule or mass. Although some features have been determined, there is still no definitive diagnostic radiologic finding. Because 18F‐fluorodeoxyglucose (FDG) positron emission tomography‐computed tomography (PET‐CT) is widely used to evaluate cancer staging or screening, the chance of false positive interpretation has increased. We report a case of surgically diagnosed PSP demonstrating a positive result for lung malignancy on the preoperative 18F‐FDG PET/CT scan.
Case report
In January 2015, a 36‐year‐old woman visited our hospital for evaluation of a solitary pulmonary nodule in her left lung. She had no respiratory symptoms or a history of smoking or previous pulmonary tuberculosis. The nodule had been detected in December 2013 in another hospital (Fig 1a). At that time, a percutaneous needle biopsy revealed no evidence of malignancy. High‐resolution CT scans were taken every six months; a scan taken in December 2014 revealed an increase in nodule diameter to 3 cm (Fig 1c). The mass was a solitary, well‐circumscribed, heterogeneously enhanced feature located in the anteromediobasal segment of the left lower lobe. There was no evidence of enlarged lymph node or pleural effusion.
Figure 1.
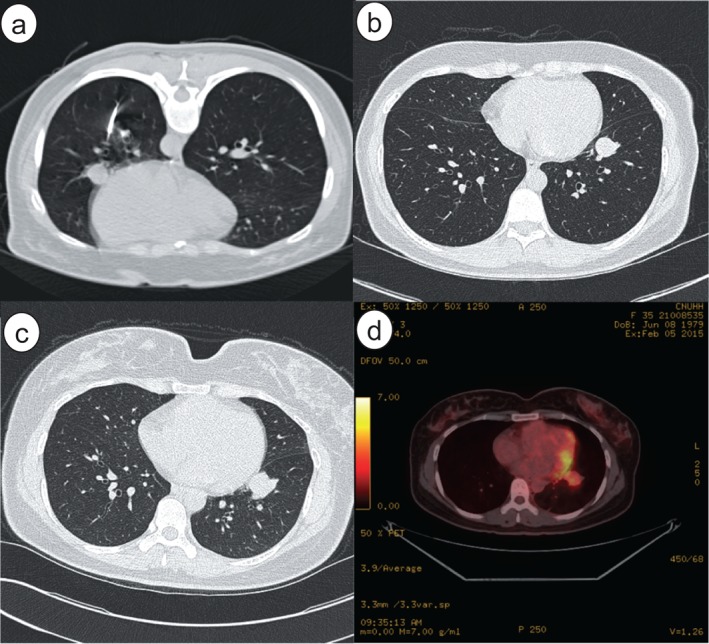
(a) Computed tomography (CT) obtained at another hospital during percutaneous needle biopsy shows a 1.5 cm round nodule in the left lower lobe, adjacent to the left cardiac border. (b) CT performed six months later revealed a slightly increased nodule diameter of 2 cm. (c) The nodule was solitary, well‐circumscribed, and round‐shaped in the anteromediobasal segment of the left lower lobe. (d) 18F‐ fluorodeoxyglucose (FDG) positron emission tomography‐CT revealed high FDG uptake (maximum standardized uptake value = 3.9) at the nodule without regional or distant metastasis.
At the time of the patient's visit to our hospital, serum carcinoembryonic antigen (CEA) and cancer antigen (CA) 19‐9 levels were within normal range (0.78 ng/mL and 6.2 U/mL, respectively). 18F‐FDG PET‐CT demonstrated high uptake of FDG at the nodule (maximum standardized uptake value [SUVmax] = 3.9; Fig 1d). Malignancy was suspected, with no metabolic evidence of regional or distant metastasis. Bronchoscopy showed no gross specific finding, except for mild edematous mucosa. A wedge resection followed by video‐assisted thoracic surgery was planned to make a definitive diagnosis and determine treatment for preoperative clinical stage IIB (T2aN0M0) lung cancer.
No malignant cells were evident in the frozen sections of tissue retrieved during the operation. This finding did not correspond with hamartoma, which is the most common benign tumor of the lung. The surgery ended with only a wedge resection of the anteromedial basal segment of the left lung. A permanent section biopsy revealed that the tumor was composed of monotonous, round tumor cells covered by hyperchromatic spindle cells (Figs 2, 3). Immunohistochemical stains were positive for cytokeratin7 (CK7), epithelial membrane antigen (EMA), thyroid transcription factor 1 (TTF‐1), progesterone receptor (PR), and negative for estrogen receptor (ER), CD56, and synaptophysin.
Figure 2.
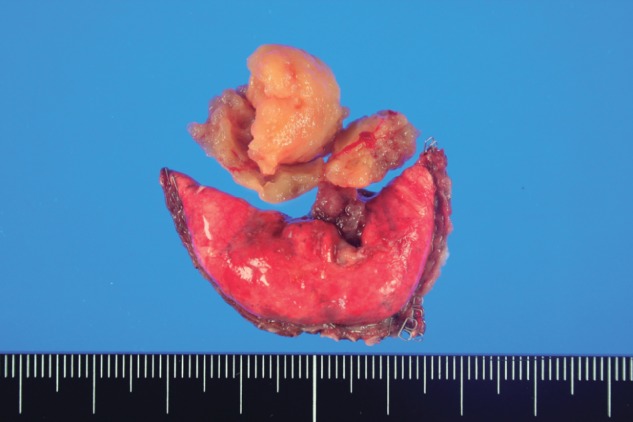
After surgical resection, a solid, yellow mass about 2.5 cm in size was separated by normal lung parenchyma.
Figure 3.
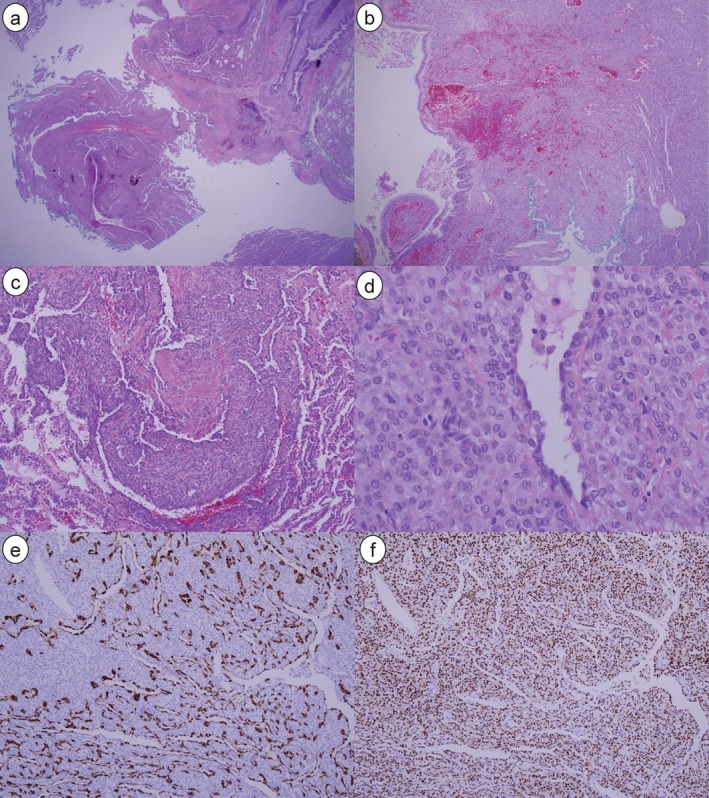
(a) A well‐demarcated solid mass was easily separated by normal lung parenchyma (hematoxylin and eosin [H&E], × 20). (b) The hemorrhagic mass is situated in the sub‐bronchial area (H&E, × 40). (c) Focal sclerotic change was shown within the tumor (H&E, × 100). (d) The tumor was composed of monotonous, round tumor cells covered by hyperchromatic spindle cells (H&E, × 400). (e) Immunohistochemical staining detected epithelial membrane antigen in the surface of the tumor cells, but not in the round cells (× 100). (f) Thyroid transcription factor‐1 was detected in both the surface tumor cells and the round cells (× 100).
After PSP was confirmed, no adjuvant therapy was needed because the tumor was benign. The patient was discharged without any complication. Follow‐up high‐resolution CT scans have not detected recurrence.
Discussion
Pulmonary sclerosing hemangioma is not a vascular tumor. The tumor is derived from primitive respiratory epithelial cells that express TTF‐1.4 PSP is a tumor of pneumocytic origin with a dual population of surface cells resembling type II pneumocytes and round cells, with slightly different histogenetic profiles. Therefore, sclerosing hemangioma is classified as sclerosing pneumocytoma, moved from “miscellaneous tumors” in the 1999 and 2004 World Health Organization Classification to “adenomas” in the 2015 World Health Organization Classification.4
Pulmonary sclerosing pneumocytoma is a benign tumor with low prevalence. Patients are usually women with a mean age of 46 years. The most common presenting form is an asymptomatic solitary pulmonary nodule that can grow up to 7 cm in diameter, although 73% of lesions are smaller than 3 cm.2, 5 PSP typically presents on chest radiograph as a peripheral, solitary, well‐defined, homogenous nodule or mass without predilection for a particular lobe. Calcification or cavitation is hard to see in PSP.6 Enhanced CT of the chest demonstrates a round to oval shaped lesion with smooth margins, but these findings are not specific.7, 8 A recent study reported that most patients had a single lesion (92.1%), smooth boundary (65.8%), and oval shape (65.8%), with a mean diameter of 2.27 cm.9 CT signs included marginal pseudocapsule (50%), overlying vessel (26.3%), air gap (2.6%), and halo sign (17.1%).9 Generally, 18F‐FDG PET/CT scan results are interpreted as positive for malignancy when the FDG uptake of a lung nodule is greater than the background mediastinal blood pool activity on qualitative assessment, or when the SUVmax values exceed 2.5.10 , 11 In the present case, 18F‐FDG PET/CT showed a false positive result, indicating malignancy. The majority of sclerosing hemangiomas show increased FDG uptake, with SUVmax values significantly correlated with tumor size. Sclerosing hemangioma ≥ 2 cm can frequently be falsely interpreted as malignant by FDG‐PET‐CT.12
Pulmonary sclerosing pneumocytoma can be very challenging to diagnose in frozen sections, small biopsies, and cytology where they can easily be mistaken for adenocarcinoma or carcinoid tumors.4 These tumors typically have a benign clinical course, with metastasis being exceedingly rare.2 Treatment of PSP involves surgical resection and the prognosis is favorable; recurrence after surgery or death as a result of PSP has not been reported.13
This case of PSP highlights the difficulty in diagnosing this rare tumor. Clinicians should be aware of the possibility of a false positive result for slow‐growing, solitary pulmonary nodules with a diameter exceeding 2 cm, even if a 18F‐FDG PET‐CT scan indicates hypermetabolic malignancy.
Disclosure
No authors report any conflict of interest.
References
- 1. Liebow AA, Hubbell DS. Sclerosing hemangioma (histiocytoma, xanthoma) of the lung. Cancer 1956; 9: 53–75. [DOI] [PubMed] [Google Scholar]
- 2. Devouassoux‐Shisheboran M, Hayashi T, Linnoila RI, Koss MN, Travis WD. A clinicopathologic study of 100 cases of pulmonary sclerosing hemangioma with immunohistochemical studies: TTF‐1 is expressed in both round and surface cells, suggesting an origin from primitive respiratory epithelium. Am J Surg Pathol 2000; 24: 906–916. [DOI] [PubMed] [Google Scholar]
- 3. Illei PB, Rosai J, Klimstra DS. Expression of thyroid transcription factor‐1 and other markers in sclerosing hemangioma of the lung. Arch Pathol Lab Med 2001; 125: 1335–1339. [DOI] [PubMed] [Google Scholar]
- 4. Travis WD, Brambilla E, Nicholson AG et al The 2015 World Health Organization Classification of Lung Tumors: Impact of genetic, clinical and radiologic advances since the 2004 classification. J Thorac Oncol 2015; 10: 1243–1260. [DOI] [PubMed] [Google Scholar]
- 5. Cardemil G, Fernández E, Riffo P et al [Sclerosing hemangioma presenting as a solitary lung nodule. Report of one case.] Rev Med Chil 2004; 132: 853–856. (In Spanish.) [DOI] [PubMed] [Google Scholar]
- 6. Kim KH, Sul HJ, Kang DY. Sclerosing hemangioma with lymph node metastasis. Yonsei Med J 2003; 44: 150–154. [DOI] [PubMed] [Google Scholar]
- 7. Chung MJ, Lee KS, Han J, Sung YM, Chong S, Kwon OJ. Pulmonary sclerosing hemangioma presenting as solitary pulmonary nodule: Dynamic CT findings and histopathologic comparisons. AJR Am J Roentgenol 2006; 187: 430–437. [DOI] [PubMed] [Google Scholar]
- 8. Cheung YC, Ng SH, Chang JW, Tan CF, Huang SF, Yu CT. Histopathological and CT features of pulmonary sclerosing haemangiomas. Clin Radiol 2003; 58: 630–635. [DOI] [PubMed] [Google Scholar]
- 9. Shin SY, Kim MY, Oh SY et al Pulmonary sclerosing pneumocytoma of the lung: CT characteristics in a large series of a tertiary referral center. Medicine (Baltimore) 2015; 94: e498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yap CS, Schiepers C, Fishbein MC, Phelps ME, Czernin J. FDG‐PET imaging in lung cancer: How sensitive is it for bronchioloalveolar carcinoma? Eur J Nucl Med Mol Imaging 2002; 29: 1166–1173. [DOI] [PubMed] [Google Scholar]
- 11. Lowe VJ, Fletcher JW, Gobar L et al Prospective investigation of positron emission tomography in lung nodules. J Clin Oncol 1998; 16: 1075–1084. [DOI] [PubMed] [Google Scholar]
- 12. Lee E, Park CM, Kang KW et al 18F‐FDG PET/CT features of pulmonary sclerosing hemangioma. Acta Radiol 2013; 54: 24–29. [DOI] [PubMed] [Google Scholar]
- 13. Kalhor N, Staerkel GA, Moran CA. So‐called sclerosing hemangioma of lung: Current concept. Ann Diagn Pathol 2010; 14: 60–67. [DOI] [PubMed] [Google Scholar]


