Abstract
Objectives. Vitamin D deficiency plays a role in insulin resistance and the pathogenesis of type 2 diabetes mellitus. Little information is available about the association between vitamin D status and insulin resistance in the Chinese population. Currently, vitamin D status is evaluated by the concentrations of serum 25-hydroxyvitamin D [25(OH)D]. This study explores the relationship between insulin resistance and serum 25-hydroxyvitamin D concentrations in Chinese patients with type 2 diabetes mellitus. Subjects and Methods. This study included 117 patients with type 2 diabetes. The following variables were measured: 25-hydroxyvitamin D [25(OH)D], glycosylated hemoglobin A1c (HbA1c), fasting blood glucose (FBS), fasting blood insulin (FINS), fasting blood C-peptide, serum creatinine (SCr), glomerular filtration rate (eGFR), body mass index (BMI), and homeostatic model estimates of insulin resistance (HOMA-IR). Results. The cases were divided into three groups: Group 1 (G1) with 25(OH)D ≤ 20 ng/mL [≤50 nmol/L], Group 2 (G2) with 25(OH)D values from 20 ng/mL [50 nmol/L] to 30 ng/mL [75 nmol/L], and Group 3 (G3) with 25(OH)D ≥ 30 ng/mL [≥75 nmol/L], with 52.6%, 26.3%, and 21.1% of subjects in Groups 1–3, respectively. There was a negative correlation between 25(OH)D and HOMA-IR (β = −0.314, p = 0.001) adjusted by age, BMI, and eGFR. Conclusion. Better vitamin D status may be protective of glucose homeostasis since 25(OH)D was negatively associated with insulin resistance in Chinese patients with type 2 diabetes.
1. Introduction
Diabetes is becoming a chronic, global epidemic with accelerated morbidity, increased and earlier mortality, and increased healthcare costs. Total global diabetes prevalence is ~9.7%, with 92.4 million adults with diabetes [1]. China is one of the countries with the heaviest diabetes burden, and the prevention and control of this disease are difficult tasks for Chinese society. Type 2 diabetes is a progressive and chronic disease characterized by both β-cell dysfunction and increased insulin resistance, defined as the inadequate response of skeletal muscle, liver, and adipose tissue to endogenous insulin secretions, and few drugs ameliorate increases in insulin resistance.
Recently, considerable interest has been generated on the extraskeletal and nonclassical effects of vitamin D [2, 3] based on the presence of vitamin D receptors (VDRs) and the activating hydroxylase in target tissues other than bone, gut, and kidney. Animal and human studies suggest that vitamin D may play a role in modulation of the risk of diabetes [4]. Subjects at risk of diabetes had lower 25(OH)D concentrations than those without risk [5, 6]. Vitamin D deficiency was related to the presence and prospective development of type 2 diabetes mellitus [4, 7–11], and compared to subjects without diabetes, type 2 diabetes patients had lower serum 25(OH)D [12]. The relationship between vitamin D status and the risk of type 2 diabetes or insulin resistance was reported in several studies. Vitamin D deficiency can play a role in insulin resistance and the pathogenesis of type 2 diabetes, through effects on both β-cell function and insulin sensitivity [13, 14]. Several roles of vitamin D deficiency are reported affecting insulin resistance through various mechanisms including increasing related proinflammatory cytokines and acute phase reactants, as found in vitamin D deficiency, mediating low-grade inflammation [15–17]. However, data on the inverse association between 25(OH)D concentrations and the development of insulin resistance (IR) is conflicting. In Europeans with metabolic syndrome, vitamin D status may not correlate with insulin activity or secretion [18], and relationships between vitamin D status and IR differ among different racial groups. Moreover, little research is available on the association between insulin resistance and vitamin D status in the Chinese population. As a result, this relationship needs to be specifically explored in different populations, including the Chinese. The aim of this research, therefore, was to clarify the relationship between vitamin D status and insulin resistance in Chinese people with T2DM. Currently, vitamin D status is evaluated by the concentration of total 25-hydroxyvitamin D [25(OH)D] [19]. According to Endocrine Society Clinical Practice Guidelines on vitamin D deficiency, serum circulating 25-hydroxyvitamin D [25(OH)D] concentrations were measured to evaluate vitamin D status [20]. We hypothesise that 25(OH)D concentrations negatively correlate with insulin resistance status based on Scragg et al. [21] and Chonchol and Scragg [22] researches.
2. Subjects and Methods
2.1. Materials and Methods
Based on Chiu et al.'s research [14], we identified 117 patients with type 2 diabetes who were treated in the outpatient Department of Endocrinology and Metabolism of Xiamen Second Hospital from January 2014 to March 2014. Three of the included subjects refused 25(OH)D concentration and intact parathyroid hormone test. The following criteria were used to include patients: (i) age ranging from 20 to 70 years, (ii) history less than 10 years, (iii) serum parathyroid hormone concentration ranging from 15.0 to 65.0 pg/mL, (iv) a serum calcium concentration less than 2.45 mmol/L, (v) normal routine blood tests of liver function, serum creatinine, and normal electrolytes, and not being treated with insulin or with thiazolidinedione (TZD), vitamin D, or drugs modulating vitamin D efficacy. Major reasons for excluding individuals included (i) absence of type 2 diabetes or presence of diabetic ketoacidosis, ketonuria, or diabetic hyperosmolar syndrome, (ii) serum phosphorus > 1.60 mmol/L, (iii) acute infection, (iv) tumors, and (v) pregnant or nursing women.
2.2. Anthropometric and Biochemical Analysis
Data were gathered on age, sex, and family history of type 2 diabetes. Participants' weights and heights were recorded, and body mass index (BMI) was calculated. The body weights and heights were measured while the subjects wore light clothing without shoes. The BMI was calculated as the weight in kilograms divided by the height in meters squared. Systolic blood pressure (SBP) and diastolic blood pressure (DBP) were measured while sitting, after a 5 minute rest, and again after a 10 minute interval, and the mean values were recorded. Hypertension was also diagnosed by a history of hypotensive medication. Blood samples were drawn between 08:00 am and 09:00 am for laboratory analysis of biochemical variables [25-hydroxyvitamin D (25(OH)D), glycated hemoglobin A1c (HbA1c), fasting blood glucose (FBS), fasting insulin (FINS), fasting serum C-peptide, total cholesterol (TC), triglyceride (TG), high-density lipoprotein (HDL-C), low-density lipoprotein (LDL-C), intact parathyroid hormone (iPTH), urea nitrogen (BUN), serum creatinine (SCr), serum calcium (Ca), and serum phosphorus (P)]. Intact parathyroid hormone (iPTH) (ntact parathyroid hormone kits, Beckman Coulter; Beckman DXI800 access immunoassay system), FINS [insulin kits, Roche Diagnostics (Shanghai); Roche Cobas 6000 analyzer], C-peptide (C-peptide kits, Roche Diagnostics; Roche Cobas 6000 analyzer), and serum 25(OH)D (25-hydroxyvitamin D kits, Roche Diagnostics; Roche Cobas 6000 analyzer) concentrations were determined by the electrochemical luminescence method. Fasting blood glucose (blood glucose kits, Beijing Leadman; Beckman DXI800 access immunoassay system) was measured by the oxygen electrode method. Serum creatinine (creatinine kits, Beijing Leadman Biochemistry; Siemens ADVIA 2400 automatic biochemical analyzer), TC (total cholesterol kits, Beijing Leadman Biochemistry; Siemens ADVIA 2400 automatic biochemical analyzer), TG (triglyceride kits, Beijing Leadman Biochemistry; Siemens ADVIA 2400 automatic biochemical analyzer), LDL (low-density lipoprotein kits, Sekisui Medical Japan; Siemens ADVIA 2400 automatic biochemical analyzer), and HDL (high-density lipoprotein kits, Randox UK; Siemens ADVIA 2400 automatic biochemical analyzer) were measured by the enzymatic method. HbA1c (D-10 glycosylated hemoglobin kits, Bio-Rad; Bio-Rad Dias TAT glycosylated hemoglobin analyzer) was measured by High Performance Liquid Chromatography (HPLC). BUN (urea nitrogen kits, Ningbo Medical System; Siemens ADVIA 2400 automatic biochemical analyzer) was measured by the urease method. Serum calcium (calcium kits, Ningbo Medical System; Siemens ADVIA 2400 automatic biochemical analyzer) and serum phosphorus (phosphorus kits, Siemens China; Siemens ADVIA 2400 automatic biochemical analyzer) were measured by the ion selective electrode method. Vitamin D deficiency was defined as 25(OH)D of ≤20 ng/mL [≤50 nmol/L], vitamin D insufficiency was defined as a 25(OH)D between 20 ng/mL [50 nmol/L] and 30 ng/mL [75 nmol/L]. The normal serum 25(OH)D concentration was defined as ≥30 ng/mL [≥75 nmol/L], according to Endocrine Society Clinical Practice Guideline of vitamin D deficiency [20]. HOMA-IR was calculated from fasting insulin and fasting glucose. HOMA-IR had the formula fasting glucose (mmol/L) × fasting insulin (pmol/L)/22.5 [23]. eGFR was calculated by the MDRD GFR equation [24]. As this was a retrospective study, and the data were anonymously analysed, informed consent was unnecessary.
2.3. Statistics
SPSS 19.0 software was used for statistical analysis. Continuous variables were expressed as the mean ± SD (mean standard deviation). Least-Significant Difference test (LSD-t) was used to analyse the comparison of 25(OH)D and β-cell function indices (FINS, HbA1c) for G1, G2, and G3 subjects. Independent-sample t test was used to compare 25(OH)D, HOMA-IR, and glucose metabolism indices (FBS, FINS, and HbA1c) in male and female subjects. Multiple linear regression analysis was used to examine the association between serum 25(OH)D concentration and insulin resistance, HOMA-IR, analysed as dependent variable with the other significantly associated variables [25(OH)D, eGFR, BMI, and age] as independent variables, and a p value < 0.05 was considered significant.
3. Results
3.1. Baseline Characteristics
A total of 117 patients were enrolled in this study; three of them refused 25(OH)D concentration and iPTH test. Table 1 shows the characteristics of the study subjects and their metabolic index levels. The population had an even sex distribution (53.85% male) and a normal BMI (mean BMI = 24.90 ± 3.24 kg/m2) and was middle aged (mean age = 50.38 ± 13.47 years). The average serum 25(OH)D value was 21.40 ± 10.68 ng/mL (53.1 ± 26.35 nmol/L), below the sufficiency cutoff value of 30 ng/mL (75 nmol/L). The prevalence of vitamin D deficiency [25(OH)D < 50 nmol/L] was 52.6% of the total study subjects.
Table 1.
Characteristics of the study population.
| Variables | n | |
|---|---|---|
| Age (years)a | 50.38 ± 13.47 | 117 |
| Menc | 53.85 | 63 |
| Body mass index (kg/m2)a | 24.90 ± 3.24 | 117 |
| Systolic blood pressure (mmHg)a | 125.76 ± 14.79 | 117 |
| Diastolic blood pressure (mmHg)a | 80.85 ± 9.30 | 117 |
| Fasting plasma glucose (mmol/L)a | 8.23 ± 3.28 | 117 |
| HOMA-IRa | 5.79 ± 2.14 | 117 |
| C-peptide (μIU/mL)a | 2.09 ± 1.04 | 117 |
| 25(OH)D (ng/mL)a | 21.40 ± 10.68 (53.1 ± 26.35 nmol/L) | 114 |
| Vitamin D deficiencyc | 52.6 | 60 |
| Vitamin D insufficiencyc | 26.3 | 30 |
| Normal 25(OH)D valuec | 21.1 | 24 |
| HbA1c (%)a | 9.51 ± 2.81 | 117 |
| iPTH (pg/mL)a | 43.07 ± 14.21 | 114 |
| TC (mmol/L)a | 5.14 ± 1.12 | 117 |
| LDL (mmol/L)a | 3.06 ± 1.01 | 117 |
| Ca (mmol/L)a | 2.33 ± 0.14 | 117 |
| P (mmol/L)a | 1.36 ± 0.15 | 117 |
| eGFR (mL/min·1.73 m2)b | 111.70 [93.85–131.35] | 117 |
| History (year)b | 3.00 [0.00–8.00] | 117 |
| FINSb | 117.10 [72.30–156.52] | 117 |
| TGb | 1.65 [1.06–2.82] | 117 |
| HDLb | 1.23 [1.11–1.46] | 117 |
| BUNb | 4.81 [4.13–5.69] | 117 |
| SCrb | 56.50 [48.05–70.45] | 117 |
Notes: amean ± SD, bmedian [interquartile range], and cpercentage.
HOMA-IR: homeostatic model estimates of insulin resistance, 25(OH)D: 25-hydroxyvitamin D, TC: total cholesterol, LDL: low-density lipoprotein, Ca: serum calcium, P: serum phosphorus, eGFR: glomerular filtration rate, FINS: fasting blood insulin, TG: triglyceride, HDL: high-density lipoprotein, BUN: blood urea nitrogen, SCr: serum creatinine, and HbA1c: glycated hemoglobin A1c, iPTH: intact parathyroid hormone.
3.2. Comparison of G1, G2, and G3 of HOMA-IR and Glucose Metabolism Indices (FINS, HbA1c)
The subjects were divided into three groups according to serum 25(OH)D value: (G1) vitamin D deficiency [25(OH)D ≤ 20 ng/mL (50 nmol/L)], (G2) vitamin D insufficiency [25(OH)D value between 20 ng/mL (50 nmol/L) and 30 ng/mL (75 nmol/L)], and (G3) normal vitamin D status [25(OH)D ≥ 30 ng/mL (75 nmol/L)]. The results of the analyses comparing data for G1, G2, and G3 are given for the mean ± SD in Table 2; the mean HOMA-IR was significantly different between all the three groups, as shown in Table 2, and both HOMA-IR and fasting insulin values were lower with higher mean 25(OH)D concentrations.
Table 2.
Comparison of G1, G2, and G3 of HOMA-IR and glucose metabolism indices (FINS, HbA1c).
| G1 | G2 | G3 | p G1G2 value | p G2G3 value | p G1G3 value | |
|---|---|---|---|---|---|---|
| HOMA-IR | 6.44 ± 1.70∗※ | 5.00 ± 1.94∗ | 4.80 ± 2.57※ | 0.006 | 0.204 | 0.000 |
| FINS (μU/mL) | 141.20 ± 72.59※ | 128.14 ± 56.79# | 93.23 ± 65.82#※ | 0.590 | 0.010 | 0.001 |
| HbA1c (%) | 10.08 ± 2.78∗ | 7.87 ± 2.34∗# | 9.99 ± 2.89# | 0.000 | 0.003 | 0.955 |
Notes: ∗comparison of G1 and G2 is significant; #comparison of G2 and G3 is significant; ※comparison of G1 and G3 is significant; HOMA-IR: homeostatic model estimates of insulin resistance; FINS: fasting blood insulin; HbA1c: glycated hemoglobin A1c; p G1G2: p value of G1 and G2 comparison; p G2G3: p value of G2 and G3 comparison; p G1G3: p value of G1 and G3 comparison.
3.3. Comparison of Male and Female of 25(OH)D, Glucose Metabolism Indices (FBS, FINS, and HbA1c), and HOMA-IR
The values for serum 25(OH)D and for insulin resistance and glucose indices (fasting blood glucose, fasting insulin levels, and HbA1c) by gender are presented in Table 3. 25(OH)D values, HOMA-IR, fasting blood glucose, and fasting insulin levels were not significantly different in male and female subjects, but the mean HbA1c was significantly higher in men than that in women, as shown.
Table 3.
Comparison of male and female of 25(OH)D, glucose metabolism indices (FBS, FINS, and HbA1c), and HOMA-IR.
| Male | Female | p value | |
|---|---|---|---|
| 25(OH)D (ng/mL) | 20.46 ± 11.76 | 22.55 ± 9.16 | 0.301 |
| HOMA-IR | 5.78 ± 2.16 | 5.81 ± 2.12 | 0.953 |
| FBS (mmol/L) | 8.03 ± 3.76 | 8.47 ± 2.64 | 0.452 |
| FINS (μU/mL) | 134.72 ± 75.47 | 118.78 ± 59.14 | 0.203 |
| HbA1c (%) | 10.09 ± 3.09 | 8.83 ± 2.28 | 0.013 |
Notes: 25(OH)D: 25-hydroxyvitamin D, HOMA-IR: homeostatic model estimates of insulin resistance, FBS: fasting blood glucose, FINS: fasting blood insulin, and HbA1c: glycated hemoglobin A1c.
3.4. Relationship between Serum 25(OH)D Levels and HOMA-IR
In the multiple linear regression analysis (shown in Table 4), vitamin D status was a predictor of HOMA-IR, as the dependent variable, but not eGFR, BMI or age, as independent variables. The Pearson correlation coefficient showed that 25(OH)D had a negative correlation with HOMA-IR (regression coefficient r = −0.327, p = 0.000; see Figure 1).
Table 4.
Multiple linear regression analysis between HOMA-IR and 25(OH)D, eGFR, BMI, and age.
| HOMA-IR | ||
|---|---|---|
| β | p value | |
| 25(OH)D | −0.314 | 0.001 |
| Age | 0.077 | 0.446 |
| eGFR | −0.059 | 0.546 |
| BMI | 0.191 | 0.043 |
Note: HOMA-IR used as dependent variable and 25(OH)D, eGFR, BMI, and age used as independent variables.
Figure 1.
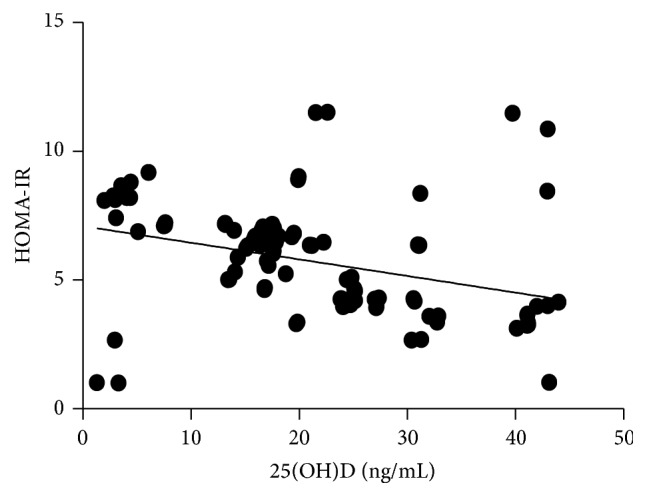
Inverse association between 25(OH)D and HOMA-IR (Pearson correlation = −0.327, p = 0.000).
4. Discussion
The prevalence of type 2 diabetes mellitus (T2DM) has increased significantly and globally over the last few decades. Type 2 diabetes results from insulin resistance linked to dyslipidemia, hyperglycemia, obesity, and hypertension. Few drugs are clinically effective in ameliorating insulin resistance. Vitamin D is a well-known vitamin responsible for bone and calcium metabolism [7]. Its deficiency results in rickets in children and osteomalacia in adults. Cutaneous synthesis and dietary ingestion are the main sources of vitamin D [25, 26]. The proportion of serum vitamin D intake from the diet is small [27, 28], and it is mainly provided by sun exposure during outdoor activities. In recent years, the extraskeletal effects of vitamin D have attracted much interest [2, 3]. Lower serum 25(OH)D concentrations are associated with higher risks for the development of type 2 diabetes mellitus [8]. In addition, more type 2 diabetes patients than control subjects had vitamin D insufficiency and deficiency [29, 30]. 25(OH)D values were lower in type 2 diabetes mellitus patients than in control group, and poor vitamin D status [25(OH)D values] was associated with less good glycemic control in type 2 diabetes mellitus patients [5, 25]. The Australian diabetes study showed that 25(OH)D data were inversely associated with type 2 diabetes risk in their population [31]. Many in vitro studies suggest repletion of vitamin D insufficiency may improve insulin secretion and sensitivity [32, 33]. However, reports on associations between insulin secretion or insulin resistance and 25(OH)D have been inconsistent among different racial groups. Vitamin D deficiency has also been suggested to be related to insulin resistance and to the risks of type 2 diabetes mellitus, as well as those of metabolic syndrome, but 25(OH)D values may not correlate with insulin activity or secretion [4, 7–11]. Moreover, few studies are available on the association between insulin resistance and vitamin D status in the Chinese population. Most research has investigated vitamin D metabolism in Chinese women with gestational diabetes or with metabolic syndrome [34–36], but one study from Cai et al. found no correlation between 25(OH)D values and insulin resistance [37], though exclusion criteria, such as treatment with insulin and thiazolidinedione administration, were omitted in that cross-sectional study which may have affected the results.
Our cross-sectional study suggests that, amongst our subjects with type 2 diabetes, the proportions with vitamin D deficiency group (G1), vitamin D insufficiency (G2), and normal vitamin D status (G3) were 52.6%, 26.3%, and 21.1%, respectively. All the subjects lived in southern China (northern latitude 24.27°, east longitude 118.06°) and, despite sufficient sunlight, more than half of the subjects were vitamin D deficient, and only 21.1% were “replete.” The prevalence of vitamin D deficiency in patients with type 2 diabetes will probably be higher in north China as this area gets less sunshine than southern China. Both HOMA-IR and fasting insulin values decreased with higher 25(OH)D concentrations, in all the three groups. Insulin resistance and the associated biomarkers were negatively associated with 25(OH)D status in our representative population of Chinese patients with type 2 diabetes, other than eGFR, BMI, and age. Similar data has been found in other racial groups. An inverse association of insulin resistance with 25(OH)D concentration has been found for 25(OH)D values between 16 and 36 ng/mL [38]. Another study suggested that 25(OH)D possibly modulated glycemic responses, both in impaired glycemia and in healthy subjects [39]. Other studies have indicated a role for vitamin D supplementation in modulation of insulin resistance and improvement of its resultant complications. In Von Hurst et al., insulin resistance was reduced but only if serum 25(OH)D on supplementation reached >80 nmol/L (>32 ng/mL) [40]; and although few data demonstrated that vitamin D insufficiency was associated with insulin resistance [21, 41–43], it was suggested that adequate vitamin D status may be helpful for prevention of increases in insulin resistance and of subsequent T2DM.
The role of vitamin D deficiency in insulin resistance is thought to have several potential mechanisms, including increasing the formation of proinflammatory cytokines and acute phase reactants, likely to increase low-grade inflammation, as well as the well-known promotion of insulin secretion from beta-cells [14, 15, 17]. The efficacy of vitamin D in stimulating insulin release can be affected by vitamin D axis gene polymorphisms, such as those for the activating enzyme (vitamin D 1α-hydroxylase; CYP27B1) and the transport protein (vitamin D binding protein), as well as for vitamin D receptors (VDR) [44–49]. Moreover, hypocalcemia worsens effects of vitamin D deficiency, including decreasing further the glucose-stimulated release of insulin from β-cells [50]. Lower serum 25(OH)D concentrations also increase serum parathyroid hormone (PTH), itself leading to decreased glucose uptake by liver, muscle, and adipose cells [51]. In another study, variants of the vitamin D receptors (VDRs) in pancreatic β-cells, skeletal muscle, and adipose tissue were associated with variation in the effects of vitamin D on glucose metabolism. Insulin sensitivity was affected by the presence of 1α-hydroxylase in β-cells and the presence of vitamin D response element in the human insulin receptor gene promoter region. The transcription of peroxisome proliferator activator receptor (PPAR) γ and of the human insulin receptor gene was directly activated by calcitriol; in vitro, vitamin D acceleration of glucose transport was mediated by insulin and vitamin D activated insulin receptor gene transcription [52–55]. Certain vitamin D binding proteins and allelic variations in the vitamin D receptor gene might also affect insulin secretion and glucose tolerance, which, in turn, might contribute to the genetic risk of type 2 diabetes [56]. Data on the MRC-Ely prospective study demonstrated that the baseline 25(OH)D concentration was inversely related to insulin sensitivity and glycemia [57, 58]. Further research is needed to explore whether vitamin D deficiency results in insulin resistance and whether supplementing with vitamin D or its active metabolite may prevent, or ameliorate, insulin resistance in healthy people or in Chinese people with type 2 diabetes.
5. Conclusion
Vitamin D status possibly plays a role in maintaining glucose homeostasis. There is a negative correlation between circulating levels of 25(OH)D and insulin resistance in Chinese people with type 2 diabetes.
Acknowledgments
The authors acknowledge all the participants in this study. This work was supported by grants from (1) the project of Natural Science Foundation of Xiamen Medical College (K2015-02) and (2) united key task project of important disease of Xiamen Science and Technology Commission and Xiamen Health and Family Planning Commission (3502Z20149032).
Competing Interests
The authors declare no conflict of interests.
References
- 1.Yang W., Lu J., Weng J., et al. Prevalence of diabetes among men and women in China. The New England Journal of Medicine. 2010;362(12):1090–1101. doi: 10.1056/nejmoa0908292. [DOI] [PubMed] [Google Scholar]
- 2.Holick M. F. The vitamin D deficiency pandemic and consequences for nonskeletal health: mechanisms of action. Molecular Aspects of Medicine. 2008;29(6):361–368. doi: 10.1016/j.mam.2008.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Holick M. F. Vitamin D: extraskeletal health. Endocrinology and Metabolism Clinics of North America. 2010;39(2):381–400. doi: 10.1016/j.ecl.2010.02.016. [DOI] [PubMed] [Google Scholar]
- 4.Pittas A. G., Lau J., Hu F. B., Dawson-Hughes B. The role of vitamin D and calcium in type 2 diabetes. A systematic review and meta-analysis. The Journal of Clinical Endocrinology & Metabolism. 2007;92(6):2017–2029. doi: 10.1210/jc.2007-0298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mitri J., Dawson-Hughes B., Hu F. B., Pittas A. G. Effects of vitamin D and calcium supplementation on pancreatic β cell function, insulin sensitivity, and glycemia in adults at high risk of diabetes: the Calcium and Vitamin D for Diabetes Mellitus (CaDDM) randomized controlled trial. American Journal of Clinical Nutrition. 2011;94(2):486–494. doi: 10.3945/ajcn.111.011684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Talaei A., Mohamadi M., Adgi Z. The effect of vitamin D on insulin resistance in patients with type 2 diabetes. Diabetology & Metabolic Syndrome. 2013;5, article 8 doi: 10.1186/1758-5996-5-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pittas A. G., Sun Q., Manson J. E., Dawson-Hughes B., Hu F. B. Plasma 25-hydroxyvitamin D concentration and risk of incident type 2 diabetes in women. Diabetes Care. 2010;33(9):2021–2023. doi: 10.2337/dc10-0790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pittas A. G., Nelson J., Mitri J., et al. Plasma 25-hydroxyvitamin D and progression to diabetes in patients at risk for diabetes: an ancillary analysis in the diabetes prevention program. Diabetes Care. 2012;35(3):565–573. doi: 10.2337/dc11-1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mitri J., Muraru M. D., Pittas A. G. Vitamin D and type 2 diabetes: a systematic review. European Journal of Clinical Nutrition. 2011;65(9):1005–1015. doi: 10.1038/ejcn.2011.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chagas C. E. A., Borges M. C., Martini L. A., Rogero M. M. Focus on vitamin D, inflammation and type 2 diabetes. Nutrients. 2012;4(1):52–67. doi: 10.3390/nu4010052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lim S., Kim M. J., Choi S. H., et al. Association of vitamin D deficiency with incidence of type 2 diabetes in high-risk Asian subjects. The American Journal of Clinical Nutrition. 2013;97(3):524–530. doi: 10.3945/ajcn.112.048496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scragg R., Holdaway I., Singh V., Metcalf P., Baker J., Dryson E. Serum 25-hydroxyvitamin D3 levels decreased in impaired glucose tolerance and diabetes mellitus. Diabetes Research and Clinical Practice. 1995;27(3):181–188. doi: 10.1016/0168-8227(95)01040-K. [DOI] [PubMed] [Google Scholar]
- 13.Deleskog A., Hilding A., Brismar K., Hamsten A., Efendic S., Östenson C.-G. Low serum 25-hydroxyvitamin D level predicts progression to type 2 diabetes in individuals with prediabetes but not with normal glucose tolerance. Diabetologia. 2012;55(6):1668–1678. doi: 10.1007/s00125-012-2529-x. [DOI] [PubMed] [Google Scholar]
- 14.Chiu K. C., Chu A., Go V. L. W., Saad M. F. Hypovitaminosis D is associated with insulin resistance and β cell dysfunction. The American Journal of Clinical Nutrition. 2004;79(5):820–825. doi: 10.1093/ajcn/79.5.820. [DOI] [PubMed] [Google Scholar]
- 15.García-Bailo B., Roke K., Mutch D. M., El-Sohemy A., Badawi A. Association between circulating ascorbic acid, -tocopherol, 25-hydroxyvitamin D, and plasma cytokine concentrations in young adults: a cross-sectional study. Nutrition & Metabolism. 2012;9, article 102 doi: 10.1186/1743-7075-9-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garcia-Bailo B., El-Sohemy A., Haddad P. S., et al. Vitamins D, C, and E in the prevention of type 2 diabetes mellitus: modulation of inflammation and oxidative stress. Biologics: Targets and Therapy. 2011;5:7–19. doi: 10.2147/btt.s14417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Badawi A., Klip A., Haddad P., et al. Type 2 diabetes mellitus and inflammation: prospects for biomarkers of risk and nutritional intervention. Diabetes, Metabolic Syndrome and Obesity: Targets and Therapy. 2010;3:173–186. doi: 10.2147/dmsott.s9089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gulseth H. L., Gjelstad I. M. F., Tierney A. C., et al. Serum vitamin D concentration does not predict insulin action or secretion in European subjects with the metabolic syndrome. Diabetes Care. 2010;33(4):923–925. doi: 10.2337/dc09-1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yousefzadeh P., Shapses S. A., Wang X. Vitamin D binding protein impact on 25-hydroxyvitamin D levels under different physiologic and pathologic conditions. International Journal of Endocrinology. 2014;2014 doi: 10.1155/2014/981581.981581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Holick M. F., Binkley N. C., Bischoff-Ferrari H. A., et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. The Journal of Clinical Endocrinology and Metabolism. 2011;96:1911–1930. doi: 10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]
- 21.Scragg R., Sowers M., Bell C. Serum 25-hydroxyvitamin D, diabetes, and ethnicity in the third national health and nutrition examination survey. Diabetes Care. 2004;27(12):2813–2818. doi: 10.2337/diacare.27.12.2813. [DOI] [PubMed] [Google Scholar]
- 22.Chonchol M., Scragg R. 25-Hydroxyvitamin D, insulin resistance, and kidney function in the Third National Health and Nutrition Examination Survey. Kidney International. 2007;71(2):134–139. doi: 10.1038/sj.ki.5002002. [DOI] [PubMed] [Google Scholar]
- 23.Bonora E., Formentini G., Calcaterra F., et al. HOMA-estimated insulin resistance is an independent predictor of cardiovascular disease in type 2 diabetic subjects: prospective data from the Verona Diabetes Complications Study. Diabetes Care. 2002;25(7):1135–1141. doi: 10.2337/diacare.25.7.1135. [DOI] [PubMed] [Google Scholar]
- 24.Levey A. S., Bosch J. P., Lewis J. B., Greene T., Rogers N., Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Annals of Internal Medicine. 1999;130(6):461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 25.Christakos S., Ajibade D. V., Dhawan P., Fechner A. J., Mady L. J. Vitamin D: metabolism. Endocrinology and Metabolism Clinics of North America. 2010;39(2):243–253. doi: 10.1016/j.ecl.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gallagher J. C., Peacock M., Yalamanchili V., Smith L. M. Effects of vitamin D supplementation in older African American women. Journal of Clinical Endocrinology and Metabolism. 2013;98(3):1137–1146. doi: 10.1210/jc.2012-3106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Andersen R., Brot C., Jakobsen J., et al. Seasonal changes in vitamin D status among Danish adolescent girls and elderly women: the influence of sun exposure and vitamin D intake. European Journal of Clinical Nutrition. 2013;67(3):270–274. doi: 10.1038/ejcn.2013.3. [DOI] [PubMed] [Google Scholar]
- 28.Freedman D. M., Cahoon E. K., Rajaraman P., et al. Sunlight and other determinants of circulating 25-hydroxyvitamin D levels in black and white participants in a nationwide US study. American Journal of Epidemiology. 2013;177(2):180–192. doi: 10.1093/aje/kws223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kostoglou-Athanassiou I., Athanassiou P., Gkountouvas A., Kaldrymides P. Vitamin D and glycemic control in diabetes mellitus type 2. Therapeutic Advances in Endocrinology and Metabolism. 2013;4(4):122–128. doi: 10.1177/2042018813501189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bachali S., Dasu K., Ramalingam K., Naidu J. N. Vitamin D deficiency and insulin resistance in normal and type 2 diabetes subjects. Indian Journal of Clinical Biochemistry. 2013;28(1):74–78. doi: 10.1007/s12291-012-0239-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gagnon C., Lu Z. X., Magliano D. J., et al. Serum 25-hydroxyvitamin D, calcium intake, and risk of type 2 diabetes after 5 years: results from a national, population-based prospective study (the Australian diabetes, obesity and lifestyle study) Diabetes Care. 2011;34(5):1133–1138. doi: 10.2337/dc10-2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bourlon P.-M., Billaudel B., Faure-Dussert A. Influence of vitamin D3 deficiency and 1,25 dihydroxyvitamin D3 on de novo insulin biosynthesis in the islets of the rat endocrine pancreas. The Journal of Endocrinology. 1999;160(1):87–95. doi: 10.1677/joe.0.1600087. [DOI] [PubMed] [Google Scholar]
- 33.Maestro B., Campión J., Dávila N., Calle C. Stimulation by 1,25-dihydroxyvitamin D3 of insulin receptor expression and insulin responsiveness for glucose transport in U-937 human promonocytic cells. Endocrine Journal. 2000;47(4):383–391. doi: 10.1507/endocrj.47.383. [DOI] [PubMed] [Google Scholar]
- 34.Li L.-H., Yin X.-Y., Yao C.-Y., Zhu X.-C., Wu X.-H. Serum 25-hydroxyvitamin D, parathyroid hormone, and their association with metabolic syndrome in Chinese. Endocrine. 2013;44(2):465–472. doi: 10.1007/s12020-013-9885-2. [DOI] [PubMed] [Google Scholar]
- 35.Wang O., Nie M., Hu Y. Y., et al. Association between vitamin D insufficiency and the risk for gestational diabetes mellitus in pregnant Chinese women. Biomedical and Environmental Sciences. 2012;25(4):399–406. doi: 10.3967/0895-3988.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 36.Lu L., Yu Z., Pan A., et al. Plasma 25-hydroxyvitamin D concentration and metabolic syndrome among middle-aged and elderly Chinese individuals. Diabetes Care. 2009;32(7):1278–1283. doi: 10.2337/dc09-0209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cai X., Hu Z., Chen L., Han X., Ji L. Analysis of the associations between vitamin D and albuminuria or β-cell function in Chinese type 2 diabetes. BioMed Research International. 2014;2014:5. doi: 10.1155/2014/640909.640909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Heaney R. P., French C. B., Nguyen S., et al. A novel approach localizes the association of vitamin D status with insulin resistance to one region of the 25-Hydroxyvitamin D continuum. Advances in Nutrition. 2013;4(3):303–310. doi: 10.3945/an.113.003731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Badawi A., Sayegh S., Sadoun E., Al-Thani M., Arora P., Haddad P. S. Relationship between insulin resistance and plasma vitamin D in adults. Diabetes, Metabolic Syndrome and Obesity: Targets and Therapy. 2014;7:297–303. doi: 10.2147/DMSO.S60569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Von Hurst P. R., Stonehouse W., Coad J. Vitamin D supplementation reduces insulin resistance in South Asian women living in New Zealand who are insulin resistant and vitamin D deficient-a randomised, placebo-controlled trial. British Journal of Nutrition. 2010;103(4):549–555. doi: 10.1017/S0007114509992017. [DOI] [PubMed] [Google Scholar]
- 41.Ford E. S., Ajani U. A., McGuire L. C., Liu S. Concentrations of serum vitamin D and the metabolic syndrome among U.S. adults. Diabetes Care. 2005;28(5):1228–1230. doi: 10.2337/diacare.28.5.1228. [DOI] [PubMed] [Google Scholar]
- 42.Brenner D. R., Arora P., Garcia-Bailo B., et al. Plasma vitamin D levels and risk of metabolic syndrome in Canadians. Clinical and Investigative Medicine. 2011;34(6):E377–E384. doi: 10.25011/cim.v34i6.15899. [DOI] [PubMed] [Google Scholar]
- 43.Kayaniyil S., Vieth R., Retnakaran R., et al. Association of vitamin D with insulin resistance and beta-cell dysfunction in subjects at risk for type 2 diabetes. Diabetes Care. 2010;33(6):1379–1381. doi: 10.2337/dc09-2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Baier L. J., Dobberfuhl A. M., Pratley R. E., Hanson R. L., Bogardus C. Variations in the vitamin D-binding protein (Gc locus) are associated with oral glucose tolerance in nondiabetic Pima Indians. The Journal of Clinical Endocrinology and Metabolism. 1998;83(8):2993–2996. doi: 10.1210/jc.83.8.2993. [DOI] [PubMed] [Google Scholar]
- 45.McDermott M. F., Ramachandran A., Ogunkolade B. W., et al. Allelic variation in the vitamin D receptor influences susceptibility to IDDM in Indian Asians. Diabetologia. 1997;40(8):971–975. doi: 10.1007/s001250050776. [DOI] [PubMed] [Google Scholar]
- 46.Pani M. A., Knapp M., Donner H., et al. Vitamin D receptor allele combinations influence genetic susceptibility to type 1 diabetes in Germans. Diabetes. 2000;49(3):504–507. doi: 10.2337/diabetes.49.3.504. [DOI] [PubMed] [Google Scholar]
- 47.Malecki M. T., Klupa T., Wolkow P., Bochenski J., Wanic K., Sieradzki J. Association study of the vitamin D: 1Alpha-hydroxylase (CYP1alpha) gene and type 2 diabetes mellitus in a Polish population. Diabetes and Metabolism. 2003;29(2):119–124. doi: 10.1016/s1262-3636(07)70017-4. [DOI] [PubMed] [Google Scholar]
- 48.García-Bailo B., Jamnik J., Da Costa L. A., Badawi A., El-Sohemy A. Genetic variation in the Vitamin D receptor, plasma 25-hydroxyvitamin D, and biomarkers of cardiometabolic disease in caucasian young adults. Journal of Nutrigenetics and Nutrigenomics. 2013;6(4-5):256–267. doi: 10.1159/000354729. [DOI] [PubMed] [Google Scholar]
- 49.Cade C., Norman A. W. Vitamin D3 improves impaired glucose tolerance and insulin secretion in the vitamin D-deficient rat in vivo. Endocrinology. 1986;119(1):84–90. doi: 10.1210/endo-119-1-84. [DOI] [PubMed] [Google Scholar]
- 50.Beaulieu C., Kestekian R., Havrankova J., Gascon-Barre M. Calcium is essential in normalizing intolerance to glucose that accompanies vitamin D depletion in vivo. Diabetes. 1993;42(1):35–43. doi: 10.2337/diab.42.1.35. [DOI] [PubMed] [Google Scholar]
- 51.Teegarden D., Donkin S. S. Vitamin D: emerging new roles in insulin sensitivity. Nutrition Research Reviews. 2009;22(1):82–92. doi: 10.1017/s0954422409389301. [DOI] [PubMed] [Google Scholar]
- 52.Bischoff H. A., Borchers M., Gudat F., et al. In situ detection of 1,25-dihydroxyvitamin D3 receptor in human skeletal muscle tissue. The Histochemical Journal. 2001;33(1):19–24. doi: 10.1023/a:1017535728844. [DOI] [PubMed] [Google Scholar]
- 53.Ashraf A., Alvarez J. A. Role of vitamin D in insulin secretion and insulin sensitivity for glucose homeostasis. International Journal of Endocrinology. 2010;2010:18. doi: 10.1155/2010/351385.351385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Norman A. W. Minireview: vitamin D receptor: new assignments for an already busy receptor. Endocrinology. 2006;147(12):5542–5548. doi: 10.1210/en.2006-0946. [DOI] [PubMed] [Google Scholar]
- 55.Li J., Byrne M. E., Chang E., et al. 1α,25-Dihydroxyvitamin D hydroxylase in adipocytes. The Journal of Steroid Biochemistry and Molecular Biology. 2008;112(1–3):122–126. doi: 10.1016/j.jsbmb.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ogunkolade B.-W., Boucher B. J., Prahl J. M., et al. Vitamin D Receptor (VDR) mRNA and VDR protein levels in relation to vitamin D status, insulin secretory capacity, and VDR genotype in Bangladeshi Asians. Diabetes. 2002;51(7):2294–2300. doi: 10.2337/diabetes.51.7.2294. [DOI] [PubMed] [Google Scholar]
- 57.Forouhi N. G., Luan J., Cooper A., Boucher B. J., Wareham N. J. Baseline serum 25-hydroxy vitamin d is predictive of future glycemic status and insulin resistance: the medical research council ely prospective study 1990-2000. Diabetes. 2008;57(10):2619–2625. doi: 10.2337/db08-0593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pinelli N. R., Jaber L. A., Brown M. B., Herman W. H. Serum 25-hydroxy vitamin D and insulin resistance, metabolic syndrome, and glucose intolerance among Arab Americans. Diabetes Care. 2010;33(6):1373–1375. doi: 10.2337/dc09-2199. [DOI] [PMC free article] [PubMed] [Google Scholar]


