Abstract
Background. Buruli ulcer (BU) is a necrotizing cutaneous infection caused by Mycobacterium ulcerans. Early diagnosis is crucial to prevent morbid effects and misuse of drugs. We review developments in laboratory diagnosis of BU, discuss limitations of available diagnostic methods, and give a perspective on the potential of using aptamers as point-of-care. Methods. Information for this review was searched through PubMed, web of knowledge, and identified data up to December 2015. References from relevant articles and reports from WHO Annual Meeting of the Global Buruli Ulcer initiative were also used. Finally, 59 articles were used. Results. The main laboratory methods for BU diagnosis are microscopy, culture, PCR, and histopathology. Microscopy and PCR are used routinely for diagnosis. PCR targeting IS2404 is the gold standard for laboratory confirmation. Culture remains the only method that detects viable bacilli, used for diagnosing relapse and accrued isolates for epidemiological investigation as well as monitoring drug resistance. Laboratory confirmation is done at centers distant from endemic communities reducing confirmation to a quality assurance. Conclusions. Current efforts aimed at developing point-of-care diagnostics are saddled with major drawbacks; we, however, postulate that selection of aptamers against MU target can be used as point of care.
1. Introduction
Buruli ulcer disease (BUD) is a neglected tropical disease caused by the environmental pathogen Mycobacterium ulcerans (MU). The disease is characterized by necrotizing, ulcerative lesions of subcutaneous fat and the overlying skin and is prevalent in poor regions of Africa, the Americas, Asia, and the Western Pacific [1]. The exact mode of transmission of MU remains unclear, but accruing data suggests that, probably, different modes of transmission occur in different geographic areas and epidemiological settings [2]. BUD begins with a preulcerative stage characterized by a firm nontender nodule, edema, or plaque with large areas of indurated skin, which is then followed by ulceration due to extensive skin cell destruction leading to the typically undermined edges [3, 4]. If left untreated, self-healing may occur which often leads to loss of vital organs and contractures. Even though mortality is low, morbidity and subsequent functional disability can be severe [5–8]. The main virulence factor responsible for the pathology of BUD is mycolactone. Mycolactone, an immunosuppressive and cytotoxic macrocyclic polyketide, is widely distributed within infected human lesions and has been postulated as a marker for diagnosis of BUD [9]. The social and economic burden of BUD can be high, particularly in impoverished rural regions. The disease affects both sexes equally and all age groups, but it is particularly common in children under the age of 15 [10].
Previously, BUD was treated by wide surgical excision followed by skin grafting; however, a study initiated by WHO and conducted in Ghana indicated that BU lesions can be sterilized by treatment with streptomycin and rifampicin [11]. Following that, the mainstay treatment protocol for BU is daily oral rifampicin plus intramuscular injection of streptomycin for 56 days, reducing surgery as an adjunct for correction of deformities [3, 12]. With the introduction of this antimycobacterial treatment, confirmation of clinically suspected cases is even more crucial for the clinical management of BU to prevent misdiagnosis and hence administration of unnecessary antibiotics. Previous reports of individuals treated for BU but were later found not to be BU by laboratory confirmation are available in literature [13–15].
Laboratory diagnosis of BU is multifaceted and has evolved over the years. There are currently four main methods that are being used for the laboratory confirmation of BUD and include microscopy for detecting acid-fast bacilli, culture to isolate viable organism, PCR for detecting pathogen specific DNA which is usually IS2404, and histopathology. The WHO recommends two laboratory tests to confirm BUD. However, in endemic settings, one may consider one positive test result from PCR or microscopy appropriate for the confirmation of clinical diagnosis because of the high positive predictive values for PCR (100%) and microscopy (97%) [3, 16]. In this review, we describe developments in the field of laboratory diagnosis of BUD, discuss applications and limitations of currently available diagnostic methods, and provide data on positivity and sensitivity ratios. This review further gives a perspective on the potential of selecting aptamers against MU targets for the development of a point-of-care diagnostics for BUD.
2. Methods
2.1. Search Strategy and Selection Criteria
Searched information for this review was done through PubMed, web of knowledge and Embase databases, and identified data up to December, 2015. References from relevant articles together with other published data from the WHO website and unpublished data presented at annual WHO advisory group meetings on Buruli ulcer were also used. The literature search was done using the following keywords: Mycobacterium ulcerans, laboratory diagnosis and confirmation, and methods for BU diagnosis and BU.
2.2. Assessment and Data Extraction
Articles in the full-text review were classified as containing original laboratory diagnostic methods for Buruli ulcer including sample collection methods, microscopy, culture, molecular techniques (PCR and its offshoots), and histopathology. Figure 1 illustrates how the review articles were searched and selected.
Figure 1.
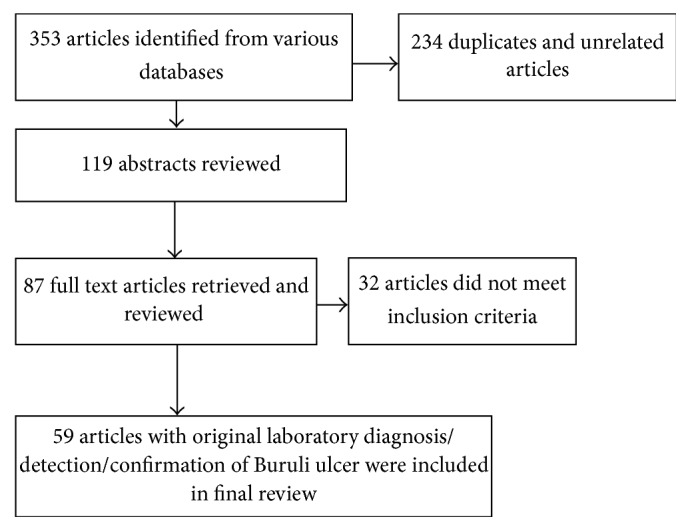
Schematic selection of review articles.
3. Results and Discussion
3.1. Samples for Laboratory Confirmation of Buruli Ulcer
Samples for laboratory diagnosis of BUD include swabs and tissue specimens [17] from punch biopsies, surgical excision [18], and fine needle aspirates (FNA) [19]. FNA and tissues are used for analysis of nonulcerative lesions, whilst all other specimen types can be collected from ulcerative tissues [20]. However, with the advent of chemotherapy, FNA and swabs are becoming the preferred sample for laboratory confirmation. Recommendations for sample collection include the following: (1) swabs should be collected by circling the entire undermined edge of ulcerative lesions to maximize cell collection as MU is not uniformly distributed in the ulcers [21]. A good sample collection can be achieved through collection of at least two swabs per lesion; (2) FNA should be collected from the weakest part of the lesion to increase the chance of collecting MU cells; and (3) tissue samples from ulcerative lesions should be taken from the edge of the lesion, preferably below the end of the undermined edge, and should contain necrotic tissue. For nonulcerative lesions, tissue samples should be collected from the center of the lesion. Tissue samples must always contain subcutaneous adipose tissue. All the samples including FNA should be evaluated by microscopy, PCR, and cultivation [22, 23]. Laboratory confirmation of osteomyelitis cases requires whole bone samples (e.g., from amputation specimens) or curetted bone samples [17, 24, 25]. Table 1 summarizes the various types of specimen and transport media used for diagnosing BUD.
Table 1.
Summary of types of specimen and transport media for BU diagnosis.
| Materials for diagnosis | Types | Country of origin | Reference |
|---|---|---|---|
| Specimen | Swabs | Ghana | Yeboah-Manu et al. [26]; de Souza et al. [27] |
| Togo | Bretzel et al. [28] | ||
| Punch biopsy | Ghana | de Souza et al. [27]; Phillips et al. [29] | |
| Australia | O'Brien et al. [30] | ||
| Togo | Bretzel et al. [28] | ||
| Benin | Ruf et al. [31] | ||
| Biopsy | Ghana | Stienstra et al. [32] | |
| Fine needle aspirate | Ghana | Ablordey et al. [33]; Yeboah-Manu et al. [26] | |
| Togo | Bretzel et al. [28] | ||
| Benin | Eddyani et al. [19] | ||
| Whole bone or curetted bone samples | Ghana | Herbinger et al. [17]; Bretzel et al. [24] | |
|
| |||
| Transport media | Modified Dubos medium (P5 medium) | Ghana | Stienstra et al. [32]; Yeboah-Manu et al., [34] |
| Liquid Middlebrook 7H9 broth | Benin | Eddyani et al. [19]; Dobos et al. [35]; | |
| 10% OADC augmented with PANTA | Ghana | Wansbrough-Jones and Phillips [9] | |
| Solid transport media (STM) | Benin | Eddyani et al. [19] | |
| Liquid Nitrogen | Ghana | Rondini et al. [21]; Beissner et al. [36] | |
|
| |||
| Decontamination methods | Oxalic acid | Ghana | Mensah-Quainoo et al. [37]; Yeboah-Manu et al. [34] |
| N-Acetyl-cysteine-NaOH technique | Ghana | Schunk et al. [8] | |
| Reversed Petroff technique | Ghana | O'Brien et al. [30] | |
| Benin | Eddyani et al. [19] | ||
|
| |||
| DNA extraction method | Commercial | Ghana | de Souza et al. [27] |
| In-house | Ghana | Ablordey et al. [33] | |
| Modified Boom DNA extraction procedure | Ghana | Durnez et al. [38]; Affolabi et al. [39] | |
| Commercial Maxwell 16 DNA extraction | Ghana | Affolabi et al. [39] | |
| One tube cell lysis (OT) | Ghana | Durnez et al. [38] | |
| FastPrep procedure | Ghana | Durnez et al. [38] | |
3.2. Microscopy
Microscopy is a quick, comparatively simple, and low-cost approach for the laboratory confirmation of suspected BUD cases and can be done with FNA, tissue, or swabs specimen. Microscopic diagnoses by direct smear examination with Ziehl-Neelsen staining to detect the presence of acid-fast bacilli are done using the quantification of smears in accordance with the method locally used for the diagnosis of TB [25]. The technological simplicity and requirement of low infrastructure allow microscopy to be conducted at all levels of health care delivery, even in less resourced countries. However, recorded sensitivity in literature is quite low and therefore undermines the overreliance of microscopy for case confirmation. Studies in Ghana and Benin which used microscopy as a first-line diagnosis of BU reported positivity rates between 40% and 78% [24, 37, 40].
Tissue smears prepared from ground samples can also be used for microscopy as well as from material hitherto subjected to decontamination procedures for culture. Nevertheless, according to a recent study in Benin, grounding of tissue does not increase the sensitivity of tissue smears (56.7%) compared with direct smears prepared from unground tissue (sensitivity, 59.4%) [40]. Whilst ZN staining is used in most of the studies, some other studies have suggested that Kinyoun and auramine-rhodamine staining techniques can also be applied to MU [8, 40].
3.3. Cultivation of Mycobacterium ulcerans from Clinical Specimen
Isolation of viable MU by culture is the final proof method among the diagnostics; however, due to the technological and infrastructure demand such as biosafety cabinets, cultures are done mainly at research centers of endemic and northern countries. Cultivation of MU from swabs and punch biopsies is normally transported in Middlebrook 7H9 broth supplemented with polymyxin B, azlocillin, amphotericin B, nalidixic acid, and trimethoprim (PANTA, Becton Dickinson Biosciences, NJ, USA). Additional supplementation with 0.5% agar yields a semisolid transport medium (STM) and preserves positive samples for up to 21 days [25, 41]. Although a number of culture media have been evaluated [34, 42, 43], Lowenstein-Jensen is considered the most appropriate medium for MU [42, 44]. Cultures are typically positive within 9–12 weeks of incubation at 29–33°C. Yet still, longer incubation times of up to 9 months have been observed [8]. Culturing MU from clinical samples is difficult and has a low sensitivity of about 35–60% [45]. The bacteria are extremely slow growing (6–8 weeks) and culture media are repeatedly contaminated with other faster growing species [7, 12, 26, 46]. This makes cultures unsuitable for quick laboratory confirmation and is limited to laboratory facilities with class II safety cabinets. The contamination effect of fast growing species are, however, counteracted by decontaminating the sample with either an acid and or a base to remove the unwanted fast growers using protocols such as the modified Petroff method (sodium hydroxide) [8], and the reversed Petroff technique (“Fortep” technique) [44]. In a decontamination protocol study conducted in Ghana, three different decontamination procedures were evaluated and concluded that a simple oxalic acid decontamination method produces high recovery rates [26, 34].
Notwithstanding these drawbacks, cultures are considered the only currently available valid confirmatory test for detection of viable bacilli in clinically suspected relapses and patients with nonhealing lesions after antimycobacterial treatment [24]. Furthermore, cultures are required for speciation, susceptibility testing, and other downstream applications [41]. Culture positivity ratios of 3–80% and sensitivities of 45–70% have been reported [6, 37, 46, 47]. The isolation of acid-fast bacilli from BUD patients alone does not offer adequate proof of the presence of MU. A cohort study in Ghana, indicated that a number of patients harbor other nontuberculous mycobacteria [37]. It is thus imperative that a confirmation of cultured isolates should be done. The main methods that have been used for isolate confirmation include sequence analysis and/or PCR detection of the insertion sequences IS2404, IS2606, ketoreductase gene of the giant plasmid, rpoB gene, the 16S–23S ribosomal RNA (rRNA) internal transcribed spacer gene, the 16S rRNA gene, VNTR, and the 65-kDa hsp gene, [17, 48–51].
3.4. Histopathology
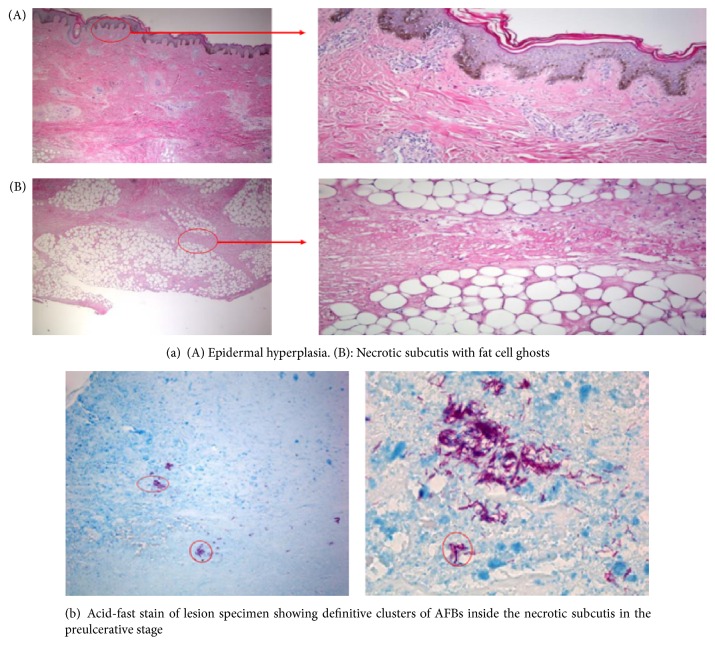
Histopathology as a diagnostic method for BUD provides a fairly rapid result with a very high sensitivity (about 90%) [25]. It is also useful in establishing differential diagnosis and monitoring response to treatment. Histopathological analysis is carried out on tissue specimens in 10% neutral or buffered (pH 7.4) formalin stained with hematoxylin and eosin, Ziehl-Neelsen, or Kinyoun, and auramine-rhodamine. Distinctive histopathological features of BUD comprise the presence of acid-fast bacilli, (AFB) hyperplasia of the epidermis, elastolysis, inflammation, vascular variations of the dermis, and fat necrosis of the subcutis [25, 44]. In nonulcerated lesions, the epidermis is unbroken but hyperplastic. The upper dermis is intact but shows several stages of degeneration with infiltration of inflammatory cells. There is also clotting necrosis of the lower dermis, subcutaneous tissue, and underlying fascia with oedema. Vasculitis is common in the subcutaneous tissue. The ZN stain reveals large numbers of extracellular AFB in clusters, confined to the necrotic areas. In ulcerative lesions, ulcers are undermined with reepithelialization of the edges of the lesion and undersurface of the superimposing flap of the dermis. Neighboring epidermis is usually hyperplastic with AFB located at the base of the central slough and necrotic subcutaneous tissue [25]. Many studies have suggested that histopathology can identify about 30% additional cases than other confirmatory tests combined, mainly from paucibacillary late or healing stages of the disease [20, 24, 47, 52]. However, histopathological features cannot always provide clear-cut identification, as granulomas diffuse mixed cellular infiltrates and dense lymphocyte aggregates in the locality of vessels during antibiotic treatment [53]. Moreover, the method is expensive to perform and requires a sophisticated laboratory and highly trained personnel. Furthermore, the technique is invasive as it requires 3 mm to 4 mm in diameter punch biopsies. Figure 2(a)(A and B) indicates epidermal hyperplasia and necrotic subcutis with fat cell ghost, respectively, whilst Figure 2(b) indicates acid-fast stain of lesion specimen showing characteristic clusters of AFB in the preulcerative stage.
Figure 2.
Histopathological images of Buruli ulcer disease.
3.5. Polymerase Chain Reaction (PCR)
Polymerase chain reaction (PCR) methods have been developed for BU diagnosis based on the insertion sequence IS2404 [54], 16S rRNA gene [45], and the hsp-65 gene [55]. The most routinely used PCR methods are conventional single-step gel-based PCR and real-time PCR targeting the insertion element IS2404. The insertion sequence IS2404 is present in high copy numbers in the MU genome and it is considered as the gold standard because it has the highest sensitivity [56] and results are accessible within a short time. A positive PCR result is considered sufficient evidence to commence antimycobacterial treatment; moreover, real-time PCR is being considered for monitoring antimycobacterial treatment. However, the technique is expensive, requires sophisticated laboratory, and expertise, a strict quality control, and does not distinguish between viable and nonviable organism [3, 57]. A WHO report further encourages endemic countries to confirm at least 50% of all cases of PCR, either locally or with an external PCR reference laboratory [16].
DNA extraction is a crucial step in PCR processes and different methods involving in-house as well as commercial kits are being used. Methods involving mechanical homogenization in a digestion buffer followed by proteinase K digestion and purification by the guanidinium thiocyanate-diatoms methods have been applied successfully [29, 58]. Durnez et al. compared two adapted extraction methods, the modified Boom (MB) DNA extraction procedure with a commercial Maxwell® 16 DNA extraction procedure (M16, Promega, WI, USA), based on enzymatic lysis and paramagnetic separation, and demonstrated the superiority of the MB in terms of IS2404 PCR sensitivity with clinical samples [38]. Another study compared semiautomated DNA extraction method using Maxwell kit with a modified Boom method and observed that Maxwell extraction method, performed on nondecontaminated suspensions, is the best for the molecular diagnosis of MU [39]. Other promising methods include heat and alkaline lysis by NaOH and sodium dodecyl sulphate followed by phenol-chloroform purification [26, 34, 59]. Many commercially available kits particularly Gentra systems and Puregene Genomic DNA purification kits have successfully been used with proteinase k to extract DNA from swabs, FNA, and tissue samples [17, 24, 44, 47]. It is recommended that DNA extraction is performed in a separate area using dedicated reagents and equipment to reduce the possibility of contamination.
Samples for PCR can be processed within hours to a day without prior storage in transport media [8] or stored at −20°C until processing or stored in transport buffers which is compatible with the extraction method [29, 58]. Many studies used transport media enriched with OADC, supplemented with PANTA and 0.5% agar [22, 42, 48, 57, 60, 61]. Transport of samples in liquid nitrogen has also been reported [21], dried swabs are also being used for DNA extraction, and positive PCR has been achieved after two weeks. PCR can also be done on paraffin-embedded tissue specimens using xylene-based deparaffinization for 10 minutes at room temperature [16, 54].
The initial primer design used for detecting MU insertion sequence IS2404 was MU1 and MU2 for amplification of a 569 bp fragment. These primers were burdened with spurious banding and were improved with MU5 and MU6 primers which amplify the 492 bp fragment [54]. This was tested with a panel of 45 mycobacteria and other organisms and obtained 100% specificity and detection sensitivity of at least 0.1 genome equivalents [59, 62, 63]. Primers used in nested IS2404-based PCR include MU1 and MU2 for amplification of a 569 bp fragment of IS2404 and PGP3 and PGP4 for amplification of a 217 bp product [32, 57]. Primers PU4F and PU7Rbio with a modified PCR protocol for amplification of a 154 bp product of IS2404 have also been described [29, 58]. For real-time PCR, TaqMan primer sequences are mostly used.
Most endemic countries are tropical and hence the development of a dry reagent based PCR (DRB-PCR) which uses lyophilized reagents (PuReTaq Ready-To-Go-Beads, Amersham, UK) and primers have been employed to simplify the process and reduce incidence of false positives [47, 56] and requirement for elaborate infrastructure for PCR. Specific real-time PCR assay allows quantitative valuation and distribution of MU in BUD lesions and has exhibited much higher sensitivity than the conventional single-run gel-based IS2404 PCR. Moreover, the enhanced TaqMan real-time PCR assay shows 12.5% higher diagnostic sensitivity compared with cultures; the assay reduces contamination and turnaround times for diagnosis and has been used routinely in Australia [61, 64]. Fyfe et al. developed two TaqMan Multiplex real-time PCR assays targeting three independent repeated sequences in the M. ulcerans genome, two multicopy insertion sequences (IS2404, IS2606), and a multicopy sequence encoding the ketoreductase B domain (KR-B) [22]. Affolabi et al. compared a single-step PCR, a nested PCR, and a real-time quantitative PCR on 74 surgical specimens from patients with clinically suspected Buruli ulcer and observed that real-time PCR after the modified Boom extraction method and a single-run PCR assay after the Maxwell extraction method, performed on nondecontaminated suspensions, are the best for the molecular diagnosis of BUD [39]. Guimaraes-Peres et al. assessed two nested PCRs, the nested IS2404-based PCR and the nested 16S rRNA gene-based PCR, and observed that the 16S rRNA gene-based PCR was positive for both MU and M. marinum; they suggested that the use of IS2404-based PCR showed better specificity, required less time, and was less costly than the 16S rRNA gene-based PCR [57]. Stienstra et al. also evaluated the IS2404-based nested PCR to detect MU from 143 BUD patients in Ghana. They further compared it with culture and histopathology results and recommended that small tissue samples might be sufficient for case confirmation in future studies [32]. Phillips et al. also used IS2404 PCR with punch biopsy specimen and obtained a positivity ratio of 98% from 70 clinically diagnosed BUD patients [29]. Among 162 clinically diagnosed BUD patients with ulcerative lesions from Cameroon, 83% were confirmed by IS2404 PCR [32]. In another study in Democratic Republic of Congo, IS2404 PCR was used to diagnose 51 BUD patients with positivity ratio of 75% [6]. In a similar study in Ghana, DRB-PCR was used to clinically confirm 67% out of a cohort of 161 BUD patients. In this study, the positivity ratio for swab samples was 66%; analysis of tissue samples produced 57% positive results for ulcerative and 63% for nonulcerative lesions [24]. In another cohort study of 230 clinically diagnosed BUD patients from Ghana, DRB-PCR positivity ratios of 61% were determined for both swab and tissue samples [47].
In a related study in Togo, out of 202 suspected BUD cases, 109 BUD patients (54%) were PCR confirmed over a period of three years [28]. These findings indicate that PCR is considered the most sensitive method for the laboratory confirmation of BUD; however, protracted persistence of mycobacterial DNA in patients on antimycobacterial treatment makes PCR not applicable for monitoring of treatment success [17].
In an attempt to overcome the drawback of PCR, Beissner et al. developed a MU specific RNA-based viability assay combining a 16S rRNA reverse transcriptase real-time PCR (RT-qPCR) to determine bacterial viability with an IS2404 quantitative real-time PCR (qPCR) for increased specificity and concurrent quantification of bacilli [36]. This technique has previously been applied for the detection of viable mycobacteria in patients with tuberculosis and leprosy [65, 66]. Conversely, the current test format requires well equipped laboratory with real-time PCR facilities and the costs per test limit its applicability. The reliance on PCR for diagnostic and research purposes in the field of BU requires the continued demonstration of its accuracy, reliability, and reproducibility. To this effect, Eddyani et al. established a multicenter external quality assessment program for PCR detection of BUD in clinical and environmental samples and reported an improved performance among participating laboratories [67].
3.6. Diagnostic Methods in Development
There is the need for simpler diagnostic that is both sensitive and specific and can be used at the point of care. The loop mediated isothermal amplification (LAMP) technique has previously been evaluated in many diseases, including malaria, and has been employed. The reported protocol employs four sets of primers, targeting sequences of the mycolactone encoding plasmid [27]. To overcome the requirement of cold-chains for transport and storage of reagents, Beissner et al. [68] recently establish a dry-reagent-based LAMP (DRB-LAMP) assay employing lyophilized reagents and clinically validated 140 clinical samples from 91 suspected BUD cases by routine assays, that is, IS2404 dry-reagent-based (DRB) PCR, conventional IS2404 PCR (cPCR), and IS2404 qPCR, compared to cLAMP. Case confirmation and positivity rates of DRB-PCR or cPCR and cLAMP (62.64% and 52.86%) were comparable and there was no significant difference between the sensitivity of the assays (DRB-PCR and cPCR, 86.76%; cLAMP, 83.82%). Moreover, the sensitivity of cLAMP (95.83%) and the sensitivity of DRB-LAMP (91.67%) were comparable. However, all the reported studies used sophisticated equipment which cannot be employed in the field and there is the need for further work to use simpler equipment in low-resourced laboratory settings; moreover, obtaining purified DNA, as well as generating isothermal conditions, remains a major challenge for the use of the LAMP method under field conditions [33].
Another approach has been serological assays; however, currently available identified MU specific antigens such as the one detecting 85kda protein cannot differentiate between BU patients and exposed control individuals [69–71]. MUL-3720 protein has been identified as a promising target for antigen capture-based detection assays. It is highly expressed by MU and has no orthologs in other pathogenic mycobacteria. However, quest to use anti-MUL_3720 antibodies in a sandwich-ELISA format was found to be of insufficient sensitivity to make it suitable for the development of antigen capture-based diagnostic tests [72]. Thin layer chromatography for detecting mycolactone in clinical specimen has also been employed. TLC is comparatively simple but can be complicated by the presence of other lipids in the specimen. This step was informed by a study that demonstrated the presence of intact mycolactone in punch biopsies before and during antibiotic therapy using thin layer chromatography and mass spectrophotometry [73]. The group further provided proof of concept that indicated assays based on mycolactone detection in serum and ulcer exudates can form the basis of BU diagnostic tests. Fluorescent TLC had sensitivity of 73.2% and specificity of 85.7% when compared with PCR [68, 74]. A method using a boronate-assisted fluorogenic chemosensor in TLC was employed by Converse et al., to selectively detect mycolactone when visualized under UV light. They concluded that F-TLC may offer a new tool for confirmation of suspected clinical lesions and may be more specific than smear microscopy, faster than culture, and simpler than PCR [75]. Recently, Wadagni and colleagues evaluated fluorescent thin layer chromatography (fTLC) for detection of mycolactone in skin samples from patients with Buruli ulcer and compared them with samples from non-Buruli ulcer lesions that gave a negative result in the standard PCR test for MU [76]. However, further studies are needed to determine the feasibility of detecting mycolactone from samples obtained routinely. Table 2 summarizes the various diagnostic techniques and their positivity ratios.
Table 2.
Summary of various diagnostic techniques for BU.
| Techniques | Number | +ve | −ve | Positivity ratio (%) | Geographic origin | Reference |
|---|---|---|---|---|---|---|
| Microscopy | 39 | 23 | 16 | 58.9% | Australia | O'Brien et al. [30] |
| 31 | 7 | 24 | 22.5% | Benin | ||
| 202 | 43 | 159 | 21.3% | Togo | Bretzel et al. [28] | |
| 24 | 11 | 13 | 45.8% | Ghana | Beissner et al. [36] | |
| 99 | 78 | 21 | 78.8% | Ghana | Mensah-Quainoo et al. [37] | |
| 41 | 32 | 9 | 78.0% | Ghana | Yeboah-Manu et al. [34] | |
| 44 | 15 | 29 | 34.1% | Ghana | Rondini et al. [21] | |
| 65 | 19 | 46 | 29.2% | Benin/Ghana | Guimaraes-Peres et al. [57] | |
| 164 | 38 | 126 | 23.2% | Cameroon | Noeske et al. [60] | |
| 36 | 22 | 14 | 61.1% | DRC | Phanzu et al. [6] | |
| 94 | 28 | 66 | 29.8 | Ghana | Bretzel et al. [28] | |
|
| ||||||
| Culture | 33 | — | 33 | — | Australia | O'Brien et al. [30] |
| 143 | 56 | 87 | 39.2% | Ghana | Stienstra et al. [32] | |
| 41 | 32 | 9 | 78.0% | Ghana | Yeboah-Manu et al. [34] | |
| 97 | 77 | 20 | 79.4% | Ghana | Mensah-Quainoo et al. [37] | |
| 65 | 22 | 43 | 33.8% | Benin/Ghana | Guimaraes-Peres et al. [57] | |
|
| ||||||
| Histopathology | 12 | 12 | — | 100.0% | Benin | Ruf et al. [31] |
| 143 | 78 | 65 | 54.5% | Ghana | Stienstra et al. [32] | |
| 36 | 27 | 9 | 75.0% | DRC | Phanzu et al. [6] | |
|
| ||||||
| IS2404 PCR | 30 | 21 | 9 | 70.0% | Ghana | Ablordey et al. [33] |
| 26 | 23 | 3 | 88.5% | Australia | O'Brien et al. [30] | |
| 143 | 107 | 36 | 74.8% | Ghana | Stienstra et al. [32] | |
| 202 | 109 | 93 | 54.0% | Togo | Bretzel et al. [28] | |
| 24 | 18 | 6 | 75.0% | Ghana | Beissner et al. [36] | |
| 65 | 55 | 10 | 84.6% | Benin/Ghana | Guimaraes-Peres et al. [57] | |
| 162 | 135 | 27 | 83.3% | Cameroon | Noeske et al. [60] | |
| 36 | 27 | 9 | 75.0% | DRC | Phanzu et al. [6] | |
| 94 | 62 | 32 | 66.0% | Ghana | Bretzel et al. [28] | |
|
| ||||||
| DRB-PCR | 230 | 139 | 91 | 60.6% | Ghana | Siegmund et al. [47] |
|
| ||||||
| Real-time qPCR | 18 | 15 | 3 | 83.3% | Ghana | Beissner et al. [36] |
| 44 | 29 | 15 | 65.9% | Ghana | Rondini et al. [21] | |
| 74 | 44 | 30 | 59.5% | Benin | Affolabi et al. [39] | |
|
| ||||||
| Nested PCR | 21 | 21 | 0 | 100.0% | Ghana | Stienstra et al. [32] |
| 65 | 52 | 13 | 80.0% | Benin/Ghana | Guimaraes-Peres et al. [57] | |
| 74 | 33 | 41 | 44.6% | Benin | Affolabi et al. [39] | |
|
| ||||||
| Others | ||||||
|
| ||||||
| LAMP assay | 20 | 6 | 14 | 30.0% | Ghana | de Souza et al. [27] |
| 30 | 9 | 21 | 30.0% | Ghana | Ablordey et al. [33] | |
| 20 | 13 | 7 | 65% | Ghana | de Souza et al. [27] | |
|
| ||||||
| TLC | 10 | 5 | 5 | 50.0% | Ghana | Sarfo et al. [73] |
|
| ||||||
| Serology | 61 | 43 | 18 | 70.5% | Ghana | Dobos et al. [35] |
|
| ||||||
| Faecal | 67 | 0 | 67 | 0.0% | Ghana | Sarfo et al. [74] |
4. Conclusion and Future Perspective
Molecular techniques for the diagnosis of BUD have proven to be effective. Notably, real-time PCR offers a consistent quantitative and rapid tool for diagnosis and can be used for monitoring of treatment response of BUD. The development and application of reverse transcriptase PCR assays for the detection of viable MU would provide a valuable alternative for conventional mycobacterial cultures and thus considerably improve the clinical management of BUD. Culture remains the only method that detects viable bacilli. However, low sensitivity, long generation time and failure to distinguish between MU and other mycobacterial infections without extra confirmatory diagnostic tools, makes cultures unsuitable to support clinical management decisions timely. Furthermore, the application of molecular species identification assays, such as internal transcribed spacer length polymorphism or PCR restriction analysis of partial rpoB or hsp-65 genes [45, 55, 56, 63], would allow the distinction of MU from other nontuberculous mycobacteria. Most of these DNA-based techniques are present only in referenced and specialized centers. Conscious efforts should be channeled towards the formation of multicenter collaborative research programs. This will ensure reliability and reproducibility of test results and further allow validation, refinement, and adjustment of the application of molecular tools to specific clinical and epidemiological questions. The nonimmunogenic nature of mycolactone and other MU proteins have thwarted effort for serological assays. A general statement with respect to the performance of the various tests is not feasible since the positivity and sensitivity ratios are influenced by the quality of clinical diagnosis, duration of disease, pretreatment history of BUD patients, type and quality of diagnostic specimen and the duration of transport to the laboratory and transport conditions. It is evidenced that all currently available BU diagnostic techniques cannot be used as point of care and the need for a diagnostic test that can be used in the field cannot be overemphasized. Experimental studies on the use of aptamers against MU diagnostic target like mycolactone could be the key to the development of a point of care for BUD.
Acknowledgments
The authors gratefully thank Therese Ruf of Swiss TPH and Dorothy Yeboah-Manu for granting us permission to use their histopathology slides and Mr. Enoch Odame for providing technical assistance.
Competing Interests
Authors declare that there are no competing interests.
References
- 1.Amofah G., Bonsu F., Tetteh C., et al. Buruli ulcer in Ghana: results of a national case search. Emerging Infectious Diseases. 2002;8(2):167–170. doi: 10.3201/eid0802.010119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Johnson P. D. R., Stinear T., Small P. L. C., et al. Buruli ulcer (M. ulcerans infection): new insights, new hope for disease control. PLoS Medicine. 2005;2(4, article e108) doi: 10.1371/journal.pmed.0020108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.WHO. Buruli ulcer: progress report, 2004–2008. Weekly Epidemiological Record. 2008;83(17):145–154. [Google Scholar]
- 4.van der werf T. S., van der Graaf W. T. A., Tappero J. W., Asiedu K. Mycobacterium ulcerans infection. The Lancet. 1999;354(9183):1013–1018. doi: 10.1016/s0140-6736(99)01156-3. [DOI] [PubMed] [Google Scholar]
- 5.Ellen D. E., Stienstra Y., Teelken M. A., Dijkstra P. U., Van Der Graaf W. T. A., Van Der Werf T. S. Assessment of functional limitations caused by Mycobacterium ulcerans infection: towards a Buruli ulcer functional limitation score. Tropical Medicine & International Health. 2003;8(1):90–96. doi: 10.1046/j.1365-3156.2003.00976.x. [DOI] [PubMed] [Google Scholar]
- 6.Phanzu D. M., Bafende E. A., Dunda B. K., et al. Mycobacterium ulcerans disease (Buruli ulcer) in a rural hospital in Bas-Congo, Democratic Republic of Congo, 2002–2004. The American Journal of Tropical Medicine and Hygiene. 2006;75(2):311–314. [PubMed] [Google Scholar]
- 7.WHO. Buruli Ulcer: Prevention of Disability (POD). Manual for Health Care Providers. Geneva, Switzerland: WHO; 2006. [Google Scholar]
- 8.Schunk M., Thompson W., Klutse E., et al. Outcome of patients with buruli ulcer after surgical treatment with or without antimycobacterial treatment in Ghana. The American Journal of Tropical Medicine and Hygiene. 2009;81(1):75–81. [PubMed] [Google Scholar]
- 9.Wansbrough-Jones M., Phillips R. Buruli ulcer: emerging from obscurity. The Lancet. 2006;367(9525):1849–1858. doi: 10.1016/s0140-6736(06)68807-7. [DOI] [PubMed] [Google Scholar]
- 10.Asiedu K., Scherpbier R., Raviglione M., editors. Buruli ulcer: Mycobacterium ulcerans Infection. Geneva, Switzerland: World Health Organization; 2000. (WHO/CDS/CPE/GBUI/2000.1). [Google Scholar]
- 11.Junghanss T., Boock A. U., Vogel M., Schuette D., Weinlaeder H., Pluschke G. Phase change material for thermotherapy of Buruli Ulcer: a prospective observational single centre proof-of-principle trial. PLoS Neglected Tropical Diseases. 2009;3(2, article e380) doi: 10.1371/journal.pntd.0000380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.WHO. Provisional Guidance on the Role of Specific Antibiotics in the Management of Mycobacterium Ulcerans Disease (Buruli ulcer). Manual for Health Care Providers. Geneva, Switzerland: WHO; 2004. [Google Scholar]
- 13.Evans M. R. W., Phillips R., Etuaful S. N., et al. An outreach education and treatment project in Ghana for the early stage of Mycobacterium ulcerans disease. Transactions of the Royal Society of Tropical Medicine and Hygiene. 2003;97(2):159–160. doi: 10.1016/s0035-9203(03)90105-2. [DOI] [PubMed] [Google Scholar]
- 14.Novales-Santa Coloma J., Navarrete-Franco G., Iribe P., López-Cepeda L. D. Ulcerative cutaneous mycobacteriosis due to mycobacterium ulcerans: report of two Mexican cases. International Journal of Leprosy and Other Mycobacterial Diseases. 2005;73(1):5–12. doi: 10.1489/1544-581X(2005)73[5:UCMDTM]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 15.Semret M., Koromihis G., Maclean J. D., Libman M., Ward B. J. Mycobacterium ulcerans infection (Buruli ulcer): First reported case in a traveler. The American Journal of Tropical Medicine and Hygiene. 1999;61(5):689–693. doi: 10.4269/ajtmh.1999.61.689. [DOI] [PubMed] [Google Scholar]
- 16.WHO. Meeting of the WHO Technical Advisory Group on Buruli ulcer. Geneva, Switzerland: WHO; 2008. [Google Scholar]
- 17.Herbinger K.-H., Adjei O., Awua-Boateng N.-Y., et al. Comparative study of the sensitivity of different diagnostic methods for the laboratory diagnosis of buruli ulcer disease. Clinical Infectious Diseases. 2009;48(8):1055–1064. doi: 10.1086/597398. [DOI] [PubMed] [Google Scholar]
- 18.Nienhuis W. A., Stienstra Y., Thompson W. A., et al. Antimicrobial treatment for early, limited Mycobacterium ulcerans infection: a randomised controlled trial. The Lancet. 2010;375(9715):664–672. doi: 10.1016/s0140-6736(09)61962-0. [DOI] [PubMed] [Google Scholar]
- 19.Eddyani M., Fraga A. G., Schmitt F., et al. Fine-needle aspiration, an efficient sampling technique for bacteriological diagnosis of nonulcerative Buruli ulcer. Journal of Clinical Microbiology. 2009;47(6):1700–1704. doi: 10.1128/JCM.00197-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beissner M., Herbinger K.-H., Bretzel G. Laboratory diagnosis of Buruli ulcer disease. Future Microbiology. 2010;5(3):363–370. doi: 10.2217/fmb.10.3. [DOI] [PubMed] [Google Scholar]
- 21.Rondini S., Horsfield C., Mensah-Quainoo E., Junghanss T., Lucas S., Pluschke G. Contiguous spread of Mycobacterium ulcerans in Buruli ulcer lesions analysed by histopathology and real-time PCR quantification of mycobacterial DNA. The Journal of Pathology. 2006;208(1):119–128. doi: 10.1002/path.1864. [DOI] [PubMed] [Google Scholar]
- 22.Fyfe J. A. M., Lavender C. J., Johnson P. D. R., et al. Development and application of two multiplex real-time PCR assays for the detection of Mycobacterium ulcerans in clinical and environmental samples. Applied and Environmental Microbiology. 2007;73(15):4733–4740. doi: 10.1128/aem.02971-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Portaels F. Laboratory Diagnosis of Buruli Ulcer: A Manual for Health-Care Providers. Geneva, Switzerland: World Health Organization; 2014. (WHO/HTM/NTD/IDM). [Google Scholar]
- 24.Bretzel G., Siegmund V., Nitschke J., et al. A stepwise approach to the laboratory diagnosis of Buruli ulcer disease. Tropical Medicine and International Health. 2007;12(1):89–96. doi: 10.1111/j.1365-3156.2006.01761.x. [DOI] [PubMed] [Google Scholar]
- 25.WHO. Diagnosis of Mycobacterium ulcerans Disease. Manual for Health Care Providers. Geneva, Switzerland: WHO; 2001. [Google Scholar]
- 26.Yeboah-Manu D., Danso E., Ampah K., Asante-Poku A., Nakobu Z., Pluschke G. Isolation of Mycobacterium ulcerans from swab and fine-needle-aspiration specimens. Journal of Clinical Microbiology. 2011;49(5):1997–1999. doi: 10.1128/JCM.02279-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.de Souza D. K., Quaye C., Mosi L., Addo P., Boakye D. A. A quick and cost effective method for the diagnosis of Mycobacterium ulcerans infection. BMC Infectious Diseases. 2012;12, article 8 doi: 10.1186/1471-2334-12-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bretzel G., Huber K. L., Kobara B., et al. Laboratory confirmation of buruli ulcer disease in togo, 2007–2010. PLoS Neglected Tropical Diseases. 2011;5(7, article e1228) doi: 10.1371/journal.pntd.0001228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Phillips R., Horsfield C., Kuijper S., et al. Sensitivity of PCR targeting the IS2404 insertion sequence of Mycobacterium ulcerans in an assay using punch biopsy specimens for diagnosis of buruli ulcer. Journal of Clinical Microbiology. 2005;43(8):3650–3656. doi: 10.1128/jcm.43.8.3650-3656.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.O'Brien D. P., Robson M., Friedman N. D., et al. Incidence, clinical spectrum, diagnostic features, treatment and predictors of paradoxical reactions during antibiotic treatment of Mycobacterium ulcerans infections. BMC Infectious Diseases. 2013;13(1, article 416) doi: 10.1186/1471-2334-13-416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ruf M.-T., Sopoh G. E., Brun L. V., et al. Histopathological changes and clinical responses of buruli ulcer plaque lesions during chemotherapy: a role for surgical removal of necrotic tissue? PLoS Neglected Tropical Diseases. 2011;5(9) doi: 10.1371/journal.pntd.0001334.e1334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stienstra Y., Van der Werf T. S., Guarner J., et al. Analysis of an IS2404-based nested PCR for diagnosis of Buruli ulcer disease in regions of Ghana where the disease is endemic. Journal of Clinical Microbiology. 2003;41(2):794–797. doi: 10.1128/jcm.41.2.794-797.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ablordey A., Amissah D. A., Aboagye I. F., et al. Detection of Mycobacterium ulcerans by the loop mediated isothermal amplification method. PLoS Neglected Tropical Diseases. 2012;6(4, article e1590) doi: 10.1371/journal.pntd.0001590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yeboah-Manu D., Bodmer T., Mensah-Quainoo E., Owusu S., Ofori-Adjei D., Pluschke G. Evaluation of decontamination methods and growth media for primary isolation of Mycobacterium ulcerans from surgical specimens. Journal of Clinical Microbiology. 2004;42(12):5875–5876. doi: 10.1128/jcm.42.12.5875-5876.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dobos K. M., Spotts E. A., Marston B. J., Horsburgh C. R., Jr., King C. H. Serologic response to culture filtrate antigens of Mycobacterium ulcerans during Buruli ulcer disease. Emerging Infectious Diseases. 2000;6(2):158–164. doi: 10.3201/eid0602.000208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Beissner M., Symank D., Phillips R. O., et al. Detection of viable mycobacterium ulcerans in clinical samples by a novel combined 16S rRNA reverse transcriptase/IS2404 real-time qPCR assay. PLoS Neglected Tropical Diseases. 2012;6(8, article e1756) doi: 10.1371/journal.pntd.0001756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mensah-Quainoo E., Yeboah-Manu D., Asebi C., et al. Diagnosis of Mycobacterium ulcerans infection (Buruli ulcer) at a treatment centre in Ghana: a retrospective analysis of laboratory results of clinically diagnosed cases. Tropical Medicine & International Health. 2008;13(2):191–198. doi: 10.1111/j.1365-3156.2007.01990.x. [DOI] [PubMed] [Google Scholar]
- 38.Durnez L., Stragier P., Roebben K., Ablordey A., Leirs H., Portaels F. A comparison of DNA extraction procedures for the detection of Mycobacterium ulcerans, the causative agent of Buruli ulcer, in clinical and environmental specimens. Journal of Microbiological Methods. 2009;76(2):152–158. doi: 10.1016/j.mimet.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 39.Affolabi D., Sanoussi N., Vandelannoote K., et al. Effects of decontamination, DNA extraction, and amplification procedures on the molecular diagnosis of Mycobacterium ulcerans disease (Buruli ulcer) Journal of Clinical Microbiology. 2012;50(4):1195–1198. doi: 10.1128/jcm.05592-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Affolabi D., Bankolé H., Ablordey A., et al. Effects of grinding surgical tissue specimens and smear staining methods on Buruli ulcer microscopic diagnosis. Tropical Medicine & International Health. 2008;13(2):187–190. doi: 10.1111/j.1365-3156.2007.01989.x. [DOI] [PubMed] [Google Scholar]
- 41.Eddyani M., Debacker M., Martin A., et al. Primary culture of Mycobacterium ulcerans from human tissue specimens after storage in semisolid transport medium. Journal of Clinical Microbiology. 2008;46(1):69–72. doi: 10.1128/jcm.00301-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Palomino J. C., Obiang A. M., Realini L., Meyers W. M., Portaels F. Effect of oxygen on growth of Mycobacterium ulcerans in the BACTEC system. Journal of Clinical Microbiology. 1998;36(11):3420–3422. doi: 10.1128/jcm.36.11.3420-3422.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Palomino J. C., Portaels F. Effects of decontamination methods and culture conditions on viability of Mycobacterium ulcerans in the BACTEC system. Journal of Clinical Microbiology. 1998;36(2):402–408. doi: 10.1128/jcm.36.2.402-408.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Guarner J., Bartlett J., Spotts Whitney E. A., et al. Histopathologic features of Mycobacterium ulcerans infection. Emerging Infectious Diseases. 2003;9(6):651–656. doi: 10.3201/eid0906.020485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Portaels F., Agular J., Fissette K., et al. Direct detection and identification of Mycobacterium ulcerans in clinical specimens by PCR and oligonucleotide-specific capture plate hybridization. Journal of Clinical Microbiology. 1997;35(5):1097–1100. doi: 10.1128/jcm.35.5.1097-1100.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Affolabi D., Tanimomo-Kledjo B., Anyo G., Johnson R. C., Anagonou S. Y., Portaels F. Setting up a national reference laboratory for Buruli ulcer: The case of Benin. Tropical Medicine and International Health. 2008;13(3):365–368. doi: 10.1111/j.1365-3156.2008.02011.x. [DOI] [PubMed] [Google Scholar]
- 47.Siegmund V., Adjei O., Nitschke J., et al. Dry reagent-based polymerase chain reaction compared with other laboratory methods available for the diagnosis of Buruli ulcer disease. Clinical Infectious Diseases. 2007;45(1):68–75. doi: 10.1086/518604. [DOI] [PubMed] [Google Scholar]
- 48.Kim B.-J., Lee S.-H., Lyu M.-A., et al. Identification of mycobacterial species by comparative sequence analysis of the RNA polymerase gene (rpoB) Journal of Clinical Microbiology. 1999;37(6):1714–1720. doi: 10.1128/jcm.37.6.1714-1720.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kim H., Kim S.-H., Shim T.-S., et al. PCR restriction fragment length polymorphism analysis (PRA)-algorithm targeting 644 bp Heat Shock Protein 65 (hsp65) gene for differentiation of Mycobacterium spp. Journal of Microbiological Methods. 2005;62(2):199–209. doi: 10.1016/j.mimet.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 50.Roth A., Reischl U., Streubel A., et al. Novel diagnostic algorithm for identification of mycobacteria using genus-specific amplification of the 16S–23S rRNA gene spacer and restriction endonucleases. Journal of Clinical Microbiology. 2000;38(3):1094–1104. doi: 10.1128/jcm.38.3.1094-1104.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Talaat A. M., Reimschuessel R., Trucksis M. Identification of mycobacteria infecting fish to the species level using polymerase chain reaction and restriction enzyme analysis. Veterinary Microbiology. 1997;58(2–4):229–237. doi: 10.1016/s0378-1135(97)00120-x. [DOI] [PubMed] [Google Scholar]
- 52.Siegmund V., Adjei O., Racz P., et al. Dry-reagent-based PCR as a novel tool for laboratory confirmation of clinically diagnosed Mycobacterium ulcerans-associated disease in areas in the tropics where M. ulcerans is endemic. Journal of Clinical Microbiology. 2005;43(1):271–276. doi: 10.1128/jcm.43.1.271-276.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schütte D., Um-Boock A., Mensah-Quainoo E., Itin P., Schmid P., Pluschke G. Development of highly organized lymphoid structures in Buruli ulcer lesions after treatment with rifampicin and streptomycin. PLoS Neglected Tropical Diseases. 2007;1(1, article e02) doi: 10.1371/journal.pntd.0000002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ross B. C., Marino L., Oppedisano F., Edwards R., Robins-Browne R. M., Johnson P. D. R. Development of a PCR assay for rapid diagnosis of Mycobacterium ulcerans infection. Journal of Clinical Microbiology. 1997;35(7):1696–1700. doi: 10.1128/jcm.35.7.1696-1700.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Roberts B., Hirst R. Immunomagnetic separation and PCR for detection of Mycobacterium ulcerans . Journal of Clinical Microbiology. 1997;35(10):2709–2711. doi: 10.1128/jcm.35.10.2709-2711.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stinear T., Ross B. C., Davies J. K., et al. Identification and characterization of IS2404 and IS2606: two distinct repeated sequences for detection of Mycobacterium ulcerans by PCR. Journal of Clinical Microbiology. 1999;37(4):1018–1023. doi: 10.1128/jcm.37.4.1018-1023.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Guimaraes-Peres A., Portaels F., De Rijk P., et al. Comparison of two PCRs for detection of Mycobacterium ulcerans . Journal of Clinical Microbiology. 1999;37(1):206–208. doi: 10.1128/jcm.37.1.206-208.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Phillips R. O., Sarfo F. S., Osei-Sarpong F., et al. Sensitivity of PCR targeting Mycobacterium ulcerans by use of Fine-needle aspirates for diagnosis of Buruli ulcer. Journal of Clinical Microbiology. 2009;47(4):924–926. doi: 10.1128/jcm.01842-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sizaire V., Nackers F., Comte E., Portaels F. Mycobacterium ulcerans infection: control, diagnosis, and treatment. The Lancet Infectious Diseases. 2006;6(5):288–296. doi: 10.1016/s1473-3099(06)70464-9. [DOI] [PubMed] [Google Scholar]
- 60.Noeske J., Kuaban C., Rondini S., et al. Buruli ulcer disease in Cameroon rediscovered. American Journal of Tropical Medicine and Hygiene. 2004;70(5):520–526. [PubMed] [Google Scholar]
- 61.Rondini S., Mensah-Quainoo E., Troll H., Bodmer T., Pluschke G. Development and application of real-time PCR assay for quantification of Mycobacterium ulcerans DNA. Journal of Clinical Microbiology. 2003;41(9):4231–4237. doi: 10.1128/jcm.41.9.4231-4237.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Johnson P. D. R., Hayman J. A., Quek T. Y., et al. Consensus recommendations for the diagnosis, treatment and control of Mycobacterium ulcerans infection (Bairnsdale or Buruli ulcer) in Victoria, Australia. Medical Journal of Australia. 2007;186(2):64–68. doi: 10.5694/j.1326-5377.2007.tb00802.x. [DOI] [PubMed] [Google Scholar]
- 63.Kim B.-J., Lee K.-H., Park B.-N., et al. Differentiation of mycobacterial species by PCR-restriction analysis of DNA (342 base pairs) of the RNA polymerase gene (rpoB) Journal of Clinical Microbiology. 2001;39(6):2102–2109. doi: 10.1128/JCM.39.6.2102-2109.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Saxegaard F. Isolation of Mycobacterium paratuberculosis from intestinal mucosa and mesenteric lymph nodes of goats by use of selective dubos medium. Journal of Clinical Microbiology. 1985;22(2):312–313. doi: 10.1128/jcm.22.2.312-313.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Desjardin L. E., Perkins M. D., Wolski K., et al. Measurement of sputum Mycobacterium tuberculosis messenger RNA as a surrogate for response to chemotherapy. American Journal of Respiratory and Critical Care Medicine. 1999;160(1):203–210. doi: 10.1164/ajrccm.160.1.9811006. [DOI] [PubMed] [Google Scholar]
- 66.Martinez A. N., Lahiri R., Pittman T. L., et al. Molecular determination of Mycobacterium leprae viability by use of real-time PCR. Journal of Clinical Microbiology. 2009;47(7):2124–2130. doi: 10.1128/JCM.00512-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Eddyani M., Lavender C., De Rijk W. B., et al. Multicenter external quality assessment program for PCR detection of Mycobacterium ulcerans in clinical and environmental specimens. PLoS ONE. 2014;9(2, article e89407) doi: 10.1371/journal.pone.0089407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Beissner M., Phillips R. O., Battke F., et al. Loop-mediated isothermal amplification for laboratory confirmation of Buruli ulcer disease—towards a point-of-care test. PLoS Neglected Tropical Diseases. 2015;9(11) doi: 10.1371/journal.pntd.0004219.e0004219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Diaz D., Döbeli H., Yeboah-Manu D., et al. Use of the immunodominant 18-kilodalton small heat shock protein as a serological marker for exposure to Mycobacterium ulcerans. Clinical and Vaccine Immunology. 2006;13(12):1314–1321. doi: 10.1128/CVI.00254-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pidot S. J., Porter J. L., Marsollier L., et al. Serological evaluation of mycobacterium ulcerans Antigens identified by comparative genomics. PLoS Neglected Tropical Diseases. 2010;4(11, article e872) doi: 10.1371/journal.pntd.0000872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yeboah-Manu D., Röltgen K., Opare W., et al. Sero-epidemiology as a tool to screen populations for exposure to Mycobacterium ulcerans . PLoS Neglected Tropical Diseases. 2012;6(1) doi: 10.1371/journal.pntd.0001460.e1460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dreyer A., Röltgen K., Dangy J. P., et al. Identification of the Mycobacterium ulcerans Protein MUL_3720 as a promising target for the development of a diagnostic test for buruli ulcer. PLoS Neglected Tropical Diseases. 2015;9(2, article e0003477) doi: 10.1371/journal.pntd.0003477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sarfo F. S., Phillips R. O., Rangers B., et al. Detection of mycolactone A/B in Mycobacterium ulcerans—infected human tissue. PLoS Neglected Tropical Diseases. 2010;4, article e577 doi: 10.1371/journal.pntd.0000577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sarfo F. S., Chevalier F., Aka N., et al. Mycolactone diffuses into the peripheral blood of buruli ulcer patients—implications for diagnosis and disease monitoring. PLoS Neglected Tropical Diseases. 2011;5(7, article e1237) doi: 10.1371/journal.pntd.0001237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Converse P. J., Xing Y., Kim K. H., et al. Accelerated detection of mycolactone production and response to antibiotic treatment in a mouse model of Mycobacterium ulcerans disease. PLoS Neglected Tropical Diseases. 2014;8(1) doi: 10.1371/journal.pntd.0002618.e2618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wadagni A., Frimpong M., Phanzu D. M., et al. Simple, rapid mycobacterium ulcerans disease diagnosis from clinical samples by fluorescence of mycolactone on thin layer chromatography. PLoS Neglected Tropical Diseases. 2015;9(11) doi: 10.1371/journal.pntd.0004247.e0004247 [DOI] [PMC free article] [PubMed] [Google Scholar]