Abstract
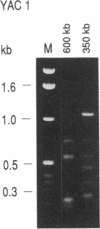
The genetic defects in Prader-Willi syndrome (PWS) and Angelman syndrome (AS) map to 15q11-13. Using microdissection, we have recently isolated several DNA probes for the critical region. Here we report that microclone MN7 detects multiple loci in 15q11-13 and 16p11.2. Eight yeast artificial chromosome (YAC) clones, two genomic phage clones, and two placenta cDNA clones were isolated to analyze these loci in detail. Two of the YAC clones map to 16p. Six YAC clones and two genomic phage clones contain a total of four or five different MN7 copies, which are spread over a large distance within 15q11-13. One cDNA clone is from chromosome 15 and one is from chromosome 16. The chromosome 15 cDNA detects transcripts of 14 and 8 kilobases in various human tissues. The presence of multiple copies of the MN7 gene family in proximal 15q may conceivably be related to the instability of this region and thus to the etiology of associated disorders.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brownstein B. H., Silverman G. A., Little R. D., Burke D. T., Korsmeyer S. J., Schlessinger D., Olson M. V. Isolation of single-copy human genes from a library of yeast artificial chromosome clones. Science. 1989 Jun 16;244(4910):1348–1351. doi: 10.1126/science.2544027. [DOI] [PubMed] [Google Scholar]
- Buiting K., Neumann M., Lüdecke H. J., Senger G., Claussen U., Antich J., Passarge E., Horsthemke B. Microdissection of the Prader-Willi syndrome chromosome region and identification of potential gene sequences. Genomics. 1990 Mar;6(3):521–527. doi: 10.1016/0888-7543(90)90481-9. [DOI] [PubMed] [Google Scholar]
- Butler M. G., Meaney F. J., Palmer C. G. Clinical and cytogenetic survey of 39 individuals with Prader-Labhart-Willi syndrome. Am J Med Genet. 1986 Mar;23(3):793–809. doi: 10.1002/ajmg.1320230307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler M. G. Prader-Willi syndrome: current understanding of cause and diagnosis. Am J Med Genet. 1990 Mar;35(3):319–332. doi: 10.1002/ajmg.1320350306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carle G. F., Olson M. V. Separation of chromosomal DNA molecules from yeast by orthogonal-field-alternation gel electrophoresis. Nucleic Acids Res. 1984 Jul 25;12(14):5647–5664. doi: 10.1093/nar/12.14.5647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaillet J. R., Knoll J. H., Horsthemke B., Lalande M. The syntenic relationship between the critical deletion region for the Prader-Willi/Angelman syndromes and proximal mouse chromosome 7. Genomics. 1991 Nov;11(3):773–776. doi: 10.1016/0888-7543(91)90090-2. [DOI] [PubMed] [Google Scholar]
- Donlon T. A., Lalande M., Wyman A., Bruns G., Latt S. A. Isolation of molecular probes associated with the chromosome 15 instability in the Prader-Willi syndrome. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4408–4412. doi: 10.1073/pnas.83.12.4408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green E. D., Olson M. V. Systematic screening of yeast artificial-chromosome libraries by use of the polymerase chain reaction. Proc Natl Acad Sci U S A. 1990 Feb;87(3):1213–1217. doi: 10.1073/pnas.87.3.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horsthemke B., Greger V., Barnert H. J., Höpping W., Passarge E. Detection of submicroscopic deletions and a DNA polymorphism at the retinoblastoma locus. Hum Genet. 1987 Jul;76(3):257–261. doi: 10.1007/BF00283619. [DOI] [PubMed] [Google Scholar]
- Kaplan L. C., Wharton R., Elias E., Mandell F., Donlon T., Latt S. A. Clinical heterogeneity associated with deletions in the long arm of chromosome 15: report of 3 new cases and their possible genetic significance. Am J Med Genet. 1987 Sep;28(1):45–53. doi: 10.1002/ajmg.1320280107. [DOI] [PubMed] [Google Scholar]
- Knoll J. H., Nicholls R. D., Magenis R. E., Graham J. M., Jr, Lalande M., Latt S. A. Angelman and Prader-Willi syndromes share a common chromosome 15 deletion but differ in parental origin of the deletion. Am J Med Genet. 1989 Feb;32(2):285–290. doi: 10.1002/ajmg.1320320235. [DOI] [PubMed] [Google Scholar]
- Lüdecke H. J., Johnson C., Wagner M. J., Wells D. E., Turleau C., Tommerup N., Latos-Bielenska A., Sandig K. R., Meinecke P., Zabel B. Molecular definition of the shortest region of deletion overlap in the Langer-Giedion syndrome. Am J Hum Genet. 1991 Dec;49(6):1197–1206. [PMC free article] [PubMed] [Google Scholar]
- Magenis R. E., Brown M. G., Lacy D. A., Budden S., LaFranchi S. Is Angelman syndrome an alternate result of del(15)(q11q13)? Am J Med Genet. 1987 Dec;28(4):829–838. doi: 10.1002/ajmg.1320280407. [DOI] [PubMed] [Google Scholar]
- Malcolm S., Clayton-Smith J., Nichols M., Robb S., Webb T., Armour J. A., Jeffreys A. J., Pembrey M. E. Uniparental paternal disomy in Angelman's syndrome. Lancet. 1991 Mar 23;337(8743):694–697. doi: 10.1016/0140-6736(91)90278-w. [DOI] [PubMed] [Google Scholar]
- Naylor S. L., McGill J. R., Zabel B. U. In situ hybridization of metaphase and prometaphase chromosomes. Methods Enzymol. 1987;151:279–292. doi: 10.1016/s0076-6879(87)51024-2. [DOI] [PubMed] [Google Scholar]
- Nelson D. L., Ledbetter S. A., Corbo L., Victoria M. F., Ramírez-Solis R., Webster T. D., Ledbetter D. H., Caskey C. T. Alu polymerase chain reaction: a method for rapid isolation of human-specific sequences from complex DNA sources. Proc Natl Acad Sci U S A. 1989 Sep;86(17):6686–6690. doi: 10.1073/pnas.86.17.6686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholls R. D., Knoll J. H., Butler M. G., Karam S., Lalande M. Genetic imprinting suggested by maternal heterodisomy in nondeletion Prader-Willi syndrome. Nature. 1989 Nov 16;342(6247):281–285. doi: 10.1038/342281a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pembrey M., Fennell S. J., van den Berghe J., Fitchett M., Summers D., Butler L., Clarke C., Griffiths M., Thompson E., Super M. The association of Angelman's syndrome with deletions within 15q11-13. J Med Genet. 1989 Feb;26(2):73–77. doi: 10.1136/jmg.26.2.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich D. C., Witkowski C. M., Summers K. M., van Tuinen P., Ledbetter D. H. Highly polymorphic locus D15S24 (CMW-1) maps to 15pter-q13. [HGM9 provisional no. D15S24]. Nucleic Acids Res. 1988 Sep 12;16(17):8740–8740. doi: 10.1093/nar/16.17.8740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Second Annual Prader-Willi Syndrome Scientific Conference. Houston, June 17, 1987. Proceedings and abstracts. Am J Med Genet. 1987 Dec;28(4):779–924. doi: 10.1002/ajmg.1320280402. [DOI] [PubMed] [Google Scholar]
- Siebenlist U., Ravetch J. V., Korsmeyer S., Waldmann T., Leder P. Human immunoglobulin D segments encoded in tandem multigenic families. Nature. 1981 Dec 17;294(5842):631–635. doi: 10.1038/294631a0. [DOI] [PubMed] [Google Scholar]
- Wagstaff J., Knoll J. H., Fleming J., Kirkness E. F., Martin-Gallardo A., Greenberg F., Graham J. M., Jr, Menninger J., Ward D., Venter J. C. Localization of the gene encoding the GABAA receptor beta 3 subunit to the Angelman/Prader-Willi region of human chromosome 15. Am J Hum Genet. 1991 Aug;49(2):330–337. [PMC free article] [PubMed] [Google Scholar]
- Wigler M., Pellicer A., Silverstein S., Axel R., Urlaub G., Chasin L. DNA-mediated transfer of the adenine phosphoribosyltransferase locus into mammalian cells. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1373–1376. doi: 10.1073/pnas.76.3.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams C. A., Gray B. A., Hendrickson J. E., Stone J. W., Cantú E. S. Incidence of 15q deletions in the Angelman syndrome: a survey of twelve affected persons. Am J Med Genet. 1989 Mar;32(3):339–345. doi: 10.1002/ajmg.1320320313. [DOI] [PubMed] [Google Scholar]