Abstract
Inflammatory bowel disease (IBD) is a chronic nonspecific intestinal inflammatory disease, including ulcerative colitis (UC) and Crohn’s disease (CD). Its pathogenesis remains not yet clear. Current researchers believe that after environmental factors act on individuals with genetic susceptibility, an abnormal intestinal immune response is launched under stimulation of intestinal flora. However, previous studies only focused on adaptive immunity in the pathogenesis of IBD. Currently, roles of innate immune response in the pathogenesis of intestinal inflammation have also drawn much attention. In this study, IBD related innate immunity and adaptive immunity were explained, especially the immune mechanisms in the pathogenesis of IBD.
Keywords: Inflammatory bowel disease, innate immunity, adaptive immunity, pathogenesis
Introduction
Inflammatory bowel disease (IBD) is an autoimmune disease with unclear etiology, and characterized by alternative recurrence and alleviation period. Abdominal pain, diarrhea, bloody stool and weight loss are main clinical manifestations of IBD. Other autoimmune diseases, such as primary sclerosing cholangitis, psoriasis and ankylosing spondylitis, often occur in IBD patients [1,2]. There are few epidemiological data about IBD patients in developing countries. However, incidence of IBD increases significantly all over the world according to surveys, indicating that IBD is a global disease [3]. Gastrointestinal mucosa is continuously exposed to microorganisms with different antigenicity. The immune system maintains in low reactions to symbiotic bacteria under normal circumstances, and only initiates protective immune responses when facing pathogens [4]. Inflammation happens when any link in the immune system is abnormal, as seen in Figure 1.
Figure 1.
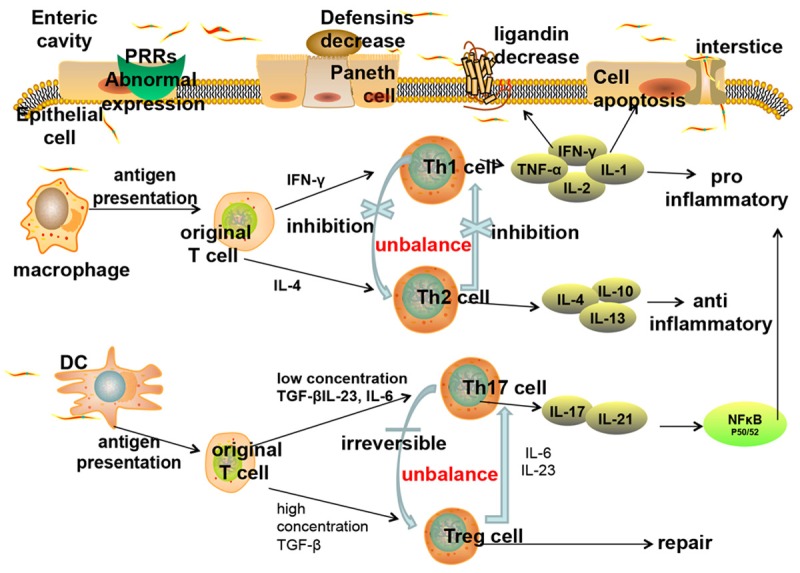
Inflammatory bowel disease related immune disorders. The innate immune abnormalities of inflammatory bowel disease lead to adaptive immune disorders (Th1/Th2 regulation imbalance and Th17/Treg transformation imbalance). The inflammatory cytokines in turn increase the innate immune damages (apoptosis of intestinal epithelial cells, reduction of connection protein expression, decrease of antibacterial peptides secreted by Paneth cells, etc.), abate the intestinal barrier function, and aggravate inflammation. Therefore a vicious circle forms.
The immunity
Immunity is one of physiological functions in humans, which can be divided into innate and adaptive immunity. The human body relies on immunity to recognize ‘self’ and ‘non-self’ components, then the immunity protects human against invasion of pathogens. Two major processes of the innate immunity are usage of a large set of different pattern recognition receptors (PRRs) and a system for random and nonselective generation of antigen specific receptors. The adaptive immunity has been viewed as a complement to the innate immunity and a definitive solution to pathogen recognition [5]. However, there are some differences between the innate and adaptive immunity, as shown in Table 1.
Table 1.
The differences between the innate and adaptive immunity
| Item | Innate immunity | Adaptive immunity |
|---|---|---|
| Acquired form | Inherent (or congenital) | Acquired |
| Do not need to contact the antigens | Need to contact the antigens | |
| Time to play roles | Early, rapid (minutes-4 days) | 4-5 days |
| Immune recognition receptors | Pattern recognition receptor | Specific antigen recognition receptors |
| Immune memory | Nothing | Generation of memory cells |
| Examples | Antibacterial substances, bactericidal substances, inflammatory cytokines, phagocytic cells, NK cells, NK T cells | T cells (cell immunity) |
| B cells (humoral immunity) |
Innate immunity
The innate intestinal immune system consists of intestinal mucosal epithelial barrier, natural immune cells, innate immune molecules, etc. Unlike adaptive immunity, innate immune is neither specific nor memorial, but it is a cornerstone of adaptive immunity. Innate immune is involved in adaptive immunity, and presents antigens for original T cells to provide the activation signals. It is also involved in maturity, differentiation and homing of immune cells in adaptive immunity, therefore regulating immune functions [6].
Intestinal mucosal epithelial barrier
Apoptosis of intestinal epithelial cells
PRRs identify pathogen-associated molecular pattern (PAMPs) on surface of pathogens, nuclear transcription factors, such as NF-κB, are activated by normal intestinal epithelial cells, and then gene expression of related factors is started. Paneth cells are activated to release defensin, and immune cells release pro-inflammatory or anti-inflammatory factors, thus gastric mucosa was protected from pathogens [7]. Immune cells in inflammatory intestinal mucosa of IBD patients produce inflammation factors, such as TNF-α and IFN-γ, which induce expression of epithelial cell apoptosis related proteins, such as Caspase-1, and inhibit expression of anti-apoptotic proteins, such as Bcl-2, resulting in induced epithelial cell apoptosis, weakened function of epithelial cells resisting pathogens and increased permeability of intestinal mucosa [8].
Increase of intestinal epithelial permeability
Intestinal mucosal epithelial contains a complete set of epithelial cells and cell connection parts of lateral margin. Cells are connected via adherence junction, tight junction and the desmosome. Tight junction locates at the top of intestinal epithelial cells and forms the top junctional complex by binding to the adjacent adherence, therefore regulating intestinal epithelial permeability [9]. During an active stage of IBD, junction protein and its corresponding mRNA in intestinal tissue decrease significantly, while the reduction is not obvious in an inactive phage, indicating that an abnormality of tight junction protein in epithelial cells is closely related to intestinal inflammation [10]. During the active stage of IBD, pathogens within enteric cavity shift to mucosa lamina propria, and activate immune cells in lamina propria after antigen presenting processes. A large number of inflammatory cytokines are produced and regulate expression of proteins in tight junction, thus impairing intestinal mucosal barrier function and increasing intestinal epithelial permeability [11].
Innate immune cells
Macrophages
Macrophages decompose engulfed pathogens into specific antigenic determinants, such as peptides and lipopolysaccharide, using proteases and oxygen free radicals. A pathogen antigen-major histocompatibility complex is a combination of antigenic determinants and major histocompatibility antigens on cell membrane. After the complex binds with antigen receptors in T cells, antigenic information is presented to T cells [12]. During the acute phage of IBD, quantity of macrophages in intestinal mucosa dramatically increases. The macrophages express a large number of T cell co-stimulating molecules (CD40, CD80, CD86, etc.) and triggering receptor expressed on myeloid cells-1 (TREM-1) [13]. TREM-1 promotes secretion of macrophage pro-inflammatory factors. Expression of TREM-1 is low in normal intestinal tissue, but higher in an experimental colitis model and IBD patients [14], indicating that abnormal innate immunity dominated by macrophages plays a vital role in pathogenesis of IBD.
Dendritic cells (DCs)
Mature DCs contain surface antigen presenting molecules. They induce immune activation, raise co-stimulating factors, increase effective areas that interact with T cells, increase expression of chemokine receptors, accelerate DCs movement towards lymphoid tissues, and then activate original T cells to secrete cytokines, thus inducing immune responses. In colitis animal model, DCs from colons were co-cultured with autologous T cells of lamina propria and T cells were stimulated to produce IFN-γ and IL-6, quantity of which was significantly higher than that produced by DCs co-cultured with homologous or heterologous spleen T cells [15]. In colonic mucosa of IBD patients, interactions between DCs and T cells promote production of inflammatory cytokines and cause inflammation [15]. In addition, there is a regulation circuit between DCs and Treg cells: Treg cells maintain a normal amount of DCs, while a loss of DCs leads to a decrease of Treg cells, thus IBD intestinal inflammation is aggravated. Therefore, interventions of this path may provide a new treatment for IBD [16].
Natural killer (NK) cells
NK cells can control viral and bacterial infections, secrete cytokines, kill tumor cells, and establish a relationship between innate immunity and acquired immunity [17]. NK cells exhibit cytotoxic damage functions without participation of T cell surface receptor or immunoglobulin signals. Response processes of NK cells act through two receptor systems, one is recognizing antigens and inhibiting immune responses via class I HLA; another is killing target cells by non-HLA molecules [18]. More NK and natural killer T (NKT) cells exist in intestinal mucosa of IBD patients. They express more immune active molecules, such as CD25, CD28 and CD69, than normal intestinal mucosa [19]. It is speculated that function of NK cells maintaining steady intestinal epithelium is associated with specific cytokines. For example, NK cells that express IL-22 have a protective effect on initiation of IBD [20]. And IL-21 in vitro can strengthen cytotoxic killing activity of NK cells and increase secretion of pro-inflammatory factors such as TNF-α and IFN-γ [21].
Innate immune molecules
Defensins
Defensins, which contain two types: α and β, possess a broad spectrum antimicrobial activity and chemotactic function, and induce T cells, monocytes and DCs gathering in inflammatory responses quickly, thus acting as a bridge between innate immunity and acquired immunity and playing a significant role in cell immunity [22,23]. Therefore, a reduction of defensins not only weakens innate immunity, but also affects initiation and regulation of acquired immunity.
Defensin-α consists of HD-5 and HD-6, which is secreted by Paneth cells. A reduction of defensin-α is associated with IBD susceptible gene NOD2/CARD15 mutations [24]. Expression of HD-5 and HD-6 in patients with ileum type Crohn’s disease (CD) is significantly less than that in normal and non-ileum CD patients, and the reduction level is closely related to NOD2 gene mutations. The expression of HD-5 and HD-6 increases in CD patients without NOD2 gene mutations [25]. And mRNA expression level of HD-5 and HD-6 in colon epithelial cells of patients with ulcerative colitis (UC) is significantly higher [26]. Therefore, CD is considered as a defensin deficiency syndrome. A loss of mucosal defensins induces more bacteria invasion of intestinal mucosa, leading to inflammation [27].
HBD1 in intestinal defensin-β exists in normal intestinal mucosa epithelial cells and is not affected by inflammation or bacterial infections, thus playing a basic defensive role. But HBD1 mRNA reduces in colon of IBD patients [28]. Unlike small intestine type CD, abnormal expression of HBD-2, HBD-3 and HBD-4 mainly occurs in colon type CD [29]. HBD-2 in intestine of UC patients is induced to express, and its level is higher than normal and CD patients [26]. HBD-2 is induced to express in infections, but its expression is low without any infections, speculating that the excessive expression of HBD-2 may aggravate UC inflammatory responses. HD expression in UC patients is closely related to pathological changes in UC patients, but not in CD patients [30].
PRRs
PRRs consist of Toll-like receptors (TLRs) and nucleotide-binding oligomerization domain like receptors (NLRs). They can identify recognition molecules of pathogen associated molecular patterns (PAMPs), activate innate immunity, produce cytokines such as IL, TNF and IFN, and fight against pathogens. Activation of PRRs promotes maturation of antigen presenting cells, and then activates the adaptive immunity. Therefore, PRRs also act as a bridge between innate immunity and adaptive immunity.
TLRs are the most important compositions of PRRs, and a variety of them express in intestinal epithelial cells. TLR2 and TLR4 exist in peripheral lymphatic endothelial cells of small intestine. DCs are activated under mediation of TLRs, induce NF-κB through MyD88 dependent or in-dependent signal transduction pathways, then activate adaptive immune responses, and clear pathogens in lumens. TLR3 is continuously expressed while TLR5, TLR2 and TLR4 are only expressed in normal intestinal epithelial cells. But the expression of TLR2 and TLR4 is also detected in intestinal epithelial cells surface of IBD patients [31,32].
After highly expressed TLR4 binds with its ligand LPS, downstream cytokines are activated to recruit inflammatory cells and cause inflammation. In a study of TLR4 Asp299Gly polymorphism allele frequency in IBD population, the frequency was significantly higher in IBD patients than that in healthy controls, prompting that abnormal TLR4 expression was involved in pathogenesis of IBD [33]. Recent studies have shown that a lack of NOD2 gene or CD-like mutations strengthened activation of NF-κB mediated by TLR2 and increased activities of Th1 cells. CD is characterized by excessive activation of Th1 cells and production of cytokines, such as TNF-α, IFN-γ and IL-2.
NLRs are cytoplasm type PRRs, and responsible for “internal” signal recognition. Their ligand recognition and signal transduction is partially overlapped with TLRs. NOD2 identifies acyl dipeptide of cell walls (MDP) which exists in both gram-positive bacteria and gram-negative bacteria cell walls. After the identification, NOD2 together with receptor binding protein RIP2 constitutes oligomer and induces ubiquitination, eventually activates NF-κB, and induces production of inflammatory cytokines. NOD2 is expressed in mononuclear cells and Paneth cells, and plays an important role in processes of antibacterial peptide produced by cells. NOD2 gene mutations significantly increase CD susceptibility, probably because the mutations are not able to identify MDP, resulting in a reduction of secretion of IL-23 and IL-1 in DCs, thus decreasing inhibitory effects of Th17 cells [34], indicating that NOD2 gene is closely related to intestinal mucosal immune responses in CD patients [35].
Adaptive immunity
In contrast to the innate immunity, adaptive immunity possesses specificities and immune memory abilities. Adaptive immunity and innate immune supplement each other and mutually resist invasion of pathogenic microorganisms. The original T cells amplify and are differentiated into different subsets such as Th1, Th2, Th17 and Treg cells after stimulation of antigens. Th1 cells eliminate pathogens inside cells; Th2 cells protect human body from harmful parasites and adjust allergic reactions; Th17 cells remove extracellular bacteria and fungi; Treg cells promote tissue repairs. However, disorders of T cell responses and imbalance of T cell subsets induce excessive releases of cytokines and chemokines, which lead to inflammation.
Th1/Th2 balance
Th1 cells
IFN-γ secreted by antigen presenting cells acts on original Th cell signal transduction and transcription activating factor 1 (STAT-1). STAT-1 translocates into nuclei to activate specific transcription factor T-bet, and prompts differentiation original Th cells into Th1 cells. Th1 cells secrete various pro-inflammatory cytokines, such as IL-1, IL-2, IL-6, IL-8, IL-12, TNF-α, IFN-γ, etc. In CD mice models induced by trinitro-benzene-sulfonic acid, Th1 cells and expression of IFN-γ were significantly elevated in local intestinal mucosa and spleen [36]. There are a lot of Thl cytokines, such as TNF, IL-12 and IL-18, in intestinal mucosa of CD patients. IL-18 promotes Thl cell differentiation via AP-1 and NF-κB pathways [37], demonstrating that Th1 cells play important roles in pathogenesis of CD.
Th2 cells
IL-4 secreted by antigen presenting cells acts on receptors on surface of original Th cells and activates STAT-6. STAT-6 moves into nuclei to activate a specific transcription activation factor GATA3, induces expression of downstream genes and promotes transformation of original Th cells into Th2 cells. IL-13, together with TNF-α, acts on IL-13 Rβ2 receptor on surface of Th2 cells, activates STAT6 and promotes proliferation of Th2 cells. Inflammatory factors, such as IL-4, IL-5 and IL-13, are secreted by Th2 cells. Expression of IL-5 and IL-13 increases in inflammatory region in UC patients [38], thus it can be inferred that Th2 cells are associated with pathogenesis of UC. While some scholars believe that UC is a result of combined effects of Thl and Th2 cells. Thl reaction is enhanced in the early phage, while Th2 reaction is dominant in the late phage. Therefore, it should be further researched which one is closer with UC [39].
Th1/Th2 adjustment
Thl and Th2 cells are in dynamic equilibrium under normal conditions, and are regulated and inhibited by cytokines secreted by each other. IFN-γ secreted by Thl cells inhibits proliferation of Th2 cells, while IL-4, IL-10 and IL-13 secreted by Th2 cells inhibit Thl reactions. Immune adjustment of imbalance of Th1/Th2 subsets is involved in pathogenesis of a variety of autoimmune diseases and inflammatory diseases, and also plays an important role in IBD development [40]. Pro-inflammatory cytokines, such as IL-1, IL-2, IL-6 and IL-8, secreted by Th1 cells participate in cellular immune responses. Anti-inflammatory cytokines, such as IL-4, IL-10 and IL-13 secreted by Th2 cells are involved in humoral immune responses. Therefore, balance of Thl and Th2 cells determines balance of pro-inflammatory and anti-inflammatory cytokines, which then determines whether and which kinds of immune reactions occur. Thus, key cytokines of adjusting Thl and Th2 cells balance need to be explored to provide new ideas for treatments of IBD in the future [41].
Balance of Th17/Treg cells
Th17 cells
IL-23 acts on IL-23 receptors on the original Th cell surface and activates cytoplasmic signal transduction and transcriptional activation factor 3 (STAT-3) with presences of TGF-β, IL-6 or IL-21. STAT-3 transfers into nuclei and activates transcription factor vitamin A acid related orphan receptor γt (RORγt), resulting in differentiations of original Th cells into Th17 cells. Activated Th17 cells mainly function through secreting cytokines, such as IL-17, IL-21 and IL-22. IL-17 induces immune cells transferring into peripheral tissues and binding with IL-17 receptors, and then activates NF-κB and promotes releases of a variety of pro-inflammatory factors. Clinical studies have found that inflammatory bowel mucosa of CD and UC patients contains a much higher level of Th17 cells and IL-17 than the normal ones. Th17 cells mainly distribute in intestinal mucosa lamina propria at the active phage of UC patients, while they distribute in mucosa, submucosa and muscularis at the active phage of CD patients [42]. Serum IL-17 is also significantly higher in CD and UC patients [43]. And RNA expression of IL-17A and IL-17F increases significantly in IBD patients [44], demonstrating that Th17 cells play an important role in pathogenesis of IBD.
Treg cells
Treg cells inhibit other Th cells such as Th1, Th2 and Th17 via direct contacts with cells and releases of cytokines such as IL-10 and TGF-β to maintain immune tolerances. Transcription factor Foxp3 is a specific surface marker of Treg cells. According to different sources, Treg cells are mainly divided into two categories: natural regulatory T cells (nTreg) and adaptability regulatory T cells (iTreg). nTreg cells reduce immune responses, induce immune tolerances and suppress occurrence of autoimmune diseases. Injections of naive T cells without CD4+ and CD25+ Treg cells into mice with T cell defection induce high responses to symbiotic bacteria in intestine, leading to autoimmune colitis. While injections of all T cells inhibit inflammation [45]. When CD4+ and CD25+ Treg cells are injected into mice models of IBD pathological injuries, these cells are recruited to intestinal lymphatic tissues and lamina propria, and then transfer into spleen to play a role in immune regulation and treatments of IBD [46]. Treg cells reductions are also found in peripheral blood and colonic mucosa of IBD patients [47], suggesting that reductions of Treg cells are associated with pathogenesis of IBD [48].
Transformation imbalance of Th17/Treg cells
Th17 cells and Treg cells are in dynamic equilibrium under normal circumstances. The balance is broken with over-increases of Th17 cells, over-enhancements of immunogenicity and decreases or abnormal functions of Treg cells, causing intestinal mucosal damages. The original T cells differentiate into Th17 cells when concentrations of TGF-β are low with the presence of IL-6, and inhibit generation of Treg cells. Productions of Th17 cells are inhibited when TGF-β is at a high concentration, and meanwhile generation of Treg cells is promoted [49]. Although Treg cells effectively repair inflammatory bowel mucosa of IBD patients, they transform into pathogenic Th17 cells with presence of IL-6 and/or IL-23 [50]. There are no reports found about Th17 cells transforming into Treg cells, indicating that conversions of Treg cells into Th17 cells are irreversible [1]. Th17 cells increase while Treg cells decrease in peripheral blood of IBD patients. The ratio of Th17/Treg is significantly higher, suggesting that transformation imbalance of Th17/Treg plays an important role on pathogenesis of IBD [51]. Therefore, regulation of balance between Th17/Treg may become a new method for treatments of IBD.
Anyhow, abnormal innate immune responses induce adaptive immunity imbalance, inflammatory cytokines produced by which increase innate immune damages, abate intestinal barrier functions and aggravate inflammation, and thus a vicious cycle forms. Comprehensive analyses of innate and adaptive immunity and exploration of the “bridge” between them exhibit an important guiding significance for treatments of IBD.
Disclosure of conflict of interest
None.
References
- 1.Geremia A, Biancheri P, Allan P, Corazza GR, Di Sabatino A. Innate and adaptive immunity in inflammatory bowel disease. Autoimmun Rev. 2014;13:3–10. doi: 10.1016/j.autrev.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 2.Bernstein CN, Wajda A, Blanchard JF. The clustering of other chronic inflammatory diseases in inflammatory bowel disease: a population-based study. Gastroenterology. 2005;129:827–836. doi: 10.1053/j.gastro.2005.06.021. [DOI] [PubMed] [Google Scholar]
- 3.Molodecky NA, Soon IS, Rabi DM, Ghali WA, Ferris M, Chernoff G, Benchimol EI, Panaccione R, Ghosh S, Barkema HW, Kaplan GG. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology. 2012;142:46–54. e42. doi: 10.1053/j.gastro.2011.10.001. quiz e30. [DOI] [PubMed] [Google Scholar]
- 4.Shale M, Ghosh S. How intestinal epithelial cells tolerise dendritic cells and its relevance to inflammatory bowel disease. Gut. 2009;58:1291–1299. doi: 10.1136/gut.2006.098475. [DOI] [PubMed] [Google Scholar]
- 5.McCurley N, Hirano M, Das S, Cooper MD. Immune related genes underpin the evolution of adaptive immunity in jawless vertebrates. Curr Genomics. 2012;13:86–94. doi: 10.2174/138920212799860670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xavier RJ, Podolsky DK. Unravelling the pathogenesis of inflammatory bowel disease. Nature. 2007;448:427–434. doi: 10.1038/nature06005. [DOI] [PubMed] [Google Scholar]
- 7.Uehara A, Fujimoto Y, Fukase K, Takada H. Various human epithelial cells express functional Toll-like receptors, NOD1 and NOD2 to produce anti-microbial peptides, but not proinflammatory cytokines. Mol Immunol. 2007;44:3100–3111. doi: 10.1016/j.molimm.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 8.Bouma G, Strober W. The immunological and genetic basis of inflammatory bowel disease. Nat Rev Immunol. 2003;3:521–533. doi: 10.1038/nri1132. [DOI] [PubMed] [Google Scholar]
- 9.Huang Y, Xiao S, Jiang Q. Role of Rho kinase signal pathway in inflammatory bowel disease. Int J Clin Exp Med. 2015;8:3089–3097. [PMC free article] [PubMed] [Google Scholar]
- 10.Gassler N, Rohr C, Schneider A, Kartenbeck J, Bach A, Obermuller N, Otto HF, Autschbach F. Inflammatory bowel disease is associated with changes of enterocytic junctions. Am J Physiol Gastrointest Liver Physiol. 2001;281:G216–228. doi: 10.1152/ajpgi.2001.281.1.G216. [DOI] [PubMed] [Google Scholar]
- 11.Shen L, Su L, Turner JR. Mechanisms and functional implications of intestinal barrier defects. Dig Dis. 2009;27:443–449. doi: 10.1159/000233282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu L, Liang L. Inflammatory bowel disease and intestinal mucosal immunity cells. World Chin J Digestol. 2008;16:3181–3186. [Google Scholar]
- 13.Schenk M, Bouchon A, Seibold F, Mueller C. TREM-1--expressing intestinal macrophages crucially amplify chronic inflammation in experimental colitis and inflammatory bowel diseases. J Clin Invest. 2007;117:3097–3106. doi: 10.1172/JCI30602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baj-Krzyworzeka M, Szatanek R, Weglarczyk K, Baran J, Urbanowicz B, Branski P, Ratajczak MZ, Zembala M. Tumour-derived microvesicles carry several surface determinants and mRNA of tumour cells and transfer some of these determinants to monocytes. Cancer Immunol Immunother. 2006;55:808–818. doi: 10.1007/s00262-005-0075-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Drakes ML, Blanchard TG, Czinn SJ. Colon lamina propria dendritic cells induce a proinflammatory cytokine response in lamina propria T cells in the SCID mouse model of colitis. J Leukoc Biol. 2005;78:1291–1300. doi: 10.1189/jlb.0605342. [DOI] [PubMed] [Google Scholar]
- 16.Darrasse-Jeze G, Deroubaix S, Mouquet H, Victora GD, Eisenreich T, Yao KH, Masilamani RF, Dustin ML, Rudensky A, Liu K, Nussenzweig MC. Feedback control of regulatory T cell homeostasis by dendritic cells in vivo. J Exp Med. 2009;206:1853–1862. doi: 10.1084/jem.20090746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Colucci F, Caligiuri MA, Di Santo JP. What does it take to make a natural killer? Nat Rev Immunol. 2003;3:413–425. doi: 10.1038/nri1088. [DOI] [PubMed] [Google Scholar]
- 18.Shen M, Liu Z. NK cells activation and effect response in inflammatory bowel diseases patients. World Chin J Digestol. 2008;16:3173–3177. [Google Scholar]
- 19.Liu Z, Jiu J. IL-21 receptor signaling enhances NKcell cytolytic activity and induces proinflammatory cytokine production in inflammatory bowel disease. Gastroenterol. 2008;134:A645. [Google Scholar]
- 20.Zenewicz LA, Yancopoulos GD, Valenzuela DM, Murphy AJ, Stevens S, Flavell RA. Innate and adaptive interleukin-22 protects mice from inflammatory bowel disease. Immunity. 2008;29:947–957. doi: 10.1016/j.immuni.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang L, Li Y. Dendritic cells and inflammatory bowel disease. Int Diges Dis J. 2007;27:355–363. [Google Scholar]
- 22.Ganz T. Defensins: antimicrobial peptides of innate immunity. Nat Rev Immunol. 2003;3:710–720. doi: 10.1038/nri1180. [DOI] [PubMed] [Google Scholar]
- 23.Yang D, Chertov O, Bykovskaia SN, Chen Q, Buffo MJ, Shogan J, Anderson M, Schroder JM, Wang JM, Howard OM, Oppenheim JJ. Beta-defensins: linking innate and adaptive immunity through dendritic and T cell CCR6. Science. 1999;286:525–528. doi: 10.1126/science.286.5439.525. [DOI] [PubMed] [Google Scholar]
- 24.Wehkamp J, Harder J, Weichenthal M, Schwab M, Schaffeler E, Schlee M, Herrlinger KR, Stallmach A, Noack F, Fritz P, Schroder JM, Bevins CL, Fellermann K, Stange EF. NOD2 (CARD15) mutations in Crohn’s disease are associated with diminished mucosal alpha-defensin expression. Gut. 2004;53:1658–1664. doi: 10.1136/gut.2003.032805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fahlgren A, Hammarstrom S, Danielsson A, Hammarstrom ML. beta-Defensin-3 and -4 in intestinal epithelial cells display increased mRNA expression in ulcerative colitis. Clin Exp Immunol. 2004;137:379–385. doi: 10.1111/j.1365-2249.2004.02543.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wehkamp J, Harder J, Weichenthal M, Mueller O, Herrlinger KR, Fellermann K, Schroeder JM, Stange EF. Inducible and constitutive beta-defensins are differentially expressed in Crohn’s disease and ulcerative colitis. Inflamm Bowel Dis. 2003;9:215–223. doi: 10.1097/00054725-200307000-00001. [DOI] [PubMed] [Google Scholar]
- 27.Wehkamp J, Fellermann K, Stange EF. Human defensins in Crohn’s disease. Chem Immunol Allergy. 2005;86:42–54. doi: 10.1159/000086672. [DOI] [PubMed] [Google Scholar]
- 28.Fahlgren A, Hammarstrom S, Danielsson A, Hammarstrom ML. Increased expression of antimicrobial peptides and lysozyme in colonic epithelial cells of patients with ulcerative colitis. Clin Exp Immunol. 2003;131:90–101. doi: 10.1046/j.1365-2249.2003.02035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fellermann K, Wehkamp J, Herrlinger KR, Stange EF. Crohn’s disease: a defensin deficiency syndrome? Eur J Gastroenterol Hepatol. 2003;15:627–634. doi: 10.1097/00042737-200306000-00008. [DOI] [PubMed] [Google Scholar]
- 30.Wehkamp J, Schmid M, Fellermann K, Stange EF. Defensin deficiency, intestinal microbes, and the clinical phenotypes of Crohn’s disease. J Leukoc Biol. 2005;77:460–465. doi: 10.1189/jlb.0904543. [DOI] [PubMed] [Google Scholar]
- 31.Cario E, Podolsky DK. Differential alteration in intestinal epithelial cell expression of toll-like receptor 3 (TLR3) and TLR4 in inflammatory bowel disease. Infect Immun. 2000;68:7010–7017. doi: 10.1128/iai.68.12.7010-7017.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Toiyama Y, Araki T, Yoshiyama S, Hiro J, Miki C, Kusunoki M. The expression patterns of Toll-like receptors in the ileal pouch mucosa of postoperative ulcerative colitis patients. Surg Today. 2006;36:287–290. doi: 10.1007/s00595-005-3144-y. [DOI] [PubMed] [Google Scholar]
- 33.Franchimont D, Vermeire S, El Housni H, Pierik M, Van Steen K, Gustot T, Quertinmont E, Abramowicz M, Van Gossum A, Deviere J, Rutgeerts P. Deficient host-bacteria interactions in inflammatory bowel disease? The toll-like receptor (TLR)-4 Asp299gly polymorphism is associated with Crohn’s disease and ulcerative colitis. Gut. 2004;53:987–992. doi: 10.1136/gut.2003.030205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van Beelen AJ, Zelinkova Z, Taanman-Kueter EW, Muller FJ, Hommes DW, Zaat SA, Kapsenberg ML, de Jong EC. Stimulation of the intracellular bacterial sensor NOD2 programs dendritic cells to promote interleukin-17 production in human memory T cells. Immunity. 2007;27:660–669. doi: 10.1016/j.immuni.2007.08.013. [DOI] [PubMed] [Google Scholar]
- 35.Souza HS, Tortori CJ, Castelo-Branco MT, Carvalho AT, Margallo VS, Delgado CF, Dines I, Elia CC. Apoptosis in the intestinal mucosa of patients with inflammatory bowel disease: evidence of altered expression of FasL and perforin cytotoxic pathways. Int J Colorectal Dis. 2005;20:277–286. doi: 10.1007/s00384-004-0639-8. [DOI] [PubMed] [Google Scholar]
- 36.Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, Cua DJ, Littman DR. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 37.Allez M, Brimnes J, Shao L, Dotan I, Nakazawa A, Mayer L. Activation of a unique population of CD8(+) T cells by intestinal epithelial cells. Ann N Y Acad Sci. 2004;1029:22–35. doi: 10.1196/annals.1309.004. [DOI] [PubMed] [Google Scholar]
- 38.Strober W, Fuss IJ. Proinflammatory cytokines in the pathogenesis of inflammatory bowel diseases. Gastroenterology. 2011;140:1756–1767. doi: 10.1053/j.gastro.2011.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pang Y, Zheng C. Relationship between Thl/Th2 cells and inflammatory bowel disease. World Chin J Digestol. 2004;12:1922–1924. [Google Scholar]
- 40.Kanai T, Kawamura T, Dohi T, Makita S, Nemoto Y, Totsuka T, Watanabe M. TH1/TH2-mediated colitis induced by adoptive transfer of CD4+CD45RBhigh T lymphocytes into nude mice. Inflamm Bowel Dis. 2006;12:89–99. doi: 10.1097/01.MIB.0000197237.21387.mL. [DOI] [PubMed] [Google Scholar]
- 41.Xie C, Zhuang Y, Luan Y. The research progress of the immune factors in the pathogenesis of ulcerative colitis. Cellul Mol Immunol. 2013;29:889–892. [Google Scholar]
- 42.Fujino S, Andoh A, Bamba S, Ogawa A, Hata K, Araki Y, Bamba T, Fujiyama Y. Increased expression of interleukin 17 in inflammatory bowel disease. Gut. 2003;52:65–70. doi: 10.1136/gut.52.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kobayashi T, Okamoto S, Hisamatsu T, Kamada N, Chinen H, Saito R, Kitazume MT, Nakazawa A, Sugita A, Koganei K, Isobe K, Hibi T. IL23 differentially regulates the Th1/Th17 balance in ulcerative colitis and Crohn’s disease. Gut. 2008;57:1682–1689. doi: 10.1136/gut.2007.135053. [DOI] [PubMed] [Google Scholar]
- 44.Seiderer J, Elben I, Diegelmann J, Glas J, Stallhofer J, Tillack C, Pfennig S, Jurgens M, Schmechel S, Konrad A, Goke B, Ochsenkuhn T, Muller-Myhsok B, Lohse P, Brand S. Role of the novel Th17 cytokine IL-17F in inflammatory bowel disease (IBD): upregulated colonic IL-17F expression in active Crohn’s disease and analysis of the IL17F p. His161Arg polymorphism in IBD. Inflamm Bowel Dis. 2008;14:437–445. doi: 10.1002/ibd.20339. [DOI] [PubMed] [Google Scholar]
- 45.O’Garra A, Vieira P. Regulatory T cells and mechanisms of immune system control. Nat Med. 2004;10:801–805. doi: 10.1038/nm0804-801. [DOI] [PubMed] [Google Scholar]
- 46.Izcue A, Coombes JL, Powrie F. Regulatory T cells suppress systemic and mucosal immune activation to control intestinal inflammation. Immunol Rev. 2006;212:256–271. doi: 10.1111/j.0105-2896.2006.00423.x. [DOI] [PubMed] [Google Scholar]
- 47.Boden EK, Snapper SB. Regulatory T cells in inflammatory bowel disease. Curr Opin Gastroenterol. 2008;24:733–741. doi: 10.1097/mog.0b013e328311f26e. [DOI] [PubMed] [Google Scholar]
- 48.Li Y, Sun K, Zhao C. Mononuclear cells Foxp3 mRNA expression level in peripheral blood of ulcerative colitis patients. World Chin J Digestol. 2006;14:810–813. [Google Scholar]
- 49.Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 Cells. Annu Rev Immunol. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- 50.Kitani A, Xu L. Regulatory T cells and the induction of IL-17. Mucosal Immunol. 2008;1(Suppl 1):S43–46. doi: 10.1038/mi.2008.51. [DOI] [PubMed] [Google Scholar]
- 51.Elson CO, Cong Y, Weaver CT, Schoeb TR, McClanahan TK, Fick RB, Kastelein RA. Monoclonal anti-interleukin 23 reverses active colitis in a T cell-mediated model in mice. Gastroenterology. 2007;132:2359–2370. doi: 10.1053/j.gastro.2007.03.104. [DOI] [PubMed] [Google Scholar]


