Abstract
Non-small cell lung cancer (NSCLC) is one of the leading causes of cancer-related deaths in the world. F-box/WD repeat-containing protein 7 (FBW7) plays important roles in human cancers, such as gastric cancer, breast cancer, and hepatocellular carcinoma. In this study, we found that high levels of FBW7 expression were associated with increased doxorubicin sensitivity in NSCLC cells. Down-regulation of FBW7 reduced the chemosensitivity in tumor cells. Twist is a critical transcription factor in epithelial-mesenchymal transition (EMT), and NSCLC cells with silenced Twist showed increased doxorubicin sensitivity. Treatment of cells with doxorubicin or hypoxia was shown to trigger EMT as evidenced by decreased E-cadherin and increased Vimentin. In contrast, ectopic expression of FBW7 prevented doxorubicin-or hypoxia-induced EMT. In addition, FBW7 was identified as a functional target of miR-223 in NSCLC cells. These findings define a critical role of miR-223/FBW7 pathway in regulating EMT and chemosensitivity in NSCLC cells.
Keywords: Non-small cell lung cancer, drug resistance, miR-223, FBW7, epithelial-mesenchymal transition
Introduction
Although a large number of clinical trials are performed aimed at improving patient survival, lung cancer has still remained as the leading cause of cancer-related death in the world [1]. Approximately 80% of all lung cancer cases are categorized as non-small cell lung cancer (NSCLC), which is typically diagnosed at advanced stages [2]. Despite recent advances in diagnosis and treatment, the prognosis for NSCLC is still unsatisfactory due to the low rate of 5-year overall survival [3]. Up to date, surgical resection and chemotherapy have been the most frequently options for NSCLC. However, drug resistance has become a great bottleneck as a result of increased application of chemotherapeutics [4]. Therefore, it is urgent to elucidate the molecular mechanisms underlying the drug resistance in NSCLC.
F-box/WD repeat-containing protein 7 (FBW7) is a member of F-box protein family located at 4q31 in humans. It is the substrate recognition component of an evolutionarily conserved SCF (SKP1, CUL1, and F-box protein) E3 ligase complex [5]. Several studies point out that FBW7 play a critical roles in regulation of multiple oncoprotein substrates such as Notch, c-Myc, cyclin E and c-Jun [6,7]. Thus, the altered expression of FBW7 has been considered to be one of the major causes involved in cancer initiation and progression. Previous study suggested that reduced expression of FBW7 was associated with poor prognoses in glioma, breast cancer, and gastric cancer [8-10]. Conversely, overexpression of FBW7 was reported to suppress cell proliferation and promote apoptosis in osteosarcoma cells, suggesting that FBW7 may be a potential tumor suppressor in human tumorigenesis [11]. In addition, FBW7 has been identified to be a target of miRNAs including miR-92a, miR-223, and miR-25, highlighting the post-transcriptional regulation of FBW7 in cancer pathogenesis [12].
It is commonly accepted that carcinogenesis of lung cancer is a multistep process regulated by aberrantly protein expression and alterations of morphological and molecular features during malignant progression [13]. Initiation of epithelial-mesenchymal transition (EMT) is a critical procedure in regulation of drug resistance in lung cancers [14]. In the current study, we aimed to investigate the miR-223/FBW7 axis in regulation of EMT and chemosensitivity in NSCLC.
Materials and methods
Cell culture
Human NSLCL cell lines A549, NCI-H358, NCI-H1299 and HCC827 were purchased from the ATCC (Manassas, VA, USA) and cultured in DMEM (Gibco, Carlsbad, CA, USA) supplemented with 10% FBS and 1% penicillin/streptomycin. All cells were maintained at 37°C in 5% CO2 incubator.
CCK-8 analysis
Cultured cells were seeded onto 96-well plates at 3000 cells/well. After 48 h, 10 µL/well CCK8 solution (Dojindo, Kumamoto, Japan) was added, the plates incubated for 3 h, and absorbance was measured at 450 nm using an MRX II microplate reader (Dynex, Chantilly, VA, USA).
EdU incorporation assay
Cell growth was determined as a percentage of untreated control. Measurement of inhibitive rate of cell proliferation was carried out using a Click-iT EdU Imaging Kit following the manufacture’s instruction.
Real time PCR
miR-223 expression was measured by real time PCR and normalized to that of U6.
Real time-PCR reactions were run using ABI 7500. The relative amounts of miR-223 was determined using the 2-ΔΔCT method and the reactions were performed in triplicate.
Immunofluorescence
NSCLC cells were washed with PBS, fixed in 4% paraformaldehyde, and incubated in 3% H2O2 at 37°C. After incubation with anti-E-cadherin or anti-Vimentin (Abcam, Cambridge, MA, USA) antibodies, the cells were washed with PBS and incubated with the appropriate secondary antibody (Abcam, Cambridge, MA, USA) for 1 h at room temperature. Cell nuclei were stained with DAPI (Sigma) and cells were observed using fluorescence confocal microscopy (Olympus, Tokyo, Japan).
Western blot analysis
Cells were harvested and washed twice in phosphate-buffered saline and lysed in lysis buffer (Cell Signaling, Danvers, MA, USA). 10 mg protein sample was subjected to 10% SDS-PAGE, transferred onto a polyvinylidene difluoride membrane (Millipore, Billerica, MA, USA), and incubated with monoclonal antibodies against FBW7, E-cadherin, Vimentin, Twist, and GAPDH (Abcam, Cambridge, MA, USA). The membranes were incubated with secondary antibodies and signals were detected using the ECL Detection System (GE Healthcare, Little Chalfont, UK).
Statistical analysis
All experiments were repeated at least three times. Data were expressed as the means ± SD. A P-value <0.05 was considered as statistically significant. All statistical analyses were performed using the SPSS 16 software program (SPSS, Inc., Chicago, IL, USA).
Results
Different FBW7 expression in NSCLC cells
Firstly, we examined the cell viabilities of four NSCLC cell lines including A549, NCI-H358, NCI-H1299 and HCC827 incubated with doxorubicin for 48 h. Data from CCK-8 assay showed that doxorubicin sensitivity varied among different cell lines, i.e., HCC827>NCI-H358>A549>NCI-H1299 (Figure 1A).
Figure 1.
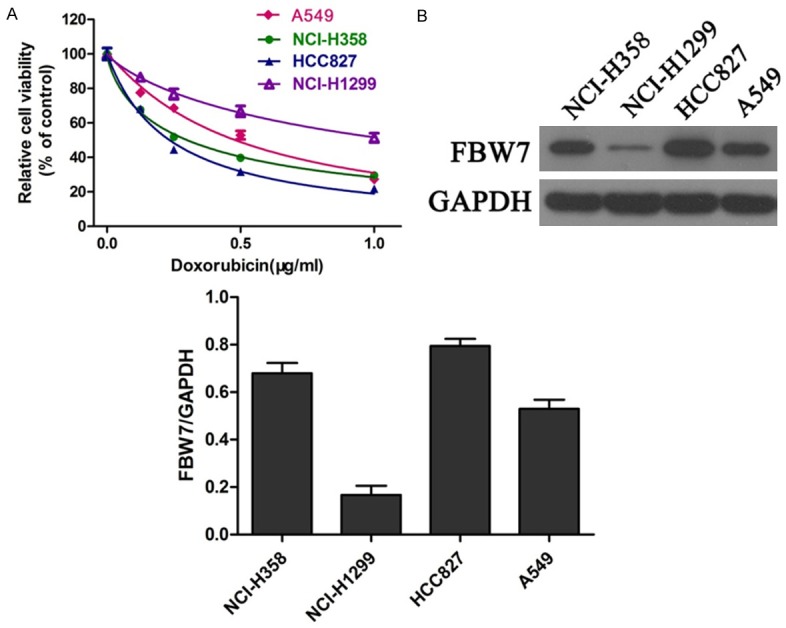
Different FBW7 expression in NSCLC cells. NSCLC cells were exposed to doxorubicin for 48 h. Cell viability was determined using CCK-8 assay (A). Protein expression of FBW7 was measured by western blot in four NSCLC cell lines (B).
In addition, the protein expression of FBW7 was examined in different cell lines incubated with doxorubicin. Interestingly, western blot revealed that FBW7 expression was significantly higher in HCC827 cells compared with NCI-H358, A549 and NCI-H1299 cells. Conversely, NCI-H1299 cells showed the lowest FBW7 level among these four cell lines (Figure 1B). Collectively, these data implied that FBW7 may be involved in doxorubicin sensitivity in NSCLC cells.
Effects of doxorubicin on EMT in NSCLC cells
In order to detect whether doxorubicin incubation could trigger EMT in lung cancer cells, the expression of epithelial/mesenchymal markers were examined by western blot and immunofluorescence. As shown in Figure 2A, the protein expression of E-cadherin was obviously reduced in the presence of doxorubicin. On the contrary, doxorubicin treatment dramatically promoted the expression of Vimentin in four NSCLC cell lines (A549, NCI-H358, HCC827, NCI-H1299). Consistently, immunofluorescent staining also showed that incubation with doxorubicin led to down-regulation of E-cadherin (epithelial marker) and up-regulation of Vimentin (mesenchymal marker) (Figure 2B). These results indicated that doxorubicin could induce EMT in lung cancer cells.
Figure 2.

Doxorubicin induced EMT in NSCLC cells. EMT markers including E-cadherin and Vimentin were determined by western blot (A) and immunofluorescence (B) after treatment with doxorubicin for 48 h. *P<0.05.
Down-regulation of FBW7 reduced the doxorubicin sensitivity in NSCLC cells
In order to confirm the role of FBW7 in doxorubicin sensitivity, four NSCLC cell lines were transfected with FBW7-siRNA to interfere with FBW7 expression. Data from CCK-8 assay revealed that cells treated with FBW7-siRNA showed a reduced sensitivity to doxorubicin compared with control group, indicating that down-regulation of FBW7 decreased the drug cytotoxocity in NSCLC cells (Figure 3A-D). Consistently, EdU incorporation assay also revealed the attenuated chemosensitivity of doxorubicin after down-regulation of FBW7 in NSCLC cells (Figure 3E-H).
Figure 3.
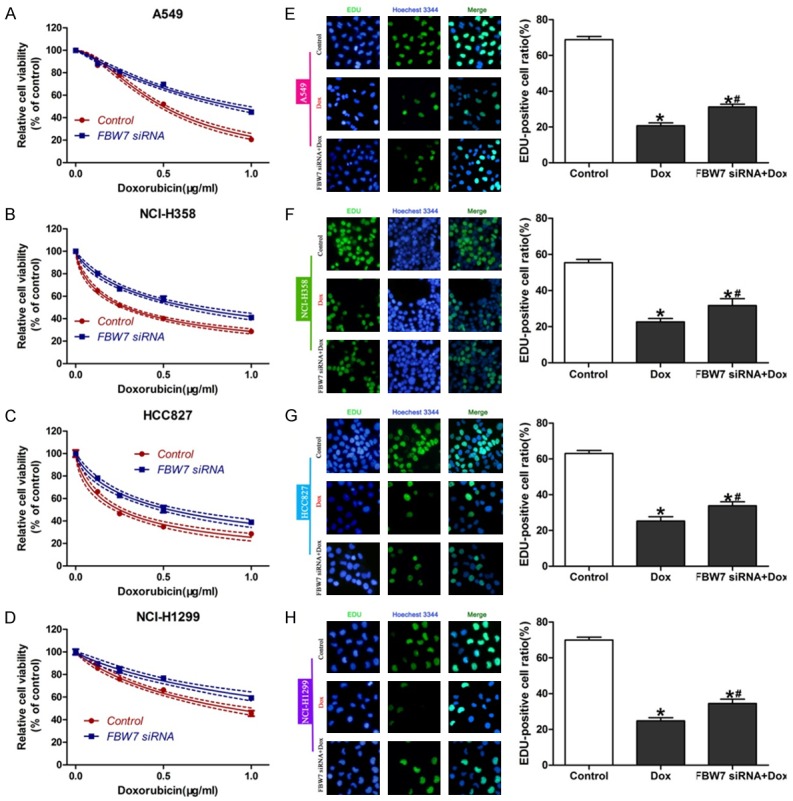
FBW7 knockdown reduced doxorubicin sensitivity. Four NSCLC cell lines were transfected with FBW7 siRNA. CCK-8 assay (A-D) and Edu incorporation assay (E-H) were performed to detect cell growth and DNA synthesis. *P<0.05, compared with control; #P<0.05, compared with Dox.
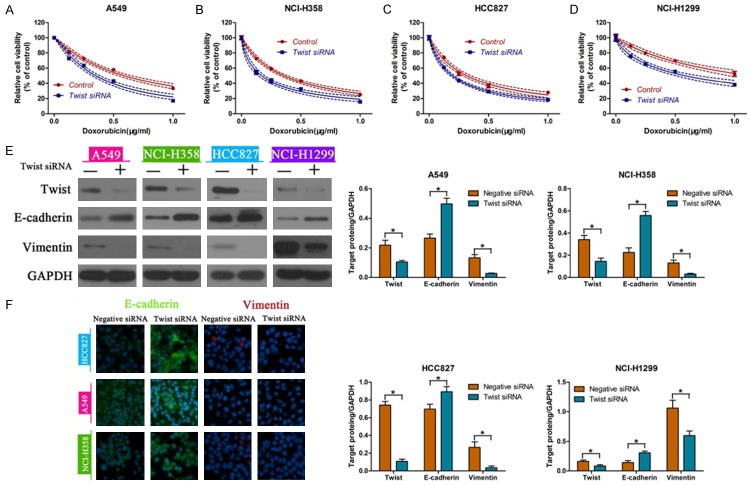
Twist knockd own enhanced the doxorubicin sensitivity in NSCLC cells
Since Twist has been shown to be a critical transcription factor in EMT, we analyzed the changes in doxorubicin sensitivity in NSCLC cells treated with or without Twist-siRNA. We observed that the cellular responses to doxorubicin were remarkably increased in four NSCLC cells treated with Twist siRNA (Figure 4A-D). In addition, western blot showed that knockdown of Twist promoted the expression of E-cadherin, and inhibited the Vimentin levels in A549, NCI-H358, HCC827, NCI-H1299 cells (Figure 4E). Consistently, immunofluorescence staining indicated the up-regulation of E-cadherin and down-regulation of Vimentin in NSCLC cells with silenced Twist (Figure 4F).
Figure 4.
Down-regulation of Twist enhanced the doxorubicin sensitivity. Analysis of cell viability after Twist siRNA transfection into A549 (A), NCI-H358 (B), HCC827 (C), NCI-H1299 (D). E-cadherin and Vimentin were determined by western blot (E) and immunofluorescence (F) in Twist siRNA transfected NSCLC cells. *P<0.05.
FBW7 reversed EMT in NSCLC cells under hypoxic conditions
Hypoxia could induce EMT in a variety of tumor cells. We found that NCI-H358 and HCC827 cells become more resistant to doxorubicin under hypoxic conditions. However, ectopic expression of FBW7 recovered the doxorubicin sensitivity in NSCLC cells even under hypoxia conditions (Figure 5A and 5B). We also found that hypoxic treatment led to down-regulation of FBW7 and E-cadherin and up-regulation of Vimentin in NCI-H358 and HCC827 cells. Conversely, we detected no obvious differences in the expression of EMT markers after overexpression of FBW7 in NSCLC cells even under hypoxia conditions (Figure 5C). In addition, immunofluorescence staining also revealed that hypoxia triggered EMT in NSCLC cells, which was reversed by ectopic expression of FBW7 (Figure 5D). Taken together, these data suggested that FBW7 could reverse hypoxia-induced EMT in NSCLC cells.
Figure 5.
FBW7 reversed EMT under hypoxic conditions. Determination of cell viability in NCI-H358 (A) and HCC827 (B) cells transfected with or without FBW7 plasmids under hypoxic conditions. The expression levels of EMT-related markers E-cadherin and Vimentin were determined by western blot (C) and immunofluorescence (D) in FBW7 plasmids transfected NSCLC cells under hypoxic conditions.
FBW7 prevented doxorubicin-induced EMT in NSCLC cells
Western blot and immunofluorescence were performed to determine the expression of EMT-related markers in doxorubicin-treated cells with or without overexpression of FBW7. We found that incubation with doxorubicin alone led to a obvious reduction in the expression of FBW7 and contributed to EMT in NCI-H358 and HCC827 cells. Nevertheless, ectopic expression of FBW7 reversed EMT as defined by up-regulation of E-cadherin and down-regulation of Vimentin (Figure 6A). Additionally, immunofluorescence staining also showed that overexpression of FBW7 induced a dramatic decrease in E-cadherin and increase in Vimentin (Figure 6B). In order to ascertain the regulatory role of FBW7 on EMT, Twist-siRNA and FBW7-siRNA were transfected into NSCLC cells and down-regulation of Twist and FBW7 was confirmed by western blot (Figure 7C and 7D). As a result, we found no significant differences in cell viability treated with or without doxorubicin (Figure 7A and 7B). Collectively, these results indicated that FBW7 prevented EMT triggered by doxorubicin in NSCLC cells.
Figure 6.
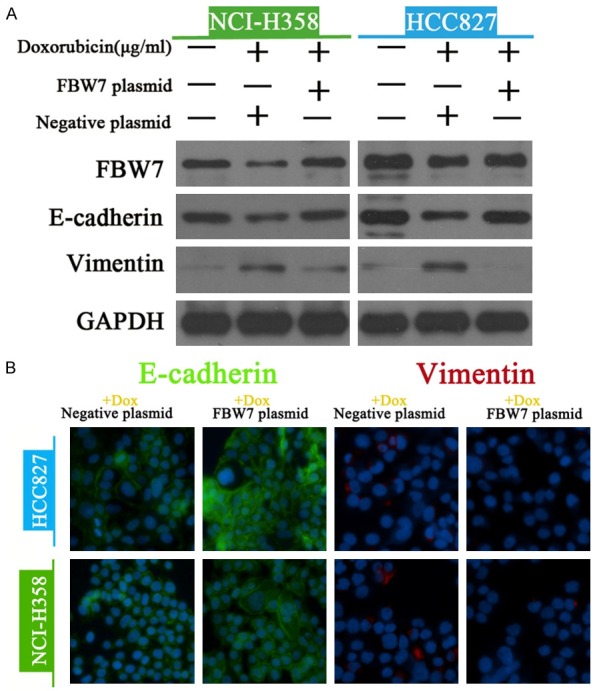
FBW7 prevented doxorubicin-induced EMT in NSCLC cells. The expression of FBW7 and EMT-related markers (E-cadherin and Vimentin) were measured western blot (A) and immunofluorescence (B) in doxorubicin-treated NSCLC cells with or without ectopic expression of FBW7.
Figure 7.
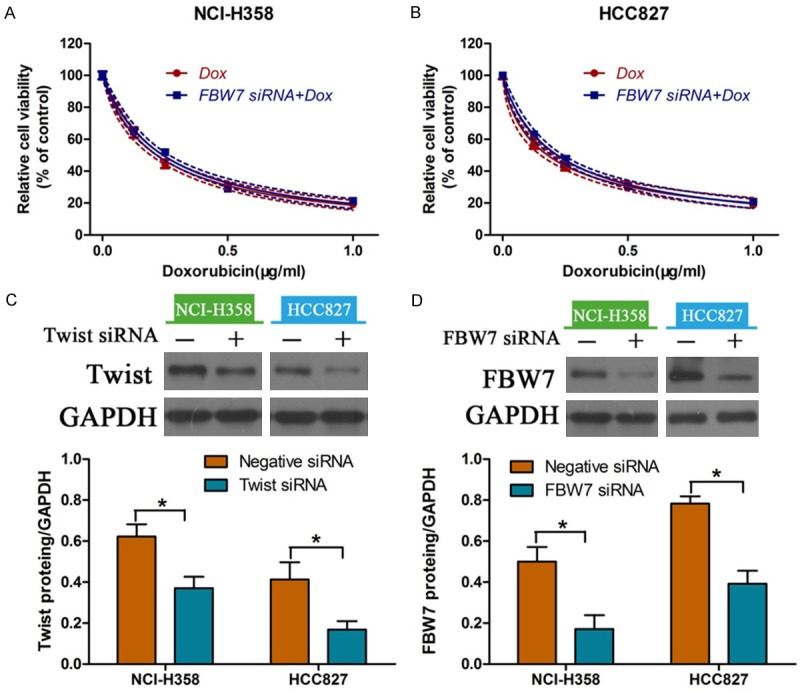
Effects of down-regulation of Twist and FBW7 on doxorubicin sensitivity. Determination of cell viability in NCI-H358 (A) and HCC827 (B) cells transfected with Twist-siRNA and FBW7-siRNA. Down-regulation of Twist (C) and FBW7 (D) as determined by western blot. *P<0.05.
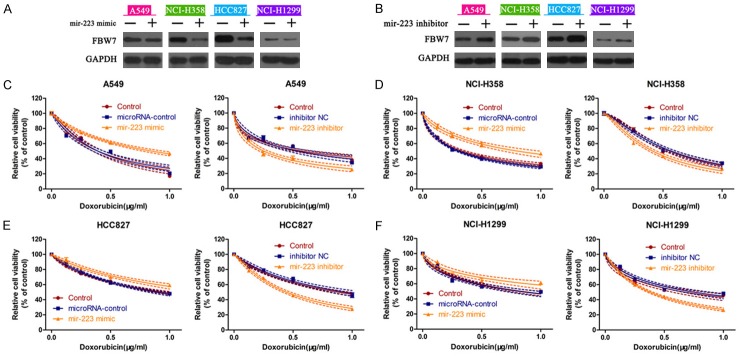
miR-223 targeted FBW7 in NSCLC cells
Bioinformatics analysis with Target scan software revealed that FBW7 was a potential target gene of miR-223 (Figure S1). Four NSCLC cell lines were used to evaluate the effects of miR-223 on FBW7 expression. We found that FBW7 protein expression was obviously decreased when cells were transfected with miR-223 mimic, compared with those transfected with the negative control (Figure 8A). Conversely, transfection of miR-223 inhibitor into cells enhanced the expression of FBW7 in A549, NCI-H358, NCI-H1299 and HCC827 cells (Figure 8B). Furthermore, we determined the role of miR-223 on cellular responses to doxorubicin by CCK-8 assay. miR-223 mimic-treated cells exhibited lower sensitivity to doxorubicin, while miR-223 inhibitor increased the doxorubicin sensitivity in A549 cells (Figure 8C). Similar results were also observed in NCI-H358 (Figure 8D), HCC827 (Figure 8E) and NCI-H1299 (Figure 8F) cells. In addition, miR-223 was differently expressed in NSCLC cells (i.e. HCC827>NCI-H358>A549>NCI-H1299) (Figure 9A). We also found that the expression of miR-223 was positively correlated with E-cadherin and negatively related with Vimentin (Figure 9B). Treatment of cells with doxorubicin inhibited the expression of miR-223 in four tumor cell lines, indicating the regulatory role of miR-223 in EMT pattern (Figure 9C). Furthermore, HCC827 and NCI-H358 cells with silenced FBW7 showed no significant differences to doxorubicin with or without miR-223 inhibitor (Figure 9D). Taken together, these data demonstrated that miR-223/FBW7 regulated cellular phenotype and chemosensitivity in NSCLC cells.
Figure 8.
miR-223 targeted FBW7 in NSCLC cells. Four NSCLC cells were incubated with miR-223 mimic or miR-223 inhibitor. The expression of FBW7 was measured by western blot (A and B). CCK-8 assay was then performed to determine the changes in cell viability in A549 (C), NCI-H358 (D), HCC827 (E) and NCI-H1299 (F) cells.
Figure 9.

Doxorubicin treatment decreased the expression of miR-223. Expression levels of miR-223 (A) and EMT markers (B) in four NSCLC cell lines. (C) After doxorubicin treatment, miR-223 was measured using real time PCR. (D) Measurement of cell viability in NCI-H358 and HCC827 cells transfected FBW7-siRNA and miR-223 inhibitor. *P<0.05.
Discussion
Chemotherapy has been shown to be effective in the postoperative therapy of a variety of cancers. Nevertheless, acquisition of drug resistance to the currently available chemotherapeutics becomes a bottleneck for successful chemotherapy [4]. Hence, it is urgent to find novel therapeutic targets to reverse drug resistance. In the present study, we found that miR-223-FBW7 axis increased the doxorubicin sensitivity via regulation of EMT pattern in NSCLC cells.
FBW7 is a F-box-containing protein in the SCF E3 ligase complex, which determines target specificity via recognition and binding of target proteins for ubiquitination and degradation [7,15]. A variety of studies have demonstrated that FBW7 is a potential tumor suppressor and responsible for carcinogenesis or cancer development through regulation of cellular processes including cell growth, apoptosis, migration and cell cycle [16-18]. In our study, HCC827 cells were most sensitive to doxorubicin among four NSCLC cell lines. Interestingly, the protein level of FBW7 was obviously higher in HCC827 cells compared with that in NCI-H358, A549 and NCI-H1299 cells. These findings led us to hypothesize that FBW7 might be involved in the doxorubicin cytotoxicity in NSCLC cells. Furthermore, FBW7 deficiency is considered to be involved in drug resistance in human cancers [19]. A previous study also supported the role of FBW7 in regulation of chemotherapeutic sensitivity and prognosis in NSCLC [20]. Consistently, we transfected NSCLC cells with FBW7-siRNA, resulting in a reduced drug sensitivity in all the four tumor cell lines. Collectively, these results indicated that FBW7 played an important role in drug resistance of NSCLC cells.
A large amount of evidences support the critical role of EMT in the modulation of tissue regeneration, embryonic development, inflammatory response, and tumor invasion [21,22]. In addition, it has also been well-recognized that EMT is one of the critical causes of drug resistance in cancers [23]. EMT consists a complex and reversible events which result in the acquisition of a mesenchymal phenotype and loss of epithelial cell adhesion [22]. We found that incubation with doxorubicin could induce EMT in NSCLC cells, and knockdown of Twist, an important E-cadherin repressor, prevented EMT as evidenced by up-regulation of E-cadherin and down-regulation of Vimentin.
F-box proteins are reported to be implicated in EMT via regulating its transcription factors and controlling its inducers [24,25]. A recent study suggested that FBW7 inhibited EMT and metastatic potential of cholangiocarcinoma cells [26]. Up-regulation of FBW7 altered EMT patterns and thus enhanced chemotherapeutics cytotoxicity in NSCLC cells and hepatocellular carcinoma cells [27,28]. In our study, tumor cells become more resistant to doxorubicin under hypoxia, which is a stimulator of EMT. Meanwhile, hypoxic treatment decreased the expression of FBW7 and E-cadherin, and increased the Vimentin level. On the contrary, plasmid-mediated upregulation of FBW7 led to no differences in the expression of EMT markers and doxorubicin sensitivity. In addition, ectopic expression of FBW7 also reversed doxorubicin-induced EMT as defined by a reduction in E-cadherin and an increase in Vimentin. siRNA-mediated silencing of FBW7 and Twist had no effects on doxorubicin sensitivity. These results suggested that FBW7 regulated EMT patterns in NSCLC cells.
miRNAs, a class of noncoding endogenous RNAs with 18-24 nt length, regulate gene expression by post-transcriptional inhibition of mRNA [29]. They have been recognized as important markers in diagnostic, prognostic and therapeutic applications in lung cancer [30,31]. It has been shown that miR-223 have critical roles in various human biological processes, such as viability, invasion, apoptosis and metastasis [32,33]. Both bioinformatics and experimentation confirmed that FBW7 is a functional target of miR-223 in gastric cancer, oesophageal squamous cell carcinoma and acute lymphoblastic leukemia [34-36]. In our study, FBW7 was decreased in miR-223 mimic-transfected cells. In contrast, transfection with miR-223 inhibitor enhanced FBW7 level, suggesting that FBW7 was a target gene of miR-223 in NSCLC cell. Moreover, miR-223 was most abundant in epithelial HCC827 cells and its expression was reduced after doxorubicin administration. In addition, down-regulation of FBW7 led to no significant differences to doxorubicin with or without miR-223 inhibitor, suggesting that miR-223/FBW7 modulated doxorubicin sensitivity in NSCLC cells.
In conclusion, our current study demonstrated that miR-223/FBW7 axis plays important roles in regulation of EMT and chemosensitivity in lung cancer cells. Thus, miR-223/FBXW7 signature might represent potential targets for therapeutic intervention in chemotherapy-resistant tumors.
Acknowledgements
This study was supported by the Natural Science Foundation of Zhejiang Province (LY15H160029), Projects of Medical and Health Technology Program of Zhejiang Province (2010KY101099), Projects of Education of Zhejiang Province (Y201223954 and Y201534626), Program of Medical Science and Technology of Zhejiang Province (2015KWB150) and Projects of Administration of Traditional Chinese Medicine of Zhejiang Province (2015ZA047).
Supporting Information
References
- 1.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.Lortet-Tieulent J, Soerjomataram I, Ferlay J, Rutherford M, Weiderpass E, Bray F. International trends in lung cancer incidence by histological subtype: adenocarcinoma stabilizing in men but still increasing in women. Lung Cancer. 2014;84:13–22. doi: 10.1016/j.lungcan.2014.01.009. [DOI] [PubMed] [Google Scholar]
- 3.Yang P. Epidemiology of lung cancer prognosis: quantity and quality of life. Methods Mol Biol. 2009;471:469–486. doi: 10.1007/978-1-59745-416-2_24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thomas RK. Overcoming drug resistance in ALK-rearranged lung cancer. N Engl J Med. 2014;370:1250–1251. doi: 10.1056/NEJMe1316173. [DOI] [PubMed] [Google Scholar]
- 5.Mao JH, Kim IJ, Wu D, Climent J, Kang HC, DelRosario R, Balmain A. FBXW7 targets mTOR for degradation and cooperates with PTEN in tumor suppression. Science. 2008;321:1499–1502. doi: 10.1126/science.1162981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Teixeira LK, Reed SI. Ubiquitin ligases and cell cycle control. Annu Rev Biochem. 2013;82:387–414. doi: 10.1146/annurev-biochem-060410-105307. [DOI] [PubMed] [Google Scholar]
- 7.Welcker M, Clurman BE. FBW7 ubiquitin ligase: a tumour suppressor at the crossroads of cell division, growth and differentiation. Nat Rev Cancer. 2008;8:83–93. doi: 10.1038/nrc2290. [DOI] [PubMed] [Google Scholar]
- 8.Hagedorn M, Delugin M, Abraldes I, Allain N, Belaud-Rotureau MA, Turmo M, Prigent C, Loiseau H, Bikfalvi A, Javerzat S. FBXW7/hCDC4 controls glioma cell proliferation in vitro and is a prognostic marker for survival in glioblastoma patients. Cell Div. 2007;2:9. doi: 10.1186/1747-1028-2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ibusuki M, Yamamoto Y, Shinriki S, Ando Y, Iwase H. Reduced expression of ubiquitin ligase FBXW7 mRNA is associated with poor prognosis in breast cancer patients. Cancer Sci. 2011;102:439–445. doi: 10.1111/j.1349-7006.2010.01801.x. [DOI] [PubMed] [Google Scholar]
- 10.Yokobori T, Mimori K, Iwatsuki M, Ishii H, Onoyama I, Fukagawa T, Kuwano H, Nakayama KI, Mori M. p53-Altered FBXW7 expression determines poor prognosis in gastric cancer cases. Cancer Res. 2009;69:3788–3794. doi: 10.1158/0008-5472.CAN-08-2846. [DOI] [PubMed] [Google Scholar]
- 11.Li Z, Xiao J, Hu K, Wang G, Li M, Zhang J, Cheng G. FBXW7 acts as an independent prognostic marker and inhibits tumor growth in human osteosarcoma. Int J Mol Sci. 2015;16:2294–2306. doi: 10.3390/ijms16022294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kitagawa K, Kitagawa M. The SCF-type E3 Ubiquitin Ligases as Cancer Targets. Curr Cancer Drug Targets. 2016;16:119–29. doi: 10.2174/1568009616666151112122231. [DOI] [PubMed] [Google Scholar]
- 13.Brambilla E, Gazdar A. Pathogenesis of lung cancer signalling pathways: roadmap for therapies. Eur Respir J. 2009;33:1485–1497. doi: 10.1183/09031936.00014009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu F, Li J, Jang C, Wang J, Xiong J. The role of Axl in drug resistance and epithelial-to-mesenchymal transition of non-small cell lung carcinoma. Int J Clin Exp Pathol. 2014;7:6653–6661. [PMC free article] [PubMed] [Google Scholar]
- 15.Wang Z, Inuzuka H, Zhong J, Wan L, Fukushima H, Sarkar FH, Wei W. Tumor suppressor functions of FBW7 in cancer development and progression. FEBS Lett. 2012;586:1409–1418. doi: 10.1016/j.febslet.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen J, Wang H, Wang J, Huang S, Zhang W. STAT1 inhibits human hepatocellular carcinoma cell growth through induction of p53 and Fbxw7. Cancer Cell Int. 2015;15:111. doi: 10.1186/s12935-015-0253-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xiang J, Hang JB, Che JM, Li HC. miR-25 is up-regulated in non-small cell lung cancer and promotes cell proliferation and motility by targeting FBXW7. Int J Clin Exp Pathol. 2015;8:9147–9153. [PMC free article] [PubMed] [Google Scholar]
- 18.Takeishi S, Nakayama KI. Role of Fbxw7 in the maintenance of normal stem cells and cancer-initiating cells. Br J Cancer. 2014;111:1054–1059. doi: 10.1038/bjc.2014.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang Z, Fukushima H, Gao D, Inuzuka H, Wan L, Lau AW, Liu P, Wei W. The two faces of FBW7 in cancer drug resistance. Bioessays. 2011;33:851–859. doi: 10.1002/bies.201100101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yokobori T, Yokoyama Y, Mogi A, Endoh H, Altan B, Kosaka T, Yamaki E, Yajima T, Tomizawa K, Azuma Y, Onozato R, Miyazaki T, Tanaka S, Kuwano H. FBXW7 mediates chemotherapeutic sensitivity and prognosis in NSCLCs. Mol Cancer Res. 2014;12:32–37. doi: 10.1158/1541-7786.MCR-13-0341. [DOI] [PubMed] [Google Scholar]
- 21.Denlinger CE, Ikonomidis JS, Reed CE, Spinale FG. Epithelial to mesenchymal transition: the doorway to metastasis in human lung cancers. J Thorac Cardiovasc Surg. 2010;140:505–513. doi: 10.1016/j.jtcvs.2010.02.061. [DOI] [PubMed] [Google Scholar]
- 22.Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119:1420–1428. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Voulgari A, Pintzas A. Epithelial-mesenchymal transition in cancer metastasis: mechanisms, markers and strategies to overcome drug resistance in the clinic. Biochim Biophys Acta. 2009;1796:75–90. doi: 10.1016/j.bbcan.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 24.Su J, Yin X, Zhou X, Wei W, Wang Z. The functions of F-box proteins in regulating the epithelial to mesenchymal transition. Curr Pharm Des. 2015;21:1311–1317. doi: 10.2174/1381612821666141211144203. [DOI] [PubMed] [Google Scholar]
- 25.Diaz VM, de Herreros AG. F-box proteins: Keeping the epithelial-to-mesenchymal transition (EMT) in check. Semin Cancer Biol. 2016;36:71–9. doi: 10.1016/j.semcancer.2015.10.003. [DOI] [PubMed] [Google Scholar]
- 26.Yang H, Lu X, Liu Z, Chen L, Xu Y, Wang Y, Wei G, Chen Y. FBXW7 suppresses epithelial-mesenchymal transition, stemness and metastatic potential of cholangiocarcinoma cells. Oncotarget. 2015;6:6310–6325. doi: 10.18632/oncotarget.3355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yu HG, Wei W, Xia LH, Han WL, Zhao P, Wu SJ, Li WD, Chen W. FBW7 upregulation enhances cisplatin cytotoxicity in non-small cell lung cancer cells. Asian Pac J Cancer Prev. 2013;14:6321–6326. doi: 10.7314/apjcp.2013.14.11.6321. [DOI] [PubMed] [Google Scholar]
- 28.Yu J, Zhang W, Gao F, Liu YX, Chen ZY, Cheng LY, Xie SF, Zheng SS. FBW7 increases chemosensitivity in hepatocellular carcinoma cells through suppression of epithelial-mesenchymal transition. Hepatobiliary Pancreat Dis Int. 2014;13:184–191. doi: 10.1016/s1499-3872(14)60029-1. [DOI] [PubMed] [Google Scholar]
- 29.Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136:215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yanaihara N, Caplen N, Bowman E, Seike M, Kumamoto K, Yi M, Stephens RM, Okamoto A, Yokota J, Tanaka T, Calin GA, Liu CG, Croce CM, Harris CC. Unique microRNA molecular profiles in lung cancer diagnosis and prognosis. Cancer Cell. 2006;9:189–198. doi: 10.1016/j.ccr.2006.01.025. [DOI] [PubMed] [Google Scholar]
- 31.Cao M, Seike M, Soeno C, Mizutani H, Kitamura K, Minegishi Y, Noro R, Yoshimura A, Cai L, Gemma A. MiR-23a regulates TGF-beta-induced epithelial-mesenchymal transition by targeting E-cadherin in lung cancer cells. Int J Oncol. 2012;41:869–875. doi: 10.3892/ijo.2012.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang J, Luo X, Li H, Yue X, Deng L, Cui Y, Lu Y. MicroRNA-223 functions as an oncogene in human colorectal cancer cells. Oncol Rep. 2014;32:115–120. doi: 10.3892/or.2014.3173. [DOI] [PubMed] [Google Scholar]
- 33.Pan Y, Liang H, Liu H, Li D, Chen X, Li L, Zhang CY, Zen K. Platelet-secreted microRNA-223 promotes endothelial cell apoptosis induced by advanced glycation end products via targeting the insulin-like growth factor 1 receptor. J Immunol. 2014;192:437–446. doi: 10.4049/jimmunol.1301790. [DOI] [PubMed] [Google Scholar]
- 34.Zhou X, Jin W, Jia H, Yan J, Zhang G. MiR-223 promotes the cisplatin resistance of human gastric cancer cells via regulating cell cycle by targeting FBXW7. J Exp Clin Cancer Res. 2015;34:28. doi: 10.1186/s13046-015-0145-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kurashige J, Watanabe M, Iwatsuki M, Kinoshita K, Saito S, Hiyoshi Y, Kamohara H, Baba Y, Mimori K, Baba H. Overexpression of microRNA-223 regulates the ubiquitin ligase FBXW7 in oesophageal squamous cell carcinoma. Br J Cancer. 2012;106:182–188. doi: 10.1038/bjc.2011.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mansour MR, Sanda T, Lawton LN, Li X, Kreslavsky T, Novina CD, Brand M, Gutierrez A, Kelliher MA, Jamieson CH, von Boehmer H, Young RA, Look AT. The TAL1 complex targets the FBXW7 tumor suppressor by activating miR-223 in human T cell acute lymphoblastic leukemia. J Exp Med. 2013;210:1545–1557. doi: 10.1084/jem.20122516. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.