Abstract
Large epidemiological studies suggest that there are important differences in the incidence and severity of a wide variety of cardiac diseases, between premenopausal and menopausal women. Recently, it has been demonstrated that resveratrol may has similar function as estrogen. However, whether resveratrol replacement could mimic estrogen to protect heart in ovariectomized mice remains completely unknown. Firstly, the present study has used OVX/CAL model to investigate the effect of RSV on ischemic heart. Echocardiography analysis revealed that RSV administration significantly improved cardiac contractile function in estrogen-deficient mice. RSV also significantly reduced CK and LDH release, and heart infarct size in OVX/CAL group. Secondly, mitochondrial functions, including MRC activities, MDA level, and mitochondrial swelling, were evaluated in OVX mice. It was found that supplementation with RSV could restore mitochondrial function dampened by OVX. Thirdly, these protective functions mediated by RSV were mainly attributed to the enhancement of SIRT1/AMPK activity. In summary, the results support a potential role of resveratrol in the protection of cardiac functions under estrogen depletion status.
Keywords: Resveratrol, ischemia, heart, mitochondrion, SIRT1, AMPK
Introduction
Cardiovascular disease (CVD) remains the leading cause of death in the world, with its prevalence growing dramatically [1]. There are important sex differences in the incidence and severity of CVD, which are thought to be mainly mediated through intrinsic hormonal regulation. Moreover, growing epidemiological studies revealed that the rates of CVD risks and mortality are significantly higher in menopausal women, compared with their premenopausal counterparts [2].
However, estrogen replacement therapy has been highlighted to be controversial between clinical and experimental studies [3]. In animal ovariectomized (OVX) models, exogenous estrogen administration could protect cardiac function by reducing inflammation, cardiomyocyte apoptosis and myocardial infarct area, or preventing arrhythmias, enhancing contractile function and post-ischemia function recovery. When refer to clinical trials, the outcomes of therapy seem to be dependent on timing and dosage of estrogen. In 2002, Women’s Health Initiative reported that hormone replacement therapy increased risk of coronary heart disease [4]. The adverse effects to estrogen treatment are numerous, which may involve impaired inflammatory response, glucose tolerance, increased obese insulin resistance, or lipid peroxidation. Thus, the discovery of safe estrogen substitutes, which could preserve heart function in post-menopausal females, may solve current problem.
Resveratrol (RSV; 3,5,4’-trihydroxystilbene) is a famous plant-derived polyphenolic phytoalexin widely existing in red grape skins, red wine, and peanuts [5]. It has been considered to play physiological roles such as antioxidative, neuroprotective, cardioprotective and anticancer effects both in adults and embryos. Although not fully delineated, the beneficial cardiovascular effects of RSV are mediated through activation of silent information regulator 1 (SIRT1), AMP-activated protein kinase (AMPK), and endogenous anti-oxidant enzymes. Besides, RSV has anti-inflammatory, anti-platelet, insulin-sensitizing, and lipid-lowering properties [6]. RSV-mediated modulation of glucose homeostasis has been recently suggested in LPS-stimulated mice [7].
Based on literatures, RSV owns chemical similarities with estrogen and binds to estrogen receptors and activates gene transcription. Subramanian et al [8] demonstrated that RSV could ameliorate hypertension caused by chronic exposure to estrogen. Another group performed a serial of experiments, showing that RSV inhibited carotid stenosis [9], VSMC proliferation in vitro [10], and promoted endothelial cell wound healing [11] through an estrogen receptor (ER)-α-dependent pathways. The direct effect of RSV on ischemic heart under estrogen-deficient condition has never been elucidated. Therefore, we postulated that resveratrol could be a potential alternative to estrogen for prevention of cardiac dysfunction against ischemia/reperfusion injury in OVX mice.
In the present study, using echocardiography etc, the protective effect of RSV on ischemic heart has been observed in estrogen-deficient mice. Furthermore, the cardioprotection mediated by RSV was showed to be correlated with modulation of mitochondrial function, as well as activation of SIRT1/AMPK signaling.
Materials and methods
Animals
All animals received humane care in compliance with the Guide for the Care and Use of Laboratory Animals prepared by the National Academy of Sciences and published by the National Institutes of Health (NIH Publication no. 86-23, revised in 1996). The use of animals in our experiments was approved by the Institutional Animal Care and Use Committee of Southern Medical University.
16-weeks-old female C57 mice were subjected to ovariectomized (OVX) according to a published protocol [12]. Briefly, after the mice were anesthetized, the bilateral ovaries were located and the oviducts, including the ovarian blood vessels, were ligated and then the ovaries were removed. Age-matched sham-operated mice underwent a similar procedure except the ovaries were not removed and oviducts were not ligated. The circulating level of 17β-estradiol was quantified by a commercial ELISA kit following the manufacturer’s instruction (Neogen Corporation, MI, USA).
Four weeks post OVX, ischemic heart disease was further induced by permanent coronary artery ligation (CAL) surgery, according to a classical protocol [13]. Briefly, after the mice were deeply anesthetized, the left anterior descending coronary artery (LAD) was exposed and ligated with 8-0 silk suture permanently. The sham-operated mice underwent the same procedure except for the ligation of suture around the coronary artery.
RSV replacement
RSV (trans-3,4,5-trihydroxystilbene) was obtained from Enzo Life Sciences (Lausen, Switzerland). After OVX surgery, the mice were orally given RSV (500 mg/kg, daily) for 8 weeks, while the control group was fed with standard diet only. The workflow diagram of animal treatment was summarized in Figure 1.
Figure 1.
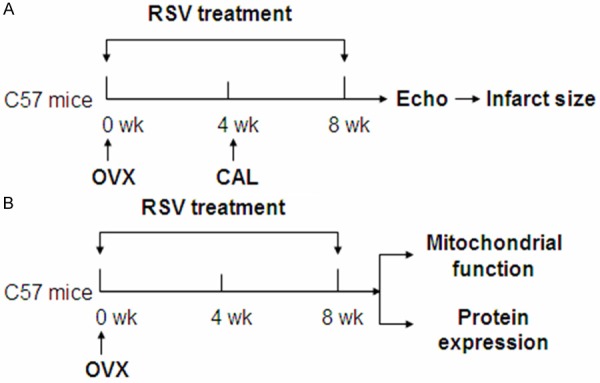
Workflow diagram. A: 4 weeks after OVX, the mice were subjected to CAL to induce myocardial ischemia. The total RSV treatment lasted 8 weeks. After echocardiographic study, the infarct size was quantified. B: Another set of OVX mice were administrated with RSV for 8 weeks and then the mitochondrial function and protein expression levels (SIRT1 etc.) were evaluated. OVX: ovariectomized; CAL: permanent coronary artery ligation surgery; RSV: resveratrol; Echo: echocardiography.
Echocardiography
At the end of RSV replacement, all the mice were performed echocardiography under light anesthesia using 1.5% isofluorane mixed with 100% oxygen. Subsequently, warmed acoustic coupling gel was applied to shaved chest and mice were fixed supine in a left lateral position on a heated pad. Images were obtained using Vevo2100 cardiovascular ultrasound system with a MS550D transducer (Visual Sonics, Toronto, Canada). Images were obtained from the B-mode long axis view and M-mode of the parasternal short-axis view to measure Left ventricular end-diastolic diameter (LVEDd), end-systolic diameter (LVEDs), end-diastolic area (LVEAd) and end-systolic area (LVEAs). LV end-diastolic volume (LVEDV) and end-systolic volume (LVESV) were calculated using the following formulae: LVEDV = 1.047 X LVEDd3 and LVESV = 1.047 X LVEDs3. Percent ejection fraction (%EF) and fractional shortening (%FS) of the LV were calculated as follows: %EF = [(LVEDV - LVESV)/LVEDV] X 100; %FS = [(LVEAd - LVEAs)/LVEAd] X 100.
Infarct size measurement
0.7 mm slices were prepared from snap-frozen hearts and incubated with 1% (w/v) 2,3,5-triphenyl-tetrazolium chloride (TTC, Sigma, St Louis, MO, USA) to visualize the infarcted area (white). Then the heart sections were scanned and the infarct size was quantified using Image J software (National Institutes of Health). Infarct area was calculated as white area/total ventricular area [14].
Lactate dehydrogenase and creatine kinase assessment
To assess the acute extent of myocardial tissue injury, 50 ul of blood was collected from tail vein and the concentrations of lactate dehydrogenas (LDH) and creatine kinase (CK) were assayed as described elsewhere [15].
Mitochondrial respiratory chain activities evaluation
Total mitochondria were isolated from fresh heart according to the protocol of a commercial kit MITOISO1 (Sigma, MO, USA). Mitochondrial respiratory chain (MRC) activities were measured as previously reported [16].
Mitochondrial swelling, as a result of mitochondrial permeability pore (mPTP) opening, was assessed as follows [17]: 0.25 mg/ml of isolated mitochondria were re-suspended in swelling buffer [KCl 120 mM, Tris-HCl 10 mM (pH7.4), MOPS 20 mM, and KH2PO4 5 Mm]. After a 5-min equilibration period, 200 mmol/L CaCl2 was added into the suspension to induce mPTP opening. The decrease of OD at A520, which indicated the opening extent of mPTP, was measured spectrophotometrically for 15 min.
The oxidative level, as indicated by lipid peroxidation product malondialdehyde (MDA) was determined with the commercial TBARS assay kit (Cayman Chemical Company, MI, USA) followed by the protocol provided by manufacturer. The results were calculated against the total protein contents in each sample.
SIRT1 activity measurements
SIRT1 activity in heart tissue lysates was determined following the instructions of a Fluorometric Drug Discovery kit (Enzo Life Sciences International Inc., Lausen, Switzerland). The fluorescence signal was detected by a microplate reader (Thermo Fisher Scientific Inc. MA, USA), with the excitation wavelength was set at 360 nm and emission detection at 460 nm. The results are expressed as fold change to ctrl values.
Western Blotting
Antibodies against total AMPK and phospho-AMPKα (Thr172) were purchased from Cell Signaling (Beverly, MA). Proteins derived from heart tissues were separated by SDS-PAGE and transferred to PVDF membranes. After overnight blocking, membranes were probed with various primary antibodies followed by secondary antibodies. Immunoreactive antibody-antigen complexes were visualized with the enhanced chemiluminescence reagents from GE Healthcare (Uppsala, Sweden).
Data analysis
The statistical calculations were performed by one-way analysis of variances (one-way ANOVA) followed by Tukey multiple comparisons using Prism version 5 (GraphPad Software; San Diego, CA, USA). All values are presented as means ± SEM. In all statistical comparisons, a P value less than 0.05 was considered to indicate significant differences.
Results
RSV administration improved cardiac contractile function in OVX/CAL mice
Four weeks post-induction of ischemia, echocardiography analysis was performed to evaluate heart performances. There were no significant differences between OVX group and their sham-operated controls (data not shown). As shown in Figure 2, both percent ejection fraction (%EF) and fractional shortening (%FS) were reduced by more than 30% in OVX+CAL group. Treatment with RSV for 8 weeks restored EF and FS levels similar to those in OVX group. End systolic left ventricular volume (LVESV) was also increased by ~40% in OVX+CAL mice, which was attenuated by RSV, while end diastolic left ventricular volume (LVEDV) showed no changes among the three groups. These results revealed that IR injury significantly decreased the systolic function estrogen-deficient mice, which could be reversed by RSV.
Figure 2.
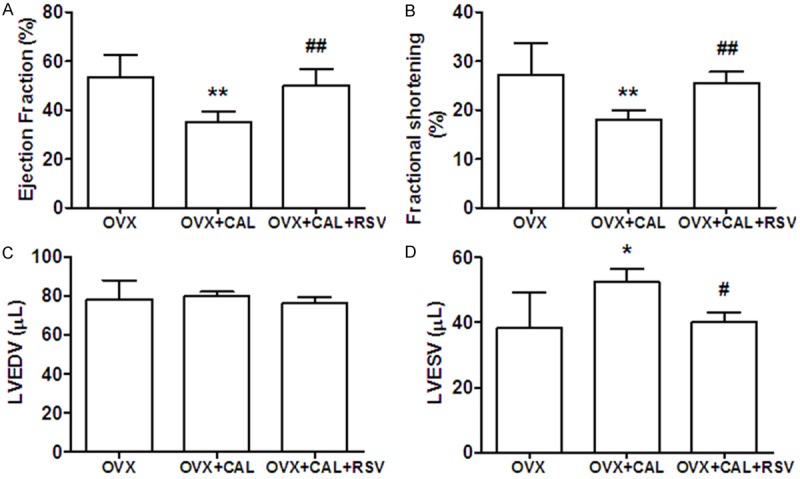
Echocardiography analysis showed RSV restored systolic contractile function. A: Ejection fraction (EF); B: Fractional shortening (FS); C: End diastolic left ventricular volume (LVEDV); D: End systolic left ventricular volume (LVESV). *, P < 0.05, **, P < 0.01 vs OVX group; #, P < 0.05, ##, P < 0.01 vs OVX+CAL group (n=8).
RSV significantly attenuated heart injury caused by OVX/CAL
In order to determine the extent of myocyte injury, serum levels of CK and LDH were measured. The results showed that acute ischemia (6 h post CAL) dramatically stimulated CK and LDH release into serum, which could be attenuated by RSV treatment. To further confirm the effects of RSV on the attenuation myocardial injury induced by IR, the infarct area size of isolated hearts were stained with TTC and quantified. It was demonstrated that infarct size of left ventricle was significantly reduced in RSV treated animals (Figure 3).
Figure 3.
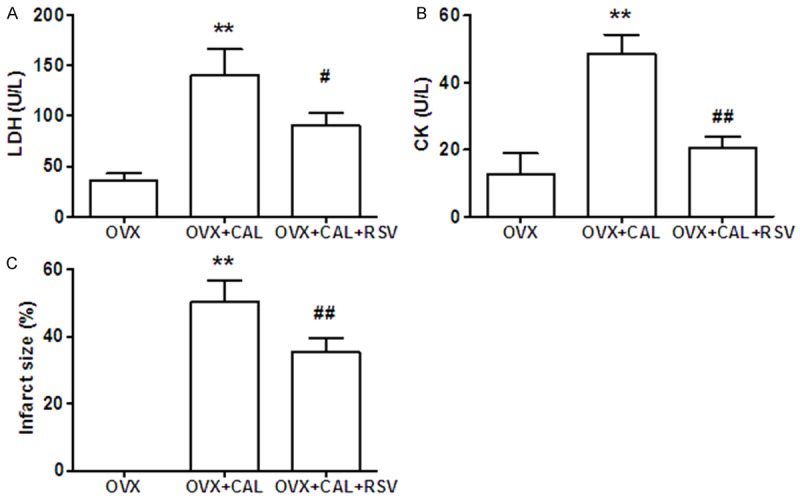
CAL induced myocytes death was ameliorated by RSV. A: LDH and B: CK release were assayed in blood collected from tail vein 6 h post CAL. C: The mice were sacrificed 4 wk post CAL and the infarct size were measured. The results are presented as percentage of infarct area vs total left ventricle area. **, P < 0.01 vs sham group; #, P < 0.05, ##, P < 0.01 vs OVX group (n=8).
RSV modulated mitochondrial function
Mitochondria play a central role in maintaining heart function. In order to dissect the underlying mechanism of RSV-mediated cardioprotection, the mitochondrial function was evaluated in Sham, OVX, OVX+RSV mice. Firstly, MRC activities were measured in mitochondria isolated from OVX mice treated with or without RSV. As shown in Figure 4A, the activities of complex I, IV and V were significantly decreased in OVX mice, when compared to sham group. RSV supplementation restored MRC activities to the level similar to those in sham-operated mice.
Figure 4.
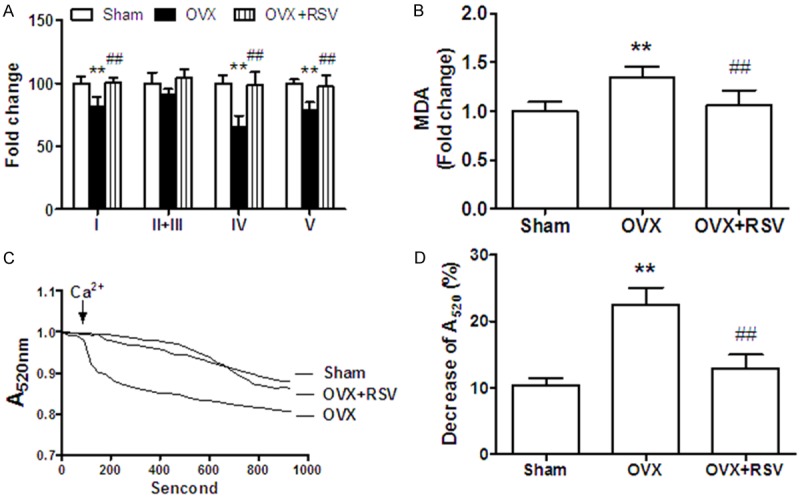
RSV improved cardiac mitochondrial function in OVX mice. (A) MRC (complex I, II+III, IV, V) activities and (B) MDA level were measured in isolated cardiac mitochondria. Data were calculated by normalization against the protein amount and presented as fold change compared to the values of sham group. (C) Pooled curves of absorbance change at 520 nm. (D) End point results of absorbance 13 min after addition of 200 μmol/L CaCl2. The percentage decreases of A520 are presented. **, P < 0.01 vs sham group; ##, P < 0.01 vs OVX group (n=8).
Next, Malondialdehyde (MDA), a lipid peroxidation product generated as a result of fatty acid overload and oxidative stress, was found to be elevated by 30% in mitochondrial fractions of OVX mice. Feeding with RSV for 8 weeks significantly reduced MDA generation to normal level (Figure 4A).
Opening of mPTP caused mitochondrial swelling, which could decrease absorbance at 520 nm (A520). In the present study, mitochondrial suspensions were prepared from heart and 200 mmol/L CaCl2 were used to induce pore opening. It was found that high concentration of calcium facilitated mPTP opening and significantly decreased A520 by 10% at the end in sham group. This effect was enlarged by 2 fold in OVX mice, which could be reversed by RSV treatment (Figure 4C and 4D).
RSV enhanced the expression and activity of SIRT1 in OVX mice
SIRT1 was reported to be an important target of RSV. Thus, we compared the protein expression level of SIRT1 in the heart tissues of OVX mice, treated with or without RSV. As shown in Figure 5A, it was found that SIRT1 level was dramatically decreased by 70% in OVX group, when compared with sham group. Supplementation with RSV for eight weeks almost reversed SIRT1 expression to normal level. Furthermore, the activity of SIRT1 was also checked in the heart tissues of these three groups. Similarly, it was showed that RSV treatment significantly enhanced SIRT1 activity (Figure 5B).
Figure 5.
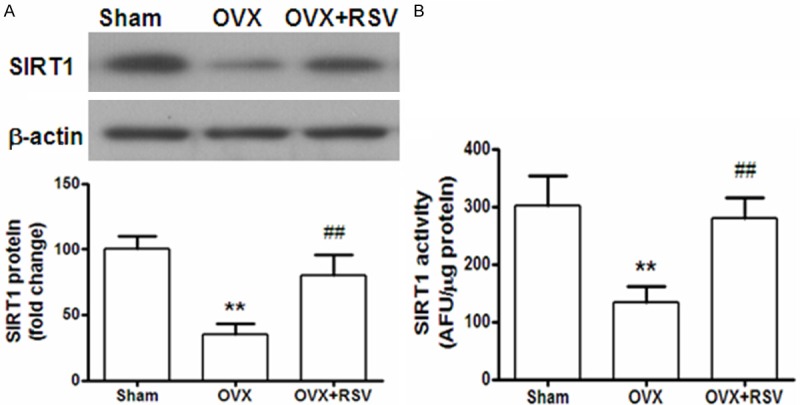
The expression and activity of SIRT1 was enhanced after RSV supplementation. A: Protein expression of SIRT1 in heart tissues were detected by Western blotting. The results are presented as fold change against the value in sham group. B: Heart tissue lysates from each group, containing 20 μg of proteins, was used for SIRT1 activity measurement by Fluorometric Drug Discovery Kit. Fluorescent readings are presented as arbitrary fluorescence unit (AFU) in equal amount of proteins for comparison. **, P < 0.01 vs sham group; ##, P < 0.01 vs OVX group (n=8).
RSV protected ischemic heart via activation of AMPK signaling in OVX mice
SIRT1 targets multiple cellular proteins, including AMP-activated protein kinase (AMPK). Activation/phosphorylation of AMPK has been suggested to be involved in cardioprotection. Therefore, we investigated the phosphorylation level of AMPK and ACC in the heart samples collected from OVX mice. It was demonstrated that the level of p-AMPKa was increased by XX% in OVX+RSV group (Figure 6A), while p-ACC decreased by % (Figure 6B).
Figure 6.
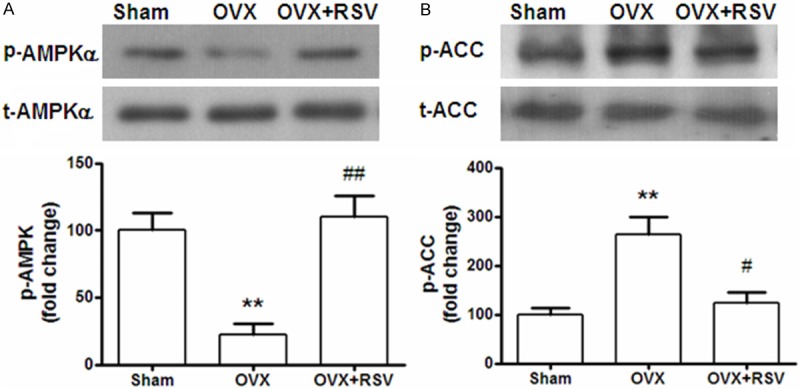
RSV-induced cardioprotection was correlated with AMPK/ACC signaling. The total protein and phosphorylation levels of AMPK (A) and ACC (B) were detected by Western blotting using specific antibodies. The results are presented as fold change against the value in sham group. **, P < 0.01 vs sham group; ##, P < 0.01 vs OVX group (n=8).
Discussion
It has been aware for years that the prevalence and severity of CVD are much higher in post-menopausal females than pre-menopausal ones. Unfortunately, although hormone replacement therapy showed effectiveness in experimental studies, the result of clinical use of estrogen is very complicated. The present study tried to find an innocuous estrogen substitute, which could mimic its cardio-protective effect. To our knowledge, this is the first investigation to demonstrate that RSV, a natural compound, significantly reduced infarct injury and improved cardiac contractile function via modulation of mitochondrial function. Further mechanistic studies revealed that SIRT1/AMPK signaling may participate in RSV-mediated protection against IR in OVX mice.
Mitochondria are critical for maintaining normal cardiac functions, involving cellular energy metabolism, myocyte contraction, Ca2+ homeostasis, reactive oxygen species generation, and apoptosis [18,19]. Mitochondrial dysfunction dramatically increases susceptibility to heart diseases. Sexual dimorphism in mitochondrial function, such as ATP generation, biogenesis, or thermogenic capacity, or antioxidant defense, has been indicated in skeletal muscle [20], adipose tissue [21], and heart [22]. A recent quantitative proteomic analysis revealed that dozens of mitochondrial proteins changed in OVX rat, among which monoamine oxidase-A (a source of oxidative stress) was the largest affected one [23]. In our study, in order to find out the precise mechanism underlying RSV-mediated cardioprotection, we evaluated mitochondrial function in OVX mice. It was found that orally treatment with RSV for 8 weeks significantly restored MRC activities, abrogated oxidative stress, and mitochondrial swelling, under estrogen-deficient condition.
SIRT1 (sirtuin1), a member of class III histone deacetylase family, is a redox-sensitive enzyme, and needs cellular NAD+ as a cofactor for its deacetylation reactivity. The cardioprotective effect of SIRT1, such as upregulation of antioxidants and downregulation of proapoptotic molecules, has been well documented [24]. It was also reported that RSV treatment could reduce myocardial injury via SIRT1 activation. Decreased SIRT1 protein levels have been observed in advanced heart failure patients [25], but whether SIRT1 expression level was diminished in menopausal females remains unknown. In the present research, both protein expression and activity of SIRT1 in heart tissues were obviously reduced in OVX group, which were reversed to normal levels after RSV supplementation.
SIRT1 targets multiple cellular proteins, such as peroxisome proliferator-activated receptor-γ and its coactivator-1α, forkhead transcriptional factors, AMP-activated protein kinase, NF-κB and protein tyrosine phosphatase. RSV has been demonstrated to activate AMPK signaling dependent on SIRT1 or not [26]. AMPK-deficient mice were showed to be resistant to the effect of RSV on enhancement of insulin sensitivity, glucose tolerance, and mitochondria biogenesis [27]. On the other hand, AMPK could enhance SIRT1 activity by increasing cellular NAD+ levels [28]. In line with previous studies, our group also confirmed that both AMPK phosphorylation level and SIRT1 activity were elevated following RSV administration. Furthermore, we also demonstrated that p-ACC expression level, a downstream molecular of AMPK, was significantly increased in OVX+RSV group. But whether RSV activated SIRT1 before AMPK warranted further study.
In conclusion, our data provide the first evidence that RSV is a potential replacement for estrogen, which contributes to protection against cardiac ischemia in OVX mice by modulation of mitochondrial function, as well as regulation of SIRT1/AMPK signaling.
Disclosure of conflict of interest
None.
References
- 1.Laslett LJ, Alagona P Jr, Clark BA 3rd, Drozda JP Jr, Saldivar F, Wilson SR, Poe C, Hart M. The worldwide environment of cardiovascular disease: prevalence, diagnosis, therapy, and policy issues: a report from the American College of Cardiology. J Am Coll Cardiol. 2012;60:S1–49. doi: 10.1016/j.jacc.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 2.Vitale C, Mendelsohn ME, Rosano GM. Gender differences in the cardiovascular effect of sex hormones. Nat Rev Cardiol. 2009;6:532–542. doi: 10.1038/nrcardio.2009.105. [DOI] [PubMed] [Google Scholar]
- 3.Sivasinprasasn S, Shinlapawittayatorn K, Chattipakorn SC, Chattipakorn N. Estrogenic Impact on Cardiac Ischemic/Reperfusion Injury. J Cardiovasc Transl Res. 2016;9:23–39. doi: 10.1007/s12265-016-9675-3. [DOI] [PubMed] [Google Scholar]
- 4.Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, Jackson RD, Beresford SA, Howard BV, Johnson KC, Kotchen JM, Ockene J. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288:321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 5.Sulaiman M, Matta MJ, Sunderesan NR, Gupta MP, Periasamy M, Gupta M. Resveratrol, an activator of SIRT1, upregulates sarcoplasmic calcium ATPase and improves cardiac function in diabetic cardiomyopathy. Am J Physiol Heart Circ Physiol. 2010;298:H833–843. doi: 10.1152/ajpheart.00418.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zordoky BN, Robertson IM, Dyck JR. Preclinical and clinical evidence for the role of resveratrol in the treatment of cardiovascular diseases. Biochim Biophys Acta. 2015;1852:1155–1177. doi: 10.1016/j.bbadis.2014.10.016. [DOI] [PubMed] [Google Scholar]
- 7.Nohr MK, Dudele A, Poulsen MM, Ebbesen LH, Radko Y, Christensen LP, Jessen N, Richelsen B, Lund S, Pedersen SB. LPS-Enhanced Glucose-Stimulated Insulin Secretion Is Normalized by Resveratrol. PLoS One. 2016;11:e0146840. doi: 10.1371/journal.pone.0146840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Subramanian M, Balasubramanian P, Garver H, Northcott C, Zhao H, Haywood JR, Fink GD, MohanKumar SM, MohanKumar PS. Chronic estradiol-17beta exposure increases superoxide production in the rostral ventrolateral medulla and causes hypertension: reversal by resveratrol. Am J Physiol Regul Integr Comp Physiol. 2011;300:R1560–1568. doi: 10.1152/ajpregu.00020.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khandelwal AR, Hebert VY, Dugas TR. Essential role of ER-alpha-dependent NO production in resveratrol-mediated inhibition of restenosis. Am J Physiol Heart Circ Physiol. 2010;299:H1451–1458. doi: 10.1152/ajpheart.00369.2010. [DOI] [PubMed] [Google Scholar]
- 10.Ekshyyan VP, Hebert VY, Khandelwal A, Dugas TR. Resveratrol inhibits rat aortic vascular smooth muscle cell proliferation via estrogen receptor dependent nitric oxide production. J Cardiovasc Pharmacol. 2007;50:83–93. doi: 10.1097/FJC.0b013e318059ae80. [DOI] [PubMed] [Google Scholar]
- 11.Yurdagul A Jr, Kleinedler JJ, McInnis MC, Khandelwal AR, Spence AL, Orr AW, Dugas TR. Resveratrol promotes endothelial cell wound healing under laminar shear stress through an estrogen receptor-alpha-dependent pathway. Am J Physiol Heart Circ Physiol. 2014;306:H797–806. doi: 10.1152/ajpheart.00892.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lizotte E, Grandy SA, Tremblay A, Allen BG, Fiset C. Expression, distribution and regulation of sex steroid hormone receptors in mouse heart. Cell Physiol Biochem. 2009;23:75–86. doi: 10.1159/000204096. [DOI] [PubMed] [Google Scholar]
- 13.Eguchi M, Xu G, Li RK, Sweeney G. Diabetes influences cardiac extracellular matrix remodelling after myocardial infarction and subsequent development of cardiac dysfunction. J Cell Mol Med. 2012;16:2925–2934. doi: 10.1111/j.1582-4934.2012.01613.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cai Z, Zhong H, Bosch-Marce M, Fox-Talbot K, Wang L, Wei C, Trush MA, Semenza GL. Complete loss of ischaemic preconditioning-induced cardioprotection in mice with partial deficiency of HIF-1 alpha. Cardiovasc Res. 2008;77:463–470. doi: 10.1093/cvr/cvm035. [DOI] [PubMed] [Google Scholar]
- 15.Banon-Maneus E, Kahbiri E, Marin JL, Pomar-Moya JL, Ramirez J, Climent F, de la Ossa PP. Increased serum creatine kinase is a reliable marker for acute transplanted heart rejection diagnosis in rats. Transpl Int. 2007;20:184–189. doi: 10.1111/j.1432-2277.2006.00410.x. [DOI] [PubMed] [Google Scholar]
- 16.Zhou M, Xu A, Tam PK, Lam KS, Chan L, Hoo RL, Liu J, Chow KH, Wang Y. Mitochondrial dysfunction contributes to the increased vulnerabilities of adiponectin knockout mice to liver injury. Hepatology. 2008;48:1087–1096. doi: 10.1002/hep.22444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gao Q, Zhang SZ, Cao CM, Bruce IC, Xia Q. The mitochondrial permeability transition pore and the Ca2+-activated K+ channel contribute to the cardioprotection conferred by tumor necrosis factor-alpha. Cytokine. 2005;32:199–205. doi: 10.1016/j.cyto.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 18.McFalls EO, Liem D, Schoonderwoerd K, Lamers J, Sluiter W, Duncker D. Mitochondrial function: the heart of myocardial preservation. J Lab Clin Med. 2003;142:141–148. doi: 10.1016/S0022-2143(03)00109-4. [DOI] [PubMed] [Google Scholar]
- 19.Crow MT, Mani K, Nam YJ, Kitsis RN. The mitochondrial death pathway and cardiac myocyte apoptosis. Circ Res. 2004;95:957–970. doi: 10.1161/01.RES.0000148632.35500.d9. [DOI] [PubMed] [Google Scholar]
- 20.Capllonch-Amer G, Sbert-Roig M, Galmes-Pascual BM, Proenza AM, Llado I, Gianotti M, Garcia-Palmer FJ. Estradiol stimulates mitochondrial biogenesis and adiponectin expression in skeletal muscle. J Endocrinol. 2014;221:391–403. doi: 10.1530/JOE-14-0008. [DOI] [PubMed] [Google Scholar]
- 21.Nadal-Casellas A, Proenza AM, Llado I, Gianotti M. Effects of ovariectomy and 17-beta estradiol replacement on rat brown adipose tissue mitochondrial function. Steroids. 2011;76:1051–1056. doi: 10.1016/j.steroids.2011.04.009. [DOI] [PubMed] [Google Scholar]
- 22.Chen Y, Zhang Z, Hu F, Yang W, Yuan J, Cui J, Hao S, Hu J, Zhou Y, Qiao S. 17beta-estradiol prevents cardiac diastolic dysfunction by stimulating mitochondrial function: a preclinical study in a mouse model of a human hypertrophic cardiomyopathy mutation. J Steroid Biochem Mol Biol. 2015;147:92–102. doi: 10.1016/j.jsbmb.2014.12.011. [DOI] [PubMed] [Google Scholar]
- 23.Lancaster TS, Jefferson SJ, Hunter JC, Lopez V, Van Eyk JE, Lakatta EG, Korzick DH. Quantitative proteomic analysis reveals novel mitochondrial targets of estrogen deficiency in the aged female rat heart. Physiol Genomics. 2012;44:957–969. doi: 10.1152/physiolgenomics.00184.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hsu CP, Zhai P, Yamamoto T, Maejima Y, Matsushima S, Hariharan N, Shao D, Takagi H, Oka S, Sadoshima J. Silent information regulator 1 protects the heart from ischemia/reperfusion. Circulation. 2010;122:2170–2182. doi: 10.1161/CIRCULATIONAHA.110.958033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lu TM, Tsai JY, Chen YC, Huang CY, Hsu HL, Weng CF, Shih CC, Hsu CP. Downregulation of Sirt1 as aging change in advanced heart failure. J Biomed Sci. 2014;21:57. doi: 10.1186/1423-0127-21-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Centeno-Baez C, Dallaire P, Marette A. Resveratrol inhibition of inducible nitric oxide synthase in skeletal muscle involves AMPK but not SIRT1. Am J Physiol Endocrinol Metab. 2011;301:E922–930. doi: 10.1152/ajpendo.00530.2010. [DOI] [PubMed] [Google Scholar]
- 27.Um JH, Park SJ, Kang H, Yang S, Foretz M, McBurney MW, Kim MK, Viollet B, Chung JH. AMP-activated protein kinase-deficient mice are resistant to the metabolic effects of resveratrol. Diabetes. 2010;59:554–563. doi: 10.2337/db09-0482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Canto C, Gerhart-Hines Z, Feige JN, Lagouge M, Noriega L, Milne JC, Elliott PJ, Puigserver P, Auwerx J. AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature. 2009;458:1056–1060. doi: 10.1038/nature07813. [DOI] [PMC free article] [PubMed] [Google Scholar]


