Abstract
Notch-1, a type-1 transmembrane protein, plays critical roles in the pathogenesis and progression of human malignancies, including breast cancer; however, the precise mechanism by which Notch-1 causes tumor cell invasion and angiogenesis remain unclear. Nuclear factor-κB (NF-κB), interleukin-8 (IL-8), vascular endothelial growth factor (VEGF), and matrix metalloproteinases (MMP) are critically involved in the processes of tumor cell invasion and metastasis, we investigated whether targeting Notch-1 could be mechanistically associated with the down-regulation of NF-κB, IL-8, VEGF, and MMP-9, resulting in the inhibition of invasion and angiogenesis of breast cancer cells. Our data showed that down-regulation of Notch-1 leads to the inactivation of NF-κB activity and inhibits the expression of its target genes, such as IL-8, VEGF and MMP-9. We also found that down-regulation of Notch-1 decreased cell invasion, and vice versa Consistent with these results, we also found that the down-regulation of Notch-1 not only decreased MMP-9 mRNA and its protein expression but also inhibited MMP-9 active form. Moreover, conditioned medium from Notch-1 siRNA-transfected breast cancer cells showed reduced levels of IL-8 and VEGF and, in turn, inhibited the tube formation of HUVECs, suggesting that down-regulation of Notch-1 leads to the inhibition of angiogenesis. Furthermore, conditioned medium from Notch-1 cDNA-transfected breast cancer cells showed increased levels of IL-8 and VEGF and, in turn, promoted the tube formation of HUVECs, suggesting that Notch-1 overexpression leads to the promotion of angiogenesis.We therefore concluded that down-regulation of Notch-1 leads to the inactivation NF-κB and its target genes (IL-8, MMP-9 and VEGF), resulting in the inhibition of invasion and angiogenesis.
Keywords: Breast cancer, Notch-1, nuclear factor-κB (NF-κB), invasion, angiogenesis
Instruction
Notch proteins are ligand-dependent transmembrane receptors that transduce extracellular signals responsible for cell fate and differentiation throughout development and across species [1,2]. Notch-1 is a heterodimeric, 300-kDa type 1 transmembrane receptor, which mediates signaling induced by cell-to-cell contact. Sequential cleavage of Notch-1 is required for activation of the full-length receptors. Intracellular domain of Notch-1 (ICN1), the active form of Notch-1, is released by proteolysis from the full-length Notch-1 and transferred to the cell nucleus to associate with DNA-binding protein to assemble a transcription complex that activates downstream target genes. Previous studies have identified a multifaceted functional role of Notch signal in diverse cellular processes such as cell differentiation, proliferation, apoptosis, adhesion, migration and epithelial-to-mesenchymal transition (EMT) [3]. Recent studies show the involvement of notch-1 signaling in cancer angiogenesis and metastasis [4-6]. However, the molecular mechanisms that underlie these effects remain largely undefined.
Efforts to reduce growth and spread of neoplasms such as breast cancer have focused recently on angiogenesis because they are dependent in part on the formation of adequate vascular support [7,8]. Vascular endothelial growth factor (VEGF) has been shown to induce proliferation of endothelial cells, increase vascular permeability, induce the production of plasminogen activator by breast cancer cells, and prolong their survival [9,10]. IL-8, a chemoattractant cytokine, has been shown to attract and activate neutrophils in inflammatory regions and to be angiogenic [11]. As is true for breast cancer, the extent of angiogenesis correlates inversely with prognosis in patients with breast cancer [12,13]. Matrix metalloproteinase-9 (MMP-9) strongly influences tumor development and metastasis, and is closely related to the most aggressive subtypes and lymph node metastasis of breast cancer [14,15].
NF-κB is a transcription factor that plays an important role in innate immunity and is a master regulator of inflammation and variety of cellular processes including angiogenesis, migration and invasion [16,17], which is most likely associated with increased expression of IL-8, MMPs and VEGF [18-23].
Recent studies have found the significant relation was showed between Notch-1 and NF-κB. In CaSki cervical cancer cells Notch-1 increased NF-κB activity through associating with the IKK signalosome through IKKalpha [24]. In breast cancer cells, Genistein (Gen) inhibited NF-κB activity via the Notch-1 pathway [25]. However, it is still unknown how the cross-talk between Notch-1 and NF-κB signaling pathways regulates the malignant behaviors of human breast cancer.
It has been shown that Notch-1 was overexpressed in breast cancer and higher Notch-1 expression was associated with transition from ductal carcinoma in situ (DCIS) to invasive cancer. Patients with Notch-1 overexpression exhibited significantly worse overall and recurrence-free survival [26]. Therefore, we sought to find novel avenues by which Notch-1 could be inactivated, which may represent a promising strategy for the development of novel and selective anticancer therapies for breast cancer.
In the present study, we provide evidence that Notch-1 plays an important role in invasiveness and angiogenesis of human breast cancer cells concomitant with decrease in the expression of NF-κB and its target genes (IL-8, MMP-9 and VEGF), resulting in the inhibition of invasion and angiogenesis.
Materials and methods
Human breast cancer cells
Breast cancer cell lines MDA-MB-231, MCF-7, SKBR-3 and T47D was purchased from ATCC (Shanghai, China). The cells were maintained in RPMI 1640 medium containing 10% FBS (MediaTech CellGro, Hangzhou China), penicillin (100 units/ml), and streptomycin (0.1 ug/ml) (Invitrogen) in a humidified atmosphere incubator with 5% CO2 at 37°C. Cells were routinely subcultured twice weekly.
Transfection with siRNAs
Notch-1 siRNA and control siRNA were obtained from Santa Cruz Biotechnology (Shanghai, China). Cells were transfected with Notch-1 siRNA using the Oligofectamine reagent following the manufacturer’s instruction. 24 hs prior to transfection, 5 × 104 MDA-MB-231 cells/well were seeded in six-well plates (corresponding to a density of 40-60% at the time of transfection) without antibiotics. The transfection mixture was prepared by mixing 175 ul of DMEM containing 6 ul of 20 uM siRNA with 15 ul of DMEM containing 3 ul of Oligofectamine reagents. Before transfection, the medium in 6-well plates was replaced with serum-free DMEM medium (800 ul/well). The transfection mixture was added to the 6-well plate within 20-40 min after mixture preparation in a total volume of 990 ul/well. The transfected cells were incubated at 37°C for 4 h, and then 500 ul of DMEM medium containing 30% fetal bovine serum was added. Cells were allowed to grow further in a CO2 incubator for 48 hs and later harvested for further analysis.
Transfection with cDNA
The Notch-1 cDNA clones was purchased from OriGene Technologies, Inc (Wuhan, China). SKBR-3 cells were transfected with Notch-1 cDNA using the Oligofectamine reagent following the manufacturer’s instruction. Cells were allowed to be transfected for 36 hs and later harvested for further analysis.
Western blot
Protein was quantified using the Bradford assay (Bio-Rad, Hercules, CA, USA), and equal amounts of protein were separated on SDS-polyacrylamide gels and transferred onto nitrocellulose membranes (Amersham Biosciences, Piscataway, NJ, USA). After blocking in 5% skim milk for 1 h at room temperature, the membranes were incubated with the indicated primary antibody at 4°C overnight, followed by a horseradish peroxidase-conjugated secondary antibody. The proteins were detected by chemiluminescence (Amersham Biosciences, Piscataway, NJ, USA). The Western blot data were quantified by measuring the intensity of the hybridization signals using an image analysis program (Fluor-ChemTM 8900, Alpha Inotech).
RT-PCR
Total RNA from transfected cells was isolated using the RNeasy Kit (Qiagen, Guangzhou, China) according to the manufacturer’s instructions. Reverse transcription was performed at 42°C for 60 min using oligo (dT)12-18 (Amersham, Little Chalfont, UK). The primers for PCR amplification used in this study were as follows: Notch-1: 5’-GCAAGAAGAAGCGGAGAG-3’ and 5’- AGCTGGCACCCTGATAGATG-3’; the Notch-1 PCR product length was 423 bp. NF-κB p65: 5’-GACCTGGCATCTGTGGACAAC-3’ and 5’-TCCGCAATGGAGGAGAAGTCT-3’, the amplified fragment length was 221 bp. IL-8: 5’-ATGACTTCCAAGCTGGCCGTGGCT-3’ and 5’-TCTCAGCCCTCTTCAAAAACTTCT-3’, the amplified fragment length was 297-bp. Control GAPDH: 5’-AGATCCACAACGGATACATT-3’ and 5’-TCCCTCAAGATTGTCAGCAA-3’, the GAPDH PCR product length was 308 bp. They all synthetized by Beijing Aoke Biotechnology Company). The PCR conditions were as follows: predenaturing at 94°C for 2 min, denaturing at 94°C for 30 s, reannealing at 53°C for 45 s, and elongation at 72°C for 30 s, for 30 cycles; and final elongation at 72°C for 10 min. The PCR products underwent 1.5% agarose gel electrophoresis.
Electrophoretic mobility shift assay (EMSA)
Nuclear protein was extracted as the manufacture’s instruction and the concentration was determined by Bradford assay, using the Bio-Rad protein assay solution (Bio-Rad Laboratories, Inc., Hercules, CA, USA). NF-κB gel-shift oligonucleotide (5’-AGTTGAGGGGACTTTCCCAGGC-3’) was labeled with [32P]-dATP (Amersham Bioscience, Piscataway, NJ, USA), using T4 polynucleotide kinase (Promega Corp). End-labeled probe was purified from unincorporated [32P]-ATP using a purification column (Amersham Biosciences, Bucking hamshire, UK) and recovered in Tris-EDTA buffer (TE). Nuclear extracts (5 μg) were preincubated in buffer containing 12% glycerol, 12 mM HEPES, pH 7.9, 4 mM Tris-HCl, pH 7.9, 1 mM EDTA, 1 mM DTT, 25 mM KCl, 5 mM MgCl2, 0.04 μg/mL Poly (dI-dC) (Amersham Bioscience), 0.4 mM PMSF, and TE. The labeled probe was added and the samples were incubated for 30 min at room temperature. The samples were subjected to electrophoretic separation at 4°C on a nondenaturing 5% acrylamide gel. The gel was dried at 80°C for 40 min and exposed to a radiography film for 6-18 h at -80°C with intensifying screens.
ELISA assay
The level of IL-8 ,MMP-9 and VEGF protein in culture supernatants was determined using a quantitative immunometric sandwich enzyme immunoassay (ELISA) kit (R&D Systems, Guangzhou, China). The absorbance of the samples was compared with the standard curve.
Invasion assay
The invasive activity of the Notch siRNA transfected or control siRNA or Cdna transfected cells was tested using the BD BioCoat Tumor Invasion Assay System (BD Biosciences) according to manufacturer’s instructions.
HUVEC tube formation assay
The Notch1 siRNA-transfected MDA-MB-231 cells or Notch-1 cDNA-transfected SKBR-3 cells were cultured in serum-free RPMI 1640 for 24 hs. The conditioned media were collected, centrifuged, transferred to fresh tubes, and stored at -20°C. HUVECs were trypsinized and seed ed (5 × 104 per well) in Matrigel-coatedwell with 250 uL of conditioned medium from Notch1 cDNA-transfected or control plasmid-transfected cells. The tube formation was assayed.
Statistical analysis
All statistical analysis were performed using SPSS.17 (IBM Corp.). Data were reported as group mean ± SEM. Statistical significance of three or more experiments was determined by performing a two-tailed, unpaired Student’s t-test for two comparisons. Significance for all statistical comparisons was set at p < 0.05.
Results
Expression of Notch-1 in human breast cancer cell lines
The baseline expression of Notch-1 was determined in a panel of human breast cancer cell lines MDA-MB-231, MCF-7, SKBR-3 and T47D. The results showed that Notch-1 protein was frequently expressed in different human breast cancer cell lines (Figure 1A). We also examined the relative mRNA levels of Notch-1 in four breast cancer cell lines by reverse transcription-PCR (RT-PCR). All the cell lines also expressed differential levels of Notch-1 mRNA (Figure 1B).
Figure 1.
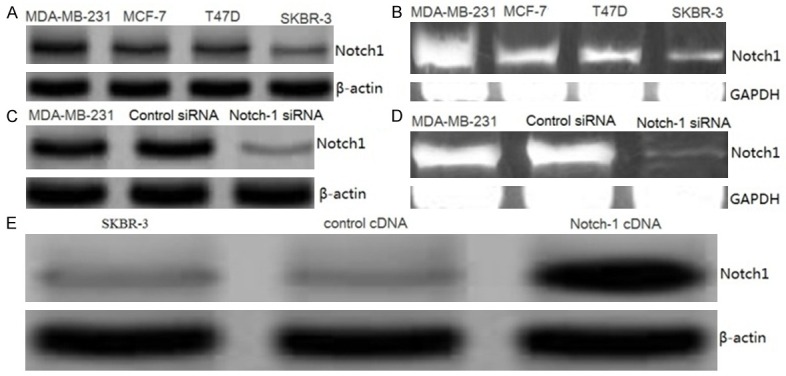
Effect of siRNA or cDNA transfection on Notch1 expression in MDA-MB-231, MCF-7, SKBR-3 and T47D cells. A. Notch1 protein expression was detected by western blot assay. B. Notch1 mRNA expression was detected by RT-PCR assay. C. Representative images showing expression of S100A4 protein in control siRNA and Notch1-siRNA transfected MDA-MB-231 cells as analyzed by Western blot assay. D. Representative images showing expression of Notch1 mRNA in control siRNA and Notch1-siRNA transfected MDA-MB-231 cells as analyzed by RT-PCR assay. E. Representative images showing expression of Notch1 protein in control cDNA and Notch1 cDNA transfected SKBR-3 cells as analyzed by Western blot assay.
Effect of siRNA on Notch-1 expression in MDA-MB-231 cells
MDA-MB-231 cell showed higher expression of Notch-1 (Figure 1A, 1B) in the four cell lines. We choose MDA-MB-231 for siRNA study. As shown in Figure 1C, MDA-MB-231 cells transfected with Notch-1 siRNA displayed significant reduction in the expression levels of Notch-1 protein by Western blot analysis. Further, the suppression of Notch-1 by siRNA in cells was confirmed by RT-PCR analysis. Cells transfected with Notch-1 siRNA exhibited a significant reduction in mRNA level of Notch-1 (Figure 1D).Control siRNA did not exhibit any effect on protein or mRNA levels of Notch-1 (Figure 1C, 1D). These data confirmed the suppression effect of siRNA and established the efficiency of siRNA transfection.
Effect of Notch-1 overexpression on SKBR-3 cells
SKBR-3 cell showed lower expression of Notch-1 (Figure 1A, 1B) in the four cell lines. We choose SKBR-3 cell for cDNA transfection study. SKBR-3 cells transfected with Notch-1 cDNA plasmid displayed a significant increase in the expression levels of Notch-1 as compared with vector control. The overexpression of Notch-1 was confirmed by performing western blot analysis 36 h after transfection (Figure 1E). Because Notch-1 expression was observed to be very high 36 h after transfection, we selected this time point for further studies.
Down-regulation of Notch-1 decreased cancer cell invasion and formation of capillary-like structures
To examine the role of Notch-1 on the invasion of human breast cancer cells, the MDA-MB-231 cells were transfected with human Notch-1 siRNA or control siRNA. We used Matrigel invasion chamber assay to examine the invasive potential of Notch-1 siRNA-transfected MDA-MB-231 cells. As illustrated in Figure 2A, Notch-1 siRNA-transfected cells showed a low level of penetration through the Matrigel-coated membrane compared with the control siRNA-transfected cells.
Figure 2.
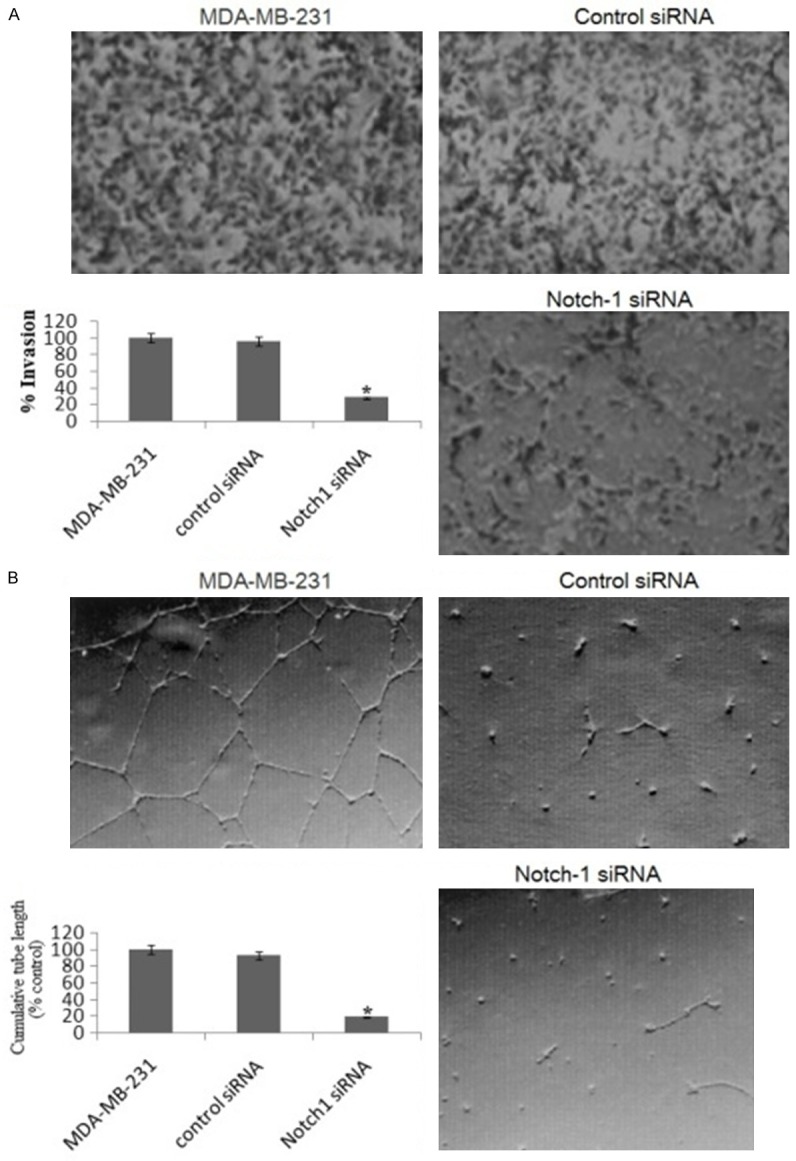
Knockdown of Notch-1 inhibits the invasion of MDA-MB-231 cells and the formation of capillary-like structures of HUVECs. A. Representative photomicrographs and histogram showing invasive capability of MDA-MB-231 cells transfected with Notch-1 siRNA in a BD BioCoat Tumor Invasion Assay System (Magnification: × 100). B. Angiogenesis induced by human umbilical vein endothelial cells (HUVECs) cultured on Matrigel matrix in 96-well plates with or without conditioned media of Notch-1 siRNA. Quantification of tubular morphogenesis induced in HUVECs cells cultured with or without Notch-1 siRNA. Tube formation was determined by the length of tube-like structures containing connected cells. Statistical significance of three or more experiments was determined by performing a two-tailed, unpaired Student’s t-test for two comparisons. Columns represents mean; bars represents SEM. *, p < 0.05.
Tumor growth and metastasis critically depend on angiogenesis; therefore inhibiting this process would inhibit tumor expansion. We hence used HUVECs plated on Matrigel-coated plates to examine the effect of siRNA on the formation of capillary tube in vitro. As shown in Figure 2B treatment of HUVECs with Notch-1 siRNA significantly inhibited tube formation when compared with control siRNA cells.
Notch-1 overexpression increased cancer cell invasion and formation of capillary-like structures
To examine the role of Notch-1 overexpression on the invasion of human breast cancer cells, the SKBR-3 cells were transfected with Notch-1 cDNA. We used Matrigel invasion chamber assay to examine the invasive potential of Notch-1 cDNA-transfected SKBR-3 cells. As illustrated in Figure 3A, Notch-1 cDNA-transfected SKBR-3 cells showed a high level of penetration through the Matrigel-coated membrane compared with the control cDNA-transfected cells. We used HUVECs plated on Matrigel-coated plates to examine the effect of cDNA on the formation of capillary tube in vitro. As shown in Figure 3B. treatment of HUVECs with Notch-1 cDNA significantly promoted tube formation when compared with control cDNA transfected SKBR-3 cells.
Figure 3.
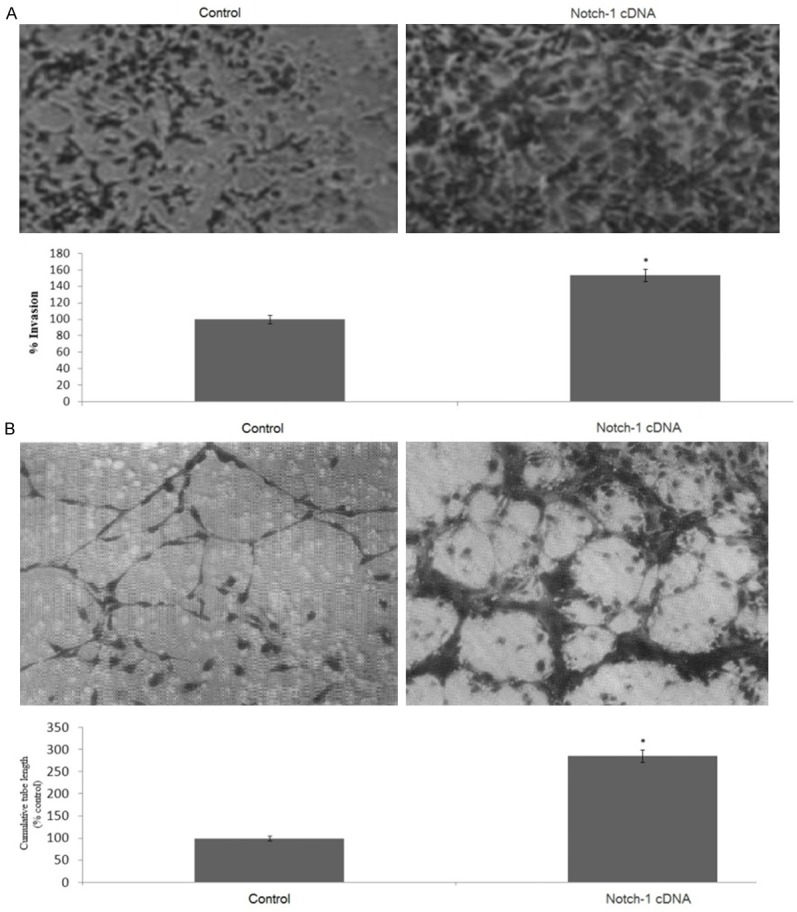
Notch1 overexpression promotes the invasion of SKBR-3 cells and the formation of capillary-like structures of HUVECs. A. Representative histogram showing invasive capability of SKBR-3 cells transfected with Notch-1 cDNA in a BD BioCoat Tumor Invasion Assay System. B. Quantification of tubular morphogenesis induced in HUVECs cells cultured with or without Notch1 cDNA. Tube formation was determined by the length of tube-like structures containing connected cells. Statistical significance of three or more experiments was determined by performing a two-tailed, unpaired Student’s t-test for two comparisons. Columns represents mean; bars represents SEM. *, p < 0.05.
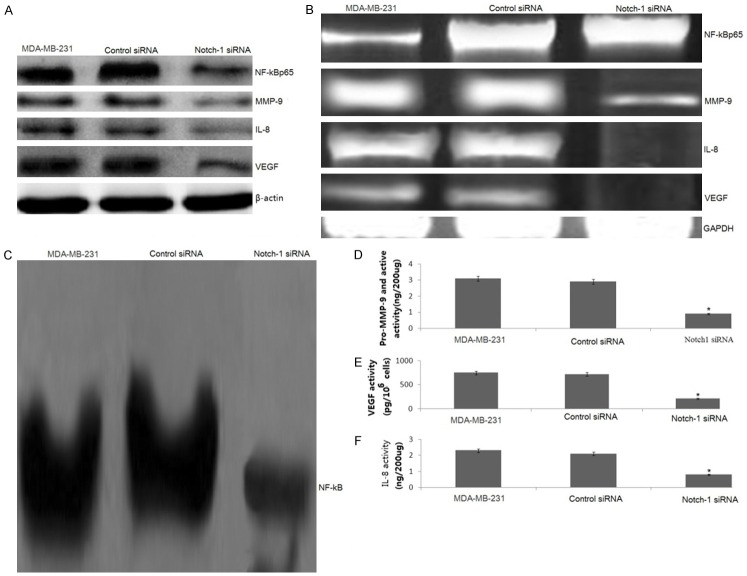
Down-regulation of Notch-1 decreased NF-κB activity and NF-κB p65 expression
The NF-κB signaling pathway is also known to regulate the expression of various genes involved in tumor cells invasion. To investigate whether Norch-1 could affect this pathway, we first examined, by Western blotting, the phosphorylation status of phospho-p65 in Notch-1 siRNA-treated MDA-MB-231 cells. We found that knockdown of Notch1 drastically inhibited the phosphorylation of NF-κB p65 (p65) (Figure 4A). RT-PCR assay showed the same results that p65 mRNA was drastically inhibited (Figure 4B). Next, we decided to measure the ability of Notch-1 siRNA on NF-κB activity. As shown in Figure 4C, low constitutive NF-κB activity was detected by EMSA in Notch-1 siRNA transfected MDA-MB-231 cells.
Figure 4.
Down-regulation of Notch-1 on NF-κB DNA-binding activity and its target genes. The MDA-MB-231 cells were transfected with human Notch-1 siRNA or control siRNA for 48 hs. The expression of IL-8, MMP-9 and VEGF was detected by western blot assay (A) and RT-PCR assay (B). NF-κB DNA-binding activity was detedted by EMSA (C). The culture supernatant of IL-8, MMP-9 and VEGF was detected by ELISA (D-F). Statistical significance of three or more experiments was determined by performing a two-tailed, unpaired Student’s t-test for two comparisons. vs control, *p < 0.05.
Down-regulation of Notch-1 decreased IL-8, MMP-9 and VEGF gene transcription and their activities
The expression of IL-8, MMP-9 and VEGF is regulated by NF-κB. We therefore investigated whether IL-8, MMP-9 and VEG F were inhibited by Notch-1 siRNA transfection. Western blotting and RT-PCR were used to detect the alteration in the expression of IL-8, MMP-9 and VEGF. We found that both IL-8, MMP-9 and VEGF protein (Figure 4A) and mRNA (Figure 4B) levels were dramatically decreased in the Notch-1 siRNA-transfected MDA-MB-231 cells. Next, we examined whether the down-regulation of Notch-1 could lead to a decrease in MMP-9 activity. There was a 2.8-fold decrease in the activity of MMP-9 (Figure 4D). Consistently, Notch-1 siRNA-transfected cells secreted significantly decreased levels of VEGF and IL-8 into the culture supernatant, as determined by quantitative IL-8 and VEGF ELISA (Figure 4E, 4F).
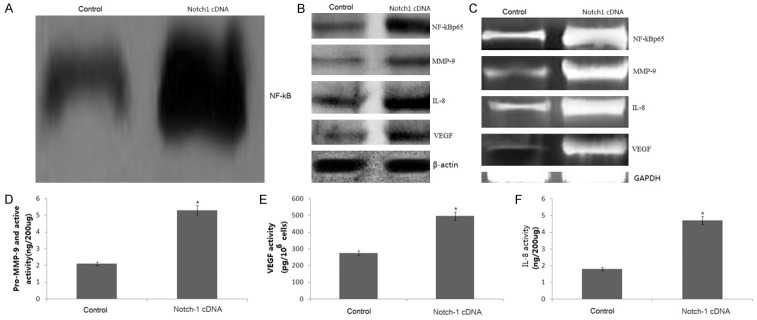
Notch-1 overexpression increased NF-κB activity and p65 expression
Notch-1 cDNA was transfected into the SKBR-3 cells. We found that Notch-1 overexpression increased NF-κB activity (Figure 5A). The specificity of NF-κB activity was confirmed by supershift. Furthermore, Notch-1 drastically increased the phosphorylation of p65 (Figure 5B) and p65 mRNA (Figure 5C).
Figure 5.
Notch-1 overexpression on NF-κB DNA-binding activity and its target genes. The SKBR-3 cells were transfected with human Notch-1 cDNA for 36 hs.The NF-κB DNA-binding activity was detedted by EMSA (A). The expression of IL-8, MMP-9 and VEGF was detected by western blot assay (B) and RT-PCR assay (C). The culture supernatant of IL-8, MMP-9 and VEGF was detected by ELISA (D-F). Statistical significance of three or more experiments was determined by performing a two-tailed, unpaired Student’s t-test for two comparisons. vs control, *p < 0.05.
Notch-1 overexpression increased IL-8, MMP-9 and VEGF gene transcription and their activities
Western blotting and RT-PCR assay showed that both the expression of IL-8, MMP-9 and VEGF protein (Figure 5B) and mRNA (Figure 5C) levels were dramatically decreased in the Notch-1 cDNA-transfected SKBR-3 cells. In addition, we found Notch-1 overexpression significantly increase the IL-8, MMP-9 and VEGF activity as determined by quantitative ELISA assay (Figure 5D-F).
Discussion
Notch-1 signaling is known to play important roles in maintaining the balance between cell proliferation, differentiation, and apoptosis [27]. The Notch-1 gene is abnormally activated in many human malignancies, including breast cancer [26]. Notch-1 is known to play critical roles in the processes of tumor cell proliferation, invasion, and angiogenesis. In the present study, we found that down-regulation of Notch-1 significantly inhibited cell invasion and angiogenesis and vice versa.
Previous studies have shown that Notch-1 activation could lead to the activation of NF-κB [24,25]. NF-κB activation has also been reported to be associated with metastatic phenotype of tumor cells by regulating the expression of a variety of important genes known to be associated with many cellular responses [28]. Because NF-κB plays important roles in many cellular processes, studies on the interaction of NF-κB activation with other cell signal transduction pathways, including the Notch-1 and NF-κB pathway, have received increased attention in recent years.
In this study, we found that Notch-1 activated the NF-κB. In addition, we also found that downregulation of Notch-1 inhibited NF-κB activity. Therefore, it is possible that Notch-1-induced cell invasion and angiogenesis is due to activation of the NF-κB activity. These data implied that the cells with Notch-1 signal activation had a greater invasive potential via regulation of NF-κB.
It has been reported that Notch-1 promotes both angiogenesis and metastasis in certain tumor models and that inhibition of Notch-1 reduces tumor cell proliferation and metastasis in breast cancer [29]. MMP-9 expression was also elevated in the Notch-1-transfected breast cancer cell line [22]. It is known that MMPs are critically involved in the processes of tumor cell invasion and metastasis and that MMP-9 is directly associated with angiogenesis and metastatic processes [30,31]. Here, we showed that overexpression of Notch-1 increased MMP -9 expression and the activity of MMP-9. Thus, these results suggest that down-regulation of Notch-1 potentiate antitumor and antimetastatic activities partly through the down-regulation of the expression of MMP-9.
Another important molecule involved in tumor cell invasion and angiogenesis is VEGF. Many studies have documented that VEGF is a critical mediator of angiogenesis and regulates most of the steps in the angiogenic cascade [32,33]. In the present study, we found a marked increase in the secreted form of VEGF in Notch-1 cDNA-transfected cells. We also found a significant reduction of VEGF secretion in the culture medium of breast cancer cells by down-regulation of Notch-1 using Notch-1 siRNA transfection. IL-8 was also an important molecule involved in cell invasion and angiogenesis in breast cancer [34]. In the present study, we found that IL-8 secretion and expression was significantly increased in Notch-1 cDNA-transfected cells, and IL-8 secretion and expression was significantly decreased in Notch-1 siRNA-transfected cells.
Because we observed that overexpression of Notch-1 increased the expression and activities of IL-8, MMP-9 and VEGF , we tested the effects of overexpression of Notch-1 on the migration and invasion of breast cancer cells and tube formation (angiogenesis) of HUVECs. We found that overexpression of Notch-1 increased migration and invasion of breast cancer cells through Matrigel and induced tube formation of HUVECs. These results are consistent with activation of IL-8, MMP-9 and VEGF by overexpression of Notch-1, resulting in the promotion of cancer cell invasion and angiogenesis. In addition, down-regulation of Notch-1 inhibited migration and invasion of breast cancer cells through Matrigel and reduced tube formation of HUVECs. Based on our results, we speculated that one possible mechanism by which Notch-1 induced invasion and angiogenesis is by the activation of NF-κB activity, which leads to upregulation of NF-κB target genes, such as IL-8, MMP-9 and VEGF. However, further in depth studies are needed to ascertain the precise molecular regulation of Notch-1 and NF-κB and their cross talks in elucidating the role of Notch-1 in cell growth, invasion, and angiogenesis of breast cancer cells in animal models and in human breast cancer.
Conclusion
In summary, we presented experimental evidence that strongly supports the role of Notch-1 sliencing as antitumor and antimetastatic mechanisms in breast cancer. Therefore, downregulation of Notch-1 could potentially be an effective therapeutic approach for the inactivation of NF-κB and its target genes, such as IL-8, MMP-9 and VEGF, which is likely to result in the inhibition of cell growth, migration, invasion, angiogenesis, and metastasis of breast cancer.
Acknowledgements
This study was supported by the LinYi Natural Science Foundation of Shandong (Grant nos. MC2014C46). This study was approved by The Ethics Committee for Animal Experiments of the Tumor Hospital of Linyi, Shandong and the experimental protocol was carried out in strict accordance with the institutional guidelines and the criteria outlined in the “Guide for Care and Use of Laboratory Animals”.
Disclosure of conflict of interest
None.
Authors’ contributions
LC and PG performed the siRNA experiments. LY ,YX and ZF performed the cDNA experiments. SC prepared the manuscript. LY and SC designed and analyzed the data. The all read and approved the final manuscript.
References
- 1.Kopan R, Ilagan MX. The canonical Notch signaling pathway: unfolding the activation mechanism. Cell. 2009;137:216–233. doi: 10.1016/j.cell.2009.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fortini ME. Notch signaling: the core pathway and its posttranslational regulation. Dev Cell. 2009;16:633–647. doi: 10.1016/j.devcel.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 3.Bolos V, Grego-Bessa J, de la Pompa JL. Notch signaling in development and cancer. Endocr Rev. 2007;28:339–363. doi: 10.1210/er.2006-0046. [DOI] [PubMed] [Google Scholar]
- 4.Gao Y, Rankin GO, Tu Y, Chen YC. Theaflavin-3, 3’-digallate decreases human ovarian carcinoma OVCAR-3 cell-induced angiogenesis via Akt and Notch-1 pathways, not via MAPK pathways. Int J Oncol. 2016;48:281–292. doi: 10.3892/ijo.2015.3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rooney P, Connolly M, Gao W, McCormick J, Biniecka M, Sullivan O, Kirby B, Sweeney C, Molloy E, Markham T, Fearon U, Veale DJ. Notch-1 mediates endothelial cell activation and invasion in psoriasis. Exp Dermatol. 2014;23:113–118. doi: 10.1111/exd.12306. [DOI] [PubMed] [Google Scholar]
- 6.Yabuuchi S, Pai SG, Campbell NR, de Wilde RF, De Oliveira E, Korangath P, Streppel MM, Rasheed ZA, Hidalgo M, Maitra A, Rajeshkumar NV. Notch signaling pathway targeted therapy suppresses tumor progression and metastatic spread in pancreatic cancer. Cancer Lett. 2013;335:41–51. doi: 10.1016/j.canlet.2013.01.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang Y, Cao S, Chen Y. Molecular Treatment of Different Breast Cancers. Anticancer Agents Med Chem. 2015;15:701–720. doi: 10.2174/1871520615666150129211901. [DOI] [PubMed] [Google Scholar]
- 8.Leite de Oliveira R, Hamm A, Mazzone M. Growing tumor vessels: more than one way to skin a cat-implications for angiogenesis targeted cancer therapies. Mol Aspects Med. 2011;32:71–87. doi: 10.1016/j.mam.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 9.Delli Carpini J, Karam AK, Montgomery L. Vascular endothelial growth factor and its relationship to the prognosis and treatment of breast, ovarian, and cervical cancer. Angiogenesis. 2010;13:43–58. doi: 10.1007/s10456-010-9163-3. [DOI] [PubMed] [Google Scholar]
- 10.Banerjee S, Dowsett M, Ashworth A, Martin LA. Mechanisms of disease: angiogenesis and the management of breast cancer. Nat Clin Pract Oncol. 2007;4:536–550. doi: 10.1038/ncponc0905. [DOI] [PubMed] [Google Scholar]
- 11.Singh JK, Simões BM, Howell SJ, Farnie G, Clarke RB. Recent advances reveal IL-8 signaling as a potential key to targeting breast cancer stem cells. Breast Cancer Res. 2013;15:210. doi: 10.1186/bcr3436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Di Salvatore M, Lo Giudice L, Rossi E, Santonocito C, Nazzicone G, Rodriquenz MG, Cappuccio S, Inno A, Fuso P, Orlandi A, Strippoli A, Capo-luongo E, Astone A, Cassano A, Barone C. Association of IL-8 and eNOS polymorphisms with clinical outcomes in bevacizu-mab-treated breast cancer patients: an exploratory analysis. Clin Transl Oncol. 2016;18:40–46. doi: 10.1007/s12094-015-1334-7. [DOI] [PubMed] [Google Scholar]
- 13.Hamed EA, Zakhary MM, Maximous DW. Apoptosis, angiogenesis, inflammation, and oxidative stress: basic interactions inpatients with early and metastatic breast cancer. J Cancer Res Clin Oncol. 2012;138:999–1009. doi: 10.1007/s00432-012-1176-4. [DOI] [PubMed] [Google Scholar]
- 14.Yousef EM, Tahir MR, St-Pierre Y, Gaboury LA. MMP-9 expression varies according to molecular subtypes of breast cancer. BMC Cancer. 2014;14:609. doi: 10.1186/1471-2407-14-609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sullu Y, Demirag GG, Yildirim A, Karagoz F, Kandemir B. Matrix metalloproteinase-2 (MMP-2) and MMP-9 expression in invasive ductal carci-noma of the breast. Pathol Res Pract. 2011;207:747–753. doi: 10.1016/j.prp.2011.09.010. [DOI] [PubMed] [Google Scholar]
- 16.Pan Q, Bao LW, Merajver SD. Tetrathiomoly bdate inhibits angiogenesis and metastasis through suppression of the NF-kappaB signaling cas-cade. Mol Cancer Res. 2003;1:701–706. [PubMed] [Google Scholar]
- 17.Helbig G, Christopherson KW 2nd, Bhat-Nakshatri P, Kumar S, Kishimoto H, Miller KD, Broxmeyer HE, Nakshatri H. NF-kappaB promotes breast cancer cell migration and metastasis by inducing the expression of the chemokine receptor CXCR4. J Biol Chem. 2003;278:21631–21638. doi: 10.1074/jbc.M300609200. [DOI] [PubMed] [Google Scholar]
- 18.Shibata A, Nagaya T, Imai T, Funahashi H, Nakao A, Seo H. Inhibition of NF-kappaB activity decreases the VEGF mRNA expression in MDA-MB-231breast cancer cells. Breast Cancer Res Treat. 2002;73:237–243. doi: 10.1023/a:1015872531675. [DOI] [PubMed] [Google Scholar]
- 19.Belugali Nataraj N, Salimath BP. Crosstalk between VEGF and novel angiogenic protein regulates tumor angiogenesisand contributes to ag-gressiveness of breast carcinoma. Cell Signal. 2013;25:277–294. doi: 10.1016/j.cellsig.2012.09.013. [DOI] [PubMed] [Google Scholar]
- 20.Malonia SK, Yadav B, Sinha S, Lazennec G, Chattopadhyay S. Chromatin remodeling protein SMAR1 regulates NF-κB depend-ent Interleukin-8 transcription in breast cancer. Int J Biochem Cell Biol. 2014;55:220–226. doi: 10.1016/j.biocel.2014.09.008. [DOI] [PubMed] [Google Scholar]
- 21.Bobrovnikova-Marjon EV, Marjon PL, Barbash O, Vander Jagt DL, Abcouwer SF. Expression of angiogenic factors vascular endothelial growth factor and interleukin-8/CXCL8 is highly responsive to ambient glutamine availability: role of nuclear factor-kappaB and activating pro-tein-1. Cancer Res. 2004;64:4858–4869. doi: 10.1158/0008-5472.CAN-04-0682. [DOI] [PubMed] [Google Scholar]
- 22.Safina A, Ren MQ, Vandette E, Bakin AV. TAK1 is required for TGF-beta 1-mediated regulation of matrix metalloproteinase-9 and metastasis. Oncogene. 2008;27:1198–207. doi: 10.1038/sj.onc.1210768. [DOI] [PubMed] [Google Scholar]
- 23.Lee CC, Liu KJ, Wu YC, Lin SJ, Chang CC, Huang TS. Sesamin inhibits macrophage-induced vascular endothelial growth factor and matrix met-alloproteinase-9 expression and proangiogenic activity in breast cancer cells. Inflammation. 2011;34:209–221. doi: 10.1007/s10753-010-9226-z. [DOI] [PubMed] [Google Scholar]
- 24.Song LL, Peng Y, Yun J, Rizzo P, Chaturvedi V, Weijzen S, Kast WM, Stone PJ, Santos L, Loredo A, Lendahl U, Sonenshein G, Osborne B, Qin JZ, Pannuti A, Nickoloff BJ, Miele L. Notch-1 associates with IKKalpha and regulates IKK activity in cervical cancer cells. Oncogene. 2008;27:5833–5844. doi: 10.1038/onc.2008.190. [DOI] [PubMed] [Google Scholar]
- 25.Pan H, Zhou W, He W, Liu X, Ding Q, Ling L, Zha X, Wang S. Genistein inhibits MDA-MB-231 triple-negative breast cancer cell growth by inhibiting NF-κB activity via the Notch-1 pathway. Int J Mol Med. 2012;30:337–343. doi: 10.3892/ijmm.2012.990. [DOI] [PubMed] [Google Scholar]
- 26.Yuan X, Zhang M, Wu H, Xu H, Han N, Chu Q, Yu S, Chen Y, Wu K. Expression of Notch1 Correlates with Breast Cancer Progression and Prognosis. PLoS One. 2015;10:e0131689. doi: 10.1371/journal.pone.0131689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miele L, Miao H, Nickoloff BJ. NOTCH signaling as a novel cancer therapeutic target. Curr Cancer Drug Targets. 2016;6:313–323. doi: 10.2174/156800906777441771. [DOI] [PubMed] [Google Scholar]
- 28.Karin M. Nuclear factor-κB in cancer development and progression. Nature. 2006;441:431–436. doi: 10.1038/nature04870. [DOI] [PubMed] [Google Scholar]
- 29.Portanova P, Notaro A, Pellerito O, Sabella S, Giuliano M, Calvaruso G. Notch inhibition restores TRAIL-mediated apoptosis via AP1-dependent upregulation of DR4 and DR5 TRAIL receptors in MDA-MB-231 breast cancer cells. Int J Oncol. 2013;43:121–30. doi: 10.3892/ijo.2013.1945. [DOI] [PubMed] [Google Scholar]
- 30.Curran S, Murray GI. Matrix metalloproteinases: molecular aspects of their roles in tumour invasion and metastasis. Eur J Cancer. 2000;36:1621–1630. doi: 10.1016/s0959-8049(00)00156-8. [DOI] [PubMed] [Google Scholar]
- 31.Nagakawa Y, Aoki T, Kasuya K, Tsuchida A, Koyanagi Y. Histologic features of venous invasion, expression of vascular endothelial growth factor and matrix metalloproteinase-2 and matrix metalloproteinase-9, and the relation with liver metastasis in pancreatic cancer. Pancreas. 2002;24:169–178. doi: 10.1097/00006676-200203000-00008. [DOI] [PubMed] [Google Scholar]
- 32.Fujioka S, Sclabas GM, Schmidt C. Inhibition of constitutive NF-κB activity by IkBaM suppresses tumorigenesis. Oncogene. 2013;22:1365–1370. doi: 10.1038/sj.onc.1206323. [DOI] [PubMed] [Google Scholar]
- 33.Wey JS, Fan F, Gray MJ. Vascular endothelial growth factor receptor-1 promotes migration and invasion in pancreatic carcinoma cell lines. Cancer. 2005;104:427–438. doi: 10.1002/cncr.21145. [DOI] [PubMed] [Google Scholar]
- 34.Singh JK, Simões BM, Howell SJ, Farnie G, Clarke RB. Recent advances reveal IL-8 signaling as a potential key to targeting breast cancerstem cells. Breast Cancer Res. 2013;15:210. doi: 10.1186/bcr3436. [DOI] [PMC free article] [PubMed] [Google Scholar]