Abstract
A series of recent studies suggested that miR-143 might involve in the tumorigenesis and metastasis of various cancer types. However, the biological function and underlying mechanisms of miR-143 in human epithelial ovarian carcinoma (EOC) remain unknown. Therefore, this study aimed to investigate the miR-143 expression and its clinical diagnosis significance in patients suffering EOC and to analyze its role and underlying molecular mechanism in EOC. Our result showed that the expression levels of miR-143 were downregulated in EOC tissues and cell lines, was associated with International Federation of Gynaecology and Obstetrics (FIGO) stage, pathological grade and lymph node metastasis (all P < 0.01) . Overexpression of miR-143 significantly inhibited EOC cell proliferation, migration, and invasion. Furthermore, computational algorithm combined with luciferase reporter assays identified connective tissue growth factor (CTGF) as the direct target of miR-143 in EOC cells. The expression level of CTGF was significantly increased in EOC tissues, was inversely correlated with miR-143 expression in clinical EOC tissues. Knockdown of CTGF mimicked the suppression effect induced by miR-143 overexpression. Restoration of CTGF expression partially reversed the suppression effect induced by miR-143 overexpression. These results suggested that miR-143 inhibited EOC cell proliferation, migration, and invasion, at least in part, via suppressing CTGF expression.
Keywords: microRNAs, miR-143, epithelial ovarian carcinoma, CTGF
Introduction
Epithelial ovarian cancer (EOC) is the most lethal of the gynecologic malignancies in women with highly aggressive clinical course, causing 125,000 deaths all over the world annually [1,2]. The main reason for the high mortality is the incomplete understanding of its pathology, the lack of diagnostic methods for early stage detection, the resistance to chemotherapy drugs, and lack of effective strategies for treatment [3-5]. Therefore, further investigations into the molecular mechanisms that involve in ovarian cancer procession and development, the discovery of robust predictive biomarkers and the development of new molecularly targeted drugs are essential for effective treatment EOC.
MicroRNAs (miRNAs), a class of endogenous small (18-24 nt) noncoding single-stranded RNAs, have recently emerged as novel regulators of various fundamental biological processes, including those relevant to tumorigenesis [6,7]. They were found to regulate gene expression in a posttranscriptional manner via binding to the 3’-untranslated regions (3’-UTR) of target expression (miRNA), thereby resulting in mRNA degradation or translational inhibition [8]. Accumulating evidence has implicated that miRNAs play crucial roles in the development of many cancer types, as either oncogenes or tumor suppressors [9,10]. Recently many miRNAs have been proven to be predictive of EOC prognosis and metastasis and may serve as molecular biomarkers for EOC detection, and as therapy agent for EOC treatment [11,12].
MicroRNA-143 (miR-143), located in a cluster within the 5q32-33 chromosomal region, has also been reported to be downregulated in several types of human cancer, such as colorectal cancer [13], osteosarcoma [14], esophageal squamous cell carcinoma [15] , breast carcinoma [16], non-small cell lung cancer [17], and glioma [18], suggesting that miR-143 might have potential roles as a tumor-suppressor miRNA in these types cancer. However, the detail biological function and underlying molecular mechanism of miR-143 in EOC remains unknown. Therefore, the aims of the present study were to investigate the miR-143 expression and its clinical diagnosis significance in patients suffering EOC and to analyze its role and underlying molecular mechanism in EOC.
Materials and methods
Patients and tissue samples
Tissue samples were obtained from the patients who underwent surgery at the First Hospital of Jilin University (Changchun, China) from June 2010 to July 2015, including 40 EOC tissues and 16 normal epithelial ovarian tissue sections. All tissue samples were immediately snapped frozen in liquid nitrogen, and stored at -80°C until use. None of the patients were treated with chemotherapy, radiotherapy or other treatment prior to surgery. The clinical characteristics of all of EOC patients were recorded and listed in Table 1. Informed consent was obtained from all patient or family before surgery. This study was approved by the ethics committee of Jilin University (Changchun, China).
Table 1.
Correlation between clinicopathological features and miR-143 expression in EOC tissues
| Variables | No. of cases | miR-143 expression | P value | |
|---|---|---|---|---|
|
| ||||
| Low (n %) | High (n %) | |||
| Age (years) | P > 0.05 | |||
| < 55 | 24 | 14 (58.3) | 10 (41.7) | |
| ≥ 55 | 16 | 9 (56.2) | 7 (43.8) | |
| Tumor size | P > 0.05 | |||
| ≥ 5 | 23 | 15 (65.2) | 8 (34.8) | |
| < 5 | 17 | 8 (47.1) | 9 (52.9) | |
| FIGO stage | P < 0.01 | |||
| I-II | 31 | 15 (48.4) | 16 (51.6) | |
| III-IV | 9 | 8 (88.9) | 2 (11.1) | |
| Histological grading | P < 0.01 | |||
| 1/2 | 29 | 14 (48.3) | 15 (51.7) | |
| 3 | 11 | 9 (81.8) | 2 (19.2) | |
| Lymph node metastasis | P < 0.01 | |||
| No | 28 | 14 (50.0) | 14 (50.0) | |
| Yes | 12 | 9 (75.0) | 2 (25.0) | |
Cell culture and transfection
Four human ovarian cancer cell lines SW626, A2780, SKOV3, OVCAR3 and a human ovarian surface epithelial cell line (HOSEpiC) were brought from the Type Culture Collection of the Chinese Academy of Sciences (Shanghai, China), and were cultured in Dulbecco’s modified eagle’s medium (DMEM, Gibco-BRL, Gaithersburg, MD, USA) supplemented with 10% fetal bovine serum (FBS, HyClone, USA), 100 IU/mL of penicillin and 100 mg/mL of streptomycin at 37°C under a humidified atmosphere containing 5% CO2.
The miR-143 mimic, negative control mimics (miR-NC), small inhibitory RNA (siRNA) for CTGF (si-CTGF), and scramble siRNA (si-Scramble) were all brought from GenePharma (Shanghai, China). The coding sequences CTGF were amplified by PCR and inserted into pcDNA3.1 vector (Invitrogen, Life Technologies, Carlsbad, USA) to generate CTGF expression vectors ppCDNA3.1-CTGF, named as pCTGF. Transfection was carried out using Lipofectamine 2000 (Invitrogen) according to the manufacturer’s instructions.
RNA extraction and real-time PCR analysis
To quantify miR-143, total RNA was extracted from tissues sample and cultured cells (HOSEpiC, SW626, A2780, SKOV3, OVCAR3) using the mir-Vana miRNA Isolation Kit (Ambion, USA) according to the manufacturer’s instructions. The purity and concentration of RNA were determined using a dual-beam ultraviolet spectrophotometer (Eppendorf, Hamburg, Germany). The All-in-OneTM miRNA qRT-PCR Detection Kit (GeneCopoeia, USA) was used to detect miR-143 expression level using specific miR-143 prime (GeneCopoeia, USA) under ABI 7900 Fast system (Applied Biosystems, Foster City, CA, USA). U6 small RNA was used as the internal control. For the detection of CTGF mRNA, total RNA was extracted from tissues or cultured cells using TRIzol reagent (Invitrogen) according to the manufacturer’s instructions. The RNA was reversely transcribed into cDNA using PrimeScript RT reagent Kit (Takara, Dalian, China). The expression levels of CTGF were quantified by Real-time PCR Mixture Reagent (Takara). β-actin was used as the internal control. The primers of CTGF and β-actin were used in this study as described previously [19]. The comparative 2-ΔΔCt method was used for the relative quantification assay.
Cell proliferation
To determine the cell proliferation capacity, MTT assay were performed. Briefly, transfected cells (5 × 103 cells/well) were seeded into 96-well plates with 100 μl of DMEM medium and cultured for 1 to 3 days. At the indicated time (24 h, 48 h, and 72 h), the cells were incubated with 20 μl MTT reagent (5 mg/ml, Sigma-Aldrich, St Louis, MO, USA), and were further cultivated for additional for 4 h at 37°C. Thereafter, the supernatant was removed, and 150 μl dimethyl sulfoxide (DMSO, Sigma-Aldrich) was added to dissolve the crystals for 10 min at 37°C. The absorbance in each well was measured at a wavelength of 570 nm by an enzyme-linked immunosorbent assay reader (Thermo Labsystems, Finland).
Cell migration and invasion assays
To determine the cell migration, Wound healing assay were performed. Briefly, transfected cells were seeded in 3.5-cm plates and grown to a density of 70 to 80%. Thereafter, an artificial homogenous wound was were created by a sterile plastic micropipette tip. After wounding, the debris was removed by washing the cells with PBS, and cell were cultured under standard conditions for 24 h. Migration of cells into the wound was observed at 0 and 24 h using an inverted light microscope (Olympus, Tokyo, Japan).
Cell invasion potential was evaluated using transwell chambers (8 μm pore; BD Biosciences). Briefly, 2 × 104 transfected cells in serum-free DMEM medium were placed into the upper side of the polycarbonate transwell filter with Matrigel (BD Biosciences, San Jose, CA, USA). Medium containing 10% FBS were added to the lower chamber to serve as chemoattractant. After incubation for 48 h in a humidified atmosphere of 5% CO2 at 37°C, noninvading cells were removed from the top well with a cotton swab, while invaded cells were fixed with 70% ethanol for 30 min and stained with 0.2% crystal violet for 10 min. Photographs of five randomly selected fields of the fixed cells were taken and counted under an inverted light microscope (Olympus, Tokyo, Japan).
Luciferase assay
3’-untranslated region (3’-UTR) regions of CTGF and the mutant 3’UTR of CTGF was chemically synthesized and inserted into the downstream of the firefly luciferase gene in a pGL3-promoter vector (Ambion, Austin, TX, USA). For luciferase assays, the SKOV3 cells were plated in 24-well plates for 24 h, and then were co-transfected with wide-type or mutant-type CTGF vector, and miR-143 mimic or miR-NC, using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s protocol. The activities of both firefly and Renilla luciferases in cell lysates were determined using the Dual Luciferase Reporter Assay System (Promega, Madison, WI, USA) at 48 h after transfection. Renilla-luciferase was used for normalization.
Western blotting
Total protein were extracted from cultured cells or tissues using RIPA buffer with 0.5% sodium dodecyl sulfate (SDS) containing proteinase inhibitor cocktail (Complete Mini; Roche Diagnostics, Basel, Switzerland). Total protein concentrations were measured by using a bicinchoninic acid protein assay kit (BCA) assay kit (Beyotime, Shanghai, China). Equal amounts of protein lysates (30 μg each lane) was separated 10% SDS-PAGE gel and then electrotransferred to polyvinylidene difluoride membranes (PVDF, Bio-Rad, Hercules, CA, USA). The membranes were probed with antibody against CTGF (1:500, Cell Signaling Technology, Boston, MA, USA) and β-actin (1:2000, Cell Signaling Technology) overnight at -4°C. The membranes were washed with PBS, and incubated with incubated with horseradish peroxidase (HRP)-conjugated secondary antibodies (1:5,000; Santa Cruz Biotechnology, CA, USA) for 2 h at room temperature. Proteins bland were observed with chemiluminescent detection system (Beyotime, Shanghai, China) and exposure with an autoradiography film (Kodak, Shanghai, China). β-actin protein was used as internal control.
Statistical analysis
The data were expressed as the mean ± SD (standard deviation) of at least three separate experiments. Comparisons between the groups were analyzed with two-tailed Student’s T-test or one-way ANOVA. Correlation between two groups was analyzed by Spearman’s rank test. All statistical analyses were performed using SPSS 11.0 software (SPSS Inc., Chicago, IL, USA). P values < 0.05 was considered statistically significant.
Results
Downregulation of miR-143 is associated with clinicopathological features of EOC patients
To determine the clinical relevance of miR-143 in human EOC, the expression levels of miR-143 in samples from primary EOC tumor tissues (n = 40) and normal ovarian epithelial tissues (n = 16) was examined. Results of by real time quantitative RT-PCR (qRT-PCR) showed that expression of miR-143 in EOC tissues was significantly downregulated compared to normal ovarian epithelial tissues (P < 0.01) (Figure 1A). In addition, the levels of miR-143 expression in four human EOC cell lines (SW626, A2780, SKOV3, OVCAR3) and a human ovarian surface epithelial cell line (HOSEpiC) were examined by qRT-PCR (Figure 1B). In all four EOC cell lines, the miR-143 expression level was lower than that of human ovarian surface epithelial cell line (HOSEpiC) (Figure 1B). The SKOV3 cell line, which possessed the lowest levels of miR-143 expression among four cell lines (Figure 1B), was selected for below study. The association between miR-143 expression and the clinicopathological parameters of the patients was assessed (Table 1). The 40 patients were divided into two group according to the median (0.351): low-miR-143 expression group (< 0.351, 23 cases) group and high-miR-143 expression group (> 0.351, 17 cases). Reduced expression of miR-141 was significantly associated with FIGO stage, histological grading and lymph node metastasis (all P < 0.01) (Table 1). However, there was no significant association between miR-143 expression and patient’s age and tumor size (Table 1). These data suggested that miR-143 might involve in EOC development.
Figure 1.
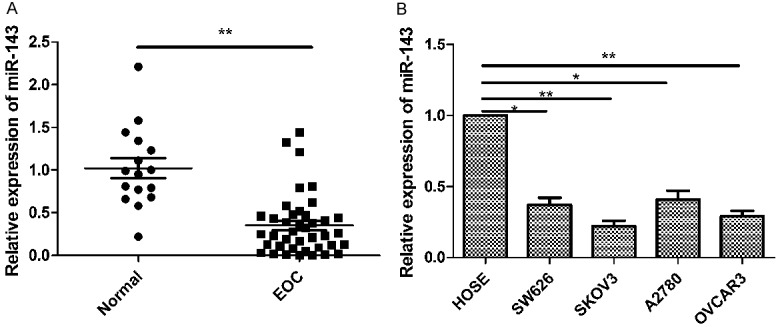
MiR-143 is downregulated in EOC tissues and cell lines. A. Relative miR-143 expression level was determined by quantitative RT- PCR (qRT-PCR) in 40 EOC tissues and in 16 normal epithelial tissues. B. Relative miR-143 expression level was determined by qRT-PCR in 4 EOC cell lines (SW626, A2780, SKOV3, OVCAR3) and human ovarian surface epithelial (HOSE cells). U6 snRNA was used as the internal control. **P < 0.01 compared to normal tissue samples or HOSE cell lines.
MiR-143 inhibits EOC cell proliferation, migration and invasion
To determine whether the miR-143 expression could affect proliferation of EOC cells, we transfected miR-143 mimic or miR-NC into SKOV3 cells, and confirmed that miR-143 expression level was significantly upregulated in SKOV3 cells transfected with miR-143 mimic compared to cells transfected with miR-NC (Figure 2A). MTT assay showed that the cells transfected with miR-143 mimic showed significantly reduced cell proliferation compared to those transfected with miR-NC (Figure 2B). To test whether miR-143 expression affect EOC cell migration and invasion, the migration and invasion were measured in SKOV3 cells after transfected with miR-143 mimic or miR-NC by wound healing assay and transwell invasion assay, respectively. It was found that overexpression of miR-143 in SKOV3 cells could significantly suppress migration (Figure 2C) and invasion (Figure 2D).
Figure 2.
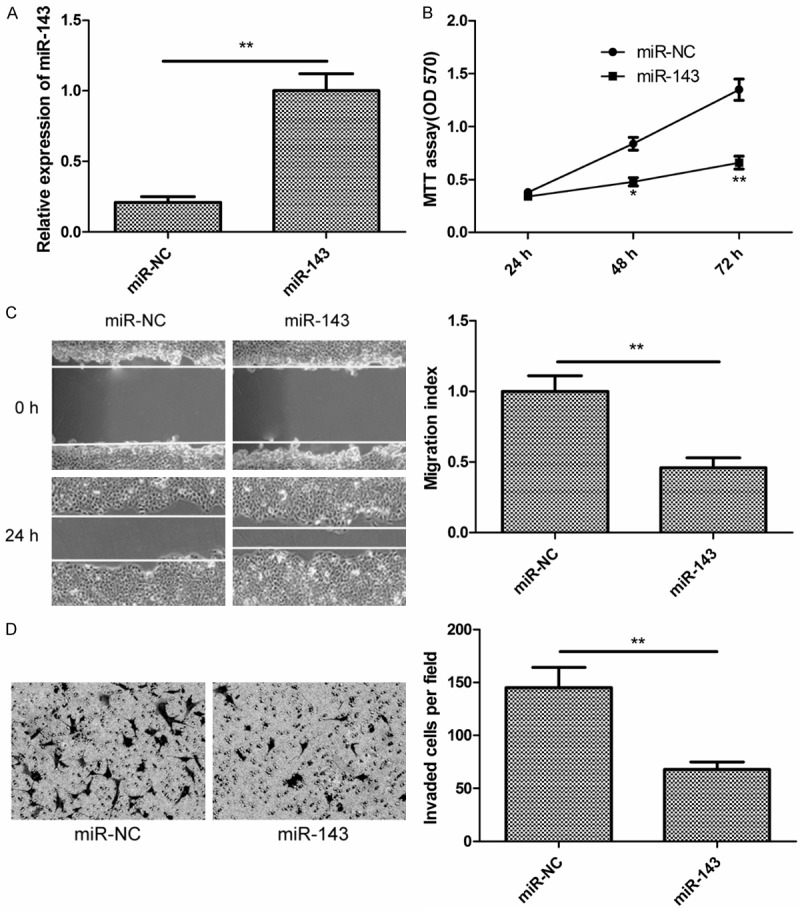
MiR-143 inhibits EOC cell proliferation, migration and invasion. A. Relative miR-143 expression level was determined by qRT-PCR in SKOV3 cells transfected with miR-143 mimic or miR-NC. B. Cell proliferation was determined by MTT assay in SKOV3 cells transfected with miR-143 mimic or miR-NC. C. Cell migration was determined by wound healing assay in SKOV3 cells transfected with miR-143 mimic or miR-NC. D. Cell invasion was determined by transwell invasion assay in SKOV3 cells transfected with miR-143 mimic or miR-NC. *P < 0.05, **P < 0.01 compared with miR-NC transfected cells.
CTGF is a direct target of miR-143
We next determined the potential targets of miR-143 by bioinformatic databases (TargetScan, PicTar, and miRanda), and found that there was a miR-143 binding site in CTGF 3’-UTR at position 53-59 (Figure 3A). In order to validate that GTGF was a direct target gene of miR-143, luciferase assay were performed. Results showed the transfection of miR-143 significantly reduced wide-type CTGF luciferase activity, while had no inhibition effect on the mutant-type CTGF luciferase activity in SKOV3 cells (Figure 3B). In addition, we detected the CTGF levels in SKOV3 cells transfected with miR-143 mimic. Consistently, miR-143 significantly reduced both the mRNA levels (Figure 3C) and the protein levels (Figure 3D) of CTGF in SKOV3 cells. Furthermore, we examined CTGF expression in 40 EOC tissues and 16 normal ovarian epithelial tissues using qRT-PCR. It was found that GTGF expression level was increased in EOC tissues compared to normal ovarian epithelial tissues (Figure 3E). With the Spearman correlation analysis, we found that there was an obvious inverse correlation between the expression levels of miR-143 and CTGF (r = -0.585, P < 0.001) (Figure 3F). These results suggested that CTGF is a direct target of miR-143.
Figure 3.
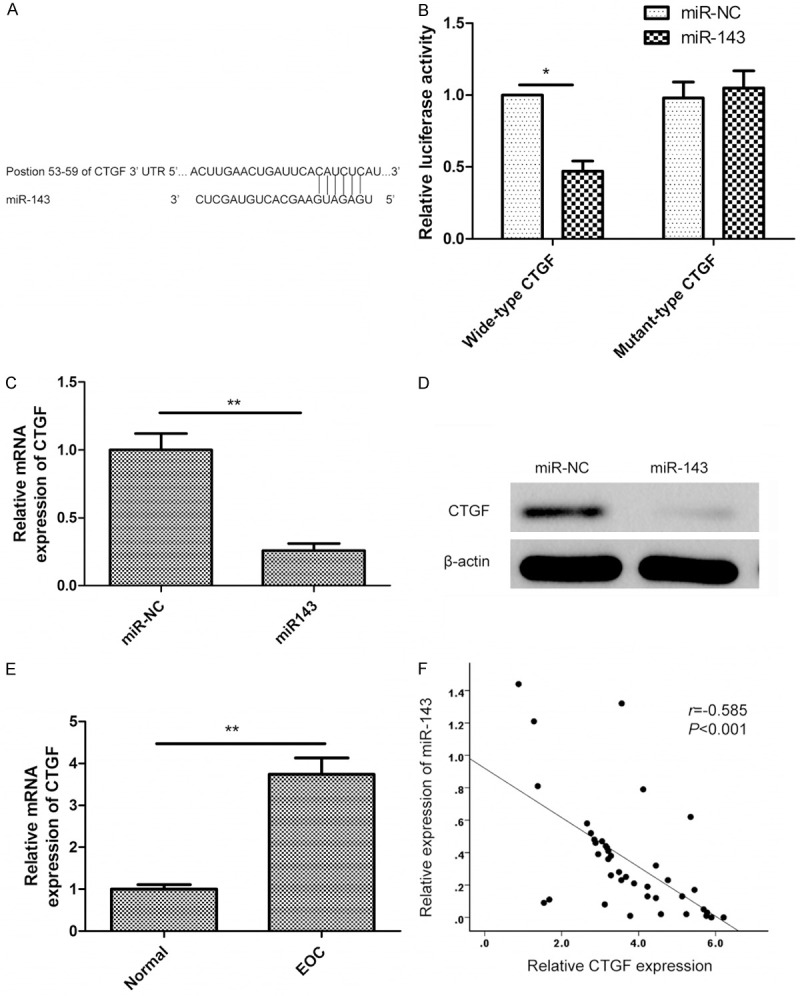
CTGF is a direct target of miR-143. A. The putative binding sites of miR-143 on the CTGF 3’-UTR region (positions 53-59). B. Luciferase activities were determined in SKOV3 48 h after co-transfected with wide-type or mutant-type CTGF 3’-UTR luciferase plasmid and miR-143 or miR-NC. *P < 0.05 compared to miR-NC transfected cells. C. Levels of CTGF mRNA were determined by qRT-PCR in SKOV3 cells transfected with miR-143 mimic or miR-NC. **P < 0.01 compared to miR-NC transfected cells. D. Levels of CTGF protein was determined by Western blot in cells transfected with miR-143 mimic or miR-NC. E. Levels of CTGF mRNA was determined by qRT-PCR in 40 EOC tissues and in 20 normal epithelial tissues. **P < 0.01 compared to normal epithelial tissues. F. The correlation of the expression levels of CTGF and miR-143 in 40 EOC tissue samples.
Inhibition of CTGF has similar effect with miR-143 overexpression in EOC cells
To investigate the biological role of CTGF in EOC, we first knocked down CTGF expression in SKOV3 cells using small interfering RNA (siRNA) targeting CTGF (si-CTGF), and confirmed the efficiency of knockdown by Western blot analysis (Figure 4A). Significantly, the knockdown of CTGF expression significantly suppressed cell proliferation (Figure 4B), migration (Figure 4C), and invasion (Figure 4D) in SKOV3 cells, which was consistent with the effect of miR-143 overexpression.
Figure 4.
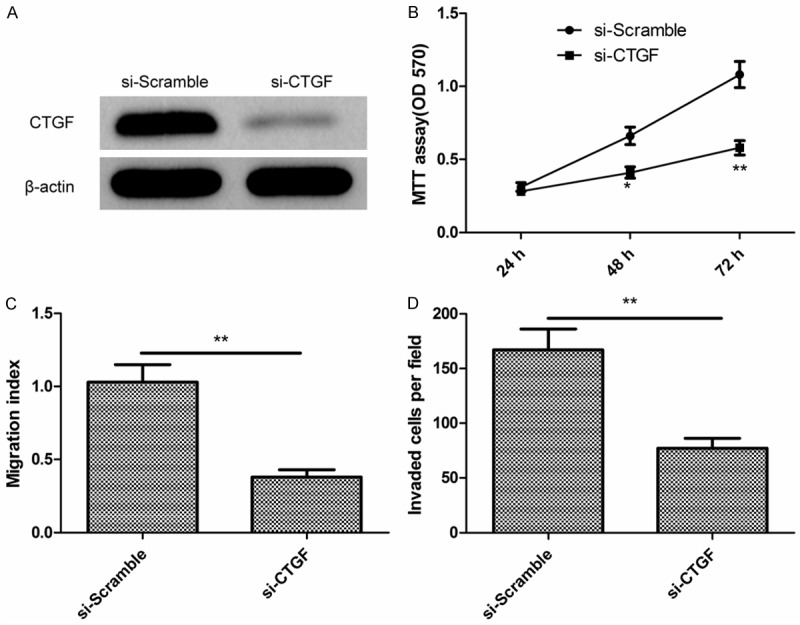
Inhibition of CTGF has similar effect with miR-143 overexpression in EOC cells. A. Levels of CTGF protein was determined by Western blot in SKOV3 cells transfected with si-CTGF or si-Scramble. B-D. Cell proliferation, migration and invasion were determined in SKOV3 cells transfected with si-CTGF or si-Scramble. **P < 0.01 compared to si-Scramble transfected cells.
Restoration of CTGF rescues the effects of miR-143 in EOC cells
To investigate a possible biological function for CTGF in miR-143-mediated suppression of EOC proliferation, migration and invasion, SKOV3 cells were simultaneously co-transfected miR-143 mimic or miR-NC and overexpression of CTGF plasmids (pCTGF). We found that overexpression of miR-143 reduced the CTGF protein expression, while co-transfection of CTGF-overexpressing plasmids could restore the CTGF protein expression (Figure 5A). Of note, transfection of CTGF-overexpressing plasmid significantly reversed the inhibition of EOC cell proliferation (Figure 5B), migration (Figure 5C), and invasion (Figure 5D) induced by the miR-143 overexpression. These results indicated that miR-143 inhibits EOC cell proliferation, migration, and invasion partially by downregulating CTGF.
Figure 5.
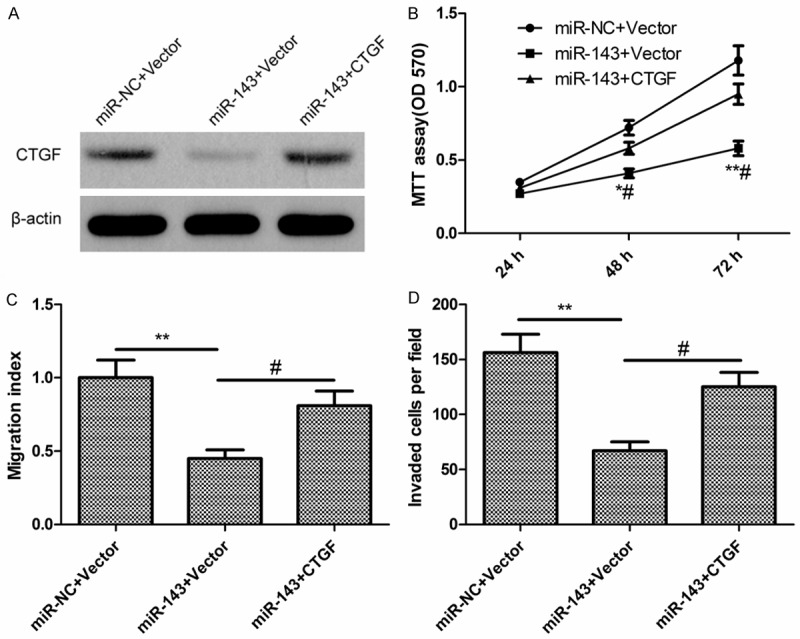
Restoration of CTGF rescues the effects of miR-143 in EOC cells. A. Levels of CTGF mRNA and protein were determined in SKOV3 cells transfected with miR-143 mimic or miR-NC, together with either blank vector (pCDNA3.1) or plasmids encoding CTGF (pCTGF). B-D. Cell proliferation, migration and invasion were determined in SKOV3 cells transfected with miR-143 mimic or miR-NC, together with either blank vector or plasmids encoding CTGF. *P < 0.05 compared with cells transfected with miR-NC together with vector control; #P < 0.05 compared with cells transfected with miR-143 together with vector control.
Discussion
Accumulating studies have identified a number of miRNAs with aberrant expression in EOC tissues or cells, and indicated that they might be involved in a variety of tumorigenic processes, including cell proliferation, apoptosis, migration and invasion [11,12]. In the current study, we investigated the miR-143 expression and its clinical diagnosis significance in patients suffering EOC and function of miR-143 in the regulation of EOC cell proliferation, migration, and invasion. Our results first showed that miR-143 expression was significantly decreased in EOC tissues and cell lines. And that miR-143 level in EOC tissue was associated with FIGO stage, pathological grade and lymph node metastasis. In addition, our results also demonstrated that restoration of miR-143 in EOC cells inhibited cell proliferation, migration and invasion by repressing CTGF. These results suggested that miR-143 might play important roles in EOC initiation and progression.
Accumulating evidence indicated that miR-143 expression was downregulated in multiple tumors, and functioned as tumor suppressor miRNAs by regulating cell proliferation, apoptosis, migration and invasion in various cancer types [13-18]. For example, Mao et al reported that miR-143 exerted a tumor-suppressing effect by inhibiting the proliferation, migration, and invasion and inducing G1/G0 phase arrest of esophageal squamous cell carcinoma cells via the negative regulation of family with sequence similarity 83 (FAM83) members expression [20]. Wang et al found that miR-143 overexpression suppressed glioma cell migration, invasion, and slowed tumor growth by inactivating N-RAS and inhibiting the phosphatidylinositol 3-kinase (PI3K)/AKT, and mitogen-activated protein kinase (MAPK)/extracellular signal regulated kinase (ERK) signaling [21]. Zhou et al showed that miR-143 exerted an inhibitory effect on cell proliferation as evidenced by decreased cell viability, increased cell apoptosis and cell cycle arrest at the G1/S transition by targeting hexokinase 2 (HK2) in prostate cancer [22]. Zhai et al demonstrated that miR-143 suppressed epithelial-mesenchymal transition(EMT) and inhibited tumor growth of breast cancer through repression of extracellular signal regulated kinase 5 (ERK5) [23]. However, the biological role and underlying molecular mechanism of miR-143 in EOC remains unclear. Here, our results demonstrated that overexpression of miR-143 impaired proliferation, colony formation, invasion and migration by repressing CTGF, suggesting that miR-143 might function as tumor suppressor in EOC.
It was well known that identification of their target gene contribute to elucidate molecular miRNAs in tumorigenesis [6]. To explore the mechanisms underlying the suppression of EOC cell proliferation and invasion mediated by miR-143, we identified CTGF as a direct functional target of miR-143 in ovarian by bioinformatic prediction. The prediction was further confirmed by luciferase assay. miR-143 overexpression reduced the expression of endogenous CTGF on mRNA level and protein level in EOC cells. These results suggested that CTGF is a target for miR-143. CTGF (also known as CCN2), a member of the CCN family of secreted proteins [24], had been suggested to acts as a multifunctional signal conductor in diverse cellular events, including fibrosis, extracellular matrix production, cell proliferation, metastasis, and angiogenesis [25,26]. CTGF expression was elevated in various tumors including glioma [27], breast cancer [28], hepatocellular carcinoma [29], esophageal squamous cell carcinomas [30], pancreatic cancers [31] and prostate cancer [32]. For EOC, previous study had been demonstrated that CTGF expression was increased in EOC tissues, and its expression correlates with stage of this disease [33], and that CTGF could promote migration and peritoneal adhesion of ovarian cancer cells [34], suggesting that CTGF serve as an oncogene in ovarian cancer. In the present study, our results showed that the expression level of CTGF was significantly increased in EOC tissues and its expression was inversely correlated with miR-143 expression in EOC tissues. Knockdown of CTGF almost perfectly mimicked the phenotype on cell proliferation, cell migration, and cell invasion induced by the overexpression of miR-143. Overexpression of CTGF partially abrogated the suppression effect induced by miR-143. These results suggested that miR-143 exerts suppression effect of in EOC, at least in part, by targeting CTGF.
Taken together, the results presented here first demonstrate that miR-143 expression level was decreased in EOC tissue and cell lines, and its expression level was significantly associated with FIGO stage, pathological grade and lymph node metastasis. And that miR-143 inhibited EOC cell proliferation, migration, and invasion by targeting CTGF. These results suggested that miR-143 might be a promising target for the treatment of EOC in the future.
Disclosure of conflict of interest
None.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Permuth-Wey J, Sellers TA. Epidemiology of ovarian cancer. Methods Mol Biol. 2009;472:413–437. doi: 10.1007/978-1-60327-492-0_20. [DOI] [PubMed] [Google Scholar]
- 3.Bristow RE. Surgical standards in the management of ovarian cancer. Curr Opin Oncol. 2000;12:474–480. doi: 10.1097/00001622-200009000-00015. [DOI] [PubMed] [Google Scholar]
- 4.Harter P, Muallem ZM, Buhrmann C, Lorenz D, Kaub C, Hils R, Kommoss S, Heitz F, Traut A, du Bois A. Impact of a structured quality management program on surgical outcome in primary advanced ovarian cancer. Gynecol Oncol. 2011;121:615–619. doi: 10.1016/j.ygyno.2011.02.014. [DOI] [PubMed] [Google Scholar]
- 5.Harries M, Gore M. Part II: chemotherapy for epithelial ovarian cancer-treatment of recurrent disease. Lancet Oncol. 2002;3:537–545. doi: 10.1016/s1470-2045(02)00847-1. [DOI] [PubMed] [Google Scholar]
- 6.Osada H, Takahashi T. MicroRNAs in biological processes and carcinogenesis. Carcinogenesis. 2007;28:2–12. doi: 10.1093/carcin/bgl185. [DOI] [PubMed] [Google Scholar]
- 7.Tie J, Fan D. Big roles of microRNAs in tumorigenesis and tumor development. Histol Histopathol. 2011;26:1353–1361. doi: 10.14670/HH-26.1353. [DOI] [PubMed] [Google Scholar]
- 8.He L, Hannon GJ. MicroRNAs: small RNAs with a big role in gene regulation. Nat Rev Genet. 2004;5:522–531. doi: 10.1038/nrg1379. [DOI] [PubMed] [Google Scholar]
- 9.Mendell JT. MicroRNAs: critical regulators of development, cellular physiology and malignancy. Cell Cycle. 2005;4:1179–1184. doi: 10.4161/cc.4.9.2032. [DOI] [PubMed] [Google Scholar]
- 10.Volinia S, Calin GA, Liu CG, Ambs S, Cimmino A, Petrocca F, Visone R, Iorio M, Roldo C, Ferracin M, Prueitt RL, Yanaihara N, Lanza G, Scarpa A, Vecchione A, Negrini M, Harris CC, Croce CM. A microRNA expression signature of human solid tumors defines cancer gene targets. Proc Natl Acad Sci U S A. 2006;103:2257–2261. doi: 10.1073/pnas.0510565103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kinose Y, Sawada K, Nakamura K, Kimura T. The role of microRNAs in ovarian cancer. Biomed Res Int. 2014;2014:249393. doi: 10.1155/2014/249393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang Y, Kim S, Kim IM. Regulation of Metastasis by microRNAs in Ovarian Cancer. Front Oncol. 2014;4:143. doi: 10.3389/fonc.2014.00143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Su J, Liang H, Yao W, Wang N, Zhang S, Yan X, Feng H, Pang W, Wang Y, Wang X, Fu Z, Liu Y, Zhao C, Zhang J, Zhang CY, Zen K, Chen X, Wang Y. MiR-143 and MiR-145 regulate IGF1R to suppress cell proliferation in colorectal cancer. PLoS One. 2014;9:e114420. doi: 10.1371/journal.pone.0114420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hirahata M, Osaki M, Kanda Y, Sugimoto Y, Yoshioka Y, Kosaka N, Takeshita F, Fujiwara T, Kawai A, Ito H, Ochiya T, Okada F. PAI-1, a target gene of miR-143, regulates invasion and metastasis by upregulating MMP-13 expression of human osteosarcoma. Cancer Med. 2016;5:892–902. doi: 10.1002/cam4.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu J, Mao Y, Zhang D, Hao S, Zhang Z, Li Z, Li B. MiR-143 inhibits tumor cell proliferation and invasion by targeting STAT3 in esophageal squamous cell carcinoma. Cancer Lett. 2016;373:97–108. doi: 10.1016/j.canlet.2016.01.023. [DOI] [PubMed] [Google Scholar]
- 16.Zhai L, Ma C, Li W, Yang S, Liu Z. miR-143 suppresses epithelial-mesenchymal transition and inhibits tumor growth of breast cancer through down-regulation of ERK5. Mol Carcinog. 2015 doi: 10.1002/mc.22445. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 17.Wei J, Ma Z, Li Y, Zhao B, Wang D, Jin Y, Jin Y. miR-143 inhibits cell proliferation by targeting autophagy-related 2B in non-small cell lung cancer H1299 cells. Mol Med Rep. 2015;11:571–576. doi: 10.3892/mmr.2014.2675. [DOI] [PubMed] [Google Scholar]
- 18.Wang L, Shi ZM, Jiang CF, Liu X, Chen QD, Qian X, Li DM, Ge X, Wang XF, Liu LZ, You YP, Liu N, Jiang BH. MiR-143 acts as a tumor suppressor by targeting N-RAS and enhances temozolomide-induced apoptosis in glioma. Oncotarget. 2014;5:5416–5427. doi: 10.18632/oncotarget.2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang MH, Lin BR, Chang CH, Chen ST, Lin SK, Kuo MY, Jeng YM, Kuo ML, Chang CC. Connective tissue growth factor modulates oral squamous cell carcinoma invasion by activating a miR-504/FOXP1 signalling. Oncogene. 2012;31:2401–2411. doi: 10.1038/onc.2011.423. [DOI] [PubMed] [Google Scholar]
- 20.Mao Y, Liu J, Zhang D, Li B. miR-143 inhibits tumor progression by targeting FAM83F in esophageal squamous cell carcinoma. Tumour Biol. 2016 doi: 10.1007/s13277-015-4760-9. [DOI] [PubMed] [Google Scholar]
- 21.Wang L, Shi ZM, Jiang CF, Liu X, Chen QD, Qian X, Li DM, Ge X, Wang XF, Liu LZ, You YP, Liu N, Jiang BH. MiR-143 acts as a tumor suppressor by targeting N-RAS and enhances temozolomide-induced apoptosis in glioma. Oncotarget. 2014;5:5416–5427. doi: 10.18632/oncotarget.2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou P, Chen WG, Li XW. MicroRNA-143 acts as a tumor suppressor by targeting hexokinase 2 in human prostate cancer. Am J Cancer Res. 2015;5:2056–2063. [PMC free article] [PubMed] [Google Scholar]
- 23.Zhai L, Ma C, Li W, Yang S, Liu Z. miR-143 suppresses epithelial-mesenchymal transition and inhibits tumor growth of breast cancer through down-regulation of ERK5. Mol Carcinog. 2015 doi: 10.1002/mc.22445. [DOI] [PubMed] [Google Scholar]
- 24.Abreu JG, Ketpura NI, Reversade B, De Robertis EM. Connective-tissue growth factor (CTGF) modulates cell signalling by BMP and TGF-beta. Nat Cell Biol. 2002;4:599–604. doi: 10.1038/ncb826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Perbal B. CCN proteins: multifunctional signalling regulators. Lancet. 2004;363:62–64. doi: 10.1016/S0140-6736(03)15172-0. [DOI] [PubMed] [Google Scholar]
- 26.Leask A, Abraham DJ. All in the CCN family: essential matricellular signaling modulators emerge from the bunker. J Cell Sci. 2006;119:4803–4810. doi: 10.1242/jcs.03270. [DOI] [PubMed] [Google Scholar]
- 27.Pan LH, Beppu T, Kurose A, Yamauchi K, Sugawara A, Suzuki M, Ogawa A, Sawai T. Neoplastic cells and proliferating endothelial cells express connective tissue growth factor (CTGF) in glioblastoma. Neurol Res. 2002;24:677–683. doi: 10.1179/016164102101200573. [DOI] [PubMed] [Google Scholar]
- 28.Lacle MM, van Diest PJ, Goldschmeding R, van der Wall E, Nguyen TQ. Expression of connective tissue growth factor in male breast cancer: clinicopathologic correlations and prognostic value. PLoS One. 2015;10:e0118957. doi: 10.1371/journal.pone.0118957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lamarca A, Mendiola M, Bernal E, Heredia V, Diaz E, Miguel M, Pastrian LG, Burgos E, Feliu J, Barriuso J. Tumoural Expression of Connective Tissue Growth Factor (CTGF) Impacts on Survival in Patients Diagnosed with Hepatocellular Carcinoma (HCC) Curr Cancer Drug Targets. 2015;15:435–444. doi: 10.2174/1568009615666150407124747. [DOI] [PubMed] [Google Scholar]
- 30.Deng YZ, Chen PP, Wang Y, Yin D, Koeffler HP, Li B, Tong XJ, Xie D. Connective tissue growth factor is overexpressed in esophageal squamous cell carcinoma and promotes tumorigenicity through beta-catenin-T-cell factor/Lef signaling. J Biol Chem. 2007;282:36571–36581. doi: 10.1074/jbc.M704141200. [DOI] [PubMed] [Google Scholar]
- 31.Kwon S, Munroe X, Crawley SC, Lee HY, Spong S, Bradham D, Gum JR Jr, Sleisenger MH, Kim YS. Expression of connective tissue growth factor in pancreatic cancer cell lines. Int J Oncol. 2007;31:693–703. [PubMed] [Google Scholar]
- 32.Yang F, Tuxhorn JA, Ressler SJ, McAlhany SJ, Dang TD, Rowley DR. Stromal expression of connective tissue growth factor promotes angiogenesis and prostate cancer tumorigenesis. Cancer Res. 2005;65:8887–8895. doi: 10.1158/0008-5472.CAN-05-1702. [DOI] [PubMed] [Google Scholar]
- 33.Gery S, Xie D, Yin D, Gabra H, Miller C, Wang H, Scott D, Yi WS, Popoviciu ML, Said JW, Koeffler HP. Ovarian carcinomas: CCN genes are aberrantly expressed and CCN1 promotes proliferation of these cells. Clin Cancer Res. 2005;11:7243–7254. doi: 10.1158/1078-0432.CCR-05-0231. [DOI] [PubMed] [Google Scholar]
- 34.Moran-Jones K, Gloss BS, Murali R, Chang DK, Colvin EK, Jones MD, Yuen S, Howell VM, Brown LM, Wong CW, Spong SM, Scarlett CJ, Hacker NF, Ghosh S, Mok SC, Birrer MJ, Samimi G. Connective tissue growth factor as a novel therapeutic target in high grade serous ovarian cancer. Oncotarget. 2015;6:44551–62. doi: 10.18632/oncotarget.6082. [DOI] [PMC free article] [PubMed] [Google Scholar]


