Abstract
Adipogenesis plays a key role in the regulation of whole-body energy homeostasis and is critically related to obesity. To overcome obesity and its associated disorders, it is necessary to elucidate the molecular mechanisms involved in adipogenesis. An adipogenesis-related miRNA array analysis demonstrated that miR-503 was differentially expressed before and after adipocyte differentiation; however, the exact role of miR-503 in adipocyte differentiation is unclear. Thus, the objective of this study was to further examine miR-503 in adipocyte differentiation. We found significantly decreased expression of miR-503 during adipocyte differentiation process. Using bioinformatic analysis, miR-503 was identified as a potential regulator of Bone Morphogenetic Protein Receptor 1a (BMPR1a). We then validated BMPR1a as the target of miR-503 using a dual luciferase assay, and found decreased miR-503 and increased BMPR1a expression during adipogenesis. Overexpression of miR-503 in preadipocytes repressed expression of BMPR1a and adipogenic-related factors such as CCAAT/enhancer binding protein a (C/EBPα), proliferator-activated receptor-gamma (PPARγ), and adipocyte protein 2 (AP2). In addition, miR-503 overexpression impaired the phosphoinositol-3 kinase (PI3K)/Akt pathway. Inhibition of miR-503 had the opposite effect. Additionally, BMPR1a interference by siRNA attenuated adipocyte differentiation and the accumulation of lipid droplets via downregulating the PI3K/Akt signaling pathway. Our study provides the first evidence of the role miR-503 plays in adipocyte differentiation by regulating BMPR1a via the PI3K/Akt pathway, which may become a novel target for obesity therapy.
Keywords: Adipogenesis, BMPR1a, miR-503, PI3K/Akt
Introduction
An abnormal or excessive accumulation of white adipose tissue (WAT) may cause a series of health problems, such as obesity, type 2 diabetes, and coronary artery disease. Since WAT is composed of numerous lipid-laden adipocytes, understanding the cellular and molecular basis of adipogenesis is necessary to develop innovative therapies that effectively treat WAT-related disorders.
There are two steps involved in adipogenesis: determination and terminal differentiation. Mesenchymal stem cells (MSCs) are first induced to become preadipocytes, which lose the ability to differentiate into non-adipocyte cells and then differentiate to mature adipocytes [1]. This process is characterized by the expression of adipocyte-specific proteins such as adipocyte protein 2 (AP2), which is regulated by two key transcriptional factors, CCAAT/enhancer binding protein α (C/EBPα) and proliferator-activated receptor-gamma (PPARγ) [1], that also act as downstream factors in the insulin-phosphoinositol-3 kinase (PI3K)/Akt pathway and play central roles in promoting adipogenesis [2].
Micro-RNAs (miRNAs) are small (21-25 nt) non-coding RNAs that regulate gene expression at a post-transcriptional level [3]. In recent years, miRNAs have been shown to regulate several cellular processes, including adipogenesis. Some examples are miR-378/378*, which promote adipogenesis by upregulating C/EBPα and -β expression [4], and miR-27b has been shown to inhibit adipogenesis by targeting PPARγ [5]. Ortega et al. showed that miR-503 was the most down-regulated miRNA during human adipocyte differentiation [6]. In our previous work, miR-503 was differentially expressed in an insulin receptor substrate-1 (IRS-1) deficient mice model characterized by abnormal adipose tissue; however, the role of miR-503 in adipogenesis and the underlying mechanism are not known. In the post-transcriptional regulation of gene expression, miRNAs bind to the 3’-UTR of their target gene and recruit the RNA induced silencing complex (RISC). Using the miRNA sequence as a guide, this RISC binds to messenger RNA (mRNA) to degrade targeted mRNAs or inhibit translation from mRNAs to proteins [7]. Using bioinformatic analysis, we predicted that the Bone Morphogenetic Protein Receptor 1a (Bmpr1a) was one of the target genes of miR-503.
Bone morphogenetic proteins (BMPs) belong to the transforming growth factor-β (TGF-β) superfamily and are known to regulate processes as diverse as cell fate determination, proliferation, apoptosis, and differentiation during both embryogenesis and adulthood [8]. Activation of BMPs requires binding to a hetero-oligomeric complex of the type 1 and type 2 BMP receptors (BMPRs). Three type 1 receptors (BMPR1a/Alk3, BMPR1b/Alk6, and ACVR1A/Alk2) and three type 2 receptors (BMPR2, ACTR2A, and ACTR2b) mediate most of the effects of BMPs [9]. Ligand binding first activates the type 2 receptor, and then trans-phosphorylates the type 1 receptor, which results in subsequent activation by phosphorylation of the receptor-regulated Smad proteins [10]. Among the different BMPR isoforms, BMPR1a is a particularly convincing candidate for involvement in adipocyte biology as it has been shown to specialize in adipocyte differentiation [11]. Truncated Bmpr1a in 2T3 cells, a murine osteoblast cell line, blocked adipocyte differentiation, and this could be reversed by BMPR1a reconstruction [11]. Overexpression of BMPR1a induced adipocyte commitment in the absence of BMP2/4 by binding to the Smad signaling pathway directly [12]. In addition to the canonical Smad pathway, the PI3K/Akt cascade is the emerging “noncanonical” signal mediated by BMPs. It was reported that insulin potentiates BMP2-induced osteogenesis via the PI3K/Akt pathway in rat spinal ligament cells [13]. Additionally, BMP2 improves osteoblast differentiation by activating PI3K/Akt signaling via BMPR threonine/serine kinase [14]. However, it is unclear how BMPs act on PI3K/Akt signaling. In this study, we validated that miR-503 regulated adipogenesis by targeting Bmpr1a, and that PI3K/Akt signaling was involved in this process in mice primary preadipocytes.
Materials and methods
Culture and differentiation of mice primary preadipocytes
Subcutaneous white adipose tissue was removed from 10-day-old C57BL/6 mice, and fat pads were cut into small pieces and digested with 1 mg/ml collagenase for 1 h in a shaking water bath.
Digested tissues were filtered through a sterile 100 µm nylon mesh to remove red blood cells. Preadipocytes were cultured in Dulbecco’s modified essential medium-F12 nutrient mixture (DMEM/F12) containing 10% fetal bovine serum at 37°C in a 5% CO2 atmosphere. To induce adipocyte differentiation, preadipocytes were grown until they reached confluence (day 0) and then induced to differentiate into mature adipocytes with 5 µg/ml insulin, 0.5 mM isobutylmethylxanthine (IBMX), and 5 µM dexamethasone (Sigma, USA) for 48 h. Then, cells were placed in growth medium, supplemented with 5 µg/ml insulin, and the medium was changed every second day.
Oil red O staining
Cells were washed twice in phosphate-buffered saline (PBS), fixed with 10% buffered formalin for 20 min, and stained for 120 min at room temperature with filtered oil red O (0.5% oil red O in isopropyl alcohol; Sigma, USA). Cells were then washed and stored with PBS and examined with a microscope (Olympus Corporation, Japan).
Adipocyte triglyceride content assay
After the adipocytes were stained with the oil red O, the dye was discarded and the cells were rinsed with distilled water. When excess water was evaporated completely, 1 ml of isopropyl alcohol was added to the stained wells for 15 min. The extracted dye was then transferred to a 96-well plate and measured using optimal absorbance at 510 nM.
Luciferase assay
Using TargetScan, miRnada, and PicTar, Bmpr1a was predicted to be the target of miR-503, we then predicted a target site for miR-503 that comprised an absolutely conserved 7-nucleotide seed sequence (CGCTGCT) within a highly-conserved region of the Bmpr1a 3’ UTR. The Bmpr1a 3’ UTR WT sequence and a mutant sequence in the seed region were isolated and ligated to pGL3 (Promega Co., Madison, WI) to form pGL3-Bmpr1a-UTR-WT and pGL3-Bmpr1a-UTR-muta (500 ng), and co-transfected into HEK293T cells with a miR-503 mimic (50 nM) (Ribobio, Guangzhou, China). In addition, pGL3-Bmpr1a-UTR-WT together with a miR-503 mimic or a miR-503 inhibitor (50 nM) (Ribobio, Guangzhou, China) were co-transfected into HEK293T cells. The normalized luciferase activity for each construct was compared.
Cell transfection in preadipocytes
Mouse Bmpr1a siRNA, a miR-503 mimic, and a miR-503 inhibitor were transfected into preadipocytes. The mouse Bmpr1a (GenBank Accession Number AY365062) specific siRNAs (mBmpr1a-siRNA) were 5’-AACTTTCGGTGAATCCTTGCA-3’ (sense) and 5’-AATGCAAGGATTCACCGAAAG-3’ (antisense). The siRNA negative control was generated using the nonspecific primers: 5’-AAACGTGACACGTTCGGAGAA-3’ (sense) and 5’-AATTCTCCGAACGTGTCACGT-3’ (antisense). The Bmpr1a siRNA (20 nM) or control siRNA (20 nM) (Qiagen, West Sussex, UK) and the miR-503 mimic/negative control (NC) or inhibitor/NC (50 nM) (Ribobio, Guangzhou, China) were incubated with Lipofectamine 2000 (Invitrogen, Paisley, UK) for 20 min to allow complete fusion, before adding the mixture to Opti-MEM I. The transfection medium was replaced after 6 h with complete media for 72 h post transfection and the cells were allowed to reach confluence (day 0). These cells were then induced for adipogenic differentiation using the methods mentioned above.
Real-time quantitative PCR (qPCR) analysis
Total RNA was isolated with Trizol Reagent (Invitrogen, USA) according to the manufacturer’s instructions and then was reverse-transcribed with a miRNA first-strand cDNA synthesis kit (Takara Bio) to generate first-strand miRNA-cDNA PCR templates. Quantitative PCR (qPCR) was performed with a LightCycler 480 II (Roche, Basel, Switzerland) and the miRcute miRNA qPCR detection kit (Takara). All PCR assays were performed in triplicate. The U6 snRNA and β-actin were used as endogenous controls for miRNA and mRNA, respectively. The sequences of all primers are shown in Table 1. Relative miRNA and mRNA expression in preadipocytes after differentiation was quantified with the -ΔΔCt method, and the fold-change was determined with the formula 2-ΔΔCt.
Table 1.
Primer sequences used for qPCR
| Gene | Sequence | |
|---|---|---|
| AP2 | Forward | AACACCGAGATTTCCTTCAA |
| Reverse | AGTCACGCCTTTCATAACACA | |
| BMPR1a | Forward | CTGCCCAGATGATGCTA |
| Reverse | GTTAATGTGGTTTCTCCCT | |
| PPARγ | Forward | ATGGTTGACACAGAGATGC |
| Reverse | GAATGCGAGTGGTCTTCC | |
| C/EBPα | Forward | CAAGAACAGCAACGAGTACCG |
| Reverse | GTCACTGGTCAACTCCAGCAC | |
| β-actin | Forward | CAACGAGCGGTTCCGATG |
| Reverse | GCCACAGGATTCCATACCCA |
Western blotting
Cells were harvested in cold RIPA extraction buffer (Sigma Aldrich, MO, USA) with protease inhibitors and a phosphatase inhibitor (Roche Diagnostics, IN, USA). Cell lysates were separated by SDS-PAGE and transferred onto a PVDF membrane, followed by overnight probing with the following primary antibodies: anti-PI3K, anti-pAkt/Akt, anti-BMPR1a, anti-PPARγ, anti-β-actin (Abcam, USA), and anti-AP2 (Cell Signaling Technology Inc, USA). Densitometric analysis was performed using Image J (NIH, USA).
Statistical analysis
All the experiments were carried out in triplicate and the results were presented as mean ± S.D. The data were analyzed using SPSS 16.0. Statistical significance was considered at p<0.05 using the Student’s t-test.
Results
MiR-503 was downregulated in mature adipocytes
To identify whether miR-503 has an effect on adipogenesis, primary preadipocytes were induced into mature adipocytes for 10 days (Figure 1A). The qPCR analysis validated that miR-503 was significantly decreased (2.56-fold) in the differentiation group compared with the preadipocytes group (Figure 1B). Thus, miR-503 was decreased during adipogenesis.
Figure 1.
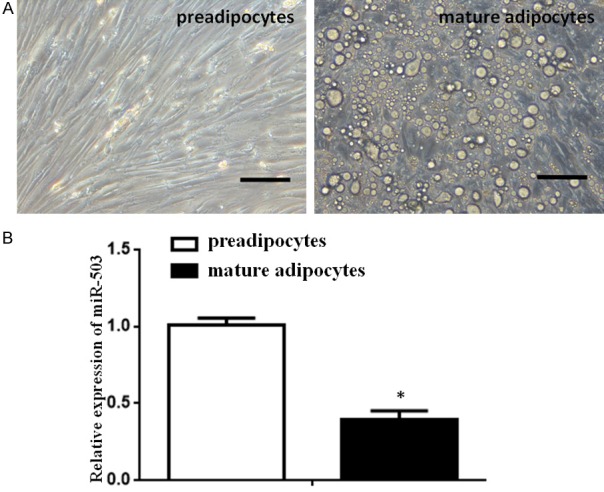
Expression of miR-503 is downregulated during preadipocyte differentiation. A. Representative microscopic images of preadipocytes (left) and mature adipocytes at day 10 (right). Size bars indicate 50 µm. B. Relative expression of miR-503 was measured by qPCR, as described in the Methods, in preadipocytes and mature adipocytes. The expression levels of miR-503 were relative to the preadipocyte level, which was defined as 1. (Data were presented as the mean ± SD, n=3; *p<0.05 compared with day 0).
MiR-503 specifically binds to the 3’-UTR of Bmpr1a
To clarify the molecular mechanisms underlying the regulation of adipogenesis by miR-503, bioinformatic prediction of miRNA targets was performed using TargetScan, miRnada, and PicTar. Although several targets of miR-503 have been verified except for Bmpr1a, such as Ptk7 [15], Rnf144b [16], and Dclk1 [17], since there has not yet been reported that they are related to adipogenesis, we predicted that there would be one binding site for miR-503 in the Bmpr1a 3’-UTR (Figure 2A). The full-length 3’-UTR of Bmpr1a mRNA was inserted downstream of the luciferase gene in the pGL3 reporter plasmid, and the seed sequence was mutated to disrupt miR-503 binding (Figure 2B). The wild-type (pGL3-Bmpr1a-UTR) or mutated (pGL3-Bmpr1a-UTR-muta) plasmid was co-transfected with a miR-503 mimic into HEK293T cells, together with the NC (no insert) for normalization. Forty-eight hours after transfection, the luciferase activity of the miR-503 group was significantly lower than that of the NC group (p<0.05), and the reduction was rescued in the mutation group (Figure 2C). Meanwhile, overexpression of miR-503 significantly reduced Bmpr1a 3’-UTR-regulated luciferase activity; conversely, inhibition of the miR-503 produced the opposite result (Figure 2D).
Figure 2.
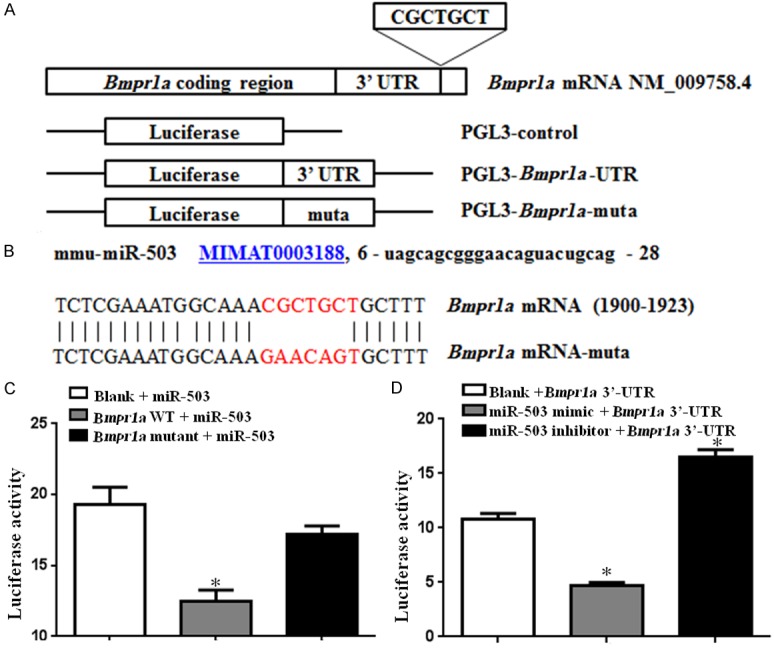
Validation that miR-503 targets the 3’-UTR of the Bmpr1a gene. A. Schematic of Bmpr1a mRNA and the luciferase reporter plasmids containing the miR-503 binding sites of Bmpr1a mRNA. The 3’-UTR sites were inserted downstream of the luciferase reporter and CGCTGCT was the predicted target site of miR-503. B. MiR-503 sequences and the predicted binding site between miR-503 and Bmpr1a mRNA. The sequence of miR-503 (www.mirbase.org) is shown. The Bmpr1a mRNA has one putative binding site for miR-503 on the 3’-UTR. The seed region is composed of seven nucleotides of the Bmpr1a 3’-UTR (red) and these were mutated using site-directed mutagenesis in Bmpr1a mRNA-muta. C. HEK293T cells were transfected with each of the constructed plasmids, together with the miR-503 luciferase reporter plasmid. D. HEK293T cells were transfected with a miR-503 mimic or inhibitor, together with the Bmpr1a 3’-UTR activated luciferase reporter plasmid (n=3; *p<0.05 vs. blank).
MiR-503 and BMPR1a expression during preadipocyte differentiation
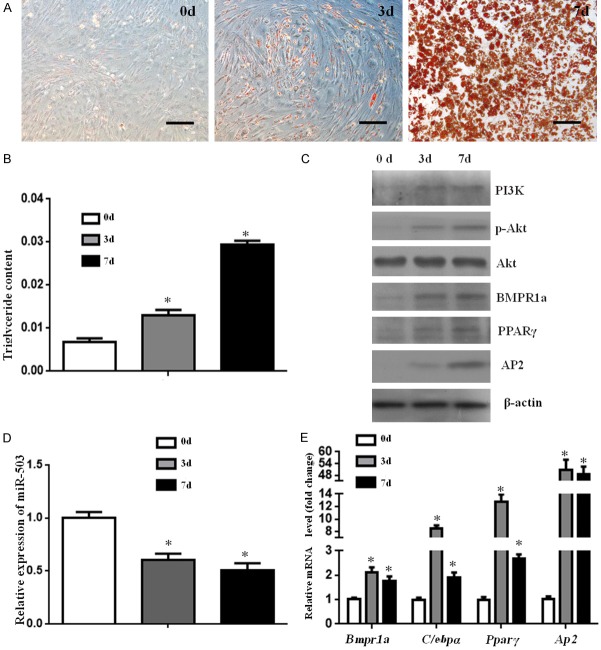
To further investigate the variation of miR-503 and BMPR1a during the process of adipogenesis, preadipocytes were obtained from 10-day-old mice and induced into mature adipocytes for 7 days. Lipid droplets gradually accumulated during this process (Figure 3A), and the triglyceride concentration dramatically increased (Figure 3B). Western blot analysis confirmed that BMPR1a, PPARγ, and AP2 progressively increased in expression between days 0 and 7 of differentiation, and the PI3K and p-Akt expression showed similar patterns (Figure 3C). We measured the time-dependent decline in the expression level of miR-503 (Figure 3D); while the transcripts of Bmpr1a, C/ebpα, Pparγ, and Ap2 exhibited an upward trend and peaked on day 3 then decreased thereafter (Figure 3E). The expression of miR-503 showed a trend contrary to that of BMPR1a, indicating that it repressed BMPR1a expression.
Figure 3.
Expression of miR-503 and BMPR1a during preadipocyte differentiation. A. Representative images of oil red O staining of primary preadipocytes (0 d), induced to differentiate (3 d) and mature (7 d) into adipocytes, characterized by lipid droplets. Size bars indicate 100 µm. B. The triglyceride concentration during adipogenesis process. C. Western blot analyses of BMPR1a, adipogenic-related markers (PPARγ, AP2), as well as PI3K and p-Akt during preadipocyte differentiation. D, E. qPCR analyses of genes and miR-503 expression in preadipocytes treated with adipogenic induction. Relative expression is shown for miR-503, Bmpr1a, and the adipocyte markers C/ebpα, Pparγ, and Ap2. Data represent mean ± SD. (n=3; *p<0.05 compared with day 0).
MiR-503 inhibited preadipocyte differentiation by down-regulating BMPR1a expression
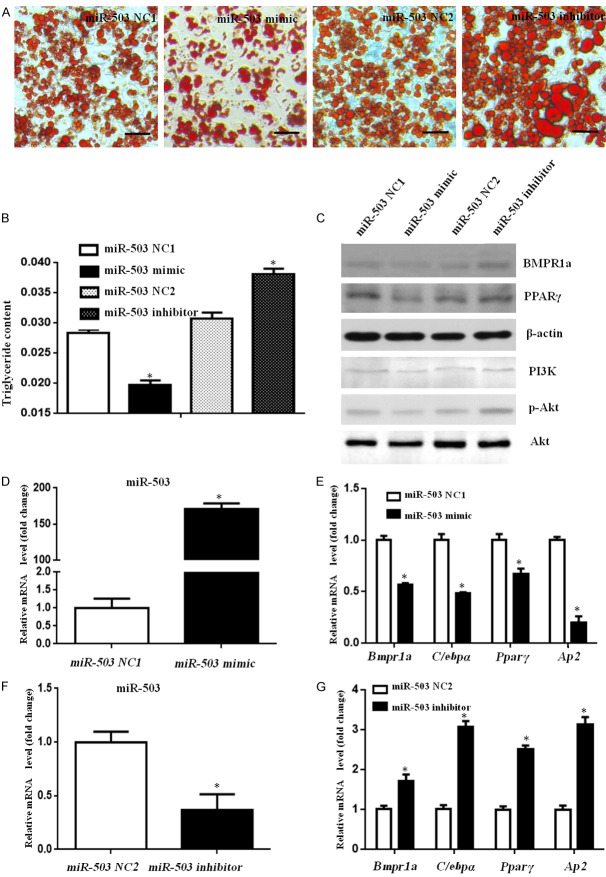
To demonstrate the adipogenic effect of miR-503, we transfected preadipocytes with a murine miR-503 mimic, a miR-503 inhibitor, or corresponding controls. We found that the miR-503 mimic significantly reduced and the miR-503 inhibitor increased lipid droplets formation in induced adipocytes (Figure 4A), which could be further confirmed by the triglyceride content (Figure 4B). The protein levels of BMPR1a and PPARγ were also reduced with the miR-503 mimic and increased with miR-503 inhibitor (Figure 4C). The PI3K and phospho-Akt at Ser-473 were inhibited by the miR-503 mimic and activated by the miR-503 inhibitor (Figure 4C). The qPCR analysis showed increased expression of miR-503 in the miR-503 mimic group (Figure 4D), and reduced miR-503 expression in the miR-503 inhibition group (Figure 4F). Furthermore, the miR-503 mimic caused significant reductions and the miR-503 inhibitor caused significant increases in mRNA expression of Bmpr1a, C/ebpα, Pparγ, and Ap2 (Figure 4E and 4G). These results indicated that miR-503 inhibited adipogenesis by down-regulating BMPR1a and the PI3K/Akt signaling pathway.
Figure 4.
MiR-503 inhibited preadipocyte differentiation by down-regulating BMPR1a expression. The miR-503 mimic/inhibitor or corresponding negative controls (NC) were transfected into preadipocytes using Lipofectamine 2000, and then cells were induced to differentiate. (A) Oil red O staining and (B) Triglyceride concentration of the four groups at day 10. Size bars indicate 50 µm. (C) Western blot of whole cell lysates of adipocytes on day 3 after induction show protein expression changes with the miR-503 mimic, inhibitor, and NCs. (D-G) The qPCR results show relative gene expression of miR-503, Bmpr1a, C/ebpα, Pparγ, and Ap2 at 72 h after transfection with a miR-503 mimic (D and E), a miR-503 inhibitor (F and G), or corresponding controls (NC). Data represent mean ± SD. (n=3; *p<0.05 compared with control).
Bmpr1a disruption inhibited adipogenesis
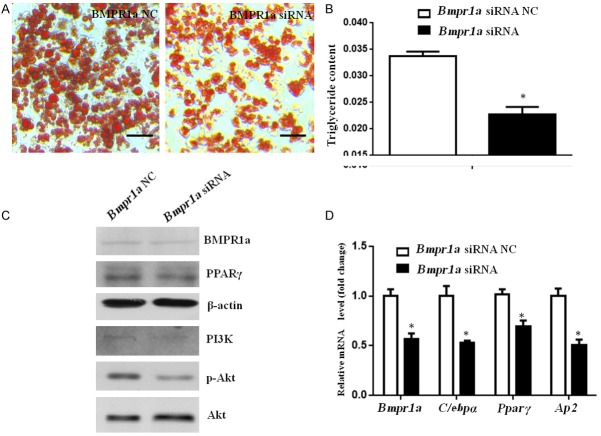
Endogenous BMPR1a expression was largely interrupted with specific siRNA in preadipocytes. The cells exhibited significantly fewer lipid droplets and the triglyceride content dramatically decreased compared with cells transfected with the NC (Figure 5A and 5B). Protein expression of BMPR1a and PPARγ was decreased in the Bmpr1a-siRNA group (Figure 5C). Accordingly, decreased Bmpr1a mRNA levels were accompanied by reductions in the expression of C/ebpα, Pparγ, and Ap2 in the Bmpr1a-siRNA group (Figure 5D). In addition, PI3K and phospho-Akt at Ser-473 were down-regulated when BMPR1a was disrupted (Figure 5C). These results indicated that BMPR1a interference resulted in lower adipogenic activity and PI3K/Akt signaling.
Figure 5.
Bmpr1a disruption inhibited adipogenesis. Bmpr1a-specific siRNA or negative control (NC) siRNA were transfected into preadipocytes. (A) Oil red O staining and (B) Triglyceride concentration of cells with control or silenced BMPR1a at day 10 after induction. Size bars indicate 50 µm. (C) Analyses of Western blots show protein expression in cells transfected with Bmpr1a siRNA or NC on day 3. Whole cell lysates were prepared and analyzed for BMPR1a, PPARγ, PI3K, phospho-Akt (Ser-473), and Akt expression. (D) The qPCR results show gene expression levels of Bmpr1a, C/ebpα, Pparγ, and Ap2 at 72 h after transfection with control (NC) or siRNA. Data represent mean ± SD. (n=3; *p<0.05 compared with control).
Discussion
Obesity and the associated metabolic syndrome represent major public health issues; to some extent, both of these are due to excessive adipose accumulation or too much adipogenesis. Adipogenesis is a complex process, involving immense communication among progenitor cells, receptors, extracellular stimuli, signaling pathways, and transcription factors. In this study, we identified miR-503 as a novel player in inhibiting adipogenesis, as evidence by its dramatic decrease during adipocyte differentiation and its ability to attenuate intracellular triglyceride accumulation. Our results are generally in agreement with recent reports that miRNAs are emerging regulators that participate in adipogenesis. This includes miR-138 [18], miR-130 [19], miR-27a, and miR-27b [5], which are all potential inhibitors of adipogenesis by suppressing PPARγ; while other miRNAs can accelerate adipogenesis, such as miR-21 [20] and miR-143 [21]. In previous studies, miR-503 was the most down-regulated miRNA before and after human adipocyte differentiation [6]. In our previous work, miR-503 was also differentially expressed in an IRS-1 deficient mice model characterized by increased brown adipose tissue and decreased white adipose tissue. Together, these data demonstrate that miR-503 is closely related to adipogenesis. This study demonstrated the exact role of miR-503 in adipogenesis for the first time.
To further elucidate the underlying mechanism by which miR-503 inhibits adipogenesis, we predicted Bmpr1a as a target of miR-503 using bioinformatic analysis (TargetScan, miRanda, and PicTar). A luciferase reporter assay showed that miR-503 functions by targeting the 3’-UTR of the Bmpr1a gene with its seed region. In both HEK293T cells and preadipocytes, the miR-503 mimic decreased BMPR1a expression; whereas, the miR-503 inhibitor resulted in elevated expression of BMPR1a. These results provide strong evidence that miR-503 inhibits BMPR1a at the post-transcriptional level.
The BMPs are involved in many aspects of adipocyte development, including adipose cell fate determination, differentiation of committed preadipocytes, and the function of mature adipocytes [8,12,22]. The effects of BMPs on adipogenesis depend on several factors such as the dosage, isoform of the BMP/BMPR involved, and the related extracellular and intracellular factors [23-25]. For instance, BMP-2 and BMP-4 promote white adipocyte differentiation, while BMP-7 is involved in brown adipogenesis [25,26]. However, BMP-2 and BMP-7 could induce adipogenesis at low concentrations; whereas, high concentrations resulted in osteogenic and chondrogenic differentiation [23,27]. The subtype of BMPR involved in intracellular signaling could also determine whether the MSCs commit to the adipogenic lineage. Of the different BMPR isoforms, BMPR1a is specialized in adipogenesis. Expression of BMPR1a increased significantly in committed preadipocytes compared with the parental C3H10T1/2 line [28]. In vivo studies showed that human WAT displayed high level of BMPR1a in overweight and obese individuals compared with lean subjects [29,30]. It is well known that among the transcriptional factors involved in adipogenesis, PPARγ and C/EBPs have key roles and govern the whole differentiation process [31]. Overexpression of either PPARγ or C/EBPα could overcome most adipogenesis blockades in preadipocytes [1]. In addition, BMP2 can promote adipogenesis by inducing PPARγ expression in 3T3-L1 and C3H10T1/2 preadipocytes [23]. Rosiglitazone, a PPARγ agonist, could enhance the BMP-2 induced terminal preadipocyte differentiation [32], suggesting BMP signaling and PPARγ cooperate with each other in adipogenesis. In accordance with the above studies, we found that the variation in BMPR1a expression increased during primary adipocyte development along with the adipogenic markers C/EBPα, PPARγ, and AP2. Interrupting BMPR1a expression resulted in reduced adipogenic activity and adipogenic markers, suggesting that BMPR1a positively regulates adipogenesis.
The BMP signaling pathway involves the transcription factors Smad1/5/8, which interact with the universal co-Smad, Smad4, to form a heterodimer that migrates into the nucleus and regulates target gene expression [10]. In addition to the Smad pathway, BMPs utilize the PI3K/Akt pathway to regulate several biological activities in numerous cell types, such as intestinal stems cells, cardiomyocytes, and follicle stem cells [14,33-35]. There was also a report that BMP7 can rescue brown adipogenesis in IRS-1 deficient cells via the PI3K/Akt signal pathway [36]. However, it is unclear whether BMP signaling regulates white adipogenesis via the PI3K/Akt cascade. In this study, we showed that the expression of PI3K and phospho-Akt increased along with increased lipid droplets; while, BMPR1a interruption resulted in reduced adipogenic ability and PI3K/Akt activity. These data described a previously unrecognized mechanism for regulation of PI3K/Akt signaling by the BMP signaling pathway in white adipogenesis. Additionally, miR-503 was reported to impair insulin/PI3K-Akt signaling and induce glucose intolerance, pancreatic β-cell dysfunction, and insulin resistance in a high-fat induced diabetic mice model [37]. In our study, miR-503 overexpression downregulated the expression of BMPR1a at both the mRNA and protein levels and reduced the adipogenic ability and lipid droplets formation. Furthermore, PI3K/Akt signaling was impaired. The miR-503 inhibition group exhibited the opposite results, emphasizing the inhibitory effect of miR-503 in adipocyte differentiation.
Taken together, our study provides evidence that miR-503 directly targets Bmpr1a and takes part in adipocyte differentiation via the PI3K/Akt signaling pathway. These findings also suggest the cross talk between BMP and the insulin signaling pathway in the adipogenic process. The mechanisms presented here might provide new therapeutic targets and strategies to deal with adipogenesis-related diseases, such as obesity, hyperlipidemia, and type 2 diabetes.
Acknowledgements
The authors gratefully thank the Metabolic Syndrome Research Center at the Second Xiangya Hospital of Central South University for technical assistance. This work was supported by grants from the National Natural Science Foundation of China (81370975, 81070278).
Disclosure of conflict of interest
None.
References
- 1.Rosen ED, MacDougald OA. Adipocyte differentiation from the inside out. Nat Rev Mol Cell Biol. 2006;7:885–96. doi: 10.1038/nrm2066. [DOI] [PubMed] [Google Scholar]
- 2.Fasshauer M, Klein J, Kriauciunas KM, Ueki K, Benito M, Kahn CR. Essential role of insulin receptor substrate 1 in differentiation of brown adipocytes. Mol Cell Biol. 2001;21:319–29. doi: 10.1128/MCB.21.1.319-329.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mallanna SK, Rizzino A. Emerging roles of microRNAs in the control of embryonic stem cells and the generation of induced pluripotent stem cells. Dev Biol. 2010;344:16–25. doi: 10.1016/j.ydbio.2010.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gerin I, Bommer GT, McCoin CS, Sousa KM, Krishnan V, MacDougald OA. Roles for miRNA-378/378* in adipocyte gene expression and lipogenesis. Am J Physiol Endocrinol Metab. 2010;299:E198–206. doi: 10.1152/ajpendo.00179.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Karbiener M, Fischer C, Nowitsch S, Opriessnig P, Papak C, Ailhaud G, Dani C, Amri EZ, Scheideler M. microRNA miR-27b impairs human adipocyte differentiation and targets PPARgamma. Biochem Biophys Res Commun. 2009;390:247–51. doi: 10.1016/j.bbrc.2009.09.098. [DOI] [PubMed] [Google Scholar]
- 6.Ortega FJ, Moreno-Navarrete JM, Pardo G, Sabater M, Hummel M, Ferrer A, Rodriguez-Hermosa JI, Ruiz B, Ricart W, Peral B, Fernández-Real JM. MiRNA expression profile of human subcutaneous adipose and during adipocyte differentiation. PLoS One. 2010;5:e9022. doi: 10.1371/journal.pone.0009022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Menor M, Ching T, Zhu X, Garmire D, Garmire LX. mirMark: a site-level and UTR-level classifier for miRNA target prediction. Genome Biol. 2014;15:500. doi: 10.1186/s13059-014-0500-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schulz TJ, Tseng YH. Emerging role of bone morphogenetic proteins in adipogenesis and energy metabolism. Cytokine Growth Factor Rev. 2009;20:523–31. doi: 10.1016/j.cytogfr.2009.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kishigami S, Mishina Y. BMP signaling and early embryonic patterning. Cytokine Growth Factor Rev. 2005;16:265–78. doi: 10.1016/j.cytogfr.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 10.Nishimura R, Hata K, Ikeda F, Matsubara T, Yamashita K, Ichida F, Yoneda T. The role of Smads in BMP signaling. Front Biosci. 2003;8:s275–84. doi: 10.2741/1049. [DOI] [PubMed] [Google Scholar]
- 11.Chen D, Ji X, Harris MA, Feng JQ, Karsenty G, Celeste AJ, Rosen V, Mundy GR, Harris SE. Differential roles for bone morphogenetic protein (BMP) receptor type IB and IA in differentiation and specification of mesenchymal precursor cells to osteoblast and adipocyte lineages. J Cell Biol. 1998;142:295–305. doi: 10.1083/jcb.142.1.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang H, Song TJ, Li X, Hu L, He Q, Liu M, Lane MD, Tang QQ. BMP signaling pathway is required for commitment of C3H10T1/2 pluripotent stem cells to the adipocyte lineage. Proc Natl Acad Sci U S A. 2009;106:12670–5. doi: 10.1073/pnas.0906266106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li H, Liu D, Zhao CQ, Jiang LS, Dai LY. Insulin potentiates the proliferation and bone morphogenetic protein-2-induced osteogenic differentiation of rat spinal ligament cells via extracellular signal-regulated kinase and phosphatidylinositol 3-kinase. Spine (Phila Pa 1976) 2008;33:2394–402. doi: 10.1097/BRS.0b013e3181838fe5. [DOI] [PubMed] [Google Scholar]
- 14.Ghosh-Choudhury N, Abboud SL, Nishimura R, Celeste A, Mahimainathan L, Choudhury GG. Requirement of BMP-2-induced phosphatidylinositol 3-kinase and Akt serine/threonine kinase in osteoblast differentiation and Smad-dependent BMP-2 gene transcription. J Biol Chem. 2002;277:33361–8. doi: 10.1074/jbc.M205053200. [DOI] [PubMed] [Google Scholar]
- 15.Andreeva A, Lee J, Lohia M, Wu X, Macara IG, Lu X. PTK7-Src signaling at epithelial cell contacts mediates spatial organization of actomyosin and planar cell polarity. Dev Cell. 2014;29:20–33. doi: 10.1016/j.devcel.2014.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ariffin JK, Kapetanovic R, Schaale K, Gatica-Andrades M, Blumenthal A, Schroder K, Sweet MJ. The E3 ubiquitin ligase RNF144B is LPS-inducible in human, but not mouse, macrophages and promotes inducible IL-1beta expression. J Leukoc Biol. 2016 doi: 10.1189/jlb.2AB0815-339R. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 17.Ito H, Tanaka S, Akiyama Y, Shimada S, Adikrisna R, Matsumura S, Aihara A, Mitsunori Y, Ban D, Ochiai T, Kudo A, Arii S, Yamaoka S, Tanabe M. Dominant Expression of DCLK1 in Human Pancreatic Cancer Stem Cells Accelerates Tumor Invasion and Metastasis. PLoS One. 2016;11:e0146564. doi: 10.1371/journal.pone.0146564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang Z, Bian C, Zhou H, Huang S, Wang S, Liao L, Zhao RC. MicroRNA hsa-miR-138 inhibits adipogenic differentiation of human adipose tissue-derived mesenchymal stem cells through adenovirus EID-1. Stem Cells Dev. 2011;20:259–67. doi: 10.1089/scd.2010.0072. [DOI] [PubMed] [Google Scholar]
- 19.Lee EK, Lee MJ, Abdelmohsen K, Kim W, Kim MM, Srikantan S, Martindale JL, Hutchison ER, Kim HH, Marasa BS, Selimyan R, Egan JM, Smith SR, Fried SK, Gorospe M. miR-130 suppresses adipogenesis by inhibiting peroxisome proliferator-activated receptor gamma expression. Mol Cell Biol. 2011;31:626–38. doi: 10.1128/MCB.00894-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kang M, Yan LM, Zhang WY, Li YM, Tang AZ, Ou HS. Role of microRNA-21 in regulating 3T3-L1 adipocyte differentiation and adiponectin expression. Mol Biol Rep. 2013;40:5027–34. doi: 10.1007/s11033-013-2603-6. [DOI] [PubMed] [Google Scholar]
- 21.Esau C, Kang X, Peralta E, Hanson E, Marcusson EG, Ravichandran LV, Sun Y, Koo S, Perera RJ, Jain R, Dean NM, Freier SM, Bennett CF, Lollo B, Griffey R. MicroRNA-143 regulates adipocyte differentiation. J Biol Chem. 2004;279:52361–5. doi: 10.1074/jbc.C400438200. [DOI] [PubMed] [Google Scholar]
- 22.Skillington J, Choy L, Derynck R. Bone morphogenetic protein and retinoic acid signaling cooperate to induce osteoblast differentiation of preadipocytes. J Cell Biol. 2002;159:135–46. doi: 10.1083/jcb.200204060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang EA, Israel DI, Kelly S, Luxenberg DP. Bone morphogenetic protein-2 causes commitment and differentiation in C3H10T1/2 and 3T3 cells. Growth Factors. 1993;9:57–71. doi: 10.3109/08977199308991582. [DOI] [PubMed] [Google Scholar]
- 24.Tang QQ, Otto TC, Lane MD. Commitment of C3H10T1/2 pluripotent stem cells to the adipocyte lineage. Proc Natl Acad Sci U S A. 2004;101:9607–11. doi: 10.1073/pnas.0403100101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jin W, Takagi T, Kanesashi SN, Kurahashi T, Nomura T, Harada J, Ishii S. Schnurri-2 controls BMP-dependent adipogenesis via interaction with Smad proteins. Dev Cell. 2006;10:461–71. doi: 10.1016/j.devcel.2006.02.016. [DOI] [PubMed] [Google Scholar]
- 26.Tseng YH, Kokkotou E, Schulz TJ, Huang TL, Winnay JN, Taniguchi CM, Tran TT, Suzuki R, Espinoza DO, Yamamoto Y, Ahrens MJ, Dudley AT, Norris AW, Kulkarni RN, Kahn CR. New role of bone morphogenetic protein 7 in brown adipogenesis and energy expenditure. Nature. 2008;454:1000–4. doi: 10.1038/nature07221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Asahina I, Sampath TK, Hauschka PV. Human osteogenic protein-1 induces chondroblastic, osteoblastic, and/or adipocytic differentiation of clonal murine target cells. Exp Cell Res. 1996;222:38–47. doi: 10.1006/excr.1996.0005. [DOI] [PubMed] [Google Scholar]
- 28.Bowers RR, Kim JW, Otto TC, Lane MD. Stable stem cell commitment to the adipocyte lineage by inhibition of DNA methylation: role of the BMP-4 gene. Proc Natl Acad Sci U S A. 2006;103:13022–7. doi: 10.1073/pnas.0605789103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Böttcher Y, Unbehauen H, Klöting N, Ruschke K, Körner A, Schleinitz D, Tönjes A, Enigk B, Wolf S, Dietrich K, Koriath M, Scholz GH, Tseng YH, Dietrich A, Schön MR, Kiess W, Stumvoll M, Blüher M, Kovacs P. Adipose tissue expression and genetic variants of the bone morphogenetic protein receptor 1A gene (BMPR1A) are associated with human obesity. Diabetes. 2009;58:2119–28. doi: 10.2337/db08-1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schleinitz D, Klöting N, Böttcher Y, Wolf S, Dietrich K, Tönjes A, Breitfeld J, Enigk B, Halbritter J, Körner A, Schön MR, Jenkner J, Tseng YH, Lohmann T, Dressler M, Stumvoll M, Blüher M, Kovacs P. Genetic and evolutionary analyses of the human bone morphogenetic protein receptor 2 (BMPR2) in the pathophysiology of obesity. PLoS One. 2011;6:e16155. doi: 10.1371/journal.pone.0016155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Farmer SR. Transcriptional control of adipocyte formation. Cell Metab. 2006;4:263–73. doi: 10.1016/j.cmet.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nishio M, Yoneshiro T, Nakahara M, Suzuki S, Saeki K, Hasegawa M, Kawai Y, Akutsu H, Umezawa A, Yasuda K, Tobe K, Yuo A, Kubota K, Saito M, Saeki K. Production of functional classical brown adipocytes from human pluripotent stem cells using specific hemopoietin cocktail without gene transfer. Cell Metab. 2012;16:394–406. doi: 10.1016/j.cmet.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 33.Ghosh-Choudhury N, Abboud SL, Chandrasekar B, Ghosh CG. BMP-2 regulates cardiomyocyte contractility in a phosphatidylinositol 3 kinase-dependent manner. FEBS Lett. 2003;544:181–4. doi: 10.1016/s0014-5793(03)00507-6. [DOI] [PubMed] [Google Scholar]
- 34.Kobielak K, Stokes N, de la Cruz J, Polak L, Fuchs E. Loss of a quiescent niche but not follicle stem cells in the absence of bone morphogenetic protein signaling. Proc Natl Acad Sci U S A. 2007;104:10063–8. doi: 10.1073/pnas.0703004104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sui X, Li D, Qiu H, Gaussin V, Depre C. Activation of the bone morphogenetic protein receptor by H11kinase/Hsp22 promotes cardiac cell growth and survival. Circ Res. 2009;104:887–95. doi: 10.1161/CIRCRESAHA.108.192328. [DOI] [PubMed] [Google Scholar]
- 36.Zhang H, Schulz TJ, Espinoza DO, Huang TL, Emanuelli B, Kristiansen K, Tseng YH. Cross talk between insulin and bone morphogenetic protein signaling systems in brown adipogenesis. Mol Cell Biol. 2010;30:4224–33. doi: 10.1128/MCB.00363-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kwon DN, Chang BS, Kim JH. MicroRNA dysregulation in liver and pancreas of CMP-Neu5Ac hydroxylase null mice disrupts insulin/PI3K-AKT signaling. Biomed Res Int. 2014;2014:236385. doi: 10.1155/2014/236385. [DOI] [PMC free article] [PubMed] [Google Scholar]