Abstract
The initiation mechanism of IgE expression has not been fully understood. Flagellin (FGN) is an important microbial factor in the regulation of immune responses in the intestine. This study tests a hypothesis that FGN plays a crucial role in the isotype switching of IgE in B cells and the initiation of food allergy. In this study, the expression of IgE in B cells was analyzed by real time RT-PCR, Western blotting and chromatin immunoprecipitation. A mouse model was developed to assess the role of Toll like receptor-5 in the development of IgE-mediated allergic reaction in the intestinal mucosa. The results showed that exposure to FGN suppressed the expression of Bcl6 in B cells via increasing the levels of histone deacetylase (HDAC) 7; the latter up regulated the levels of methylated H3K9 and H3K27, down regulated RNA polymerase II and STAT3 (signal transducer and activator of transcription 3) at the Bcl6 promoter locus. Exposure to FGN and IL-4 markedly increased the expression of IgE in B cells via activating p300, H3K4, Pol II and STAT6 at the IgE promoter locus. As compared with the sensitized wild mice, the sensitized TLR5-deficient mice showed no detectable OVA-specific IgE in the serum; mast cells in the intestinal mucosa were not activated, no apparent allergic symptoms were evoked after the specific antigen challenge. In conclusion, FGN facilitates the initiation of food allergy in mice by triggering IgE transcription in B cells in a Th2 polarization environment via activating HDAC7 and suppressing Bcl6 expression.
Keywords: Allergy, B lymphocyte, B cell lymphoma-6, Toll like receptor-5, flagellin
Introduction
Immunoglobulin (Ig) E is one of the 5 Ig types in the body, producing by plasma cells [1]. The main function of IgE is to immunity to parasites [2]. IgE also plays a key role in the type I hypersensitivity, such as allergic asthma, food allergy and allergic dermatitis [3]. By forming complexes with the high affinity IgE receptors, the antigen specific IgE makes mast cells sensitized; and the mast cells gain the capacity to release allergic mediators to initiate clinical allergic symptoms upon re-exposure to specific antigens [4]. Yet, the mechanism of the initiation of IgE expression in the body is not completely understood.
The protein of B cell lymphoma-6 (Bcl6) in humans is encoded by the BCL6 gene [5]. It is a repressing protein and has clinical significance in lymphoma [6]. Its action is negatively regulated by the gene PRDM1 encoding the transcription factor Blimp-1 [7]. It is reported that Bcl6 acts as a sequence-specific repressor of transcription, and has been demonstrated to modulate the signal transducer and activator of transcription-dependent Interleukin 4 (IL-4) responses of B cells. Bcl6 can interact with several corepressor complexes to block transcription. Bcl6 is the major transcription factor controlling the germinal center B cell program and functions to repress the IgE switch [8]. Thus, to elucidate the mechanism and to prevent Bcl6 dysfunction may open new avenues to inhibit allergic reactions triggered by IgE. Yet, factors to regulate Bcl6 expression have not been fully understood.
Some microbial products are associated with the pathogenesis of allergic diseases. Several microbial products, such as cholera toxin and Staphylococcal enterotoxin B, are employed as adjuvants in the development of animal allergy models [9,10]. We and others previously showed that the microbial component flagellin (FGN) was involved in the induction of T helper (Th) 2 inflammation in the intestine [11,12]. Yet, whether FGN modulates IgE expression has not been investigated.
Histone deacetylase (HDAC) family has 11 major submembers involving gene transcription [13]; some of which are associated with allergic diseases [14]. HDAC7 is associated with Bcl6 [15]. Bcl6 suppresses IgE isotype class switch [8]. FGN is part of the microbial products. It is reported that FGN increases nuclear factor-κB [16]; the latter is associated with regulating a number of gene transcription [17]. Based on the above information, we hypothesize that to alter HDAC activities in B cells may regulate the expression of IgE via repressing the Bcl6 expression. Thus, we stimulated B cells with FGN in a T helper (Th) 2 cytokine dominant environment. The results showed that FGN triggered the IgE expression in B cells by activating HDAC7.
Materials and methods
Reagents
The antibodies of HDAC7, Bcl6, H3K9me, H3K27me, H3K4ac, RNA polymerase, STAT3, STAT6, IgE, p300, TLR5 and shRNA kits of HDAC7 and Bcl6 were purchased from Santa Cruz Biotech (Shanghai, China). The fluorochrome-labeled antibodies of CD19, CD138, IgE, TLR5 were purchased from BD Biosciences (Shanghai, China). The immune cell isolation reagent kits were purchased from Miltenyi Biotech (Shanghai, China). The reagents for RT-qPCR, gene transfection, luciferase assay and Western blotting were purchased from Invitrogen (Shanghai, China). The ChIP kit and ovalbumin (OVA) purchased from Sigma Aldrich (Shanghai, China). The recombinant FGN (purity = 99.99%) was purchased from Biomart (Beijing, China).
Mice
Male C57BL/6 mice (6-8 week old) were purchased from the Guangzhou Experimental Animal Center. The TLR5-/- mice were purchased from The Jackson Laboratory (Bar Harbor, ME, USA). The mice were maintained in pathogen-free cages with free access to food and water. The experimental procedures were approved by the Animal Ethic Committee at Shenzhen University. The experiments were carried out in accordance with the approved guidelines.
Isolation of B cells
CD19+ CD138- B cells were isolated from the naive mouse spleen by the magnetic cell sorting (MACS) using commercial reagent kits from Miltenyi Biotech following the manufacturer’s instructions. The cell purity was greater than 96% as checked by flow cytometry.
Cell culture
Cells were cultured in RPMI1640 culture medium supplemented with 10% fetal bovine serum, 100 U/ml penicillin, 0.1 mg/ml streptomycin and 2 mM L-glutamine. For B cell culture, anti-CD40 antibody (20 ng/ml) was added to the culture to prevent B cell apoptosis. The medium was changed in every 3 days. The cell viability was checked by the Trypan blue exclusion assay.
Flow cytometry
The cells were stained with the fluorochrome-labeled antibodies for 30 min on ice. For the intracellular staining, the cells were then fixed with 2% paraformaldehyde containing 0.5% saponin for 1 h. After washing with phosphate buffered saline (PBS), the cells were stained with the fluorochrome-labeled antibodies of interest for 30 min on ice. Isotype IgG was used as a staining control. After washing, the cells were analyzed with a flow cytometer (FACSCanto II, BD Biosciences). The data were analyzed with software flowjo. Data from isotype IgG staining were used as a gating reference.
Preparation of cytosolic and nuclear extracts
Cells were incubated with lysis buffer (10 mM HEPES, pH 7.4, 10 mM NaCl, 1.5 mM MgCl2, 0.5 mM DTT, 0.2% Nonidet P-40, and 0.2 mM PMSF) at 4°C for 15 min, and centrifuged at 500 ×g for 10 min at 4°C. The supernatant was collected as the cytosolic extract. The pellet was added with nuclear extract buffer (20 mM HEPES-KOH, pH 7.9, 25% glycerol, 420 mM NaCl, 1.5 mM MgCl2, 0.2 mM EDTA, 0.5 mM DTT, 0.2 mM PMSF, and 1× protease inhibitor cocktail) and incubated for 15 min at 4°C, followed by centrifugation at 13,000 ×g for 10 min at 4°C. The supernatant was collected as the nuclear extract. The protein concentrations were determined by the Bradford method.
Western blotting
The total proteins were extracted from the B cells, fractioned by SDS-PAGE (sodium dodecyl sulfate polyacrylamide gel electrophoresis) and transferred onto a PVDF membrane. The membrane was blocked by incubation with 5% skim milk for 30 min at room temperature, and incubated with the primary antibodies of interest overnight at 4°C, and followed by incubating with the second antibodies (conjugated with peroxidase) for 1 h at room temperature. The immune blots on the membrane were developed with ECL (enhanced chemiluminescence). The results were photographed with an image system (UVI, Beijing, China).
RNA interference (RNAi)
RNAi was performed with B cells to knock down the HDAC7 gene or Bcl6 gene. The B cells were treated with commercial RNAi reagent kits following the manufacturer’s instructions. The results of RNAi were assessed by Western blotting.
Assessment of Bcl6 or IgE promoter activities
The luciferase-conjugated reporter genes (or control) of the Bcl6 and IgE promoters was constructed by Genescript (Nanjing, China). Naive B cells were isolated from the C57BL/6 mouse spleen, seeded in 24-well plates and transfected with either a control vector or a Bcl6 promoter reporter vector (or an IgE promoter reporter vector) using Lipofectamine 2000. Cells were lysed 48-h after the transfection in a lysis buffer. Luciferase assays were performed on an Orion II microplate luminometer (Berthold detection systems, Oak Ridge, TN, Germany) with commercial reagent kits following the manufacturer’s instructions.
Chromatin immunoprecipitation (ChIP)
The chromatin changes were assessed by ChIP assay. The B cells were fixed with 1% formaldehyde for 15 min. The fixed cells were lysed and sonicated. The samples were pre-cleared by incubating with protein G. The supernatant was collected by centrifugation and incubated with antibodies of interest overnight at 4°C. Protein G agarose beads were added to the samples for 3 h. The beads were collected by centrifugation. After washing, the immune complex on the beads was eluted with eluting buffer. The DNA was separated with a commercial reagent kit following the manufacturer’s instructions and analyzed by qPCR. The primers using in ChIP assay include Bcl6 promoter (cctatagtgcggatcgtggt and tcagaattccagaggccgag) and IgE promoter (aagggaacttccaaggctgctaag and ccattaattcatgcccagatggtag). The results were presented as changes in the DNA amount against the input.
Real time quantitative RT-PCR (RT-qPCR)
The total RNA was extracted from the cells with the TRIzol reagents. The cDNA was synthesized with a reverse transcription kit. qPCR was performed on a qPCR device (MiniOpticon; Bio-Rad) using SYBR Green Master Mix. The primers used in the present study are presented in Table 1. The data were analyzed by the 2-ΔΔCt method and presented as relevant changes against the controls.
Table 1.
Primers used in the present study
| Molecules | Forward | Reverse |
|---|---|---|
| Bcl6 | acaccaccagcctcttatcc | ggcgagtagatgttgctgtg |
| IgE | atccagacagtgtgaagggg | tgactgaggttccttgaccc |
| HDAC1 | gatgaggagggagaaggtgg | aacttggggagaagatgggg |
| HDAC2 | gttttgtcagctctccacgg | aattcgaggatggcaagcac |
| HDAC3 | gcctggcattgactcatagc | tgtgtaacgggagcagaact |
| HDAC4 | gtcttgggaatgtacgacgc | ctgcatgcggagtctgtaac |
| HDAC5 | catgacttgaccgccatctg | gcctcctctttctcacctgt |
| HDAC6 | cgtgaaggtgccaactttga | cacgtccagcaatgatctgg |
| HDAC7 | agcccttgagagaacagtcc | ttgcgtctctccagggattt |
| HDAC8 | gcaccaatgactcaaagcca | aagcagagatcagcccaact |
| HDAC9 | ctacatctgcaaccgtcagc | tccaccacaggcatcatcat |
| HDAC10 | agagcggatcaaagtgacca | tctgatgcctcacaagctga |
| HDAC11 | tctcaacgagctgaagtggt | tagccagtgtgatgtctgca |
Development a food allergy model
Following our established procedures [10], C57BL/6 mice were gavage-fed with a mixture of OVA (0.1 mg/mouse) and cholera toxin (0.02 mg/mouse) weekly for 4 consecutive weeks. In week 5, the mice were challenged by gavage-fed with OVA (10 mg/mouse in 0.3 ml saline) and sacrificed next day. The parameters of food allergy, including serum OVA-specific IgE, Th2 cytokines, mast cell infiltration in the intestinal mucosa, intestinal CD4+ T cell proliferation, core temperature changes and diarrhea, were assessed with our established procedures, which were published elsewhere [10].
Assessment of serum β-hexosaminidase (β-hex)
Serum β-hex was determined by ELISA to assess MC degranulation. 50 µl of serum was mixed with 50 µl of 4 mM p-nitrophenyl-N-acetyl-β-d-glucosaminide (dissolved with 0.2 M citric acid buffer, pH 4.5). The mixture was maintained at 37°C for 1 h. Glycine buffer (0.2 M, pH 10.7, 150 µl) was added to terminate the reaction. Optical density (OD) was measured at 405 nm using a microplate reader (BioTel EXL-800, Shanghai, China). The β-hex levels were calculated by a formulae: (ODserum - ODtest - blank)/(ODtest - blank - ODblank) × 100%.
Statistics
The data are presented as mean ± SD. The difference between groups was determined by the Student t test or ANOVA if more than two groups. A p<0.05 was set as a significant criterion.
Results
Flagellin (FGN) suppresses Bcl6 expression in B cells
Our previous work shows that Bcl6 is associated with immune regulation [18]. Exposure to FGN induces immune inflammation [19]. Thus, we inferred that FGN might affect the expression of Bcl6 in B cells. We firstly assessed the expression of TLR5, the receptor of FGN, in B cells. As shown by data from flow cytometry and Western blotting, about 20.8% naive B cells were TLR5 positive, which was increased to 92.8% after culturing in the presence of anti-CD40 Ab (Figure 1A, 1B). To assess the role of FGN in the regulation of Bcl6 expression in B cells, we stimulated naive B cells with both anti-CD40 and IL-6. Such a treatment markedly increased the expression of Bcl6 in the B cells, which is in line with published data [20]. The IL-6-primed B cells were then stimulated with FGN in the culture. The results showed that exposure to FGN markedly suppressed the Bcl6 levels at both mRNA and protein levels in a FGN dose-dependent manner (Figure 1C, 1D). The results suggest that FGN can suppress Bcl6 expression in B cells. To enforce the results, we transduced naive B cells with the luciferase reporter of the Bcl6 promoter (Figure 1E). The cells were stimulated with anti-CD40, IL-6 and FGN in the culture. The results showed that the presence of FGN repressed the IL-6-induced luciferase activity in the cells in a FGN dose-dependent manner (Figure 1F).
Figure 1.
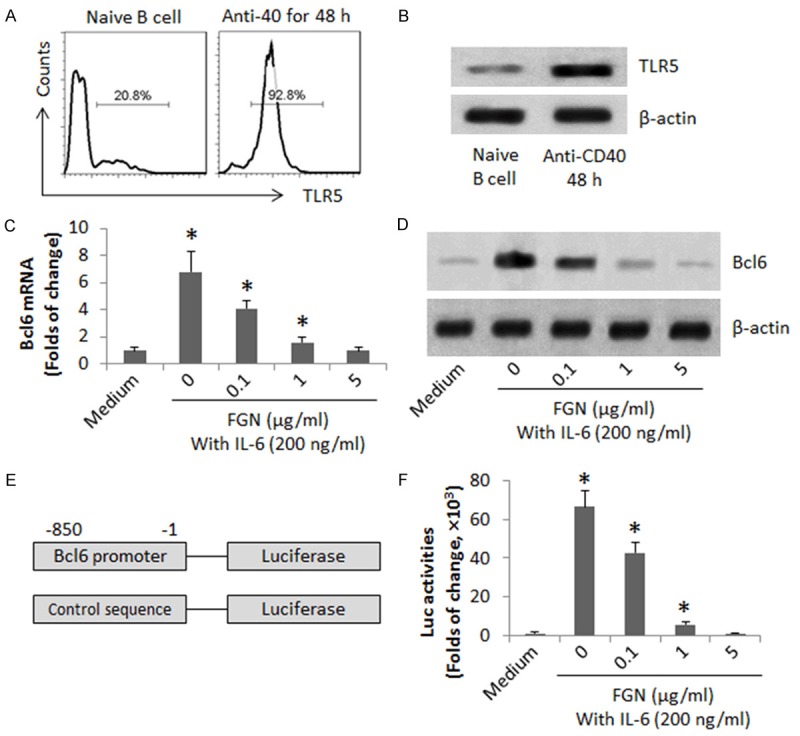
FGN suppresses Bcl6 expression in B cells. CD19+ CD138- B cells were isolated from the spleen of naïve mice by MACS. (A, B) The naive B cells were cultured for 48 h in the presence of anti-CD40 Ab (20 ng/ml). The gated histograms indicate the frequency of TLR5+ B cells (A). The immune blots indicate the TLR5 protein in the B cell extracts (B). (C, D) The naive B cells were cultured in the presence of IL-6 and anti-CD40 with or without the presence of FGN (at graded concentrations) for 48 h. The cell extracts were analyzed by RT-qPCR and Western blotting. The bars indicate the mRNA levels of Bcl6 (C). The immune blots indicate the protein levels of Bcl6 (D). (E) A sketch of Bcl6 promoter reporter gene. (F) The bars indicate the Bcl6 promoter activities. The data of bars are presented as mean ± SD. *, p<0.01, compared with the medium group. The data are representatives of 3 independent experiments.
FGN increases histone acetyltransferase (HAT) HDAC7 in B cells
The data of Figure 1 indicate that FGN modulated the Bcl6 transcription in B cells. Since HAT activities are the important factors in the initiation of gene transcription in cells, we screened 13 HATs in the B cells after exposure to FGN. As shown by the results of RT-qPCR, the exposure to FGN generally up regulated most HAT activities, but uniquely markedly increased the activities of HDAC7 (Figure 2A). To enforce the results, we stimulated the IL-6-primed B cells with FGN at graded concentrations in the culture for 48 h. The cell extracts were analyzed by Western blotting. The results showed that FGN increased HDAC7 in the B cells in a FGN dose-dependent manner (Figure 2B). The results implicate that the increase in HDAC7 may be associated with the FGN-modulating Bcl6 in B cells.
Figure 2.
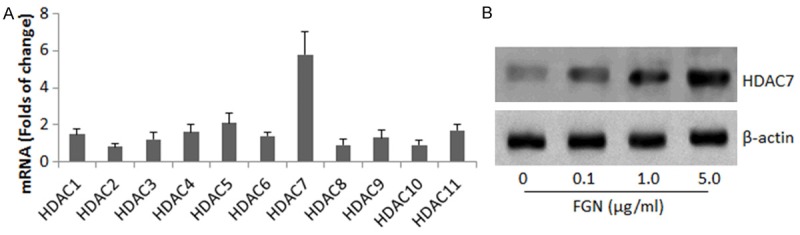
FGN increases HDAC7 in B cells. (A) CD19+ CD138- B cells were isolated from the mouse spleen. The cells were with FGN at 5 μg/ml (A), or 0-5 μg/ml (B). (A) The bars indicate the mRNA levels (mean ± SD) of 13 histone acetyltransferases in the B cells. (B) The immune blots indicate the protein levels of HDAC7 in the B cells. The data are a representative of 3 independent experiments.
FGN induces the chromatin remolding at the Bcl6 promoter locus in B cells
We further observed chromatin alterations at the Bcl6 promoter locus induced by FGN in B cells. As shown by Figure 3A-E, exposure to FGN for 6 h in the culture significantly increased the levels of pHDAC7, H3K9me and H3K27me at the Bcl6 promoter locus, while the levels of Pol II and STAT3 were suppressed. Considering the HDAC7 might be the critical factor in the FGN-induced changes at the Bcl6 promoter locus, we knocked down the HDAC7 gene in B cells (Figure 3F); the HDAC7-deficient B cells were stimulated with FGN in the culture for 6 h. The results showed that the FGN-induced changes at the Bcl6 promoter locus were abolished (Figure 3A-E). The results suggest that exposure to FGN increases HDAC7 in B cells; the HDAC7 increases H3K9me and H3K27me at Bcl6 promoter locus, which suppress the Pol II and STAT3 (the transcription factor of Bcl6 [20]) in situ.
Figure 3.
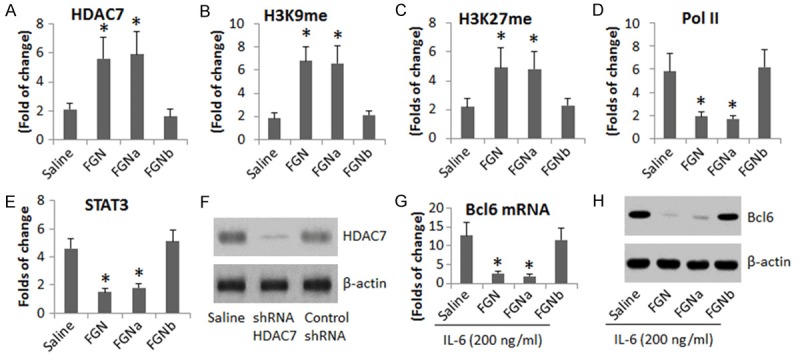
HDAC7 modulates Bcl6 gene transcription in B cells. B cells were separated from the mouse spleen and were stimulated with flagellin (FGN; 5 μg/ml) in the culture for 3 h and analyzed by ChIP assay. The bar graphs indicate the levels of pHDAC7 (A), H3K9me (B), H3K27me (C), Pol II (D) and STAT3 (E) at the Bcl6 promoter locus. (F) The immune blots indicate the results of HDAC7 gene knockdown. (G and H) Cells were treated with IL-6 (200 ng/ml) and FGN (5 μg/ml) for 48 h; the cells were analyzed by RT-qPCR and Western blotting. The bars indicate the mRNA level of Bcl6 in the B cells (G); the Western blots indicate the protein level of Bcl6 (H). FGNa and FGNb: Control shRNA (a) and HDAC7 shRNA (b) treated B cells were treated with FGN in the culture. The data of bars are presented as mean ± SD. *, p<0.01, compared with the saline group. The data are representatives of 3 independent experiments.
We also treated B cells with both IL-6 and FGN for 48 h; the cell extracts were analyzed by RT-qPCR and Western blotting. The results showed that exposure to FGN significantly suppressed the IL-6-induced Bcl6 in the B cells, which could be blocked by knockdown of the HDAC7 gene (Figure 3G-H). Collectively, the results suggest that FGN is capable of suppressing Bcl6 expression in B cells via increasing HDAC7.
FGN induces IgE expression via suppressing Bcl6 in B cells
Based on that Bcl6 is capable of suppressing IgE expression [21] and FGN represses Bcl6 expression in B cells via activating HDAC7 as shown by the present data, we inferred that FGN could initiate IgE expression in B cells in certain environments, such as in a Th2 cytokine dominant condition. To this end, we treated B cells with FGN and IL-4 in the culture for 4 days, with or without knocking down the Bcl6 gene or TLR5 gene by RNAi. As shown by Figure 4, exposure to both FGN and IL-4 induced IgE expression in the B cells, but not in those exposed to either FGN alone or IL-4 alone. Knockdown of the Bcl6 or TLR5 abolished the FGN/IL-4-induced IgE expression in the B cells.
Figure 4.

Flagellin facilitates IgE expression in B cells. (A and B) The Western blots indicate the RNAi results of Bcl6 (A; a) and TLR5 (B; b). (C and D) Naive B cells were isolated from the mouse spleen by MACS. The cells were treated in the culture as denoted on the X axis of panel C for 96 h. The bars indicate the levels of mRNA (C), the immune blots indicate the protein (D) of IgE in B cells. (E) A sketch of an IgE promoter luciferase reporter gene. (F) The bars indicate the IgE promoter activities. The label “c” indicates the cells were treated with control shRNA. (G-I) Naive B cells were treated in the culture for 48 h as denoted on the X axis. The cells were analyzed by ChIP assay. The bars indicate the levels of p300 (G), H3K4ac (H), H3K9ac (I) and STAT6 (J) at the IgE promoter locus. #, the presence of garcinol (15 μM). The data of bars are presented as mean ± SD. *, p<0.01, compared with the medium group. The data are a representative of 3 independent experiments.
Next, we transfected a luciferase reporter gene of the IgE promoter (Figure 4E) into naive B cells. The cells were treated with FGN or/and IL-4 in the culture for 6 h. The cell extracts were analyzed by luciferase assay. The results showed the exposure to FGN and IL-4 significantly increased the luciferase activities, while the exposure to either FGN alone or IL-4 did not apparently increase the luciferase activities in the B cells (Figure 4F). The results indicate that exposure to both FGN and IL-4 is capable of increasing the IgE promoter activities in B cells.
We next assessed the chromatin alteration at the IgE promoter locus in the B cells after the exposure to FGN or/and IL-4. The B cells were analyzed by ChIP assay. The results showed the exposure to FGN/IL-4 significantly increased the levels of p300, H3K4ac, H3K9ac and STAT6 at the IgE promoter locus, which did not occur in the B cells exposed to either FGN alone or IL-4 alone (Figure 4G-J). The results implicate that the increase in p300 may the occurred first; it then induces the rest alterations at the IgE promoter locus. To test the inference, in separate experiments, we added an inhibitor of p300, the garcinol, to the culture, which abolished the FGN/IL-4 induced alterations at the IgE promoter locus (Figure 4G-J).
FGN facilitates the expression of IgE in a food allergy mouse model
Based on the results reported above and the concept that IgE is the major mediator of allergic diseases, we inferred that FGN might facilitate or be required in the expression of IgE in allergic disorders. To this end, we developed a food allergy mouse model with or without supplementing with FGN in the sensitizing procedures. The results (Figure 5) showed that mice sensitized to OVA manifesting the presence of serum OVA-specific IgE, increases in the serum levels of IL-4, IL-5, IL-13 and β-hexosaminidase (β-Hex), abundant infiltration of mast cells and eosinophils in the intestinal mucosa, the presence of intestinal OVA-specific CD4+ T cells, drop of the core temperature and had diarrhea in response to the OVA-challenge. The data indicate that mice sensitized with OVA were induced the allergy-like inflammation in the intestine. On the other hand, mice treated with OVA and FGN showed even higher serum levels of OVA-specific IgE, more mast cell infiltration in the intestinal mucosa and drop of the core temperature than mice treated with OVA, while the serum Th2 cytokine levels and intestinal OVA-specific CD4+ T cell proliferation in mice treated with OVA/FGN were similar to mice treated with OVA. To take a further insight into the role of FGN in the development of allergic disorders in the intestine, we treated TLR5-/- mice with OVA, or OVA/FGN in the same procedures above. The results showed that although the serum Th2 cytokines were still higher, the OVA-specific CD4+ T cells were still detected in the sensitized mice, the serum OVA-specific IgE was below the detectable levels in TLR5-/- mice. The frequency of mast cells in the intestinal mucosa was not statistically different between the wild mice and TLR5-/- mice. Most importantly, the TLR5-/- mice did not show mast cell activation, nor the core temperature drop and diarrhea after the specific antigen challenge. The results indicate that the TLR5-/- mice can be induced the antigen-specific Th2 polarization status in the intestine, but do not have the antigen specific intestinal allergic reactions.
Figure 5.
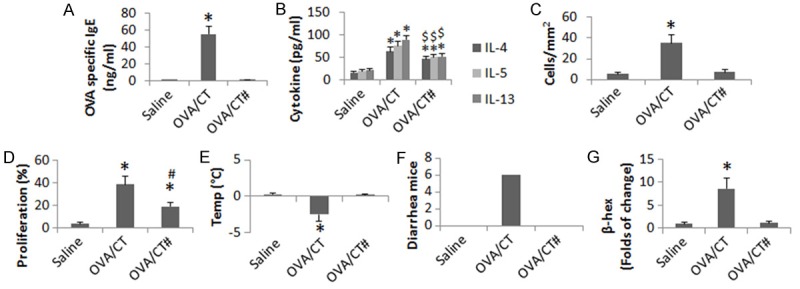
FGN activates TLR5 to initiate the allergic reaction in the intestine. C57BL/6 or TLR5-/- mice were treated with the procedures as denoted on the X axis and in the text. The bar graphs show the serum levels of OVA-specific IgE (A), serum Th2 cytokines (B), mast cell and eosinophil infiltration in the intestine (C), CD4+ T cell proliferation (D), drop of the core temperature (E) and diarrhea (F). (G) The bars indicate the levels of β-hexosaminidase (β-Hex). #, mice were deficient of TLR5. *, p<0.01, compared with the saline group. $, p<0.01, compared with the sensitized wild mice. Each group consisted of 6 mice. Samples from individual mice were processed separately.
Discussion
IgE is the most important mediator in allergic disorders. To understand its initiation and regulation is of significance. A number of previous studies showed promising results using anti-IgE antibodies, such as the omalizumab, to inhibit allergic reactions [22]. The present study has revealed that FGN, one of the microbial products, is in a position to initiate the IgE gene transcription in B cells via activating TLR5 and HDAC7 as well as modulating the chromatin structure at Bcl6 and IgE promoter loci. The important spot of the present findings is that the interaction of FGN/TLR5 functions as a checkpoint to initiate allergic reactions.
FGN is the principal substituent of the bacterial flagellum, and is present in large amounts of nearly all flagellated bacteria; it extensively exists in the intestinal tract. Our previous work indicates that FGN is involved in the induction of intestinal inflammation [11,19]. Others found that FGN is associated with the pathogenesis of allergic inflammation [16]. The present data have added further information to the studies of allergy that FGN facilitates the expression of IgE in B cells. FGN is a macromolecular protein; it has a mass of about 30,000 to 60,000 Daltons. Thus, in physiological condition, FGN may not be absorbed into the deep regions of the intestinal mucosa. This may explain why FGN does not induce the intestinal immune inflammation in healthy subjects, although it usually extensively exists in the intestinal tract. Only in certain circumstances, such as under psychological stress, the intestinal barrier function may be compromised [23]; microbial products, such as FGN, and food antigens with competent antigenicity may be absorbed into the deeper regions of the intestinal mucosa to initiate aberrant immune responses such as food allergy [24].
The HDAC7 is one member of the HDAC family, which plays an important part in the gene transcription and has multiple functions [13]. In our study, the data demonstrate that in naive B cells, HDAC7 is detectable. It is only in the certain condition, such as exposure to FGN in the culture, HDAC7 is activated to suppress Bcl6 in B cells, and to facilitate the expression of IgE by B cells. It is accepted that IgE is the principal mediator of allergic reactions [3]. Bcl6 can suppress the expression of IgE [8]. Therefore, it is conceivable of that to block the aberrant increase in HDAC7 may block the FGN-induced IgE expression in B cells.
The present results showed that, after treatment with the sensitization procedures, the wild mice showed allergy like reactions in the intestine, while the TLR5-/- mice did not have mast cell activation (the lower serum levels of β-hex and less mast cell infiltration in the intestinal mucosa as compared with the sensitized wild mice), lacked of core temperature drop and no mice had diarrhea after challenging with the specific antigen, although these mice still showed the OVA-specific CD4+ T cell in the intestine, had higher serum levels of Th2 cytokines as compared with the naive control mice. This phenomenon may be explained that the TLR5-/- mice did not have detectable OVA-specific IgE as shown by the data; the mast cells in the mice were supposed not being sensitized (supported by the less mast cell infiltration in the intestinal mucosa and the less serum levels of β-hex in the TLR5-/- mice), and thus no clinical symptoms (including core temperature did not drop and no diarrhea was observed) were induced by the specific antigen challenge.
It was reported by Lemichez et al that CT could induce the intestinal epithelial barrier dysfunction [25]. Guichard et al found the mechanism by which CT disrupted intestinal barrier integrity by disturbing the Rab11- and exocyst-dependent delivery of endocytic recycling cargo to cell-cell junctions [26]. Our previous work also showed that CT directly compromised the intestinal monolayer barrier function [27]. CT is commonly used as an adjuvant to develop food allergy animal models [10]. Therefore, the present data have expanded the current knowledge about the CT/allergen-food allergy model by showing a previous unknown aspect of CT. Not only does CT disrupt the intestinal epithelial barrier as reported before, CT also suppresses the production of Bcl6 in B cells to initiate the IgE gene transcription.
In summary, the present data show that exposure to FGN enhanced the HDAC7 activities in B cells. The pHDAC7 modulated the chromatin structure to suppress the Bcl6 expression, which promoted the IgE expression and contributed to the development of food allergy.
Acknowledgements
This study was supported by grants from the innovation of science and Technology Commission of Shenzhen Municipality (JCYJ20150402090413008, JCYJ20140418095735611 and ZDSYS201506050935272), the Natural Science Foundation of China (81373176, 31570932, 31400856, 81571790 and 81501573).
Disclosure of conflict of interest
None.
Authors’ contribution
LJL, NM, LZ, HPZ, LHM, XXL, LZX, BY, ZGL and BSF performed the experiments, analyzed data and reviewed the manuscript. PCY and PYZ organized the study and supervised the experiments. PCY designed the project and wrote the manuscript.
References
- 1.Yalcin AD. Advances in Anti-IgE Therapy. Biomed Res Int. 2015;2015:317465. doi: 10.1155/2015/317465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gaze S, Bethony JM, Periago MV. Immunology of experimental and natural human hookworm infection. Parasite Immunol. 2014;36:358–66. doi: 10.1111/pim.12088. [DOI] [PubMed] [Google Scholar]
- 3.Logsdon SL, Oettgen HC. Anti-IgE therapy: clinical utility and mechanistic insights. Curr Top Microbiol Immunol. 2015;388:39–61. doi: 10.1007/978-3-319-13725-4_3. [DOI] [PubMed] [Google Scholar]
- 4.Reber LL, Sibilano R, Mukai K, Galli SJ. Potential effector and immunoregulatory functions of mast cells in mucosal immunity. Mucosal Immunol. 2015;8:444–63. doi: 10.1038/mi.2014.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ye BH, Lista F, Lo Coco F, Knowles DM, Offit K, Chaganti RS, Dalla-Favera R. Alterations of a zinc finger-encoding gene, BCL-6, in diffuse large-cell lymphoma. Science. 1993;262:747–50. doi: 10.1126/science.8235596. [DOI] [PubMed] [Google Scholar]
- 6.Testoni M, Zucca E, Young KH, Bertoni F. Genetic lesions in diffuse large B-cell lymphomas. Ann Oncol. 2015;26:1069–80. doi: 10.1093/annonc/mdv019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johnston RJ, Poholek AC, DiToro D, Yusuf I, Eto D, Barnett B, Dent AL, Craft J, Crotty S. Bcl6 and Blimp-1 are reciprocal and antagonistic regulators of T follicular helper cell differentiation. Science. 2009;325:1006–10. doi: 10.1126/science.1175870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ye BH, Cattoretti G, Shen Q, Zhang J, Hawe N, de Waard R, Leung C, Nouri-Shirazi M, Orazi A, Chaganti RS, Rothman P, Stall AM, Pandolfi PP, Dalla-Favera R. The BCL-6 proto-oncogene controls germinal-centre formation and Th2-type inflammation. Nat Genet. 1997;16:161–70. doi: 10.1038/ng0697-161. [DOI] [PubMed] [Google Scholar]
- 9.Krysko O, Maes T, Plantinga M, Holtappels G, Imiru R, Vandenabeele P, Joos G, Krysko DV, Bachert C. The adjuvant-like activity of staphylococcal enterotoxin B in a murine asthma model is independent of IL-1R signaling. Allergy. 2013;68:446–53. doi: 10.1111/all.12102. [DOI] [PubMed] [Google Scholar]
- 10.Yang G, Geng XR, Song JP, Wu Y, Yan H, Zhan Z, Yang L, He W, Liu ZQ, Qiu S, Liu Z, Yang PC. Insulin-like growth factor 2 enhances regulatory T-cell functions and suppresses food allergy in an experimental model. J Allergy Clin Immunol. 2014;133:1702–8. e5. doi: 10.1016/j.jaci.2014.02.019. [DOI] [PubMed] [Google Scholar]
- 11.Li L, Mo L, Hao H, Yang W, Zhou Q, Xue F, Shi Z, Liu Z, Yang PC, Feng B. Flagellin modulates TIM4 expression in mast cells. Cell Biol Int. 2014;38:1330–6. doi: 10.1002/cbin.10330. [DOI] [PubMed] [Google Scholar]
- 12.Deifl S, Kitzmüller C, Steinberger P, Himly M, Jahn-Schmid B, Fischer GF, Zlabinger GJ, Bohle B. Differential activation of dendritic cells by toll-like receptors causes diverse differentiation of naive CD4+ T cells from allergic patients. Allergy. 2014;69:1602–9. doi: 10.1111/all.12501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen HP, Zhao YT, Zhao TC. Histone deacetylases and mechanisms of regulation of gene expression. Crit Rev Oncog. 2015;20:35–47. doi: 10.1615/critrevoncog.2015012997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jiang J, Liu JQ, Li J, Li M, Chen HB, Yan H, Mo LH, Qiu SQ, Liu ZG, Yang PC. Trek1 contributes to maintaining nasal epithelial barrier integrity. Sci Rep. 2015;5:9191. doi: 10.1038/srep09191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lemercier C, Brocard MP, Puvion-Dutilleul F, Kao HY, Albagli O, Khochbin S. Class II histone deacetylases are directly recruited by BCL6 transcriptional repressor. J Biol Chem. 2002;277:22045–52. doi: 10.1074/jbc.M201736200. [DOI] [PubMed] [Google Scholar]
- 16.Jeon JH, Ahn KB, Kim SK, Im J, Yun CH, Han SH. Bacterial flagellin induces IL-6 expression in human basophils. Mol Immunol. 2015;65:168–76. doi: 10.1016/j.molimm.2015.01.022. [DOI] [PubMed] [Google Scholar]
- 17.Stutz AM, Woisetschlager M. Functional synergism of STAT6 with either NF-kappa B or PU. 1 to mediate IL-4-induced activation of IgE germline gene transcription. J Immunol. 1999;163:4383–91. [PubMed] [Google Scholar]
- 18.Liu ZQ, Song JP, Liu X, Jiang J, Chen X, Yang L, Hu T, Zheng PY, Liu ZG, Yang PC. Mast cell-derived serine proteinase regulates T helper 2 polarization. Sci Rep. 2014;4:4649. doi: 10.1038/srep04649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen X, Feng BS, Zheng PY, Liao XQ, Chong J, Tang SG, Yang PC. Fc gamma receptor signaling in mast cells links microbial stimulation to mucosal immune inflammation in the intestine. Am J Pathol. 2008;173:1647–56. doi: 10.2353/ajpath.2008.080487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Choi YS, Eto D, Yang JA, Lao C, Crotty S. Cutting edge: STAT1 is required for IL-6-mediated Bcl6 induction for early follicular helper cell differentiation. J Immunol. 2013;190:3049–53. doi: 10.4049/jimmunol.1203032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang TT, Makondo KJ, Marshall AJ. p110delta phosphoinositide 3-kinase represses IgE switch by potentiating BCL6 expression. J Immunol. 2012;188:3700–8. doi: 10.4049/jimmunol.1103302. [DOI] [PubMed] [Google Scholar]
- 22.Tsabouri S, Tseretopoulou X, Priftis K, Ntzani EE. Omalizumab for the treatment of inadequately controlled allergic rhinitis: a systematic review and meta-analysis of randomized clinical trials. J Allergy Clin Immunol Pract. 2014;2:332–40. e1. doi: 10.1016/j.jaip.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 23.Wu W, Sun M, Zhang HP, Chen T, Wu R, Liu C, Yang G, Geng XR, Feng BS, Liu Z, Liu Z, Yang PC. Prolactin mediates psychological stress-induced dysfunction of regulatory T cells to facilitate intestinal inflammation. Gut. 2014;63:1883–92. doi: 10.1136/gutjnl-2013-306083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang PC, Jury J, Soderholm JD, Sherman PM, McKay DM, Perdue MH. Chronic psychological stress in rats induces intestinal sensitization to luminal antigens. Am J Pathol. 2006;168:104–14. doi: 10.2353/ajpath.2006.050575. quiz 363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lemichez E, Stefani C. Cholera Toxin Notches Epithelial Junctions. Cell Host Microbe. 2013;14:227–9. doi: 10.1016/j.chom.2013.08.009. [DOI] [PubMed] [Google Scholar]
- 26.Guichard A, Cruz-Moreno B, Aguilar B, van Sorge NM, Kuang J, Kurkciyan AA, Wang Z, Hang S, Pineton de Chambrun GP, McCole DF, Watnick P, Nizet V, Bier E. Cholera toxin disrupts barrier function by inhibiting exocyst-mediated trafficking of host proteins to intestinal cell junctions. Cell Host Microbe. 2013;14:294–305. doi: 10.1016/j.chom.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu X, Yang G, Geng XR, Cao Y, Li N, Ma L, Chen S, Yang PC, Liu Z. Microbial products induce claudin-2 to compromise gut epithelial barrier function. PLoS One. 2013;8:e68547. doi: 10.1371/journal.pone.0068547. [DOI] [PMC free article] [PubMed] [Google Scholar]


