Abstract
Background: Cardiomyocytes apoptosis under hypoxia condition contributes significantly to various cardiovascular diseases. In this study, we investigated the role of microRNA-322 (miR-322) in regulating hypoxia-induced apoptosis in neonatal murine cardiomyocytes in vitro. Method: Cardiomyocytes of C57BL/6J mice were treated with hypoxia condition in vitro. Cardiomyocyte apoptosis was measured by TUNEL assay. Gene expression pattern of miR-322 was measured by qRT-PCR. Stable downregulation of miR-322 in cardiomyocytes were achieved by lentiviral transduction, and the effect of miR-322 downregulation on hypoxia-induced cardiomyocyte apoptosis was investigated. Possible regulation of miR-322 on its downstream target gene, brain derived neurotrophic factor (BDNF) was investigated in cardiomyocytes. BDNF was then genetically silenced by siRNA to evaluate its role in miR-137 mediated cardiomyocyte apoptosis protection under hypoxia condition. Results: Under hypoxia condition, significant apoptosis was induced and miR-322 was significantly upregulated in cardiomyocytes in vitro. Through lentiviral transduction, miR-322 was efficiently knocked down in cardiomyocytes. Downregulation of miR-322 protected hypoxia-induced cardiomyocyte apoptosis. Luciferase assay showed BDNF was the target gene of miR-322. QRT-PCR showed BDNF expression was associated with miR-322 regulation on hypoxia-induced cardiomyocyte apoptosis. Silencing BDNF in cardiomyocyte through siRNA transfection reversed the protective effect of miR-322 downregulation on hypoxia-induced apoptosis. Conclusion: Our study revealed that miR-322, in association with BDNF, played important role in regulating hypoxia-induced apoptosis in cardiomyocyte.
Keywords: Cardiomyocyte, apoptosis, miR-322, BDNF
Introduction
Coronary artery disease is one of the major causes of mortality and morbidity in the world [1,2]. The most common heart diseases associated with coronary artery disease may include cardiac hypertrophy, acute myocardial infraction and myocardial ischemia [3]. During the process of myocardial ischemia or reperfusion injury, the excessive production of reactive oxygen species (ROS) may cause significant cardiomyocyte apoptosis [4,5]. In addition, both animal and human studies have shown that hypoxia-induced apoptosis was one of the major molecular mechanisms contributing to cardiomyocyte death, therefore leading to the progression of various heart diseases [6-8]. In order to seek methods of early diagnosis and develop optimal treatment plans for patients suffered from various heart diseases, it is critical to understand the underlying mechanisms of hypoxia-induced cardiomyocyte apoptosis.
MicroRNAs (miRNAs) are classes of non-coding small RNAs that pair with the 3’ untranslated regions (3’-UTR) of target genes to induce post-transcriptional gene silencing or protein degradation [9,10]. Studies have shown that miRNAs played critical roles during the progression of multiple heart diseases, including myocardial ischemia [11-13]. Among many of the heart-associated miRNAs, human miR-424, as well as its murine ortholog mmu-miR-322, has been shown to be actively regulated during the maturing process of vascular smooth muscle [14]. Also, studies have shown that miR-424/322 was upregulated during the process of cardiovascular injuries in hearts, and subsequent regulation of miR-424/322 protected vascular smooth muscles or endothelial cells from injury [14,15]. However, it is not clear whether miR-424/322 may actively regulate the cardiomyocyte apoptosis under hypoxia condition, and if so, through what signaling pathways.
Brain derived neurotrophic factor (BDNF) is a BHLH transcriptional factor that plays important role in heart development [16,17]. Under myocardial infraction in heart disease, BDNF was shown to up-regulate transient receptor potential canonical 3/6 channels to protect cardiomyocyte apoptosis [18]. It was also shown that activating BDNF/VEGF signaling pathways improved survival rates in animal model of myocardial ischemia [19]. However, the association between miR-424/322 regulation and the protective effect of BDNF in cardiac injury has never been revealed.
In the present study, we took advantage of an in vitro model of cardiomyocyte culture and investigated the role of murine miR-322 in regulating cardiomyocyte apoptosis under hypoxia condition. In addition, explored the possible functional correlation between miR-322 and BDNF gene in cardiomyocyte apoptosis protection. The results of our study may help to elucidate the underlying mechanisms of miRNA regulation in heart diseases.
Materials and methods
Ethic statement
In the present study, all experimental procedures were approved by the Animal Study and Ethics Committee at the Second Affiliated Hospital of Harbin Medical University in Harbin, China.
In vitro murine cardiomyocytes culture and hypoxia treatment
The method of maintaining in vitro explant of neonatal murine cardiomyocytes was described previously [20]. Briefly, C57BL/6J mice were sacrificed at postnatal 1 or 2 days. The hearts were quickly transferred into a petri dish filled with phosphate-buffered saline (PBS, Sigma Aldrich, USA). The ventricles were cut into small pieces by micro-scissors under microscope. After treating diced cardiac tissues with 0.5% trypsin-EDTA (Invitrogen, USA) and centrifuging, cardiomyocytes were separated from supernatants and resuspended in DMEM/F12 medium (Invitrogen, USA) supplemented with 20% FCS (Invitrogen, USA) and antibiotics. The cultures were then maintained in a tissue culture incubator at 37°C with 5% CO2. The method of hypoxia treatment was described previously [21]. Briefly, cardiomyocytes were plated in 48-well plates (3,000 cells/well) and pre-treated with serum starvation for 12 h. Then, they were treated with a gas mixture of 95% N2 and 5% CO2 for 2 h.
TUNEL assay
After hypoxia treatment, cardiomyocytes were washed 3 times in PBS and permeabilized with 0.3% TritonX-100 (Sigma-Aldrich, USA) for 15 min at room temperature. Apoptosis of cardiomyocytes was determined by a terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL) assay (Molecular Probes, USA) according to the manufacturer’s instruction. A nuclei immunohistochemistry was also carried out by DAPI staining. Using an ImageJ software (NIH, USA), TUNEL-positive cardiomyocytes were counted in each well, and then normalized to the total number of cardiomyocytes based on DAPI staining.
RNA extraction and qRT-PCR
Total RNA was extracted from cardiomyocytes using a RNA Isolation Kit (Ambion, USA). Quantitative real-time PCR (qRT-PCR) for mmu-miR-322 was performed using a SYBR Green Real-Time PCR Master Mix Kit (Applied Biosystems, USA) with u6 sRNA as internal control. QRT-PCR for murine BDNF was performed using a TaqMan MicroRNA Assays (Applied Biosystems, USA) with GAPDH as internal control. All PCR reactions were carried out on a LightCycler 480 System II (Roche Diagnostics, USA) according to the manufacturer’s instructions. Gene expression levels were calculated using 2-∆∆Ct method and presented as fold changes relative to control.
Lentiviral assay for miR-322 downregulation
Genetic knockdown of miR-322 in cardiomyocytes were carried out by lentiviral transduction. Lentivirus of mmu-miR-322 inhibitor (L-miR322-Inhibitor), as well as its negative control miRNA lentivirus (L-C-miR), were obtained from SunBio (SunBio Medical Biotechnology, China). Cardiomyocytes were then incubated with L-miR322-Inhibitor or L-C-miR in combination with 8 μg/ml polybrene at an MOI of 20~50 for 48 h. After changing in new culture medium, cardiomyocytes were continuously cultured for 48 h for stabilization. The transduction efficiency was then measured by qRT-PCR.
Dual-luciferase reporter assay
Murine BDNF gene was amplified from a mouse heart cDNA library and verified by sequencing. The 3’-untranslated region (3’-UTR) of BDNF, including the binding site of mmu-miR-322 was cloned into a pmiR-REPORT luciferase vector (Applied Biosystems, USA) and named as BDBF 3’ UTR luciferase vector. The putative miR-322 binding sequence on BDNF 3’-UTR was mutated through a Quik-Change™ Site-Directed Mutagenesis Kit (Stratagene, USA). The mutated BDNF 3’-UTR was then cloned into pmiR-REPORT and named as (m) BDNF 3’ UTR. The relative lu-ciferase activities were measured by a dual-luciferase re-porter kit (Promega, USA) on an ARVO MX plate reader (Perkin-Elmer, USA) according to the manufacturer’s instruction.
SiRNA assay for BDNF downregulation
Murine BDNF siRNA (siRNA-BDNF), as well as its control scrambled siRNA (siRNA-C) were obtained from RiboBio (RiboBio, China). Genetic knockdown of BDNF gene in cardiomyocytes was then performed by siRNA transfection using Lipofectamine 2000 reagent. 24 h after transfection, knockdown efficiency was measured by qRT-PCR.
Statistical analysis
In this study, all experiments were repeated at least three times, and data were shown as mean +/- standard errors. Statistical analysis was performed by Student’s t-test using a SPSS software. Statistical difference was determined if P < 0.05.
Results
Hypoxia induced apoptosis and miR-322 upregulation in cardiomyocytes
In our study, we took advantage of the in vitro explant model of neonatal murine cardiomyocyte to study the molecular mechanisms of cardiac hypoxia. Cardiomyocytes were isolated from postnatal 1 or 2 days old C57BL/6J mice and maintained in 48-well plates (3,000 cells/well) in vitro. The cultures were treated with a gas mixture of 95% N2 and 5% CO2 for 2 h to induce hypoxia. 24 h after hypoxia treatment, cardiomyocyte apoptosis was evaluated through a TUNEL assay. The immunostaining result showed that in cardiomyocytes (identified by DAPI immunoreactivity) under control condition, little or no apoptosis (identified by TUNEL immunoreactivity) was visible, whereas in hypoxia-treated cardiomyocytes, considerable amount of apoptotic cells were seen (Figure 1A). By comparing the averaged percentages of apoptotic cardiomyocytes (TUNEL/DAPI (%)) between control and hypoxia conditions, it was clearly demonstrated that significantly apoptosis was induced by hypoxia in murine cardiomyocytes in vitro (Figure 1B, *P < 0.05).
Figure 1.
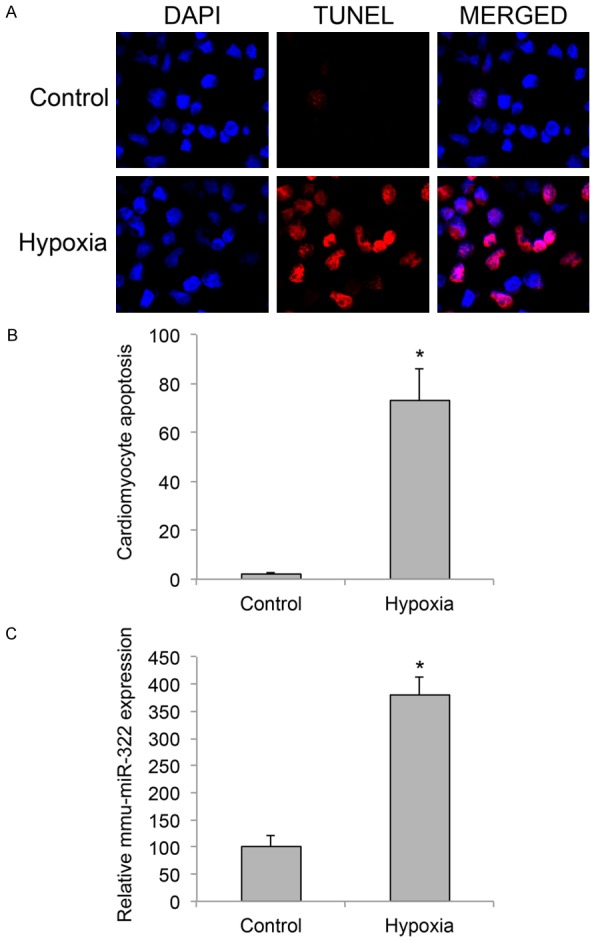
Cardiomyocyte apoptosis and miR-322 upregulation under hypoxia condition. Murine neonatal cardiomyocytes (postnatal 1~2 days old) were cultured in 48-well plates (3,000 cells/well) and treated with a gas mixture of 95% N2 and 5% CO2 for 2 h to induce hypoxia. A. 24 h after hypoxia treatment, a TUNEL assay (Red) was carried out to examine apoptosis in cardiomyocytes. Cardiomyocytes were identified by DAPI nuclear staining (Blue). B. The averaged percentages of apoptotic cardiomyocytes (DAPI/TUNEL (%)) were compared between control and hypoxia cultures (*P < 0.05). C. 24 h after hypoxia treatment, relative expression levels of mmu-miR-322 in cardiomyocytes were compared between control and hypoxia conditions (*P < 0.05).
Previous study showed that human miR-424, and its murine ortholog, mmu-miR-322 was upregulated in endothelial cells during ischemic injury [15]. Therefore, we decided to investigate the gene expression pattern of miR-322 in our hypoxia model. The results of qRT-PCR demonstrated that mmu-miR-322 was markedly upregulated in murine cardiomyocytes under hypoxia condition (Figure 1C, *P < 0.05).
Downregulation of miR-322 protected hypoxia-induced apoptosis in cardiomyocyte
Since we demonstrated that hypoxia condition induced significant apoptosis and upregulated murine miR-322 in cardiomyocyte, we wondered whether there might be an association between miR-322 regulation and hypoxia-induced cardiomyocyte apoptosis. To examine this hypothesis, we created a cardiomyocyte culture with stably downregulation of miR-322, through the method of transducing cardiomyocytes with mmu-miR-322 inhibitor lentivirus (L-miR322-Inhibitor). In control culture, cardiomyocytes were transduced with a scrambled miRNA lentivirus (L-C-miR). After lentiviral transduction was stabilized, we used qRT-PCR to confirm that gene expression level of mmu-miR-322 was indeed downregulated in cardiomyocytes transduced with L-miR322-Inhibitor, than in cardiomyocytes transduced with L-C-miR (Figure 2A, *P < 0.05).
Figure 2.
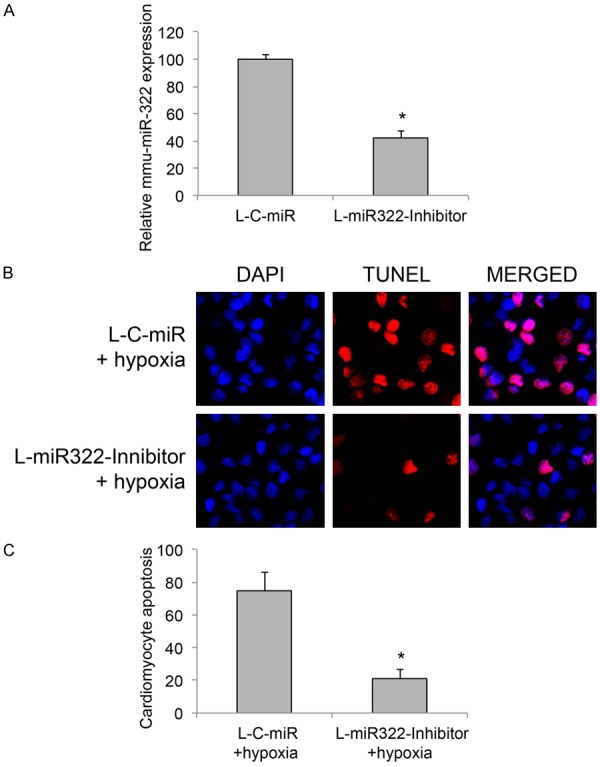
Suppressing miR-322 rescued cardiomyocyte apoptosis under hypoxia condition. A. Cardiomyocytes were transduced with a lentivirus containing mmu-miR-322 inhibitor (L-miR322-Inhibitor), or a lentivirus containing negative control scrambled miRNA (L-C-miR). After transduction was stabilized, qRT-PCR was carried out to evaluate the efficiency of miR-322 downregulation in cardiomyocyte (*P < 0.05). B. Lentiviral-transduced cardiomyocytes were then treated with hypoxia condition for 2 h. 24 h after that, a TUNEL assay was carried out and immune-fluorescent images were shown for cardiomyocyte apoptosis (Red: TUNEL; Blue: DAPI). C. The percentages of apoptotic cardiomyocytes under hypoxia condition were compared between cells transduced with L-C-miR and cells transduced with L-miR322-Inhibitor (*P < 0.05).
We then treated lentiviral-transduced cardiomyocytes with hypoxia condition and re-evaluated apoptosis. The immunostaining results of TUNEL assay showed that considerably less apoptotic immunoreactivity was observed in cardiomyocytes with miR-322 downregulation (Figure 2B). Comparison between L-miR322-Inhibitor and L-C-miR transduced cardiomyocytes showed that miR-322 downregulation (w/L-miR322-Inhibitor transduction) significantly reduced the percentages of apoptotic cardiomyocytes under hypoxia condition (Figure 2C, *P < 0.05).
BDNF is the target gene of miR-322 in cardiomyocyte under hypoxia condition
In the next step of our work, we tried to identify the molecular target gene of miR-322 in cardiomyocyte under hypoxia condition. We took advantage of several online databases to predict the downstream genes that would be directly regulated by miR-322. In searching the databases including TargetScan (www.targetscan.org) and microRNA (www.microrna.org), we noticed that BDNF gene was a possible downstream target of miR-322 in mouse (Figure 3A).
Figure 3.
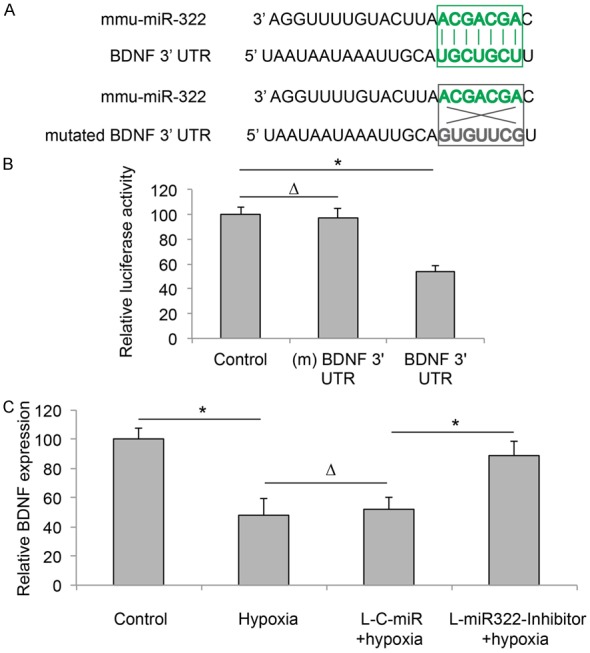
BDNF is the target gene of miR-322 in cardiomyocyte under hypoxia condition. A. 3’-UTR of BDNF gene is predicted to be bound by miR-322 (top panel). Alternatively, a mutated BDNF 3’-UTR was created to dissociate miR-322 binding (bottom panel). B. HEK293T cells were co-transfected with mmu-miR-322 mimics, and a Control luciferase reporter, a luciferase reporter expressing mutated (m) BDNF 3’-UTR, or a luciferase reporter expressing BDNF 3’-UTR. Three groups of HEK293T cells were then underwent a dual-luciferase reporter assay to measure the relative luciferase activities (*P < 0.05). C. QRT-PCR was carried out to compare the expression levels of BDNF in normally cultured cardiomyocytes (Control), cardiomyocytes under hypoxia condition (Hypoxia), L-C-miR transduced cardiomyocytes under hypoxia condition (L-C-miR + hypoxia), and L-miR322-Inhibitor transduced cardiomyocytes under hypoxia condition (L-miR322-Inhibitor + hypoxia) (*P < 0.05, ∆P > 0.05).
To verify whether BDNF is indeed directly regulated by miR-322, we carried out a dual-luciferase reporter assay. In one group of HEK293T cells, we co-transfected cells with mmu-miR-322 mimics, and a luciferase reporter expressing 3’-UTR of BDNF gene, which includes the putative binding site of mmu-miR-322. In second group of HEK293T cells, we co-transfected cells with mmu-miR-322 mimics and another luciferase reporter to express the mutated (m) 3’-UTR of BDNF gene with suppressed mmu-miR-322 binding site. The 3rd group of HEK293T cells was co-transfected with mmu-miR-322 mimics and an empty luciferase reporter. 48 h after con-transfection, fluorescent reading showed that relative luciferase activity in HEK293T cells transfected with BDNF 3’-UTR was significantly lower than in the luciferase activities in cells transfected with (m) BDNF 3’-UTR or Control luciferase reporter (Figure 3B, *P < 0.05, ∆P > 0.05).
We then investigated the regulation of miR-322 on gene expression levels of BDNF in cardiomyocytes under hypoxia condition. We found that in normal cardiomyocytes, BDNF was significantly downregulated by hypoxia (Figure 3C, Control vs. Hypoxia, *P < 0.05). The effects of hypoxia on downregulating BDNF were similar between normal cardiomyocytes and cardiomyocytes transduced with L-C-MiR (Figure 3C, Hypoxia vs. L-C-MiR + Hypoxia, ∆P > 0.05). We also found that, downregulation of miR-322 elevated BDNF gene level in cardiomyocytes while cells were under hypoxia condition (Figure 3C, L-C-MiR + Hypoxia vs. L-miR322-Inhibitor + Hypoxia, *P < 0.05).
BDNF downregulation reduced the protective effect of miR-322 on cardiomyocyte apoptosis under hypoxia condition
As we demonstrated BDNF gene was directly associated with miR-322 regulation on cardiomyocyte apoptosis, we wondered whether BDNF have functional role in it. To examine this hypothesis, we firstly transfected cardiomyocytes with BDNF specific siRNA (siRNA-BDNF) to knockdown BDNF gene. In control experiments, cardiomyocytes were transfected with a negative control scrambled siRNA (siRNA-C). 24 h after transfection, the downregulation of BDNF gene in cardiomyocyte was verified by qRT-PCR assay (Figure 4A, *P < 0.05).
Figure 4.
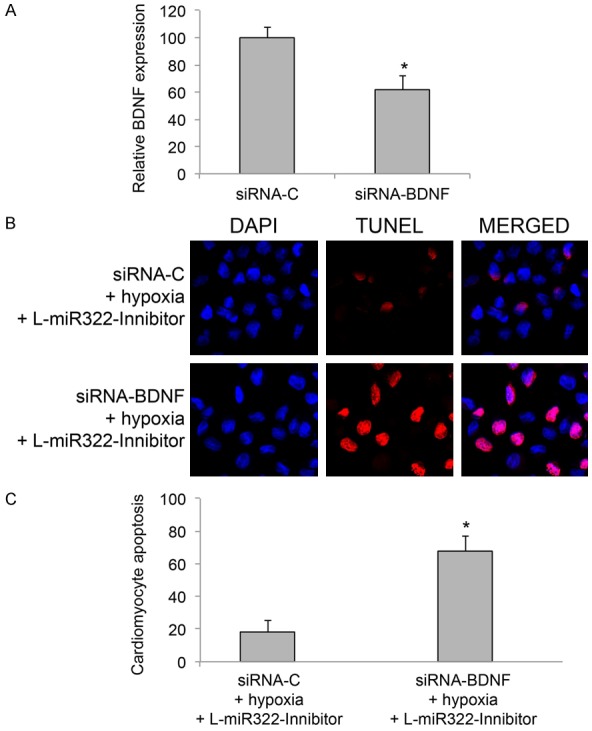
BDNF knockdown reversed the protection of miR-322 on cardiomyocyte apoptosis under hypoxia condition. A. Cardiomyocytes were transfected with murine BDNF specific siRNA (siRNA-BDNF) for 24 h. The control cardiomyocytes were transfected with a negative control scrambled siRNA (siRNA-C). 24 h after transfection, relative expression levels of BDNF were compared by qRT-PCR between two groups of cardiomyocytes (*P < 0.05). B. In cardiomyocytes with stable miR-322 downregulation (transduced with lentivirus of L-miR322-Inhibitor), cells were transfected with siRNA-BDNF or siRNA-C for 24 h, followed by hypoxia treatment. 24 h after hypoxia condition, a TUNEL assay was conducted and immune-fluorescent images were shown for cardiomyocyte apoptosis. C. The percentages of apoptotic cardiomyocytes under hypoxia condition were compared between cells transfected with siRNA-C and cells transfected with siRNA-BDNF (*P < 0.05).
We then applied siRNA transfection in cardiomyocytes whose miR-322 expression levels were downregulated by Lentiviral transduction of L-miR-322-Inhibitor. 24 h after transfection, cardiomyocytes were treated with hypoxia. Another 24 h later, a TUNEL assay was carried out to examine apoptosis in cardiomyocytes. Interestingly, results of immunostaining demonstrated that, much more apoptotic cardiomyocytes were observed while BDNF was downregulation (Figure 4B). Statistical analysis confirmed this observation, showing that BDNF downregulation significantly reduced the protection of miR-322 downregulation on cardiomyocyte apoptosis under hypoxia condition (Figure 4C, *P < 0.05). Therefore, our data strongly suggest the regulation of miR-322 on hypoxia-induced cardiomyocyte apoptosis was directly through BDNF gene.
Discussion
It has been demonstrated in the literatures that human miR-424, or murine ortholog miR-322, was upregulated in rat vascular smooth muscle cells upon pathological proliferation, or in human endothelial cells under ischemic injury [14,15]. Interestingly, the underlying mechanisms of miR-424/322 upregulation in various heart diseases could be drastically different. In Merlet’s report, miR-424/322 was proposed as a negative modulator in smooth muscle cells, that upregulation of miR-424/322 suppressing the pathological proliferation of cells [14]. However, in Ghosh’s report, miR-424/322 was positively associated with ischemia injury in human endothelial cells, that upregulation of miR-424 directly promoted angiogenesis [15]. In another recent report, Zeng and colleagues demonstrated that miR-322 was significantly upregulated in hypoxia-induced pulmonary arterial smooth muscle cells through the regulation of BMP-Smad signaling pathway, thus positively associated with hypoxic pulmonary hypertension [22]. In the present study, we demonstrated in murine cardiomyocyte, miR-322 was significantly upregulated during the process of hypoxia-induced apoptosis. Most importantly, we showed that inhibition of miR-322 protected hypoxic apoptosis in cardiomyocytes. Therefore, the results of our study favor the positive regulator role of miR-322 in cardiomyocyte apoptosis under hypoxia condition.
We also explored the association of miR-322 and BDNF gene in regulating hypoxia-induced cardiomyocyte apoptosis. Through dual-luciferase activity assay, we demonstrated that miR-322 was directly paired with the 3’-UTR of BDNF. Through qRT-PCR assay, we showed that gene expression of BDNF in cardiomyocyte was actively modulated by miR-322 upregulation under hypoxia condition. Furthermore, our functional approach revealed that, downregulation of BDNF reduced the protective effect of miR-322 downregulation on cardiomyocyte apoptosis under hypoxia condition, thus suggesting a protective role of BDNF in cardiomyocyte apoptosis under hypoxia condition. It’s worth noting that, BDNF gene has been closely associated with various hypoxic conditionings in heart. In a rodent model, Hang and colleagues showed that BDNF protected cardiomyocyte from apoptosis in myocardial ischemia through BCL-2 and transient receptor potential canonical 3/6 channels [18]. Interestingly, in another in vivo rodent model, Fulgenzi and colleagues demonstrated an appositive effect of BDNF, showing it could be a facilitator for myocardial ischemia in aged rats [17]. It seems like aging could be the obvious explanation for those two contrary reports on BDNF function in myocardial ischemia, namely protection vs. facilitation. However, further studies on the associated pathways on BDNF regulation in ischemic or hypoxic conditions, especially those correlated with miRNA regulations, are much needed to elucidate the complex molecular network underlying hypoxic apoptosis in cardiomyocytes.
In conclusion, our study identified that miR-322, by negatively modulating BDNF gene, played a critical role in regulating cardiomyocyte apoptosis under hypoxia condition. These results may broaden our knowledge of miRNA regulation in myocardial ischemia.
Acknowledgements
The manuscript was approved by Heilongjiang Province Natural Science Fund Project (Grant No. D201220).
References
- 1.Mack M, Gopal A. Epidemiology, traditional and novel risk factors in coronary artery disease. Cardiol Clin. 2014;32:323–332. doi: 10.1016/j.ccl.2014.04.003. [DOI] [PubMed] [Google Scholar]
- 2.Hanson MA, Fareed MT, Argenio SL, Agunwamba AO, Hanson TR. Coronary artery disease. Prim Care. 2013;40:1–16. doi: 10.1016/j.pop.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 3.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, de Ferranti S, Despres JP, Fullerton HJ, Howard VJ, Huffman MD, Judd SE, Kissela BM, Lackland DT, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Matchar DB, McGuire DK, Mohler ER 3rd, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Willey JZ, Woo D, Yeh RW, Turner MB. American Heart Association Statistics C and Stroke Statistics S. Heart disease and stroke statistics--2015 update: a report from the American Heart Association. Circulation. 2015;131:e29–322. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 4.Penna C, Mancardi D, Rastaldo R, Pagliaro P. Cardioprotection: a radical view Free radicals in pre and postconditioning. Biochim Biophys Acta. 2009;1787:781–793. doi: 10.1016/j.bbabio.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 5.Venardos KM, Perkins A, Headrick J, Kaye DM. Myocardial ischemia-reperfusion injury, antioxidant enzyme systems, and selenium: a review. Curr Med Chem. 2007;14:1539–1549. doi: 10.2174/092986707780831078. [DOI] [PubMed] [Google Scholar]
- 6.Kang PM, Haunstetter A, Aoki H, Usheva A, Izumo S. Morphological and molecular characterization of adult cardiomyocyte apoptosis during hypoxia and reoxygenation. Circ Res. 2000;87:118–125. doi: 10.1161/01.res.87.2.118. [DOI] [PubMed] [Google Scholar]
- 7.Elsasser A, Suzuki K, Lorenz-Meyer S, Bode C, Schaper J. The role of apoptosis in myocardial ischemia: a critical appraisal. Basic Res Cardiol. 2001;96:219–226. doi: 10.1007/s003950170052. [DOI] [PubMed] [Google Scholar]
- 8.Rodriguez M, Lucchesi BR, Schaper J. Apoptosis in myocardial infarction. Ann Med. 2002;34:470–479. doi: 10.1080/078538902321012414. [DOI] [PubMed] [Google Scholar]
- 9.Carrington JC, Ambros V. Role of microRNAs in plant and animal development. Science. 2003;301:336–338. doi: 10.1126/science.1085242. [DOI] [PubMed] [Google Scholar]
- 10.Wienholds E, Plasterk RH. MicroRNA function in animal development. FEBS Lett. 2005;579:5911–5922. doi: 10.1016/j.febslet.2005.07.070. [DOI] [PubMed] [Google Scholar]
- 11.Yu S, Li G. MicroRNA expression and function in cardiac ischemic injury. J Cardiovasc Transl Res. 2010;3:241–245. doi: 10.1007/s12265-010-9168-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sala V, Bergerone S, Gatti S, Gallo S, Ponzetto A, Ponzetto C, Crepaldi T. MicroRNAs in myocardial ischemia: identifying new targets and tools for treating heart disease. New frontiers for miR-medicine. Cell Mol Life Sci. 2014;71:1439–1452. doi: 10.1007/s00018-013-1504-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frost RJ, van Rooij E. miRNAs as therapeutic targets in ischemic heart disease. J Cardiovasc Transl Res. 2010;3:280–289. doi: 10.1007/s12265-010-9173-y. [DOI] [PubMed] [Google Scholar]
- 14.Merlet E, Atassi F, Motiani RK, Mougenot N, Jacquet A, Nadaud S, Capiod T, Trebak M, Lompre AM, Marchand A. miR-424/322 regulates vascular smooth muscle cell phenotype and neointimal formation in the rat. Cardiovasc Res. 2013;98:458–468. doi: 10.1093/cvr/cvt045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ghosh G, Subramanian IV, Adhikari N, Zhang X, Joshi HP, Basi D, Chandrashekhar YS, Hall JL, Roy S, Zeng Y, Ramakrishnan S. Hypoxia-induced microRNA-424 expression in human endothelial cells regulates HIF-alpha isoforms and promotes angiogenesis. J Clin Invest. 2010;120:4141–4154. doi: 10.1172/JCI42980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fulgenzi G, Tomassoni-Ardori F, Babini L, Becker J, Barrick C, Puverel S, Tessarollo L. BDNF modulates heart contraction force and long-term homeostasis through truncated TrkB. T1 receptor activation. J Cell Biol. 2015;210:1003–12. doi: 10.1083/jcb.201502100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cai D, Holm JM, Duignan IJ, Zheng J, Xaymardan M, Chin A, Ballard VL, Bella JN, Edelberg JM. BDNF-mediated enhancement of inflammation and injury in the aging heart. Physiol Genomics. 2006;24:191–197. doi: 10.1152/physiolgenomics.00165.2005. [DOI] [PubMed] [Google Scholar]
- 18.Hang P, Zhao J, Cai B, Tian S, Huang W, Guo J, Sun C, Li Y, Du Z. Brain-derived neurotrophic factor regulates TRPC3/6 channels and protects against myocardial infarction in rodents. Int J Biol Sci. 2015;11:536–545. doi: 10.7150/ijbs.10754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Katare RG, Kakinuma Y, Arikawa M, Yamasaki F, Sato T. Chronic intermittent fasting improves the survival following large myocardial ischemia by activation of BDNF/VEGF/PI3K signaling pathway. J Mol Cell Cardiol. 2009;46:405–412. doi: 10.1016/j.yjmcc.2008.10.027. [DOI] [PubMed] [Google Scholar]
- 20.Sreejit P, Kumar S, Verma RS. An improved protocol for primary culture of cardiomyocyte from neonatal mice. In Vitro Cell Dev Biol Anim. 2008;44:45–50. doi: 10.1007/s11626-007-9079-4. [DOI] [PubMed] [Google Scholar]
- 21.Yue R, Hu H, Yiu KH, Luo T, Zhou Z, Xu L, Zhang S, Li K, Yu Z. Lycopene protects against hypoxia/reoxygenation-induced apoptosis by preventing mitochondrial dysfunction in primary neonatal mouse cardiomyocytes. PLoS One. 2012;7:e50778. doi: 10.1371/journal.pone.0050778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zeng Y, Liu H, Kang K, Wang Z, Hui G, Zhang X, Zhong J, Peng W, Ramchandran R, Raj JU, Gou D. Hypoxia inducible factor-1 mediates expression of miR-322: potential role in proliferation and migration of pulmonary arterial smooth muscle cells. Sci Rep. 2015;5:12098. doi: 10.1038/srep12098. [DOI] [PMC free article] [PubMed] [Google Scholar]


