Abstract
Increasing evidence has suggested that discoidin domain receptor 2 (DDR2) plays an important role in cancer development and metastasis. However, the correlation between DDR2 expression and clinical outcome in ovarian cancer has not been investigated. In this study, DDR2 expression was examined by Real-time PCR in surgically resected ovarian cancer and normal ovary tissues. Besides, DDR2 expression was analyzed immunohistochemically in 103 ovarian cancer patients, and the correlation between DDR2 expression with clinicopathologic factors was analyzed. The result showed that DDR2 mRNA expression was upregulated in ovarian cancer tissues compared with normal ovary tissues. Statistical analysis revealed that DDR2 expression correlated with tumor stage (P = 0.008) and peritoneal metastasis (P = 0.009). Patients with high DDR2 expression showed poorer 5-year overall survival (P = 0.005), and DDR2 remained an independent prognostic marker for OS (P = 0.013) in multivariate analysis. Our results suggest that DDR2 might be closely associated with ovarian cancer progression and metastasis. Its high expression may serve as a potential prognostic biomarker in human ovarian cancer.
Keywords: DDR2, ovarian cancer, prognosis, metastasis
Introduction
Despite a decrease in deaths in recent decades, ovarian cancer is still one of the most leading cause of cancer-related death worldwide, with more than 225,000 new cases and nearly 142,000 deaths annually [1]. For patients with advanced stages with metastatic lesions, the clinical outcome remains rather poor, with a 5-year survival rate of <30% [2]. As clinicians move towards personalized cancer medicine, it is therefore crucial to understand the molecular mechanisms involved in the tumor progression, not only to predict ovarian cancer outcome, but also to select subset of population for potential targeted therapeutic strategies before tumor progression.
Discoidin domain receptor 2 (DDR2) belongs to the family of receptor tyrosine kinases (RTKs) that binds to and is activated by collagen in the extracellular matrix [3]. RTKs are important for the communication of cells with their microenvironment and are involved in the regulation of cell growth, differentiation and metabolism [4]. The DDR2 gene is located on chromosome 1q23.3, and the DDR2 protein is expressed in epithelial cells, particularly in the kidney, lung, gastrointestinal tract, and brain [5]. Using an exon sequencing assay, DDR2 was first identified as a potential oncogenic target in lung squamous cell cancer (SCC). The authors reported a 3.8% incidence of DDR2 point mutations in lung SCC samples [6]. Depletion of mutant DDR2 using RNA interference in lung SCC cells demonstrated oncogene addiction. In addition, DDR2 has been implicated to exhibits crosstalk with other cell surface receptors such as the integrins and RTKs resulting in diversification of downstream signal transduction networks [7]. Moreover, DDR2 has been shown to function as an adhesion receptor, which is activated by collagen, although there is limited information available on the signaling pathways activated by DDR2 upon collagen engagement. Rather, Payne et al. showed that these signaling events are independent of integrin activation by collagen and are specific to the DDR2 pathway [8]. Recently, DDR2 has been implicated in a number of cancer types to play a role in driving proliferation and metastasis. However, the biological roles of DDR2 in human ovarian cancer remain unknown.
In the present study, we first detected DDR2 expression in primary ovarian carcinoma using real-time PCR and immunohistochemistry. The aim was to evaluate the association between DDR2 overexpression and clinical pathological factors and analyze its impact on clinical survival. Then the association of DDR2 overexpression and the clinicopathological significance was investigated to clarify the role of DDR2 in ovarian cancer development and metastasis.
Materials and methods
Patients and tissue specimens
A total of 103 ovarian cancer patients who underwent surgical resection from January 2006 to February 2010 at Guangzhou Women and Children’s Medical Center were analyzed. The records of patients were reviewed in the context of clinicopathological and follow-up information. The stage of ovarian cancer was classified according to the latest International Federation of Gynecology and Obstetrics criteria [9]. The overall survival time and recurrence-free survival time was calculated starting from the date of the initial surgery to the time of death or recurrence, counting death from any cause as the end point or the last date of follow-up as the end point, if no event was documented. None of the patients received preoperative chemotherapy or radiation therapy. After surgery, resected specimens were processed routinely for macroscopic pathological assessment. Prior informed consent was obtained from each patient and this study was approved by the Research Ethics Committee of Guangzhou Medical University.
Real-time polymerase chain reaction (PCR)
The expression levels of DDR2 in ovarian cancer and normal ovary tissues were determined by Real time RT-PCR. A total of 2 ug of tissue RNA was reverse transcribed to cDNA with a high-capacity cDNA reverse transcription kit (Applied Biosystems) according to the manufacturer’s instructions. For the quantitative analysis of specific mRNA expression, CT values were calculated by 7500 SDS software, and each PCR reaction was repeated at least three times. The primers used are as follows: DDR2: 5’-CTCCCAGAAT TTGCTCCAG-3’ (sense), 5’-GCCACATCTT TTCCTGAGA-3’ (antisense); β-actin, 5’-AATCTGGCAC CACACCTTCT ACAA-3’ (sense) and 5’-TAGCACAGCC TGGATAGCAA CG-3’ (antisense). The expression ratio of DDR2 to β-actin in every sample was considered as the relative mRNA level.
Immunohistochemistry
Immunohistochemical staining was performed to assess the protein expression of DDR2 in ovarian cancer specimens. Formalin-fixed tumor tissues were embedded in paraffin, and serial sections (4 μm) were dewaxed in toluene, rehydrated in an alcohol gradient, permeabilized in citrate buffer (pH 6.0), quenched with 3% H2O2 for 5 min to eliminate endogenous peroxidase activity, washed in PBS, incubated overnight at 4°C with monoclonal rabbit antibody against DDR2 (Abcam, Cambridge, USA; 1:200). Then the tissue sections were incubated with biotinylated goat anti-rabbit IgG antibody for 15 min. After washing, sections were incubated with streptavidin-peroxidase, lightly counterstained with hematoxylin, and observed under a photomicroscope. Immunostaining was separately reviewed and scored by two independent pathologists who were blinded as to the patients. All tissue samples were assessed in a consecutive analysis to ensure maximal internal consistency. The analysis was assessed according to both the proportion and intensity of positively stained cancer cells. The extensional standards taken were as follows: (1) number of positive stained cells ≤5%, scored 0; 6-25%, scored 1; 25-50%, scored 2; 51-75%, scored 3; and >75%, scored 4; and (2) intensity of stain: colorless, scored 0; pallideflavens, scored 1; yellow scored 2; and brown, scored 3. The extensional standards (1) and (2) were multiplied, and the staining grade was stratified as absent (0 score), weak (1-4 score), moderate (5-8 score) or strong (9-12 score). Intensity and fraction of positive cell scores were multiplied and thus the scoring system was defined as low expression for scores of 0-3, and as high expression for scores of 4-12.
Statistical analysis
SPSS 16.0 software was used to perform statistical analyses. Statistical comparisons of the clinicopathological characteristics were performed by the χ2 test. Differences in mRNA levels between groups and the association between clinicopathological factors were analyzed by the Student t test. The data were expressed as mean ± standard deviation (SD). Overall survival and recurrence-free survival curves were obtained by the Kaplan-Meier method and were compared with the log-rank test. Multivariate analyses were performed by Cox proportional hazard models. P<0.05 was considered statistically significant.
Results
DDR2 is highly expressed in ovarian cancer
In order to explore the role of DDR2 in ovarian cancer, we selected 10 freshly frozen ovarian cancer tissues and 10 normal ovary tissues randomly to detect the expression level of DDR2 using real-time quantitative PCR (qPCR) analysis. It was found that the expression level of DDR2 in cancer tissues was higher than in normal ovary tissues (Figure 1).
Figure 1.
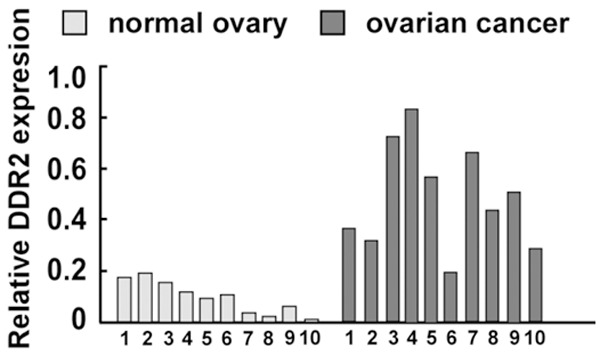
Expression of DDR2 mRNA in 10 freshly frozen ovarian cancer tissues and 10 normal ovary tissues through real-time PCR. Bars represent the means of three independent experiments; *P<0.05.
Association of DDR2 expression and clinicopathological factors
DDR2 expression in 103 primary ovarian tumor specimens was determined by immunohistochemistry. As shown in Figure 2, DDR2 protein mainly localized at cytomembrane systems. Statistical analysis further showed positive associations of DDR2 expression with tumor stage (P = 0.008) and peritoneal metastasis (P = 0.009). However, DDR2 expression showed no significant relation with age, tumor size, histological type, or differentiation (Table 1).
Figure 2.
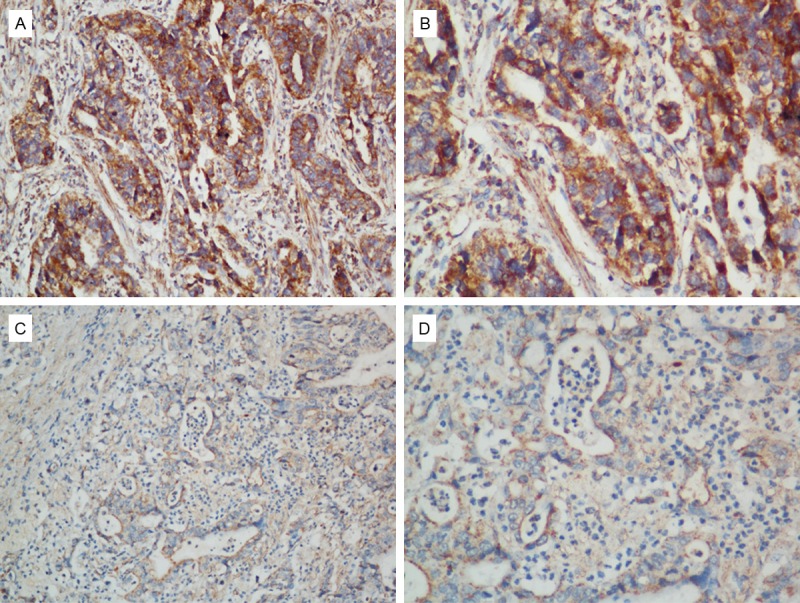
Representative images of DDR2 from immunohistochemistry assays in ovarian cancer specimens (high expression for A and B; low expression for C and D) (200x for A and C, 400x for B and D).
Table 1.
Correlation between DDR2 expression and clinicopathologic characteristics of in 103 ovarian cancer patients
| Variables | n | DDR2 expression | P | ||
|---|---|---|---|---|---|
|
| |||||
| High | Low | ||||
| Age (y) | ≤55 | 36 | 20 | 16 | 0.392 |
| >55 | 67 | 43 | 24 | ||
| FIGO stage | I-II | 42 | 16 | 16 | 0.008 |
| III-IV | 61 | 47 | 14 | ||
| Grade | 1/2 | 70 | 42 | 28 | 0.724 |
| 3 | 33 | 21 | 12 | ||
| Tumor size | ≤5 cm | 55 | 33 | 22 | 0.352 |
| >5 cm | 57 | 39 | 18 | ||
| Histological type | Serous | 67 | 42 | 25 | 0.677 |
| Others | 36 | 21 | 15 | ||
| Peritoneal metastasis | No | 45 | 21 | 24 | 0.009 |
| Yes | 58 | 42 | 16 | ||
| LN metastasis | No | 83 | 47 | 36 | 0.054 |
| Yes | 20 | 16 | 4 | ||
DDR2 expression predicted worse overcome in ovarian cancer
The 5-year disease-specific survival rate was 50.0% in the whole population. In a univariate analysis, DDR2 expression was associated with clinical outcome. Patients with a high level of DDR2 exhibited a lower 5-year overall survival rate than patients with a low level of DDR2 (P = 0.005 Figure 3). A multivariate analysis showed that DDR2 expression was an independent prognostic marker with regard to overall survival (HR 3.463, P = 0.013, Table 2).
Figure 3.
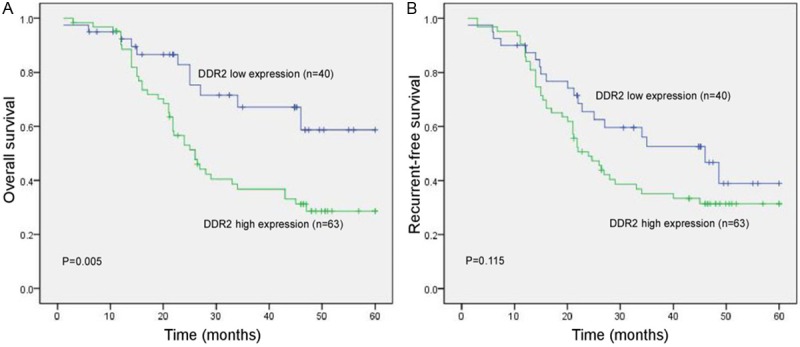
Kaplan-Meier survival analysis stratified according to DDR2 expression in ovarian cancer patients. (A) DDR2 expression and patients’ overall survival and (B) patients’ recurrent-free survival.
Table 2.
Multivariate Cox regression analysis of survival in all population
| Prognostic variables | OS | RFS | ||
|---|---|---|---|---|
|
|
|
|||
| HR (95% CI) | P | HR (95% CI) | P | |
| Age (>55 vs ≤55) | 1.843 (0.084-4.548) | 0.674 | 1.407 (0.296-5.315) | 0.709 |
| FIGO Stage (III-IV vs I-II) | 2.535 (0.897-6.593) | 0.063 | 2.776 (0.526-7.621) | 0.345 |
| Grade (3 vs 1/2) | 1.075 (0.037-4.893) | 0.529 | 1.058 (0.171-4.613) | 0.442 |
| Tumor size (>5 cm vs ≤5 cm) | 2.274 (1.042-5.457) | 0.075 | 2.638 (1.077-6.001) | 0.219 |
| Histological type (Serous vs others) | 1.347 (0.319-2.727) | 0.739 | 1.265 (0.358-3.473) | 0.527 |
| Peritoneal metastasis (+ vs -) | 2.949 (0.071-7.705) | 0.065 | 2.504 (0.662-7.833) | 0.156 |
| LN metastasis (+ vs -) | 3.395 (0.115-13.362) | 0.014 | 3.463 (0.230-10.931) | 0.028 |
| DDR2 expression (high vs low) | 3.463 (0.375-7.602) | 0.013 | 3. 405 (0.181-9.905) | 0.148 |
Discussion
Although there is limited information on the role of DDR2 and its molecular targets, it has been suggested to be crucial in driving cell proliferation. The present study investigated that DDR2 is up-regulated in ovarian cancer tissues in comparison with that in normal ovarian surface tissues. The expression level of DDR2 was significantly associated with tumor stage and peritoneal metastasis of ovarian cancer patients. Moreover, we found that high DDR2 expression was a significant indicator of poor clinical prognosis for patients with ovarian cancer. Together with our results, DDR2 represent a novel predicative marker for the prognosis of ovarian cancer patients.
DDR2 receptor belongs to a DDR family that shows tyrosine kinase activity. It is highly expressed in mesenchymal cells and is important for a variety of developmental processes, in particular bone and cartilage formation [10]. It also contributes to disease progression, including hepatic fibrosis, osteoarthritis and cancer [11]. In recent years, DDR2 has been identified in several human cancers and cancer genome sequencing efforts have showed a series of oncogenic DDR2 point mutations that occur at low frequency in lung squamous cell carcinoma [12]. Moreover, DDR2 has also been identified as a potential therapeutic target of kinase inhibitors such as dastinib [13], suggesting the potential use of DDR2 in drug resistance that may arise in dasatinib trials. However, there is some controversy regarding the role of DDR2 in cancer. While Hammerman et al. demonstrated that a subset of the DDR2 mutants are oncogenic [6], Wall et al. showed that the process of fibrillar collagen inhibited cancer cell growth is through a DDR2-dependent cell cycle arrest in melanoma and fibrosarcoma cells, suggesting that DDR2 functions in a context-dependent manner and may act as a tumor suppressor in the presence of its natural ligand collagen [14]. Zhao et al. previously found that DDR2 expression is upregulated in ovarian cancer cells, and that DDR2 expression is reversely correlated with NDRG1 expression, which is an important gene regulating tumor cell adhesion, migration and invasion activities without affecting cell proliferation [15]. In this report, DDR2 expression is significantly upregulated in ovarian cancer tissues compared with in normal ovary, implying that DDR2 may function as an oncogene in ovarian cancer.
In addition a direct role in regulating tumor cell growth, literature also exists for the role of DDR2 in supporting tumor metastasis. Ren et al. reported that DDR2 plays an indispensable role in a series of hypoxia-induced behaviors of breast cancer cells, including migration, invasion, and epithelial-mesenchymal transition (EMT) [16]. Xu et al. found that DDR2 overexpression in head and neck squamous cell carcinoma cells failed to alter cell proliferation but markedly accelerates cell invasion and migration as well as hypoxia-induced EMT, and that elevated expression of DDR2 speeds up the metastasis of cancer cells to the lung [17]. Xie revealed that DDR2 facilitates hepatocellular carcinoma cell invasion, migration and EMT via activating ERK2 and stabilizing SNAIL1 [18]. In the present study, we showed that the DDR2 expression was significantly associated with tumor stage and peritoneal metastasis of ovarian cancer patients. Thus, our present results are consistent with the previous findings in that DDR2 may play an important role in promoting cancer migration and progression.
In this study, we assessed DDR2 protein expression in 103 ovarian cancer tissues by using immunohistochemistry. We showed that DDR2 expression was significantly associated with clinical outcome; patients with DDR2-low tumors had substantially longer OS than did patients with DDR2-high tumors. Furthermore, multivariate analysis showed that high DDR2 expression was an independent marker for OS in the ovarian cancer population. The present research provided the first evidence that increased DDR2 expression in primary human ovarian cancer might be a powerful, independent predictor of clinical prognosis.
In conclusion, our study showed that DDR2 was identified as one of the overexpressed molecules in ovarian cancer. DDR2 might be closely associated with ovarian cancer progression and metastasis, and its strong expression had a negative impact on the prognosis of ovarian cancer patients. This study suggests that DDR2 may be considered as a prognostic biomarker and may serve as a novel therapeutic target in ovarian cancer.
Disclosure of conflict of interest
None.
References
- 1.Knutson KL, Karyampudi L, Lamichhane P, Preston C. Targeted immune therapy of ovarian cancer. Cancer Metastasis Rev. 2015;34:53–74. doi: 10.1007/s10555-014-9540-2. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 2.Yeung TL, Leung CS, Yip KP, Au Yeung CL, Wong ST, Mok SC. Cellular and molecular processes in ovarian cancer metastasis. A Review in the Theme: Cell and Molecular Processes in Cancer Metastasis. Am J Physiol Cell Physiol. 2015;309:C444–456. doi: 10.1152/ajpcell.00188.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leitinger B. Discoidin domain receptor functions in physiological and pathological conditions. Int Rev Cell Mol Biol. 2014;310:39–87. doi: 10.1016/B978-0-12-800180-6.00002-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fu HL, Valiathan RR, Arkwright R, Sohail A, Mihai C, Kumarasiri M, Mahasenan KV, Mobashery S, Huang P, Agarwal G, Fridman R. Discoidin domain receptors: unique receptor tyrosine kinases in collagen-mediated signaling. J Biol Chem. 2013;288:7430–7437. doi: 10.1074/jbc.R112.444158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oxnard GR, Binder A, Janne PA. New targetable oncogenes in non-small-cell lung cancer. J. Clin. Oncol. 2013;31:1097–1104. doi: 10.1200/JCO.2012.42.9829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hammerman PS, Sos ML, Ramos AH, Xu C, Dutt A, Zhou W, Brace LE, Woods BA, Lin W, Zhang J, Deng X, Lim SM, Heynck S, Peifer M, Simard JR, Lawrence MS, Onofrio RC, Salvesen HB, Seidel D, Zander T, Heuckmann JM, Soltermann A, Moch H, Koker M, Leenders F, Gabler F, Querings S, Ansen S, Brambilla E, Brambilla C, Lorimier P, Brustugun OT, Helland A, Petersen I, Clement JH, Groen H, Timens W, Sietsma H, Stoelben E, Wolf J, Beer DG, Tsao MS, Hanna M, Hatton C, Eck MJ, Janne PA, Johnson BE, Winckler W, Greulich H, Bass AJ, Cho J, Rauh D, Gray NS, Wong KK, Haura EB, Thomas RK, Meyerson M. Mutations in the DDR2 kinase gene identify a novel therapeutic target in squamous cell lung cancer. Cancer Discov. 2011;1:78–89. doi: 10.1158/2159-8274.CD-11-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iwai LK, Chang F, Huang PH. Phosphoproteomic analysis identifies insulin enhancement of discoidin domain receptor 2 phosphorylation. Cell Adh Migr. 2013;7:161–164. doi: 10.4161/cam.22572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Payne LS, Huang PH. Discoidin domain receptor 2 signaling networks and therapy in lung cancer. J Thorac Oncol. 2014;9:900–904. doi: 10.1097/JTO.0000000000000164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pecorelli S. Revised FIGO staging for carcinoma of the vulva, cervix, and endometrium. Int J Gynaecol Obstet. 2009;105:103–104. doi: 10.1016/j.ijgo.2009.02.012. [DOI] [PubMed] [Google Scholar]
- 10.Iwai LK, Payne LS, Luczynski MT, Chang F, Xu H, Clinton RW, Paul A, Esposito EA, Gridley S, Leitinger B, Naegle KM, Huang PH. Phosphoproteomics of collagen receptor networks reveals SHP-2 phosphorylation downstream of wild-type DDR2 and its lung cancer mutants. Biochem J. 2013;454:501–513. doi: 10.1042/BJ20121750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nicos M, Powrozek T, Krawczyk P, Jarosz B, Pajak B, Sawicki M, Kucharczyk K, Trojanowski T, Milanowski J. Sensitive methods for detection of the S768R substitution in exon 18 of the DDR2 gene in patients with central nervous system metastases of non-small cell lung cancer. Med Oncol. 2014;31:176. doi: 10.1007/s12032-014-0176-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ohashi K, Pao W. A new target for therapy in squamous cell carcinoma of the lung. Cancer Discov. 2011;1:23–24. doi: 10.1158/2159-8274.CD-11-0069. [DOI] [PubMed] [Google Scholar]
- 13.Day E, Waters B, Spiegel K, Alnadaf T, Manley PW, Buchdunger E, Walker C, Jarai G. Inhibition of collagen-induced discoidin domain receptor 1 and 2 activation by imatinib, nilotinib and dasatinib. Eur J Pharmacol. 2008;599:44–53. doi: 10.1016/j.ejphar.2008.10.014. [DOI] [PubMed] [Google Scholar]
- 14.Wall SJ, Werner E, Werb Z, DeClerck YA. Discoidin domain receptor 2 mediates tumor cell cycle arrest induced by fibrillar collagen. J Biol Chem. 2005;280:40187–40194. doi: 10.1074/jbc.M508226200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhao G, Chen J, Deng Y, Gao F, Zhu J, Feng Z, Lv X, Zhao Z. Identification of NDRG1-regulated genes associated with invasive potential in cervical and ovarian cancer cells. Biochem Biophys Res Commun. 2011;408:154–159. doi: 10.1016/j.bbrc.2011.03.140. [DOI] [PubMed] [Google Scholar]
- 16.Ren T, Zhang W, Liu X, Zhao H, Zhang J, Zhang J, Li X, Zhang Y, Bu X, Shi M, Yao L, Su J. Discoidin domain receptor 2 (DDR2) promotes breast cancer cell metastasis and the mechanism implicates epithelial-mesenchymal transition programme under hypoxia. J Pathol. 2014;234:526–537. doi: 10.1002/path.4415. [DOI] [PubMed] [Google Scholar]
- 17.Xu J, Lu W, Zhang S, Zhu C, Ren T, Zhu T, Zhao H, Liu Y, Su J. Overexpression of DDR2 contributes to cell invasion and migration in head and neck squamous cell carcinoma. Cancer Biol Ther. 2014;15:612–622. doi: 10.4161/cbt.28181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xie B, Lin W, Ye J, Wang X, Zhang B, Xiong S, Li H, Tan G. DDR2 facilitates hepatocellular carcinoma invasion and metastasis via activating ERK signaling and stabilizing SNAIL1. J Exp Clin Cancer Res. 2015;34:101. doi: 10.1186/s13046-015-0218-6. [DOI] [PMC free article] [PubMed] [Google Scholar]


