Abstract
Diabetes can be simply classified into type 1 diabetes mellitus and type 2 diabetes mellitus. Zinc transporter 8 (ZnT8), a novel islet autoantigen, is specifically expressed in insulin‐containing secretory granules of β‐cells. Genetic studies show that the genotypes of SLC30A8 can determine either protective or diabetogenic response depending on environmental and lifestyle factors. The ZnT8 protein expression, as well as zinc content in β‐cells, was decreased in diabetic mice. Thus, ZnT8 might participate in insulin biosynthesis and release, and subsequently involved deteriorated β‐cell function through direct or indirect mechanisms in type 1 diabetes mellitus and type 2 diabetes mellitus. From a clinical feature standpoint, the prevalence of ZnT8A is gradiently increased in type 2 diabetes mellitus, latent autoimmune diabetes in adults and type 1 diabetes mellitus. The frequency and epitopes of ZnT8‐specific T cells and cytokine release by ZnT8‐specific T cells are also different in diabetic patients and healthy controls. Additionally, the response to ZnT8 administration is also different in type 1 diabetes mellitus and type 2 diabetes mellitus. In the present review, we summarize the literature about clinical aspects of ZnT8 in the pathogenesis of diabetes, and suggest that ZnT8 might play a different role between type 1 diabetes mellitus and type 2 diabetes mellitus.
Keywords: Type 1 diabetes mellitus, Type 2 diabetes mellitus, Zinc transporter 8
Introduction
Diabetes, one of the most challenging heterogeneous diseases, can be simply classified into type 1 diabetes mellitus and type 2 diabetes mellitus1. Historically, the distinction between type 1 diabetes mellitus and type 2 diabetes mellitus has mostly depended on the clinical presentation, such as age at disease onset, the presence of ketosis and the dependence of insulin. Type 1 diabetes mellitus, appearing mainly in childhood or young adulthood, is characterized by T cells‐mediated autoimmune destruction of β‐cells, absolute insulin dependence and the need for insulin treatment. Type 2 diabetes mellitus, mainly appearing in adulthood, is the result of insulin resistance and relative insulin deficiency.
However, in clinical practice, some diabetic patients present features of both type 1 diabetes mellitus and type 2 diabetes mellitus, such as patients with latent autoimmune diabetes in adults (LADA)2. This subtype of diabetes is often positive for autoantibodies against islet autoantigens (AAbs) including insulin, glutamic acid decarboxylase 65 (GAD65), tyrosine phosphatase‐related molecules‐2 and the zinc transporter‐8 (ZnT8), which indicates that LADA is an autoimmune disease2. Among these autoantibodies, positivity of glutamic acid decarboxylase antibodies (GADA) is one of three criteria, which can distinguish LADA from phenotypic type 2 diabetes mellitus1. However, LADA patients, especially patients with low‐titer GADA, shared similar metabolic features with that of type 2 diabetes mellitus patients2, 3. Our study has shown that the frequency of HLA‐DQA1*03‐DQB1*0303, HLA‐DQA1*05‐DQB1*0201 and HLA‐DQA1*03‐DQB1*0401 haplotypes showed a gradient decrease tendency in juvenile‐onset type 1 diabetes mellitus, adult‐onset type 1 diabetes mellitus, LADA with high GADA titer, LADA with low GADA titer, type 2 diabetes mellitus and control groups4. Therefore, some authors hold the opinion that diabetes is a continuous clinical spectrum, from type 1 diabetes mellitus, through LADA, to type 2 diabetes mellitus2, 5.
ZnT8, a 369 amino acid transmembrane protein, is a novel islet autoantigen in type 1 diabetes mellitus. It is more specifically expressed in insulin‐containing secretory granules (ICSG) than that of GAD65 and tyrosine phosphatase‐related molecules‐26. A number of studies have been carried out to examine the biochemical function, genetic characteristics and immune features of ZnT8 in diabetes, including type 1 diabetes mellitus, LADA and type 2 diabetes mellitus6, 7, 8, 9, 10, 11. These studies show that the role of ZnT8 among subtypes of diabetes is complicated. In the present review, we mainly discuss the role of ZnT8 in type 1 diabetes mellitus and type 2 diabetes mellitus, and examine whether ZnT8 is the possible common molecular basis in the diabetes spectrum.
Different Role of slc30a8 Gene on the Susceptibility to Type 1 Diabetes Mellitus and Type 2 Diabetes Mellitus
ZnT8 is encoded by SLC30A8, which is located in chromosome 8q24.11. This gene has a common non‐synonymous single‐nucleotide polymorphism (SNP), rs13266634 (a C/T polymorphism), which encodes either arginine (R) by the C allele or tryptophan (W) by the T allele at aa325 of ZnT87. Notably, aa268‐369 of ZnT8, especially ZnT8‐325R and ZnT8‐325W, is the dominant epitope in type 1 diabetes mellitus12, 13, 14. The ZnT8‐325R allele is associated with autoantibody against ZnT8‐325R (ZnT8A‐325R)12, 13, which suggests that rs13266634 might be critical for humoral autoimmunity in type 1 diabetes mellitus12. A study showed that SLC30A8 genotype can stratify type 1 diabetes mellitus risk in ZnT8A‐positive children15. However, some studies found that there is no association between this polymorphism and disease progression of type 1 diabetes mellitus16, 17.
A genome‐wide association study reported that the C allele of SLC30A8 rs13266634 confers an increased risk of type 2 diabetes mellitus (odds ratio [OR] 1.18–1.53)7. A meta‐analysis also found the relationship between rs13266634 and impaired glucose tolerance (IGT; OR 1.06–1.26)17. In non‐diabetic offspring of type 2 diabetes mellitus patients, the C allele of rs13266634 was associated with decreased first‐phase insulin release and impaired proinsulin conversion, but not associated with insulin resistance18, 19, 20. These studies show that SLC30A8 rs13266634 is the common genetic background of relatives of type 2 diabetes mellitus, IGT and type 2 diabetes mellitus patients. In addition, a recent study has surprisingly shown that 12 protein‐truncating mutations in SLC30A8 could confer a 65% reduction in type 2 diabetes mellitus risk10.
Therefore, the genotypes of SLC30A8 can determine whether the responses are protective or diabetogenic in the development of type 2 diabetes mellitus. However, SLC30A8 rs13266634 might not be the susceptibility gene of type 1 diabetes mellitus, while the connection between this polymorphism and LADA has also not been reported. Questions remain about why so many patients present different types of diabetes, such as type 1 diabetes mellitus and type 2 diabetes mellitus, even when they carry the same genotype of SLC30A8 rs13266634.
Interaction Between Genetic and Environmental Factors Determines the Type of Diabetes
The development of diabetes is multifactorial, and influenced by the interaction between genetic and environmental factors1. The onset of type 2 diabetes mellitus is often influenced by lifestyle factors, such as age, obesity and high‐fat diet1. Interestingly, the function of ZnT8 is also age‐, sex‐ and diet‐dependent21, 22, 23. For example, glucose tolerance and insulin sensitivity remained unchanged when ZnT8‐knockout (ZnT8−/−) mice were fed a normal diet. However, they developed weight gain (~10%), glucose intolerance and their islets became less responsive to glucose, leading to overt diabetes in 50% of the ZnT8−/− mice after high‐fat diet feeding21, 22. The whole processes could be influenced by the C allele of SLC30A8 rs1326663422, 23, 24. Thus, the interaction between the SLC30A8 genotype and lifestyle factors might play an important role in the pathogenesis of type 2 diabetes mellitus.
Environmental factors, such as viral infection, can contribute to the risk of initiating and developing type 1 diabetes mellitus1. In type 1 diabetes mellitus patients, ZnT8A can recognize the epitopes of asymptomatic infection of Mycobacterium aviumsubspecies paratuberculosis (MAP) through food contamination25, 26. There are similar sequences between ZnT8 and MAP at least in two pairs: ZnT8186–194 (VAANIVLTV) and MAP3865c133–141 (LAANFVVAL), and ZnT8178–186 (MIIVSSCAV) and MAP3865c125–133 (MIAVALAGL)26. These similarities show that ZnT8 might be a target protein in initiating the islet autoimmunity through a molecular mimicry mechanism after bacterial infection25, 26. Additionally, ZnT8 also can be presented by islet antigen presenting cells (APCs) to T cells in NOD mice, especially under inflammation of an islet11. Thus, environmental factors can participate in type 1 diabetes mellitus development through the autoimmune response to ZnT8.
Taken together, it seems that the different role of SLC30A8 rs13266634 in type 1 diabetes mellitus and type 2 diabetes mellitus might depend on environmental factors, such as diet and bacterial infection. This means that gene–environment interactions could determine which type of diabetes occurs.
Different Role of ZnT8 in Β Cell Function in Type 1 Diabetes Mellitus and Type 2 Diabetes Mellitus
ZnT8 and Zip (SLC39A gene family) regulate zinc homeostasis, and zinc is required for insulin crystallization, storage and the exocytosis of insulin secretory vesicles9. Interestingly, both in the type 2 diabetes mellitus animal model (db/db mice) and in the maturity‐onset diabetes of the young (MODY) animal model (Akita mice), the protein expression of ZnT8 in β‐cells was significantly downregulated in the early stage of diabetes27. The zinc content in islets was also decreased with the downregulation of ZnT89, 28. These results suggest that the decrease of ZnT8 expression and/or its function can indirectly regulate insulin homeostasis through maintaining zinc homeostasis in β‐cells.
The effects of ZnT8 on β‐cell number and β‐cell function have been investigated by several studies21, 22, 23, 24, 28, 29, 30. In vitro experiments using ZnT8‐silenced INS‐1 cells showed that both insulin content and glucose‐stimulated insulin secretion (GSIS) were reduced28. However, the effects of ZnT8 on GSIS are inconsistent in ZnT8−/− mice9. Some studies showed that ZnT8 cannot regulate GSIS21, 23, whereas other studies reported impaired GSIS24, 30, or even enhanced GSIS22, 29. These inconsistent results could be explained by the differences in age, sex, diet and the SLC30A8 genotype of mice21, 22, 23. Recent studies found the significant decrease of plasma insulin concentrations24, 29 with increased plasma proinsulin levels30, indicating the defect of proinsulin to insulin conversion. Other studies found that the reduced mature dense core insulin granules were replaced by immature ‘progranules’21, 22, suggesting that ZnT8 might affect insulin crystallization. In addition, the transcription factors (Pdx1 and Mafa), the processing enzymes (prohormone convertase 1 and 2) and the adenosine triphosphate‐sensitive K+ channel (KIR6.2 protein) were reduced in islets from ZnT8−/− mice30. These results suggest that ZnT8 might regulate insulin biosynthesis directly, as the molecules are required for GSIS and insulin biosynthesis. Tamaki et al.29 recently showed that insulin clearance in the liver was increased after glucose tolerance test in mice and in humans carrying SLC30A8 rs13266634. Most studies reported that ZnT8 has no effect on islet size, islet number, β‐cell mass, cellular composition and insulin sensitivity21, 24, 30. Thus, ZnT8 has a direct effect on maintaining peripheral insulin homeostasis, whereas it has no effects on islet architecture.
The aforementioned studies show that ZnT8 deficiency or downregulation can affect insulin biosynthesis, release, and β‐cell function through direct and/or indirect mechanisms. Deterioration of β‐cell function leads to absolute or relative deficiency of insulin, which can cause type 1 diabetes mellitus and type 2 diabetes mellitus1. Genetic studies have found the association between the C allele of SLC30A8 rs13266634 SNP and residual β‐cells dysfunction in type 1 diabetes mellitus31, 32. This SNP is also associated with variance in the doses of insulin therapy33. In addition, in type 1 diabetes mellitus, the initially higher titer of ZnT8A is also associated with higher plasma levels of stimulated C‐peptide32, whereas the decreases in ZnT8A‐325W levels can predict the decline of β‐cell function 3–6 years after diagnosis34. In individuals at increased risk of type 1 diabetes mellitus, the C allele of rs13266634 was associated with decreased first‐phase insulin release and impaired proinsulin conversion18, 19, 20. However, in in type 2 diabetes mellitus and LADA patients, there are no articles to report the association between SLC30A8 genotype and β‐cell function. Thus, the role of ZnT8 on the β‐cell function in diabetic patients is still under study.
Gradient Changes of Humoral Autoimmunity, but not Cellular Autoimmunity, to ZnT8 in Diabetes Spectrum
During the process of insulin biosynthesis and secretion, the frequent exocytosis of ICSG can increase the chance of ZnT8 exposed to the β‐cell surface, which means a better β‐cell function might cause more ZnT8 antigen to be exposed32. Once ZnT8 is exposed, it can trigger or exacerbate autoimmunity in genetically susceptible individuals35. Actually, this phenomenon can occur in healthy subjects, type 1 diabetes mellitus patients and type 2 diabetes mellitus patients36, 37, 38.
AAbs, such as GADA, tyrosine phosphatase‐related molecules‐2 and ZnT8A, are useful humoral autoimmunity biomarkers for diabetes diagnosis1. The specific distribution of ZnT8A among different types of diabetes has been shown by several studies6, 39, 40, 41, 42, 43. Wenzlau et al.6 found that the positive rate of ZnT8A was 60–80% in Caucasians with new‐onset type 1 diabetes mellitus, whereas the positive rate was <3% in type 2 diabetes mellitus patients and <2% in healthy people6. Our research showed that ZnT8A‐positive rates in type 1 diabetes mellitus, LADA and type 2 diabetes mellitus are 24.1, 10.7 and 1.99%, respectively in Chinese diabetic patients39, 42, 43. Similar results were also reported in Japan40, 41. These results show that the gradient increase of ZnT8A positive rates from type 2 diabetes mellitus, through LADA, to type 1 diabetes mellitus is one of the autoimmune features of the diabetes spectrum.
The T cell responses against islet autoantigens are also an important etiological classification tool for diabetes. Several reports confirmed that ZnT8 is a major target of T cell response in patients with type 1 diabetes mellitus (>68%)8, 36, 38. It has shown that even in AAbs‐negative phenotypic type 2 diabetes mellitus patients, some people still have islet autoantigens‐specific T cell reactivity44, such as ZnT8‐reactive CD8+ T cell37. In fact, a low frequency of ZnT8‐sepcific T cell response was observed in the healthy population (<8%) or type 2 diabetes mellitus (<6/24)36, 37, 38. Additionally, based on cytokine secretion profiles, ZnT8‐specific CD4+ T cells tended to convert into T helper (Th)1 cells in type 1 diabetes mellitus patients, but into Th2 and interleukin‐10 producing cells in healthy adults38. These results show a gradient change of ZnT8‐specific T cell response from type 1 diabetes mellitus, through type 2 diabetes mellitus, to healthy subjects, but the frequency of ZnT8‐specific T cells in LADA, the bridge among diabetes spectrum, has not been shown.
Several T cell epitopes of ZnT8 have been identified, mainly mapped to the transmembrane region or the C‐terminal of ZnT8, but not overlapped with the amino acid polymorphism at position 32511, 36, 37, 45, 46, 47. Chang and Unanue45 identified aa166‐179 of ZnT8 as an epitope bound to HLA‐DQ8 molecules. In addition, the HLA‐DR4 restricted epitopes are ZnT88–22, ZnT815–29, ZnT8120–134, ZnT8134–148, ZnT8260–274, ZnT8267–281 and ZnT8295–309, whereas the HLA‐DR3 epitopes are ZnT8155–169 and ZnT8323–337 in humans36. There are differences of the predominant T cell epitope of ZnT8 between type 1 diabetes mellitus and type 2 diabetes mellitus. ZnT8186–194, ZnT8153–161, ZnT8107–115, ZnT8115–123 and ZnT8145–153 are predominantly T cell epitopes in type 1 diabetes mellitus37, 46, 47, whereas ZnT8253–261 is more frequently recognized in HLA‐A2+ type 2 diabetes mellitus patients37. These showed that the different ZnT8 epitope‐specific T cell responses might be the different molecular basis of autoimmunity in type 1 diabetes mellitus and type 2 diabetes mellitus.
Different Responses of Glucose Metabolism to Zinc Supplementation Between Type 1 Diabetes Mellitus and Type 2 Diabetes Mellitus
Given the tissue specificity of ZnT8, it might become an attractive target of diabetes treatment13. The potential ZnT8‐based diabetic treatments include ZnT8‐specific immunomodulation, ZnT8 function regulation and/or zinc supplementation. The islet autoantigen‐specific therapies, including oral and nasal insulin or alum‐formulated recombinant human GAD65, are the important immunomodulatory strategies for type 1 diabetes mellitus48. However, until now, there is no relevant literature about ZnT8‐specific immunotherapy in type 1 diabetes mellitus patients or animal models. Because of the direct diabetogenic role of ZnT811, it might induce immune tolerance similar to GAD65 in type 1 diabetes mellitus48. The most important prerequisite to induce immune tolerance and prevent type 1 diabetes mellitus is matching the diabetogenic epitope form of ZnT8 to the recipient13. It is supposed that an individual's genotype could determine the protective or immunogenic response to ZnT8‐specific immunotherapy in humans13.
Regulating ZnT8 function might be a complex treatment strategy. The results from ZnT8−/− animal models suggested that increasing ZnT8 function can improve insulin levels and glucose metabolism21, 22, 23, 24, 29, 30. However, study of loss‐of‐function mutations in SLC30A8 suggested the protective role of ZnT8 in type 2 diabetes mellitus10, which indicates that ZnT8 inhibitors can be used as a potential therapy for diabetes49. One of the possible explanations is the genotype of SLC30A8. For diabetic patients carrying the C allele of rs13266634 SNP, enhancing ZnT8 activity would be a useful therapeutic option, whereas for patients with SLC30A8 haploinsufficiency or protein‐truncating variants, inhibition of ZnT8 would be a therapeutic strategy49. However, evidence of the potential therapeutic role of regulating ZnT8 function is mostly from type 2 diabetes mellitus models10, 27, 29.
The results of several studies using zinc supplementation to treat diabetes are conflicting. A recent meta‐analysis reported that zinc supplementation (3–240 mg/day, median 30 mg/day) significantly decreased fasting glucose concentrations (−0.19 ± 0.08 mmol/L) and glycated hemoglobin (−0.64 ± 0.36%), whereas serum insulin levels remained unchanged in diabetic patients in the overall, ungrouped analysis50. However, zinc supplementation seems to have no effect on glucose concentrations in type 1 diabetes mellitus subjects50. Conversely, in non‐diabetic patients, fasting glucose concentrations tended to increase from 91.2 ± 12.13 mg/dL to 95.5 ± 18.99 mg/dL after zinc supplementation50. Therefore, the different responses to zinc supplementation in type 1 diabetes mellitus, type 2 diabetes mellitus and non‐diabetic patients show the different role of zinc in the diabetes spectrum.
Conclusions
In conclusion, we suggest that ZnT8 plays a different role on type 1 diabetes mellitus and type 2 diabetes mellitus. The differences are mainly concentrated in the different role of the genotypes of SLC30A8 in the susceptibility to type 1 diabetes mellitus and type 2 diabetes mellitus, the different outcome of gene–environment interactions, and the different response to ZnT8 administration. However, diabetes autoimmunity against ZnT813 and the multifunctional role of ZnT8 in β‐cell function show the potential mechanistic link between type 2 diabetes mellitus and type 1 diabetes mellitus, the two major forms of the diabetes spectrum. Therefore, further studies are required to verify the hypothesis that ZnT8 might be one of the molecules playing a major role in the diabetes spectrum (Figure 1). Efforts are required to examine the role and frequencies of ZnT8‐specific T cells in LADA and type 2 diabetes mellitus. The association between the SLC30A8 genotype and the risk of LADA also needs to be shown. Although ZnT8 is a diabetogenic antigen that can participate in diabetes11, the detailed mechanisms of ZnT8 autoimmunity‐induced β‐cell destruction are still unclear. It is necessary to confirm the regulatory function of ZnT8 in insulin homeostasis in NOD mice and in humans. It is also necessary to explore the cytokine expression profiles released by ZnT8‐specific T cells among different types of diabetes.
Figure 1.
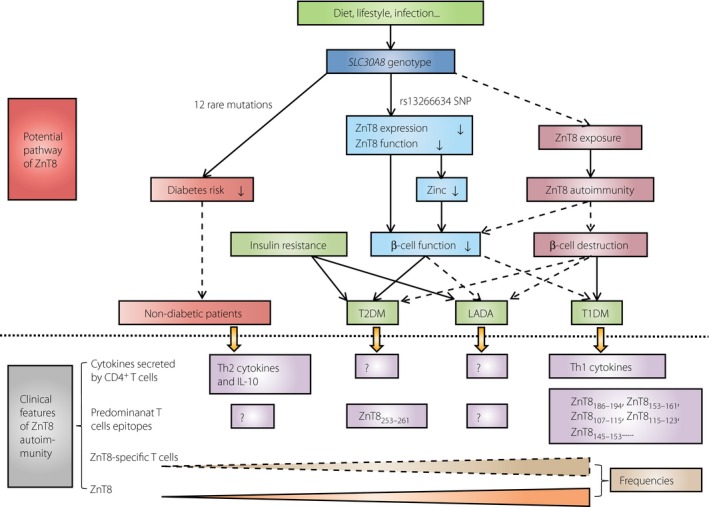
The clinical features (below the dotted line) and potential pathway (above the dotted line) of zinc transporter 8 (ZnT8)in diabetes. The initial phase of diabetes might be the interaction between genetic and environmental factors. One or more of the 12 rare mutations of SLC30A8 might be dominant to environmental factors and reduce the risk of diabetes (protect effect). In contrast, the C allele of rs13266634 single nucleotide polymorphism might downregulate ZnT8 protein expression and transporter activity causing decreased zinc concentration (indirect biological effect), and subsequently impaired β‐cell function (direct biological effect). In addition, during the biosynthesis and secretion of insulin, exocytosis of insulin granules can increase the chance of ZnT8 exposed to the cell surface, which initiates ZnT8 epitope‐specific T cells‐mediated β‐cell destruction, and cause type 1 diabetes mellitus (T1DM; autoimmune injury), or cause type 2 diabetes mellitus (T2DM) and latent autoimmune diabetes in adults (LADA; combined with insulin resistance). The prevalence of ZnT8A and ZnT8‐specific T cells is gradiently decreased from T1DM, through T2DM, then to healthy controls clinically. The cytokine secretion and T cell epitopes of ZnT8‐specific T cells are different in T1DM and T2DM. The solid lines represent certain effect. The dashed line represents the possible effect. The symbol ‘?’ represents the lack of relevant data. IL‐10, interleukin‐10; Th1, type 1 T helper; Th2, type 2 T helper.
Disclosure
The authors declare no conflict of interest.
Acknowledgments
We would thank Professor Xiangbing Wang (Division of Endocrinology, Metabolism, and Nutrition, Rutgers University‐Robert Wood Johnson Medical School, USA), Dr Zhiguo Xie, and Dr Peilin Zheng (Institute of Metabolism and Endocrinology, 2nd Xiangya Hospital, Central South University, China) for their advice and proofreading of the manuscript. This work was funded by the National Key Technology R&D program (2012BAI02B04) and Hunan Provincial Innovation Foundation for Postgraduate (CX2013B116).
J Diabetes Investig 2016; 7: 459–465
References
- 1. Tuomi T, Santoro N, Caprio S, et al The many faces of diabetes: a disease with increasing heterogeneity. Lancet 2014; 383: 1084–1094. [DOI] [PubMed] [Google Scholar]
- 2. Xiang YF, Zhao YJ, Zhou ZG. Latent autoimmune diabetes in adults: evidences for diabetes spectrum? Chin Med J (Engl) 2013; 126: 783–788. [PubMed] [Google Scholar]
- 3. Liu L, Li X, Xiang Y, et al Latent autoimmune diabetes in adults with low‐titer GAD antibodies: similar disease progression with type 2 diabetes: a nationwide, multicenter prospective study (LADA China Study 3). Diabetes Care 2015; 38: 16–21. [DOI] [PubMed] [Google Scholar]
- 4. Lin J, Zhou ZG, Wang JP, et al From Type 1, through LADA, to type 2 diabetes: a continuous spectrum? Ann N Y Acad Sci 2008; 1150: 99–102. [DOI] [PubMed] [Google Scholar]
- 5. Brooks‐Worrell B, Palmer JP. Is diabetes mellitus a continuous spectrum? Clin Chem 2011; 57: 158–161. [DOI] [PubMed] [Google Scholar]
- 6. Wenzlau JM, Juhl K, Yu L, et al The cation efflux transporter ZnT8 (Slc30A8) is a major autoantigen in human type 1 diabetes. Proc Natl Acad Sci USA 2007; 104: 17040–17045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sladek R, Rocheleau G, Rung J, et al A genome‐wide association study identifies novel risk loci for type 2 diabetes. Nature 2007; 445: 881–885. [DOI] [PubMed] [Google Scholar]
- 8. Enee E, Kratzer R, Arnoux JB, et al ZnT8 is a major CD8+ T cell‐recognized autoantigen in pediatric type 1 diabetes. Diabetes 2012; 61: 1779–1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Davidson HW, Wenzlau JM, O'Brien RM. Zinc transporter 8 (ZnT8) and beta cell function. Trends Endocrinol Metab 2014; 25: 415–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Flannick J, Thorleifsson G, Beer NL, et al Loss‐of‐function mutations in SLC30A8 protect against type 2 diabetes. Nat Genet 2014; 46: 357–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nayak DK, Calderon B, Vomund AN, et al ZnT8‐reactive T cells are weakly pathogenic in NOD mice but can participate in diabetes under inflammatory conditions. Diabetes 2014; 63: 3438–3448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kawasaki E, Uga M, Nakamura K, et al Association between anti‐ZnT8 autoantibody specificities and SLC30A8 Arg325Trp variant in Japanese patients with type 1 diabetes. Diabetologia 2008; 51: 2299–2302. [DOI] [PubMed] [Google Scholar]
- 13. Wenzlau JM, Liu Y, Yu L, et al A common nonsynonymous single nucleotide polymorphism in the SLC30A8 gene determines ZnT8 autoantibody specificity in type 1 diabetes. Diabetes 2008; 57: 2693–2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wenzlau JM, Moua O, Liu Y, et al Identification of a major humoral epitope in Slc30A8 (ZnT8). Ann N Y Acad Sci 2008; 1150: 252–255. [DOI] [PubMed] [Google Scholar]
- 15. Achenbach P, Lampasona V, Landherr U, et al Autoantibodies to zinc transporter 8 and SLC30A8 genotype stratify type 1 diabetes risk. Diabetologia 2009; 52: 1881–1888. [DOI] [PubMed] [Google Scholar]
- 16. Gohlke H, Ferrari U, Koczwara K, et al SLC30A8 (ZnT8) polymorphism is associated with young age at type 1 diabetes onset. Rev Diabet Stud 2008; 5: 25–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Xu K, Zha M, Wu X, et al Association between rs13266634 C/T polymorphisms of solute carrier family 30 member 8 (SLC30A8) and type 2 diabetes, impaired glucose tolerance, type 1 diabetes–a meta‐analysis. Diabetes Res Clin Pract 2011; 91: 195–202. [DOI] [PubMed] [Google Scholar]
- 18. Staiger H, Machicao F, Stefan N, et al Polymorphisms within novel risk loci for type 2 diabetes determine beta‐cell function. PLoS ONE 2007; 2: e832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Boesgaard TW, Zilinskaite J, Vanttinen M, et al The common SLC30A8 Arg325Trp variant is associated with reduced first‐phase insulin release in 846 non‐diabetic offspring of type 2 diabetes patients–the EUGENE2 study. Diabetologia 2008; 51: 816–820. [DOI] [PubMed] [Google Scholar]
- 20. Kirchhoff K, Machicao F, Haupt A, et al Polymorphisms in the TCF7L2, CDKAL1 and SLC30A8 genes are associated with impaired proinsulin conversion. Diabetologia 2008; 51: 597–601. [DOI] [PubMed] [Google Scholar]
- 21. Lemaire K, Ravier MA, Schraenen A, et al Insulin crystallization depends on zinc transporter ZnT8 expression, but is not required for normal glucose homeostasis in mice. Proc Natl Acad Sci USA 2009; 106: 14872–14877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nicolson TJ, Bellomo EA, Wijesekara N, et al Insulin storage and glucose homeostasis in mice null for the granule zinc transporter ZnT8 and studies of the type 2 diabetes‐associated variants. Diabetes 2009; 58: 2070–2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pound LD, Sarkar SA, Ustione A, et al The physiological effects of deleting the mouse SLC30A8 gene encoding zinc transporter‐8 are influenced by gender and genetic background. PLoS ONE 2012; 7: e40972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pound LD, Sarkar SA, Benninger RK, et al Deletion of the mouse Slc30a8 gene encoding zinc transporter‐8 results in impaired insulin secretion. Biochem J 2009; 421: 371–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Masala S, Cossu D, Piccinini S, et al Recognition of zinc transporter 8 and MAP3865c homologous epitopes by new‐onset type 1 diabetes children from continental Italy. Acta Diabetol 2014; 51: 577–585. [DOI] [PubMed] [Google Scholar]
- 26. Masala S, Paccagnini D, Cossu D, et al Antibodies recognizing Mycobacterium avium paratuberculosis epitopes cross‐react with the beta‐cell antigen ZnT8 in Sardinian type 1 diabetic patients. PLoS ONE 2011; 6: e26931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tamaki M, Fujitani Y, Uchida T, et al Downregulation of ZnT8 expression in pancreatic beta‐cells of diabetic mice. Islets 2009; 1: 124–128. [DOI] [PubMed] [Google Scholar]
- 28. Fu Y, Tian W, Pratt EB, et al Down‐regulation of ZnT8 expression in INS‐1 rat pancreatic beta cells reduces insulin content and glucose‐inducible insulin secretion. PLoS ONE 2009; 4: e5679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tamaki M, Fujitani Y, Hara A, et al The diabetes‐susceptible gene SLC30A8/ZnT8 regulates hepatic insulin clearance. J Clin Invest 2013; 123: 4513–4524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wijesekara N, Dai FF, Hardy AB, et al Beta cell‐specific Znt8 deletion in mice causes marked defects in insulin processing, crystallisation and secretion. Diabetologia 2010; 53: 1656–1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Nielsen LB, Vaziri‐Sani F, Porksen S, et al Relationship between ZnT8Ab, the SLC30A8 gene and disease progression in children with newly diagnosed type 1 diabetes. Autoimmunity 2011; 44: 616–623. [DOI] [PubMed] [Google Scholar]
- 32. Andersen ML, Vaziri‐Sani F, Delli A, et al Association between autoantibodies to the Arginine variant of the Zinc transporter 8 (ZnT8) and stimulated C‐peptide levels in Danish children and adolescents with newly diagnosed type 1 diabetes. Pediatr Diabetes 2012; 13: 454–462. [DOI] [PubMed] [Google Scholar]
- 33. Moosavi M, Seguin J, Li Q, et al The effect of type 2 diabetes risk loci on insulin requirements in type 1 diabetes. Horm Res Paediatr 2012; 77: 305–308. [DOI] [PubMed] [Google Scholar]
- 34. Sorensen JS, Vaziri‐Sani F, Maziarz M, et al Islet autoantibodies and residual beta cell function in type 1 diabetes children followed for 3‐6 years. Diabetes Res Clin Pract 2012; 96: 204–210. [DOI] [PubMed] [Google Scholar]
- 35. Arvan P, Pietropaolo M, Ostrov D, et al Islet autoantigens: structure, function, localization, and regulation. Cold Spring Harb Perspect Med 2012; 2: a007658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Dang M, Rockell J, Wagner R, et al Human type 1 diabetes is associated with T cell autoimmunity to zinc transporter 8. J Immunol 2011; 186: 6056–6063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Scotto M, Afonso G, Larger E, et al Zinc transporter (ZnT)8(186‐194) is an immunodominant CD8+ T cell epitope in HLA‐A2+ type 1 diabetic patients. Diabetologia 2012; 55: 2026–2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chujo D, Foucat E, Nguyen TS, et al ZnT8‐Specific CD4+ T cells display distinct cytokine expression profiles between type 1 diabetes patients and healthy adults. PLoS ONE 2013; 8: e55595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Huang G, Xiang Y, Pan L, et al Zinc transporter 8 autoantibody (ZnT8A) could help differentiate latent autoimmune diabetes in adults (LADA) from phenotypic type 2 diabetes mellitus. Diabetes Metab Res Rev 2013; 29: 363–368. [DOI] [PubMed] [Google Scholar]
- 40. Kawasaki E, Nakamura K, Kuriya G, et al Zinc transporter 8 autoantibodies in fulminant, acute‐onset, and slow‐onset patients with type 1 diabetes. Diabetes Metab Res Rev 2011; 27: 895–898. [DOI] [PubMed] [Google Scholar]
- 41. Kawasaki E, Nakamura K, Kuriya G, et al Differences in the humoral autoreactivity to zinc transporter 8 between childhood‐ and adult‐onset type 1 diabetes in Japanese patients. Clin Immunol 2011; 138: 146–153. [DOI] [PubMed] [Google Scholar]
- 42. Luo S, Zhang Z, Li X, et al Fulminant type 1 diabetes: a collaborative clinical cases investigation in China. Acta Diabetol 2013; 50: 53–59. [DOI] [PubMed] [Google Scholar]
- 43. Yang L, Luo S, Huang G, et al The diagnostic value of zinc transporter 8 autoantibody (ZnT8A) for type 1 diabetes in Chinese. Diabetes Metab Res Rev 2010; 26: 579–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Rolandsson O, Palmer JP. Latent autoimmune diabetes in adults (LADA) is dead: long live autoimmune diabetes!. Diabetologia 2010; 53: 1250–1253. [DOI] [PubMed] [Google Scholar]
- 45. Chang KY, Unanue ER. Prediction of HLA‐DQ8beta cell peptidome using a computational program and its relationship to autoreactive T cells. Int Immunol 2009; 21: 705–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Li S, Li H, Chen B, et al Identification of novel HLA‐A 0201‐restricted cytotoxic T lymphocyte epitopes from Zinc Transporter 8. Vaccine 2013; 31: 1610–1615. [DOI] [PubMed] [Google Scholar]
- 47. Wu X, Xu X, Gu R, et al Prediction of HLA class I‐restricted T‐cell epitopes of islet autoantigen combined with binding and dissociation assays. Autoimmunity 2012; 45: 176–185. [DOI] [PubMed] [Google Scholar]
- 48. Lernmark A, Larsson HE. Immune therapy in type 1 diabetes mellitus. Nat Rev Endocrinol 2013; 9: 92–103. [DOI] [PubMed] [Google Scholar]
- 49. Pearson E. Zinc transport and diabetes risk. Nat Genet 2014; 46: 323–324. [DOI] [PubMed] [Google Scholar]
- 50. Capdor J, Foster M, Petocz P, et al Zinc and glycemic control: a meta‐analysis of randomised placebo controlled supplementation trials in humans. J Trace Elem Med Biol 2013; 27: 137–142. [DOI] [PubMed] [Google Scholar]


