Abstract
Aims/Introduction
Dental pulp stem cells (DPSCs) are thought to be an attractive candidate for cell therapy. We recently reported that the transplantation of DPSCs increased nerve conduction velocity and nerve blood flow in diabetic rats. In the present study, we investigated the immunomodulatory effects of DPSC transplantation on diabetic peripheral nerves.
Materials and Methods
DPSCs were isolated from the dental pulp of Sprague–Dawley rats and expanded in culture. Eight weeks after the streptozotocin injection, DPSCs were transplanted into the unilateral hindlimb skeletal muscles. Four weeks after DPSC transplantation, neurophysiological measurements, inflammatory gene expressions and the number of CD68‐positive cells in sciatic nerves were assessed. To confirm the immunomodulatory effects of DPSCs, the effects of DPSC‐conditioned media on lipopolysaccharide‐stimulated murine macrophage RAW264.7 cells were investigated.
Results
Diabetic rats showed significant delays in sciatic nerve conduction velocities and decreased sciatic nerve blood flow, all of which were ameliorated by DPSC transplantation. The number of CD68‐positive monocytes/macrophages and the gene expressions of M1 macrophage‐expressed cytokines, tumor necrosis factor‐α and interleukin‐1β, were increased in the sciatic nerves of the diabetic rats. DPSC transplantation significantly decreased monocytes/macrophages and tumor necrosis factor‐α messenger ribonucleic acid expression, and increased the gene expression of the M2 macrophage marker, CD206, in the sciatic nerves of the diabetic rats. The in vitro study showed that DPSC‐conditioned media significantly increased the gene expressions of interleukin‐10 and CD206 in lipopolysaccharide‐stimulated RAW264.7 cells.
Conclusions
These results suggest that DPSC transplantation promoted macrophages polarization towards anti‐inflammatory M2 phenotypes, which might be one of the therapeutic mechanisms for diabetic polyneuropathy.
Keywords: Cell transplantation, Dental pulp stem cells, Diabetic polyneuropathy
Introduction
Diabetic polyneuropathy, the most common diabetic complications in both type 1 and type 2 diabetes, affects up to 50% of such patients1. Although drugs for symptomatic therapy for painful diabetic neuropathy are effective in improving the quality of life, fundamental therapies for the pathogenesis of diabetic polyneuropathy are still required. Cell therapy using some progenitor or stem cells is one of the candidates. We and others have reported that the transplantation of progenitor/stem cells, such as endothelial progenitor cells, mesenchymal stem cells and embryonic stem cell/induced pluripotent stem cell‐derived cells, ameliorated diabetic polyneuropathy in animal models of diabetes2, 3, 4, 5, 6, 7. Regarding the mechanisms of the therapeutic efficacy for diabetic polyneuropathy, the angiogenic and neurotrophic factors secreted by progenitor/stem cells were the first candidates.
In contrast, inflammation is the key target for obesity and diabetes, as well as diabetic complications8. Interactions between the nervous and immune systems lead to neuroendocrine immune modulations involved in neuropathology9. In diabetic polyneuropathy, there is accumulating evidence that inflammatory processes are involved in the pathogenesis of diabetic neuropathy9, 10, 11, 12. Inhibiting the release of cytokines, such as tumor necrosis factor‐α (TNF‐α) resulted in an improvement in the delayed nerve conduction velocity and structural changes in streptozotocin (STZ)‐induced diabetic rats13, 14, 15. Among progenitor and stem cells, mesenchymal stem cells are known to have strong immunosuppressive properties, which support the therapeutic efficacy of allogenic transplantation16, 17.
However, like other mature cells, not only diabetes but also aging impaired the cell functions in these progenitor and stem cells, which might reduce the efficacy of autologous cell therapy18, 19. Dental pulp stem cells (DPSCs), which are mesenchymal stem cells, are thought to be an attractive candidate for cell therapy, because these are easy to obtain from teeth extracted for orthodontic reasons without further invasive procedures. DPSCs have a high growth rate20 and multilineage differentiation ability21, 22, which are maintained even after long‐term cryopreservation23. We showed that the transplantation of cryopreserved‐DPSCs improved sciatic nerve conduction velocity (NCV) and sciatic nerve blood flow (SNBF), as well as intraepidermal nerve fiber density using STZ‐induced diabetic rats24. Furthermore, as the third molar is extracted from many people during early adolescence, we could obtain and keep DPSCs from young and prediabetic dental pulp for diabetic patients.
In the present study, we examined whether DPSCs have immunosuppressive effects on the peripheral nerve tissues in STZ‐induced diabetic polyneuropathy. Here, we showed that DPSC transplantation significantly decreased the number of macrophages in diabetic sciatic nerves, while decreasing M1 macrophage gene expressions and increasing M2 macrophage gene expressions. These findings suggest that transplantation of DPSCs could be an efficacious anti‐inflammatory cell therapy for diabetic polyneuropathy by modulating the proportions of M1/M2 macrophages.
Materials and Methods
Animals
Male Sprague–Dawley (SD) rats were obtained from Chubu Kagakushizai (Nagoya, Japan) at 6 weeks‐of‐age. Diabetes was induced by an intraperitoneal injection of STZ (Sigma Chemical Co., St. Louis, MO, USA; 60 mg/kg). Rats with blood glucose levels above 14 mmol/L were considered diabetic and selected together with age‐matched male SD rats. This study was approved by the Institutional Animal Care and Use Committees of Aichi Gakuin University.
Isolation and culture of DPSCs
The incisors teeth were dissected carefully from the mandibles of 6‐week‐old male normal SD rats and green fluorescent protein (GFP)‐transgenic SD rats (SD‐Tg[CAG‐EGFP]Cz‐0040sb; Japan SLC Inc., Hamamatsu, Japan). Dental pulp tissues were collected and suspended in phosphate‐buffered saline containing 0.1% collagenase and 0.25% trypsin‐ethylenediaminetetraacetic acid. DPSCs were cultured in an alpha modification of Eagle's medium (GIBCO Lab Inc., Grand Island, NY, USA), which glucose concentration was 5.5 mmol/L, with 20% fetal bovine serum (GIBCO) on plastic dishes at 37°C in 5% humidified CO2. Non‐adherent cells were washed off, and adherent cells were continuously expanded until passage 3.
Characterization of DPSCs
Cultured DPSCs were incubated with the R‐PE‐conjugated hamster antibody against rat CD29 (Becton Dickinson, Franklin Lakes, NJ, USA), the R‐PE‐conjugated mouse monoclonal antibodies against rat CD90 or the R‐PE‐conjugated mouse monoclonal antibodies against rat CD45 (Becton Dickinson), and were characterized by magnetic activated cell sorting (Miltenyi Biotec, Bergisch Gladbach, Germany).
Differential ability of DPSCs into adipocytes, osteocytes and chondrocytes
Differentiation into adipocytes, osteoblasts and chondrocytes of cultured DPSCs was carried out according to the manufacturer's instructions (R&D Systems, Minneapolis, MN, USA). For the detection of adipogenic differentiation, the cells were stained with oil red O (Polysciences Inc., Warrington, PA, USA) and the fatty acid‐binding protein‐4 (R&D Systems). For the detection of osteogenic differentiation, the cells were stained with osteocalcin (R&D Systems). Calcification of osteogenic monolayers was also visualized using Alizarin Red S (Merck, Darmstadt, Germany). For the detection of chondrogenic differentiation, the cells were stained with aggrecan (R&D Systems), known as the major proteoglycan in the articular cartilage.
Transplantation of DPSCs
Eight weeks after the induction of diabetes, we transplanted DPSCs from SD rats or GFP rats into the hindlimb skeletal muscles. DPSC suspension (1.0 mL in total, 1 × 106 cells) was injected at ten points in the unilateral hindlimb skeletal muscles. Vehicle (saline, 1.0 mL in total) was also injected into the contralateral hindlimb skeletal muscles as the control. At the time of transplantation and 4 weeks after the transplantation, the following parameters were bilaterally measured.
Sciatic nerve conduction velocities
Rats were deeply anesthetized with pentobarbital and body temperature was maintained at 37°C using a warming pad to relieve the animals of stress from the anesthetic. Motor nerve conduction velocity (MNCV) between the ankle and the sciatic notch, and sensory nerve conduction velocity (SNCV) between the ankle and the knee were measured. MNCV and SNCV were assessed using a Neuropak MEB‐9400 instrument (Nihon‐Koden, Osaka, Japan).
SNBF
SNBF was measured using a Laser Doppler Blood Flow Meter (FLO‐N1; Omega Wave Inc., Tokyo, Japan). After the rats were anesthetized, the femur skin was cut and an incision was made in the myofascia to expose the sciatic nerve. Then, 5 min after this procedure, a laser probe was placed just above the exposed sciatic nerve. All measurements were carried out while the rats were laid out on a heated pad in a room maintained at 25°C to maintain a constant rectal temperature of 37°C.
Tissue collection
Four weeks after the transplantation, rats were killed by an overdose of pentobarbital (15 mg/100 g). The sciatic nerves were fixed in a 4% paraformaldehyde phosphate buffer solution overnight and immersed in an optimum cutting temperature compound (Sakura Finetechnical, Tokyo, Japan). The proximal portions of the sural nerves were immersed in 2.5% glutaraldehyde (Sigma) overnight. They were postfixed in osmium tetroxide, dehydrated and embedded in Epon for morphometric analysis.
Morphological analysis of sural nerves
Semithin sections of proximal sural nerve were cut transversally (0.5‐μm thick sections), stained with toluidine blue and examined by light microscopy (Leica microsystems, Wetzlar, Germany). We assessed the total complement of sural nerve myelinated fibers as previously described. Parameters were obtained using image processing and analysis software ImageJ (version 1.49; NIH, Bethesda, MD, USA).
Detection of monocytes/macrophages in sciatic nerves
For the detection of monocytes/macrophages, the sections were incubated with the primary mouse CD68 antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA). After washing, Alexa Fluor 594‐coupled goat anti‐mouse immunoglobulin G (IgG) antibody (Invitrogen, Carlsbad, CA, USA) and 4′,6‐diamidino‐2‐phenylindole were applied.
To characterize macrophages, the sections were double‐stained with the primary mouse CD68 antibody and rabbit CD206 antibody (Santa Cruz). After washing, Alexa Fluor 594‐coupled goat anti‐mouse IgG antibody, Alexa Fluor 488‐coupled goat anti‐rabbit IgG antibody (Invitrogen) and 4′,6‐diamidino‐2‐phenylindole were applied. Slides were analyzed under a fluorescence microscope.
Messenger ribonucleic acid expressions of inflammatory cytokines in sciatic nerves
Total ribonucleic acid was extracted from the frozen samples of sciatic nerves using TRIzol Reagent (Invitrogen), and purified using a purification column (Qiagen, Valencia, CA, USA). Complementary deoxyribonucleic acid was synthesized using ReverTra Ace (Toyobo, Osaka, Japan) according to the manufacturer's instructions. Primers and probes for TNF‐α (Tnf), interleukin (IL)‐1β (Il1b), IL‐10 (Il10), CD68 (CD68), CD11c (Itgax) and CD206 (Mrc1) were purchased from TaqMan Gene Expression Assays (Applied Biosystems, Foster City, CA, USA). Real‐time quantitative polymerase chain reaction (PCR) was carried out and measured by the ABI Prism 7000 (Applied Biosystems). Relative quantity was calculated by the ΔΔCt method using β2 microglobulin as the endogenous control25.
Effects of DPSC‐conditioned media on murine macrophage RAW264.7 cells
DPSCs were maintained in Dulbecco's modified Eagle medium, which glucose concentration was 5.5 mmol/L, containing 1% fetal bovine serum (GIBCO). After 24 h, the culture media were collected, concentrated ten times using 10‐kDa centrifugal filters (Amicom Ultra‐15; Nihon Millipore, Tokyo, Japan) and frozen at −20°C until use. The murine macrophage cell line, RAW264.7 cells (American Type Culture Collection, Rockville, MD, USA), were seeded in 12‐well plates (5 × 105 cells/well), and cultured in Dulbecco's modified Eagle medium containing 1% fetal bovine serum. After 12 h, the cells were replaced with the serum‐free medium and pretreated with a one‐tenth dilution of DPSC‐conditioned media for 1 h. After the cells were incubated with lipopolysaccharide (LPS; 50 ng/mL; Sigma) for 4 h, messenger RNA (mRNA) expressions of RAW264.7 cells were investigated by real‐time quantitative PCR.
To evaluate the effects of DPSC‐conditioned media on M1 macrophage state of RAW264.7 cells, RAW264.7 cells were pre‐exposed to 50 ng/mL LPS to differentiate into M1 macrophage. After 48 h, a one‐tenth dilution of DPSC‐conditioned media was added, and the cells were incubated for 4 h. mRNA expressions were investigated by real‐time quantitative PCR.
The effect of DPSC‐conditioned media on the proliferation/viability of RAW264.7 cells were assessed by 3‐(4,5)‐dimethylthiahiazo (‐z‐y1)‐3,5‐di‐phenytetrazoliumromide (MMT; Cayman Chemical Company, Ann Arbor, MI, USA) and Cell Counting Kit‐8 (CCK‐8; Dojindo Laboratories, Kumamoto, Japan) assay according to the manufacturer's procedure. Cells were seeded in 96‐well plates (2 × 104 cells/well) and cultured in Dulbecco's modified Eagle medium containing 1% fetal bovine serum. After 12 h, the cells were incubated with a one‐tenth dilution of DPSC‐conditioned media for 3 days.
mRNA expressions of cultured DPSCs
To investigate the ability to produce bioactive basic fibroblast growth factor (bFGF), vascular endothelial growth factor (VEGFA), nerve growth factor (NGF), neurotrophin‐3 (NTF3), glial cell line‐derived neurotrophic factor (GDNF), Tnf, Il1b, Il6 (IL‐6), MCP1, Il10, Il4 (IL‐14), Il13 (IL‐13), CSF1 (also known as M‐CSF) and CSF2 (also known as GM‐CSF). Primers were purchased from TaqMan Gene Expression Assays (Applied Biosystems). mRNA expressions of cultured DPSCs were investigated by real‐time quantitative PCR. The log concentrations of the target (X) and the housekeeping gene (β2 microglobulin) were calculated (Y) from a standard curve. The expression levels were standardized and compared (X/Y) between each sample. Thus, standardizing log concentration values (X/Y) should provide the relative amount of cultured DPSCs mRNA.
Statistical analysis
Results were expressed as means ± standard error of the mean. Statistical analyses were made by one‐way anova with the Bonferroni correction for multiple comparisons. The differences were considered to be significant at P < 0.05.
Results
DPSCs expressed mesenchymal stem cell markers and differentiated into mesenchymal‐derived cells
DPSCs showed the typical spindle‐shape morphology and expressed the cell surface markers of mesenchymal stem cells, such as CD29 and CD90, and expressed no CD45, a hematopoietic lineage marker (Figure 1a). The cells induced into chondrocytes showed stainability in aggrecan. Adipogenesis was confirmed by the staining with oil red O and fatty acid‐binding protein‐4. DPSCs were also differentiated into osteoblasts stained with osteocalcin, and showed marked mineralization by Alizarin Red S staining (Figure 1b).
Figure 1.
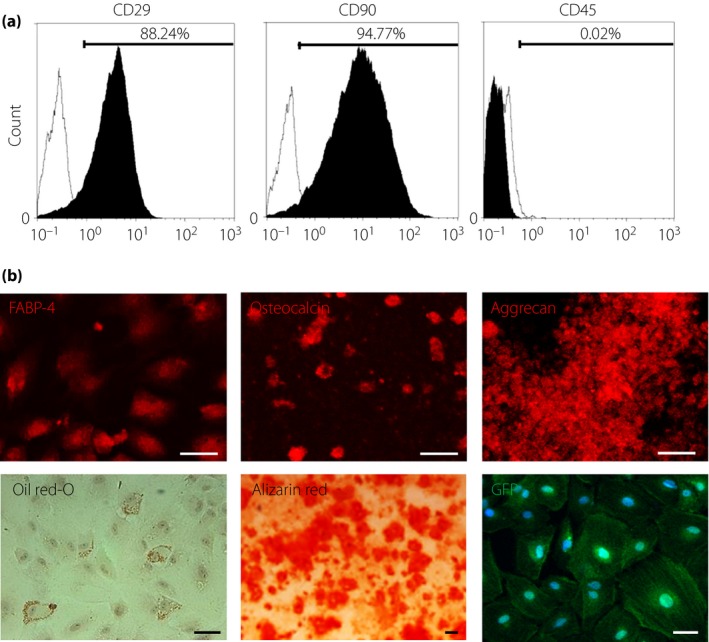
Characterization and differentiation of green fluorescent protein (GFP)‐expressing dental pulp stem cells (GFP‐DPSCs). (a) GFP‐DPSCs derived from 6‐week‐old Sprague–Dawley rats were positive for mesenchymal stem cell markers (CD29, CD90) and negative for the hematopoietic marker CD45 (black area). Isotype‐identical antibodies served as the controls (white area). (b) GFP‐DPSCs could differentiate into adipogenic, osteogenic and chondrogenic lineages in vitro. For discrimination, oil red O and fatty acid‐binding protein‐4 were used for adipocytes, Alizarin Red and osteocalcin for osteocytes, and aggrecan for chondrocytes. Scale bar, 50 μm.
Bodyweights and blood glucose levels
At 12 weeks after STZ injection, the diabetic rats showed severe hyperglycemia (normal rats 6.1 ± 0.4 mmol/L, STZ‐induced diabetic rats 26.6 ± 2.2 mmol/L; P < 0.01) and significantly reduced bodyweight (normal rats 508.3 ± 16.4 g, STZ‐induced diabetic rats 284.0 ± 34.3 g; P < 0.01). DPSC transplantation did not change the bodyweight or blood glucose in the diabetic groups (bodyweight: non‐transplanted diabetic rats 294.3 ± 33.9 g, transplanted diabetic rats 284.0 ± 34.3 g; blood glucose: non‐transplanted diabetic rats 23.8 ± 3.2 mmol/L, transplanted diabetic rats 26.6 ± 2.2 mmol/L).
DPSC transplantation improved delayed NCVs and decreased SNBF in diabetic rats
The diabetic rats showed delayed MNCV and SNCV, and decreased SNBF compared with the control side of the normal rats (Figure 2). The delay of MNCV and SNCV, and the decrease in SNBF were significantly ameliorated by the DPSC transplantation in diabetic rats.
Figure 2.
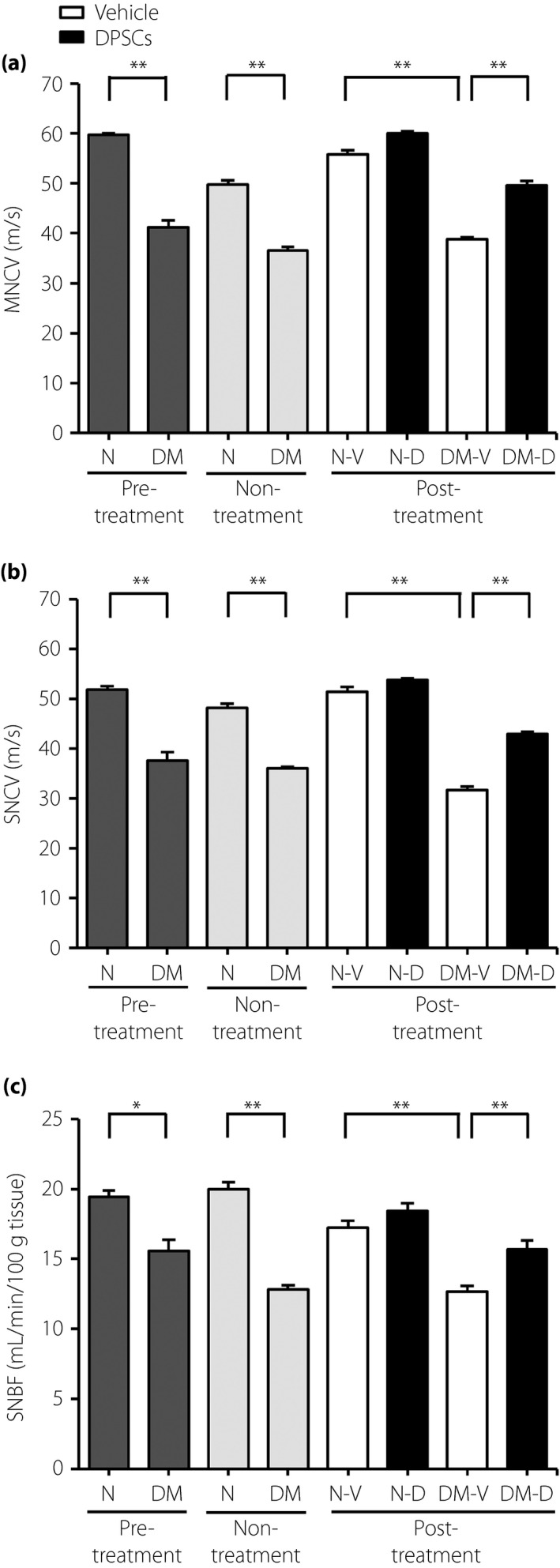
Dental pulp stem cell (DPSC) transplantation improved the delay in sciatic nerve conduction velocities and the decrease in sciatic nerve blood flow in the diabetic rats. (a) Sciatic nerve motor nerve conduction velocities (MNCV). (b) Sciatic nerve sensory nerve conduction velocities (SNCV). (c) Sciatic nerve blood flow (SNBF; n = 5). Results are mean ± standard error of the mean. **P < 0.01, *P < 0.05. DM, diabetic rats; DM‐D, dental pulp stem cell‐transplanted diabetic rats; DM‐V, vehicle‐injected diabetic rats; N, normal rat; Non‐treatment, measurements at the time of 4 weeks after the transplantation in rats without having transplantation; N‐V, vehicle‐injected normal rats; N‐D, dental pulp stem cell‐transplanted normal rats; Post‐treatment, measurements at the time of 4 weeks after the transplantation in dental pulp stem cell‐transplanted rats; Pre‐treatment, measurements at the time of transplantation in normal and diabetic rats.
DPSC transplantation ameliorated sural nerve axonal circularity in diabetic rats
The results of the myelinated fiber morphometry of sural nerves are shown in Table 1. Twelve‐week STZ‐induced diabetic rats showed no changes in fiber area, density of myelinated fibers, occupancy rate, mean myelin area, mean axonal area, axon‐to‐myelin ratio, axonal diameter and myelin thickness compared with the normal rats. The axonal circularity of the sural nerve in the control side of diabetic rats was significantly decreased compared with that of normal rats. DPSC transplantation significantly improved the axonal circularity in the diabetic rats.
Table 1.
Morphometric data of myelinated fibers in sural nerves
| Limb | Fiber area (μm2) | Density (fiber/mm2) | Occupancy rate (%) | Myelin area (μm2) | Axon area (μm2) | Axonal‐to‐myelin area ratio | Axonal diameter (μm) | Myelin thickness (μm) | Axonal circularity |
|---|---|---|---|---|---|---|---|---|---|
| Normal‐vehicle | 26.1 ± 3.2 | 16,146 ± 2,222 | 40.2 ± 2.0 | 15.2 ± 2.0 | 10.8 ± 1.3 | 0.72 ± 0.06 | 5.15 ± 0.53 | 1.69 ± 0.17 | 0.704 ± 0.006 |
| Normal‐DPSCs | 26.4 ± 2.1 | 16,289 ± 2,322 | 42.5 ± 2.7 | 15.3 ± 2.4 | 11.0 ± 0.3 | 0.74 ± 0.14 | 5.35 ± 0.15 | 1.60 ± 0.01 | 0.709 ± 0.044 |
| Diabetic‐vehicle | 26.9 ± 3.1 | 14,757 ± 1,210 | 39.0 ± 1.9 | 15.9 ± 2.0 | 11.0 ± 1.8 | 0.71 ± 0.12 | 5.87 ± 0.55 | 1.73 ± 0.07 | 0.570 ± 0.038* |
| Diabetic‐DPSCs | 27.0 ± 0.6 | 13,969 ± 445 | 37.8 ± 1.8 | 15.7 ± 1.1 | 11.3 ± 1.2 | 0.73 ± 0.13 | 5.07 ± 0.44 | 1.77 ± 0.03 | 0.722 ± 0.034** |
Data are mean ± standard error of the mean. *P < 0.05 vs normal‐vehicle rats; **P < 0.05 vs diabetic‐vehicle rats. DPSCs, dental pulp stem cells.
DPSC transplantation decreased monocytes/macrophages in sciatic nerves of diabetic rats
The number of monocytes/macrophages in the sciatic nerves was significantly increased in the control side of the diabetic rats compared with the normal rats (diabetes 31.50 ± 2.41/mm2, normal 16.81 ± 1.15/mm2, P < 0.01; Figure 3a). The transplantation of DPSCs significantly decreased monocytes/macrophages in the sciatic nerves compared with the control side of the diabetic rats (21.89 ± 0.61/mm2, P < 0.01). There was no significant difference in the number of monocytes/macrophages between the DPSC‐transplanted sides compared with the vehicle‐injected sides of normal rats (Figure 3b).
Figure 3.
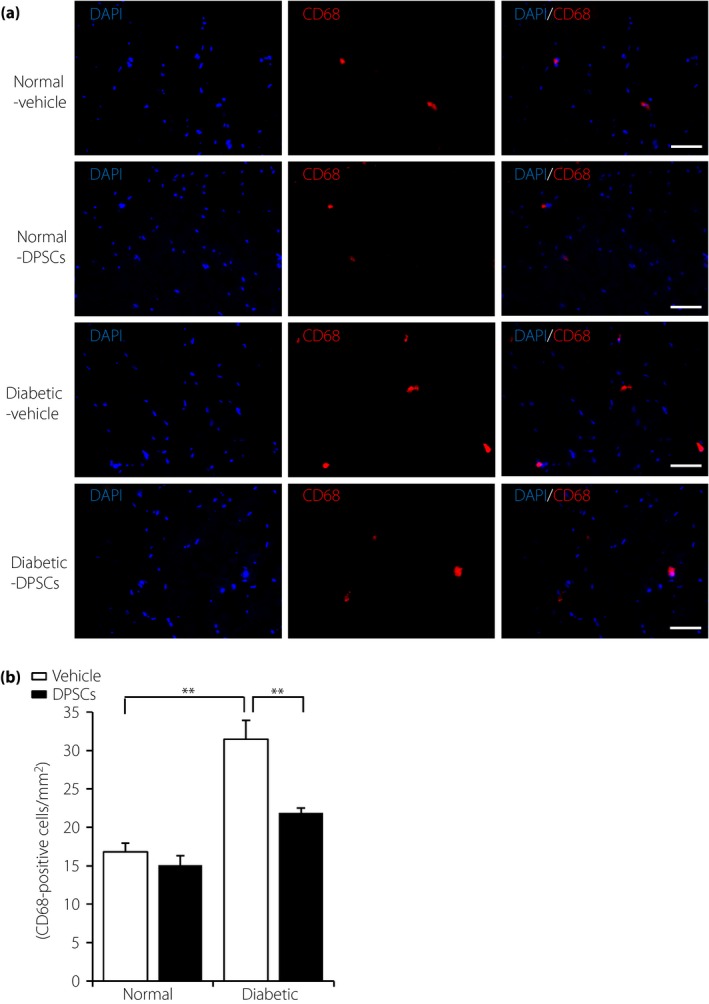
Dental pulp stem cell (DPSC) transplantation suppressed the number of CD68‐positive monocytes/macrophages in the sciatic nerves of the diabetic rats. (a) Representative photomicrographs of histological sections in the vehicle‐injected and the DPSCs‐transplanted sides of the sciatic nerves in the normal and diabetic rats. Monocytes/macrophages were detected by immunostaining for CD68. Scale bar, 50 μm. (b) Quantitative analyses for CD68‐positive cells/mm2 in the sciatic nerves of the normal and the diabetic rats (n = 5). Results are means ± standard error of the mean. **P < 0.01.
To investigate the correlation between the sciatic nerve inflammation and the neurophysiological activity, we evaluated the number of monocytes/macrophages and MNCV in each rat. We found a negative correlation between the number of monocytes/macrophages in the sciatic nerves and MNCV (Figure S1).
DPSC transplantation increased M2‐phenotype of macrophage in sciatic nerves in diabetic rats
The diabetic rats showed significant increases in pro‐inflammatory gene expressions by M1 macrophages, such as TNF‐α, IL‐1β and CD11c, in the control side of the sciatic nerves compared with the normal rats (Figure 4). Transplantation of DPSCs significantly decreased the gene expression of TNF‐α in sciatic nerves compared with the vehicle‐injected sides of diabetic rats (P < 0.01). Although the levels of the M2 macrophage‐expressed genes, CD206 and IL‐10, did not show significant differences between the normal and diabetic rats, the transplantation of DPSCs significantly increased the CD206 mRNA expression in diabetic rats (P < 0.01), together with an increased tendency of IL‐10.
Figure 4.
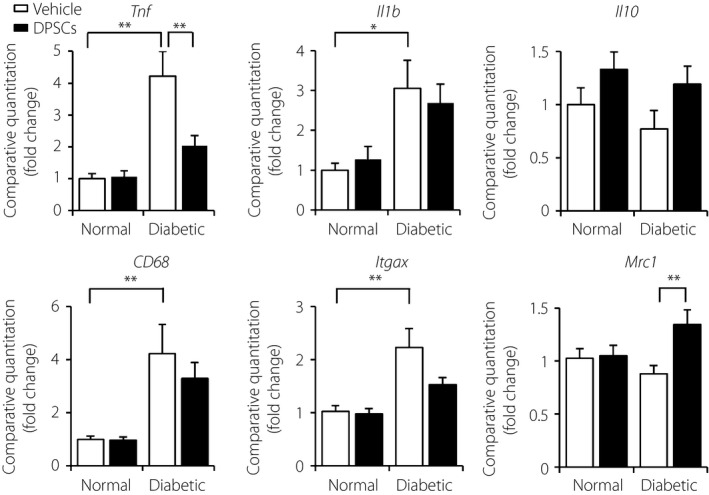
Effects of dental pulp stem cell (DPSC) transplantation on the inflammatory messenger ribonucleic acid expressions in sciatic nerves. Four weeks after the transplantation of DPSCs, messenger ribonucleic acid expressions of tumor necrosis factor‐α (TNF‐α; Tnf), interleukin (IL)‐1β (Il1b), IL‐10 (Il10), CD68 (CD68), CD11c (Itgax) and CD206 (Mrc1) in the sciatic nerves were evaluated by real‐time quantitative polymerase chain reaction (n = 8). Results are means ± standard error of the mean. **P < 0.01, *P < 0.05.
The immunohistological analysis showed that the population of CD68+CD206+ cells, which are thought to be immunosuppressive subpopulation of macrophages (M2 macrophages), was increased in the DPSC‐transplanted side of sciatic nerve (Figure S2).
DPSC‐conditioned media increased gene expressions of M2 macrophage markers in RAW264.7 cells
The effects of DPSC‐conditioned media on LPS‐induced pro‐inflammatory and anti‐inflammatory cytokine gene expressions in RAW264.7 cells were evaluated to determine the effects of factors secreted by DPSCs (Figure 5). The gene expressions of TNF‐α, IL‐1β and IL‐10 were upregulated in all cultures by LPS stimulation for 4 h. The level of LPS‐stimulated IL‐10 was significantly increased in the presence of DPSC‐conditioned media (P < 0.01). The gene expression of CD206 was unchanged by LPS stimulation, whereas it was significantly increased in the presence of DPSC‐conditioned media (P < 0.01). The LPS‐activated TNF‐α and IL‐1β levels were not affected by the DPSC‐conditioned media. The CD11c level was not affected by LPS or the DPSC‐conditioned media. These results show that DPSC‐conditioned media‐pretreatment followed by LPS‐activation does not affect M1 polarization, but increases M2 polarization of macrophages.
Figure 5.

Effects of dental pulp stem cell (DPSC)‐conditioned media (DPSCCM) on macrophage cell line, RAW264.7 cells. (a) Messenger ribonucleic acid expressions of undifferentiated RAW264.7 cells by DPSCCM pretreatment followed by lipopolysaccharide (LPS). After 12 h, the RAW264.7 cells were replaced with the serum‐free medium and pretreated with DPSCCM for 1 h. Cells were incubated with LPS for 4 h, messenger ribonucleic acid expressions of tumor necrosis factor‐α (TNF‐α; Tnf), interleukin (IL)‐1β (Il1b), IL‐10 (Il10), CD11c (Itgax) and CD206 (Mrc1) were investigated by real‐time quantitative polymerase chain reaction (n = 4). **P < 0.01. (b) RAW264.7 cells were pre‐exposed to 50 ng/mL LPS to differentiate into M1 macrophage. After 48 h, DPSCCM was added and the cells were incubated for 4 h. Messenger ribonucleic acid expressions were investigated by real‐time quantitative polymerase chain reaction (n = 6). **P < 0.01. (c) The effect of DPSCCM on the proliferation/viability of RAW264.7 cells was assessed by 3‐(4,5)‐dimethylthiahiazo(‐z‐y1)‐3,5‐di‐phenytetrazoliumromide and Cell Counting Kit‐8 assay. After cells were seeded in 96‐well plates and cultured for 12 h, the cells were incubated with DPSCCM for 3 days (n = 6). Results are means ± standard error of the mean. a.u., arbitrary unit.
To investigate the effect of DPSC‐conditioned media on M2 polarization from M1 macrophages, we added DPSC‐conditioned media to LPS‐pretreated RAW264.7 cells as M1‐like macrophages, and evaluated the change of M2/M1 phenotype expressions. As shown in Figure 5b, DPSC‐conditioned media significantly increased M2 polarization from M1‐like macrophages (Figure 5b).
DPSC‐conditioned media showed no effect on the proliferation/viability of RAW264.7 cells
The effect of DPSC‐conditioned media on RAW264.7 cell proliferation/viability was assessed by 3‐(4,5)‐dimethylthiahiazo(‐z‐y1)‐3,5‐di‐phenytetrazoliumromide and Cell Counting Kit‐8 assay. As shown in Figure 5c, the proliferation/viability in RAW264.7 cells was not enhanced by DPSC‐conditioned media.
Cultured DPSCs expressed angiogenic factors, neurotrophic factors and M2‐inducing factors
DPSCs expressed angiogenic factors and neurotrophic factors, such as bFGF, VEGF, NGF, NT3 and GDNF (Table 2). DPSCs also expressed M1 macrophage markers, such as TNF‐α and IL‐1β, and the M2 macrophage marker, IL‐10. In particular, DPSCs expressed a high level of M2 macrophage‐inducing factor M‐CSF (1.99 ± 0.42 × 10−1 expression level) compared with the M1 macrophage‐inducing factor GM‐CSF (1.20 ± 0.57 × 10−7 expression level). Other M2 macrophage‐inducing factors, such as IL‐4 and IL‐13, were not detected in cultured DPSCs, although DPSC‐expressed IL‐10 is also one of the M2 macrophage‐inducing factors.
Table 2.
Messenger ribonucleic acid expressions of cytokines in cultured dental pulp stem cells
| Arbitrary unit (×10−6) | Arbitrary unit (×10−6) | ||
|---|---|---|---|
| Tnf | 122.9 ± 27.7 | M‐CSF | 198597.6 ± 42302.4 |
| Il1b | 24.0 ± 4.5 | GM‐CSF | 0.1 ± 0.1 |
| Il6 | 70.7 ± 41.1 | bFGF | 22211.2 ± 4544.4 |
| MCP1 | 7099.3 ± 1564.3 | VEGFA | 428701.1 ± 183830.7 |
| Il10 | 3.2 ± 0.5 | NGF | 18566.9 ± 7306.2 |
| Il4 | ND | NTF3 | 13.2 ± 5.1 |
| Il13 | ND | GDNF | 4094.9 ± 1207.5 |
Data are mean ± standard error of the mean. bFGF, basic fibroblast growth factor; GDNF, glial cell line‐derived neurotrophic factor; GM‐CSF, colony stimulating factor 2; Il, interleukin; MCP1, monocyte chemoattractant protein‐1; M‐CSF, colony stimulating factor 1; ND, not detected; NGF, nerve growth factor; NTF3, neurotrophin‐3; Tnf, tumor nectrosis factor; VEGFA, vascular endothelial growth factor.
Discussion
The present study showed that the transplantation of DPSCs into the hindlimb skeletal muscles induced immunomodulatory effects on the sciatic nerves, which was accompanied by the recovery from the reduction in sciatic nerve conduction velocities, sciatic nerve blood flow and axonal circularity of the sural nerve in STZ‐induced diabetic rats. Furthermore, the in vitro study showed that DPSC‐conditioned media increased M2‐related gene expressions in the LPS‐stimulated macrophage cell line, RAW264.7 cells.
Accumulating evidence suggests the involvement of inflammatory processes in the pathogenesis of diabetic polyneuropathy9, 10, 11, 12. The local use of natural antagonists, soluble receptors, blocking antibodies and specific signaling or cytokine blockers could be promising treatment candidates15, 26. Yamagishi et al.27 showed that ED‐1 positive macrophages were increased in the sciatic nerves 12 weeks after STZ injection in rats, which was suppressed with the improvement of nerve conduction velocity by the treatment of peroxisome proliferator activated γ ligand. We showed in the present study that monocytes/macrophages and inflammatory gene expressions were increased in the sciatic nerves of the diabetic rats, which was ameliorated by DPSC transplantation with the increase of M2‐phenotype macrophages/gene expressions, as well as the recovery of nerve conduction velocity, nerve blood flow and axonal morphology in the diabetic rats. Shi et al.27 showed that the intra‐abdominal injection of TNF‐α receptor II : IgG Fc fusion protein ameliorated the nerve conduction velocity and neuropathological changes in diabetic rats15. These results suggest that the anti‐inflammation might become a crucial target for the treatment of diabetic polyneuropathy.
Macrophages are composed of two different phenotypes, classically activated M1 macrophages and alternatively activated M2 macrophages. M2 macrophages show low levels of pro‐inflammatory cytokines, but high levels of anti‐inflammatory cytokines, such as IL‐1028. Transplantation of DPSCs into the diabetic rats showed significant increases in M2 macrophage markers together with a significant decrease in M1 macrophage markers in sciatic nerves. We confirmed these effects using a culture system in which DPSC‐conditioned media markedly increased the CD206 and IL‐10 levels in LPS‐stimulated RAW264.7 cells, indicating that DPSC‐conditioned media‐pretreatment followed by LPS‐activation induced M2 polarization of macrophages. We also showed that DPSC‐conditioned media increased M2 polarization in LPS‐pretreated M1‐like macrophages. It has been shown in vitro that macrophages are polarized to the M1 state when stimulated by TNF‐α, interferon‐γ or other bacterial products, and to the M2 state when stimulated by IL‐4, IL‐13 and IL‐1029. In particular, IL‐10 reduced the production of pro‐inflammatory cytokines, which suggests that IL‐10 could have potent anti‐inflammatory activities30, 31. GM‐CSF and M‐CSF are also cytokines that modulate M1/M2 macrophage polarization32. In the present study, we identified that cultured DPSCs expressed M2‐inducing factors, such as IL‐10 and M‐CSF, suggesting that the transplanted DPSC‐produced M2‐inducing factors increased the M2 macrophages. These results showed that DPSC transplantation led to the differentiation or recruitment of M2 macrophages in the inflammatory condition of diabetic peripheral nerves.
Angiogenic factors, such as bFGF and VEGF, could be useful for the treatment of diabetic polyneuropathy33, 34. Stem/progenitor cells could have the ability to secrete bFGF and VEGF if transplanted for the treatment of diabetic polyneuropathy3, 4, 7, 24 . We showed here the high mRNA expressions of VEGF and bFGF with other cytokines and neurotrophic factors in cultured DPSCs. A reduction in neurotrophic factors plays a significant role in the pathogenesis of diabetic polyneuropathy35, 36, and treatment with NGF has been shown to exert beneficial effects on the structure and function in developing diabetic neuropathy37. Furthermore, NGF and NGF receptor in cells have attracted a great deal of attention regarding their effects on the immune response38, 39. Taken together, DPSC‐secreted angiogenic and neurotrophic factors must have added the beneficial effects of DPSC transplantation with its immunomodulatory effects in diabetic polyneuropathy.
Guimarães et al.40 intravenously transplanted DPSCs into STZ‐induced diabetic mice, and showed that DPSC transplantation improved both early diabetic hyperalgesia and late diabetic hypoalgesia. Because they only investigated thermal threshold, other physiological and pathological effects on intravenous DPSC transplantation are not known. As intravenous DPSC transplantation decreased blood glucose within 5 days after transplantation, the recovery from hyperglycemia might have a beneficial effect on thermal threshold. Here, we showed that the transplantation of DPSCs into the hindlimb skeletal muscles ameliorated the reduction in sciatic nerve conduction velocities, sciatic nerve blood flow and axonal circularity of sural nerve in STZ‐induced diabetic rats, without changing blood glucose. Differences in the therapeutic effects of DPSC transplantation on diabetic polyneuropathy should be evaluated in the future for clinical application.
In the present study, we transplanted freshly isolated and culture‐expanded DPSCs, and showed the increase of NCVs and SNBF in the diabetic rats. Although previous studies have already reported that various types of cell therapy were valid for diabetic polyneuropathy, it is important to develop non‐invasive cell‐therapy resources. It was also reported that even progenitor or stem cells taken from diabetic patients showed functional depression41, 42. DPSCs can be taken from the young before the onset of diabetes, because extraction of the third molar or premolar is normally carried out at a young age. We propose a strategy to use the cryopreserved DPSCs for the treatment of diabetic polyneuropathy.
In summary, we showed for the first time that the transplantation of DPSCs into diabetic rats has the anti‐inflammatory ability to modulate the proportions of M1/M2 macrophages in diabetic peripheral nerves, which might have beneficial effects in ameliorating diabetic polyneuropathy. The abundant immunomodulatory cytokines produced by DPSCs might play an important role in the anti‐inflammatory effects of the treatment for diabetic polyneuropathy.
Disclosure
The authors declare no conflict of interest.
Supporting information
Figure S1| The relationship between the monocytes/macrophages number in the sciatic nerves and motor nerve conduction velocity.
Figure S2| Double‐staining with CD68 and CD206 antibody in the sciatic nerves.
Acknowledgments
We thank Mr Brent Bell for reading the manuscript. This research was supported in part by a Grant‐in‐Aid for Scientific Research (21592506) from the Ministry of Education, Culture, Sports, Science and Technology (MEXT), and in part by the ‘Strategic Research AGU‐Platform Formation (2008–2012)’ Project for Private University from MEXT.
J Diabetes Investig 2016; 7: 485–496
References
- 1. Tesfaye S. Recent advances in the management of diabetic symmetrical polyneuropathy. J Diabetes Investig 2010; 2: 33–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Naruse K, Hamada Y, Nakashima E, et al Therapeutic neovascularization using cord blood‐derived endothelial progenitor cells for diabetic neuropathy. Diabetes 2005; 54: 1823–1828. [DOI] [PubMed] [Google Scholar]
- 3. Naruse K, Sato J, Funakubo M, et al Transplantation of bone marrow‐derived mononuclear cells improves mechanical hyperalgesia, cold allodynia and nerve function in diabetic neuropathy. PLoS One 2011; 6: e27458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Shibata T, Naruse K, Kamiya H, et al Transplantation of bone marrow‐derived mesenchymal stem cells improves diabetic polyneuropathy in rats. Diabetes 2008; 57: 3099–3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Himeno T, Kamiya H, Naruse K, et al Angioblast derived from ES cells construct blood vessels and ameliorate diabetic polyneuropathy in mice. J Diabetes Res 2015; 2015: 257230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Okawa T, Kamiya H, Himeno T, et al Transplantation of neural crest‐like cells derived from induced pluripotent stem cells improves diabetic polyneuropathy in mice. Cell Transplant 2013; 22: 1767–1783. [DOI] [PubMed] [Google Scholar]
- 7. Jeong JO, Kim MO, Kim H, et al Dual angiogenic and neurotrophic effects of bone marrow‐derived endothelial progenitor cells on diabetic neuropathy. Circulation 2009; 119: 699–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. King GL. The role of inflammatory cytokines in diabetes and its complications. J Periodontol 2008; 79: 1527–1534. [DOI] [PubMed] [Google Scholar]
- 9. Shepherd AJ, Downing JE, Miyan JA. Without nerves, immunology remains incomplete – in vivo veritas. Immunology 2005; 116: 145–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Schaper NC, Huijberts M, Pickwell K. Neurovascular control and neurogenic inflammation in diabetes. Diabetes Metab Res Rev 2008; 24(Suppl 1): S40–S44. [DOI] [PubMed] [Google Scholar]
- 11. Vincent AM, Callaghan BC, Smith AL, et al Diabetic neuropathy: cellular mechanisms as therapeutic targets. Nat Rev Neurol 2011; 7: 573–583. [DOI] [PubMed] [Google Scholar]
- 12. Herder C, Bongaerts BW, Rathmann W, et al Association of subclinical inflammation with polyneuropathy in the older population KORA F4 study. Diabetes Care 2013; 36: 3663–3670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yamakawa I, Kojima H, Terashima T, et al Inactivation of TNF‐alpha ameliorates diabetic neuropathy in mice. Am J Physiol Endocrinol Metab 2011; 301: E844–E852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Satoh J, Yagihashi S, Toyota T. The possible role of tumor necrosis factor‐alpha in diabetic polyneuropathy. Exp Diabetes Res 2003; 4: 65–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Shi X, Chen Y, Nadeem L, et al Beneficial effect of TNF‐α inhibition on diabetic peripheral neuropathy. J Neuroinflammation 2013; 10: 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nauta AJ, Fibbe WE. Immunomodulatory properties of mesenchymal stromal cells. Blood 2007; 110: 3499–3506. [DOI] [PubMed] [Google Scholar]
- 17. Von Bahr L, Batsis I, Moll G, et al Analysis of tissues following mesenchymal stromal cell therapy in humans indicates limited long‐term engraftment and no ectopic tissue formation. Stem Cells 2012; 30: 1575–1578. [DOI] [PubMed] [Google Scholar]
- 18. Loomans CJ, de Koning EJ, Staal FJ, et al Endothelial progenitor cell dysfunction: a novel concept in the pathogenesis of vascular complications of type 1 diabetes. Diabetes 2004; 53: 195–199. [DOI] [PubMed] [Google Scholar]
- 19. Gong M, Yu B, Wang YG, et al Bone marrow rejuvenation. An excellent potential therapy for age‐related endothelial dysfunction. Circ J 2013; 77: 2886–2888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Alge DL, Zhou D, Adams LL, et al Donor‐matched comparison of dental pulp stem cells and bone marrow‐derived mesenchymal stem cells in a rat model. J Tissue Eng Regen Med 2010; 4: 73–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hata M, Naruse K, Ozawa S, et al Mechanical stretch increases the proliferation while inhibiting the osteogenic differentiation in dental pulp stem cells. Tissue Eng Part A 2013; 19: 625–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zhang W, Walboomers XF, Shi S, et al Multilineage differentiation potential of stem cells derived from human dental pulp after cryopreservation. Tissue Eng 2006; 12: 2813–2823. [DOI] [PubMed] [Google Scholar]
- 23. Papaccio G, Graziano A, d'Aquino R, et al Long‐term cryopreservation of dental pulp stem cells (SBP‐DPSCs) and their differentiated osteoblasts: a cell source for tissue repair. J Cell Physiol 2006; 208: 319–325. [DOI] [PubMed] [Google Scholar]
- 24. Hata M, Omi M, Kobayashi Y, et al Transplantation of cultured dental pulp stem cells into the skeletal muscles ameliorated diabetic polyneuropathy: therapeutic plausibility of freshly‐isolated and cryopreserved dental pulp stem cells. Stem Cell Res Ther 2015; 6: 162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Schmittgen TD, Livak KJ. Analyzing real‐time PCR data by the comparative C(T) method. Nat Protoc 2008; 3: 1101–1108. [DOI] [PubMed] [Google Scholar]
- 26. Skundric DS, Lisak RP. Role of neuropoietic cytokines in development and progression of diabetic polyneuropathy: from glucose metabolism to neurodegeneration. Exp Diabetes Res 2003; 4: 303–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yamagishi SI, Ogasawara S, Mizukami H, et al Correction of protein kinase C activity and macrophage migration in peripheral nerve by pioglitazone, peroxisome proliferator activated‐gamma‐ligand, in insulin‐deficient diabetic rats. J Neurochem 2008; 104: 491–499. [DOI] [PubMed] [Google Scholar]
- 28. Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest 2007; 117: 175–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Martinez FO, Sica A, Mantovani A, et al Macrophage activation and polarization. Front Biosci 2008; 13: 453–461. [DOI] [PubMed] [Google Scholar]
- 30. Moore KW, de Waal Malefyt R, Coffman RL, et al Interleukin‐10 and the interleukin‐10 receptor. Annu Rev Immunol 2001; 19: 683–765. [DOI] [PubMed] [Google Scholar]
- 31. Kalampokis I, Yoshizaki A, Tedder TF. IL‐10‐producing regulatory B cells (B10 cells) in autoimmune disease. Arthritis Res Ther 2013; 15(Suppl 1): S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Fleetwood AJ, Lawrence T, Hamilton JA, et al Granulocyte‐macrophage colony‐stimulating factor (CSF) and macrophage CSF‐dependent macrophage phenotypes display differences in cytokine profiles and transcription factor activities: implications for CSF blockade in inflammation. J Immunol 2007; 178: 5245–5252. [DOI] [PubMed] [Google Scholar]
- 33. Xu W, Wang X, Xu G, et al Light‐induced retinal injury enhanced neurotrophins secretion and neurotrophic effect of mesenchymal stem cells in vitro. Arq Bras Oftalmol 2013; 76: 105–110. [DOI] [PubMed] [Google Scholar]
- 34. Zhang H, Issekutz AC. Growth factor regulation of neutrophil‐endothelial cell interactions. J Leukoc Biol 2001; 70: 225–232. [PubMed] [Google Scholar]
- 35. Andreassen CS, Jakobsen J, Flyvbjerg A, et al Expression of neurotrophic factors in diabetic muscle–relation to neuropathy and muscle strength. Brain 2009; 132: 2724–2733. [DOI] [PubMed] [Google Scholar]
- 36. Kang TH, Moon E, Hong BN, et al Diosgenin from Dioscorea nipponica ameliorates diabetic neuropathy by inducing nerve growth factor. Biol Pharm Bull 2011; 34: 1493–1498. [DOI] [PubMed] [Google Scholar]
- 37. Unger JW, Klitzsch T, Pera S, et al Nerve growth factor (NGF) and diabetic neuropathy in the rat: morphological investigations of the sural nerve, dorsal root ganglion, and spinal cord. Exp Neurol 1998; 153: 23–34. [DOI] [PubMed] [Google Scholar]
- 38. Prencipe G, Minnone G, Strippoli R, et al Nerve growth factor downregulates inflammatory response in human monocytes through TrkA. J Immunol 2014; 192: 3345–3354. [DOI] [PubMed] [Google Scholar]
- 39. Sofroniew MV, Howe CL, Mobley WC. Nerve growth factor signaling, neuroprotection, and neural repair. Annu Rev Neurosci 2001; 24: 1217–1281. [DOI] [PubMed] [Google Scholar]
- 40. Guimarães ET, Cruz GDS, Farias de Almeida T, et al Transplantation of stem cells obtained from murine dental pulp improves pancreatic damage, renal function, and painful diabetic neuropathy in diabetic type 1 mouse model. Cell Transplant 2013; 22: 2345–2354. [DOI] [PubMed] [Google Scholar]
- 41. Kim H, Han JW, Lee JY, et al Diabetic mesenchymal stem cells are ineffective for improving limb ischemia due to their impaired angiogenic capability. Cell Transplant 2014; 8: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Nakamura N, Naruse K, Kobayashi Y, et al High glucose impairs the proliferation and increases the apoptosis of endothelial progenitor cells by suppression of Akt. J Diabetes Investig 2011; 2: 262–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1| The relationship between the monocytes/macrophages number in the sciatic nerves and motor nerve conduction velocity.
Figure S2| Double‐staining with CD68 and CD206 antibody in the sciatic nerves.


