Abstract
Aims/Introduction
Oxidative stress has a key role in the pathogenesis of diabetes. Propolis and its constituents have a wide range of medicinal properties against oxidative stress. In the present study, we evaluated the anti‐oxidant effects of ethanolic extracts of propolis on kidneys in diabetes mellitus rats.
Materials and Methods
A total of 40 male Wistar rats were randomly divided into the following five groups: control, diabetes mellitus, diabetes mellitus with vehicle treatment, diabetes mellitus with propolis treatment (100 mg/kg) and diabetes mellitus with propolis treatment (200 mg/kg). Diabetes mellitus in rats was induced by intraperitoneal injection of streptozotocin (60 mg/kg). Diabetic groups were treated with vehicle or ethanolic extracts of Iranian propolis for 6 weeks. Serum concentration of malondialdehyde, superoxide dismutase and glutathione peroxidase were measured.
Results
The results showed that Iranian propolis significantly inhibited bodyweight loss in diabetes mellitus rats. The propolis extracts significantly reduced serum glucose levels and kidney weight in diabetes mellitus rats (P < 0.001). Furthermore, propolis extracts significantly reduced the malondialdehyde content, and increased the activity of superoxide dismutase and glutathione peroxidase (P < 0.001) along with the total anti‐oxidant activity in the kidney tissue of diabetes mellitus rats. In the kidneys of the diabetes mellitus and vehicle group, the glomerular basement membrane thickness and glomerular area were significantly increased. Treatment of diabetes mellitus rats with the propolis extract significantly reduced the glomerular basement membrane thickness and glomerular area.
Conclusions
The present study results showed that the Iranian propolis extract could enhance the anti‐oxidant levels and histopathological changes in the kidneys of rats. The final results showed that most of the favorable effects of propolis are mediated by a reduction of blood glucose levels in diabetic animals.
Keywords: Diabetes mellitus, Oxidative stress, Propolis
Introduction
Propolis is a resinous substance that honeybees collect from the buds of certain trees, sap flows or other botanical sources. It is used as a sealant for unwanted open spaces in the hive, and is used as a traditional herbal medicine in many countries1, 2. More than 300 compounds, such as phenolic compounds (flavonoids and aromatic compounds), terpenes, cinnamic acid, caffeic acid, several esters and essential oils, have been detected in propolis, with their structures being strongly dependent on the collection location, time and plant source2. Current studies have shown that propolis has several medical properties, including antibacterial, antifungal, antiviral, immunoregulatory, anti‐oxidative, antitumor, hepatoprotective and anti‐inflammatory. However, the pharmacological activity of propolis is highly variable depending on its geographic origin1, 2, 3. Diabetic nephropathy is a major microvascular complication of diabetes, representing the leading cause of end‐stage renal disease in the world, and a major cause of morbidity and mortality in both type 1 and type 2 diabetes patients. Clinical hallmarks of diabetic nephropathy include a progressive increase in urinary albumin excretion and a decrease in glomerular filtration rate, which occurs in association with an increase in blood pressure, ultimately leading to end‐stage renal failure1. These renal functional changes develop as a consequence of structural abnormalities, including glomerular basement membrane thickening, mesangial expansion with extracellular matrix accumulation, changes in glomerular epithelial cells (podocytes), including a decrease in number and/or density, podocyte foot process broadening and effacement, glomerulosclerosis, and tubulointerstitial fibrosis4. Type 1 diabetes accounts for 5–10% of diabetic cases2. Globally, the number of people with type 1 diabetes is unknown, although it is estimated that approximately 80,000 children develop the disease each year. Within the USA, the number of affected persons is estimated at 1–3 million2, 3, 4, 5, 6, 7, 8. The development of new cases varies by country and region; the lowest rates appear to be in Japan and China, with approximately one person per 100,000 per year; and the highest rates are found in Scandinavia, where it is closer to 35 new cases per 100,000 per year. The USA and northern Europe fall somewhere in between with eight to 17 new cases per 100,000 per year2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12 .Tao et al.3 estimated that in the USA, type 1 diabetes is responsible for $14.4 billion in medical costs each year. Type 2 diabetes (formerly non‐insulin‐dependent diabetes mellitus or adult‐onset diabetes) is a metabolic disorder that is characterized by hyperglycemia (high blood glucose) in the context of insulin resistance and hyperinsulinemia. This is in contrast to type 1 diabetes, in which there is an absolute lack of insulin as a result of a breakdown of islet cells in the pancreas3, 4, 5, 6, 7. Mohammadzadeh et al.5 showed that Iranian propolis as a natural source of anti‐oxidant compounds can be used to prevent free radical‐related diseases. Their research showed that ethanolic extract of Iranian propolis exhibited the highest ferric‐reducing ability of plasma (FRAP) value. The FRAP assay was used for assessing the ‘anti‐oxidant power’ of propolis. Flavonoid and various phenolics are the most important active constituents in propolis. It has been shown to be capable of scavenging free radicals, and thereby protecting lipids and other compounds, such as vitamin C, from being oxidized or destroyed during oxidative damage6. Iranian propolis has the highest anti‐oxidative activity, with high amounts of phenolics and poly phenolics compounds5.
Studies on Chinese and Brazilian propolis extracts showed that propolis causes a reduction of blood glucose and lipid levels in diabetes mellitus rats6. According to another study, the anti‐oxidant activity of propolis is fourfold more potent than that of vitamin E, and is 25–50‐fold more potent than that of wild fruits7. Because of the serious complications of diabetes mellitus, research has focused on herbal medicine that could control glucose levels and lower the risk of complications5. Previous findings have shown that the concentration of propolis compounds greatly depends on the geographic location, environment and type of vegetation3, 4, 5. To date, no studies have reported the effects of Iranian propolis on diabetes mellitus complications. The purpose of the present study was to assess the effects of the ethanolic extract of Iranian propolis on histopathological changes in the kidney and anti‐oxidant effects in streptozotocin‐induced diabetic rats.
Materials and methods
Animals
All procedures were carried out in the laboratory of biochemistry and stem cells research center of Semnan University of Medical Sciences (SUMS), Semnan, Iran. A total of 40 male adult Wistar rats (bodyweight 200–220 g) were obtained from the laboratory animal center of SUMS. The rats were maintained under constant conditions: free access was allowed to standard diet and water, controlled temperature (22 ± 2°C), relative humidity (55–60%), and light period (12‐h light/12‐h dark) in plastic cages. All animals were acclimatized for a minimum period of 2 weeks before the beginning of the study. Experimental procedures were approved by the ethics committee of SUMS, Semnan, Iran.
Experimental Design and Induction of Type 1 Diabetes Mellitus
A total of 40 rats were randomly assigned into five groups (8 rats per group): a non‐diabetic control group (CO), an untreated diabetes mellitus group (DM), propolis vehicle‐treated diabetic vehicle group (DV), ethanolic extracts of propolis (EEP) 100 mg/kg‐treated diabetes mellitus group (DP100) and EEP 200 mg/kg‐treated diabetes mellitus group (DP200). Rats received propolis vehicle (10% ethanol, v/v) and EEP at dose levels of 100 and 200 mg/kg bodyweight by oral gavages, daily for 6 weeks, starting after 3 days of STZ injection. For drug therapy purposes, diabetic and control animals were age‐matched.
Type 1 diabetes mellitus in animals was induced by a single‐dose intraperitoneal injection of freshly prepared 60 mg/kg streptozotocin (STZ; Sigma‐Aldrich Co., St Louis, MO, USA) dissolved in a 0.1 mol/L citrate buffer (pH = 4.8)8. Hyperglycemia was confirmed 48 h after the STZ injection by measuring tail vein blood glucose levels using a blood glucose monitoring kit (Glucocard 01; Arkray Factory Inc., Shiga, Japan). Only animals with mean plasma glucose levels >250 mg/dL were accepted as being diabetes mellitus5, 6, 7.
Drugs and Reagents
Propolis used in the present study was collected from beehives located in different parts of the Semnan province and verified by an agricultural organization. Extracts were prepared according to the method of Greenaway9. In summary, the major components of propolis were cut into small pieces, mixed (25 g) with 250 mL of 80% ethanol and incubated at room temperature for 48 h with shaking (4 g). The extract was clarified twice by Whatman grade 42 filter paper, and ethanol was evaporated using a rotary vacuum evaporator to obtain the purified propolis extract. The EEP concentrations were measured and diluted to the required dilutions (weight to volume) using 10% ethanol. The extracts were stored at 2–8°C under protective light conditions and warmed to room temperature just before injecting9.
Determination of Total Polyphenol Content
Propolis extract was re‐dissolved in 95% distilled water at a concentration of 50 mg/mL. Water extract (0.1 mL) was diluted with 95% distilled water (0.9 mL) and mixed with 5 mL of 10‐fold diluted solution of 2N Folin–Ciocalteu reagent (Sigma, Dorset, UK). Four milliliters of saturated sodium carbonate solution were added to the mixture, which was then stirred. The absorbance of the reaction mixture was measured at 765 nm after 3 h. Caffeic acid (Sigma, Munich, Germany) was used as a standard compound in the range of 100– 500 μg/mL concentration to construct a standard curve10.
Measurement of Anti‐Oxidant Activity
A total of 24 h after EEP treatments, all the rats were anesthetized by administering ketamine/xylazine (75/20 mg/kg) intraperitoneally. After opening the abdominal cavity, blood samples were collected from their hearts for serum glucose measurements. The left kidney was removed, washed with physiological saline, cleared of renal fat, weighed and homogenized (10% w/v) in ice‐cold 1.15 mol/L KCl. The homogenates were centrifuged at 20,000 g for 10 min at 4°C12. The supernatants were collected and used for assessment of FRAPS, MDA levels, SOD and GPx (Randox Laboratories, Shanghai, China) activities11, 12, 13.
Statistical Analysis
All data are expressed as mean ± standard error of the mean. The quantitative were analyzed using the spss version 16 software (SPSS, Chicago, IL, USA). Data from experiments with more than two independent variables were analyzed using analysis of variance (anova) followed by the Tukey–Kramer post‐hoc tests. Regression analysis was used for evaluation of correlation between propolis level and anti‐oxidant parameters adjusted for blood glucose. Spearman's analysis was used for evaluation of correlation between blood glucose with propolis (100, 200), and correlation between blood glucose and anti‐oxidant parameters. Statistical differences were considered significant when P < 0.05 (two‐tailed).
Results
Total Polyphenol Content (g/100 g extract)
Total polyphenol content, determined by Folin–Ciocalteu colorimetric methods was (6.4 g/100 g ± 0.03).
Effects of Propolis on Bodyweight and Kidney Weight
As shown in Table 1, there was a marked reduction in the bodyweights of diabetes mellitus rats compared with that of the control group (260.75 ± 7.9 g vs 367.60 ± 9.5 g, P < 0.05). In addition, kidney weights in the diabetes mellitus groups were significantly higher compared with that of the control group (1.07 ± 0.09 g vs 0.82 ± 0.04 g, P < 0.05). Treatment with EEP (100 and 200 mg/kg) resulted in a significant increase in the bodyweight (313.00 ± 7.82 g and 330.17 ± 6.21 g) and reduction in the kidney weight compared with untreated or vehicle‐treated diabetes mellitus rats (0.94 ± 0.11 g and 0.91 ± 0.07 g vs 1.07 ± 0.09 g, respectively, P < 0.05).
Table 1.
Effect of ethanol extract of propolis on the bodyweight and kidney weight of diabetic rats
| Groups | Bodyweight (g) | Kidney weight (g) | |
|---|---|---|---|
| First day | 40th day | ||
| CO | 217.2 ± 2.24 | 367.6 ± 9.57 | 0.82 ± 0.04 |
| DM | 218.75 ± 3.31 | 260.75 ± 7.92* | 1.07 ± 0.09* |
| DV | 219.25 ± 1.71 | 266.75 ± 8.47* | 1.02 ± 0.05* |
| DP100 | 219.60 ± 2.46 | 313 ± 7.82† | 0.94 ± 0.11 |
| DP200 | 220 ± 1.78 | 330.17 ± 6.21† | 0.91 ± 0.07† |
Data are mean ± standard error of the mean. P < 0.05. *Compared with the control (CO) group. †Compared with the diabetes mellitus (DM) group and diabetic vehicle (DV) group. Bodyweights were measured at the beginning (1st day) and end (40th day) of the experiment. DP100, diabetes mellitus treated with the ethanolic extract of propolis 100 mg/kg; DP200, diabetes mellitus treated with the ethanolic extract of propolis 200 mg/kg.
Effects of Propolis on Blood Glucose
As shown in Figure 1, 6 weeks after STZ administration, type 1 diabetes mellitus rats in both the DM and DV groups had hyperglycemia compared with the control rats (546 ± 49.3 mg/dL and 522 ± 51.1 mg/dL vs 96.67 ± 6.6 mg/dL, P < 0.001). Treatment with EEP (100 and 200 mg/kg) significantly reduced the serum glucose levels in diabetic rats (297.43 ± 68.3 and 264.14 ± 47.2, P < 0.01). Correlation analysis showed a significant correlation between blood glucose and level of propolis in groups of DP100 and DP 200 (P < 0.001).
Figure 1.
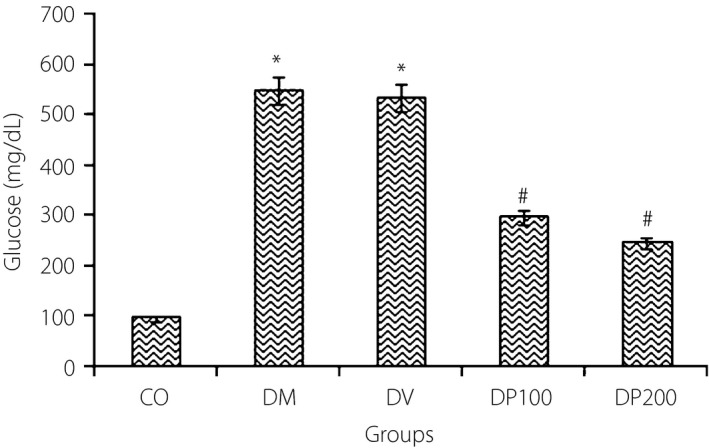
Changes in blood glucose levels in control (CO), diabetes mellitus (DM), diabetic vehicle (DV) group, and diabetes mellitus treated rats with the ethanolic extract of propolis100 and 200 mg/kg (DP100, DP200). The data are expressed as mean ± standard error of the mean. *Compared with the CO group. #Compared with the DM group and DV group. P < 0.05
Effects of Propolis on Glomerular Area and Basement Membrane Thickness
The glomerular area and glomerular basement membrane (GBM) thickness in diabetes mellitus and diabetes vehicle rats with and without adjusting are shown in Table 2. The GBM thickness was significantly higher in the diabetes mellitus and diabetes vehicle rats than in the control group (0.67 ± 0.07 μm and 0.66 ± 0.07 μm vs 0.47 ± 0.07 μm, P < 0.001). EEP significantly decreased the GBM thickness with both doses of 100 and 200 mg/kg in the treated diabetes mellitus group (100 and 200 mg/kg) compared with the untreated or diabetes mellitus rats (0.49 ± 0.05 μm and 0.47 ± 0.04 μm vs 0.67 ± 0.01 μm, P < 0.001). The results also show a dose of 200 mg/kg EEP causes a significant reduction in GA compared with the untreated or diabetes mellitus rats (3641.61 ± 12.6 μm vs 4641.75 ± 11.9 μm). There was no significant difference between groups in terms of GBM thickness and GA adjusting for blood glucose level (Table 2).
Table 2.
Effects of ethanol extract of propolis on renal parameters/anti‐oxidant activities indexes with and without adjusting for plasma glucose levels in experimental groups
| Without adjusting | Adjusting for plasma glucose level | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Index | Group | Mean | SD | P * | (95% Confidence interval) | ||||
| Mean | SE | Lower Bound | Upper B | P ** | |||||
| GA | CO | 3619.30 | 507.21 | 3881.21 | 576.74 | 268.81 | 5080.60 | ||
| DM | 4641.75 | 543.57 | 4381.01 | 574.53 | 3186.21 | 5575.82 | |||
| DV | 4723.17 | 380.08 | < 0.001 | 4558.16 | 400.31 | 3725.65 | 5390.66 | 0.131 | |
| DP100 | 4536.72 | 382.34 | 4566.08 | 206.37 | 4136.90 | 4995.26 | |||
| DP200 | 3641.60 | 538.57 | 3784.78 | 294.72 | 3135.87 | 4361.70 | |||
| GBM | CO | 0.472 | 0.051 | 0.537 | 0.076 | 0.378 | 0.695 | ||
| DM | 0.674 | 0.075 | 0.609 | 0.076 | 0.451 | 0.768 | |||
| DV | 0.668 | 0.078 | <0.001 | 0.627 | 0.053 | 0.517 | 0.737 | 0.370 | |
| DP100 | 0.495 | 0.056 | 0.502 | 0.27 | 0.445 | 0.559 | |||
| DP200 | 0.471 | 0.056 | 0.498 | 0.039 | 0.417 | 0.579 | |||
| MDA | CO | 1.900 | 0.122 | 2.194 | 0.140 | 1.902 | 2.486 | ||
| DM | 2.640 | 0.0114 | 2.347 | 0.140 | 2.057 | 2.638 | |||
| DV | 2.520 | 0.109 | <0.001 | 2.335 | 0.97 | 2.132 | 2.537 | 0.648 | |
| DP100 | 2.316 | 0.160 | 2.350 | 0.050 | 2.245 | 2.454 | |||
| DP200 | 2.133 | 0.121 | 2.254 | 0.072 | 2.104 | 2.403 | |||
| FRAP | CO | 2.780 | 0.109 | 2.491 | 0.170 | 2.137 | 2.845 | ||
| DM | 2.100 | 0.158 | 2.388 | 0.170 | 2.035 | 2.740 | |||
| DV | 2.200 | 0.141 | <0.001 | 2.382 | 0.118 | 2.137 | 2.628 | 0.097 | |
| DP100 | 2.266 | 0.206 | 2.234 | 0.061 | 2.108 | 2.361 | |||
| DP200 | 2.550 | 0.104 | 2.432 | 0.087 | 2.251 | 2.613 | |||
| SOD | CO | 5.580 | 0.277 | 5.161 | 0.254 | 4.633 | 5.688 | ||
| DM | 4.150 | 0.180 | 4.744 | 0.176 | 4.378 | 5.110 | |||
| DV | 4.480 | 0.342 | 4.744 | 0.176 | 4.378 | 5.110 | |||
| DP100 | 4.683 | 0.172 | <0.001 | 4.636 | 0.091 | 4.448 | 4.825 | 0.027 | |
| DP200 | 5.300 | 0.089 | 5.129 | 0.130 | 4.859 | 5.398 | |||
| GPx | CO | 16.3400 | 1.11490 | 14.713 | 0.771 | 13.111 | 16.316 | ||
| DM | 11.7600 | 0.30496 | 13.379 | 0.771 | 11.783 | 14.976 | |||
| DV | 12.1400 | 1.03102 | <0.001 | 13.165 | 0.535 | 12.052 | 14.277 | 0.490 | |
| DP100 | 14.083 | 0.44907 | 13.901 | 0.276 | 13.328 | 14.474 | |||
| DP200 | 15.1333 | 0.25820 | 14.468 | 0.394 | 13.349 | 15.287 | |||
P < 0.05. *One way analysis of variance. **Two way analysis of variance. Correlation between renal parameters/anti‐oxidant activities indexes with and without adjusting for level of glucose in all experimental groups. CO, control; DM, diabetes mellitus; DV, diabetic vehicle; DP100, diabetes mellitus treated with the ethanolic extract of propolis 100 mg/kg; DP200, diabetes mellitus treated with the ethanolic extract of propolis 200 mg/kg; FRAP, ferric‐reducing ability of plasma; GBM, glomerular basement membrane (μm); GPx, glutathione peroxidase; MDA, malondialdehyde; SD, standard deviation; SE, standard error; SOD, superoxide dismutase.
Effects of Propolis on the Lipid Peroxidation and Anti‐Oxidant Activity
The EEP showed a strong effect on lipid peroxidation and anti‐oxidant parameters. A significant increase in MDA and reduction in SOD, GPx and FRAP concentration were observed in the diabetes mellitus rats as compared with the control group (P < 0.001; Figure2). The increase in MDA concentration in renal tissue of the diabetic animals showed an exacerbated oxidative stress. The treatment of rats with EEP (200 mg/kg) causes a significant reduction in MDA. Furthermore, treatment with EEP (100 and 200 mg/kg) followed by an upregulation of SOD and GPx levels in the kidney tissue compared with the untreated or vehicle‐treated diabetes mellitus rats (DM and DV groups) (P < 0.001). The FRAP concentration in renal tissue was significantly increased only in rats that received EEP with doses of 200 mg/kg (P < 0.001). Correlation analysis showed a significant correlation between blood glucose and anti‐oxidant parameters (P < 0.05). Although without adjusting between groups, all experimental indexes were significant (P < 0.001), but with adjusting blood glucose level, the significance for all of variables except SOD (P = 0.027) were impaired (Table 2).
Figure 2.
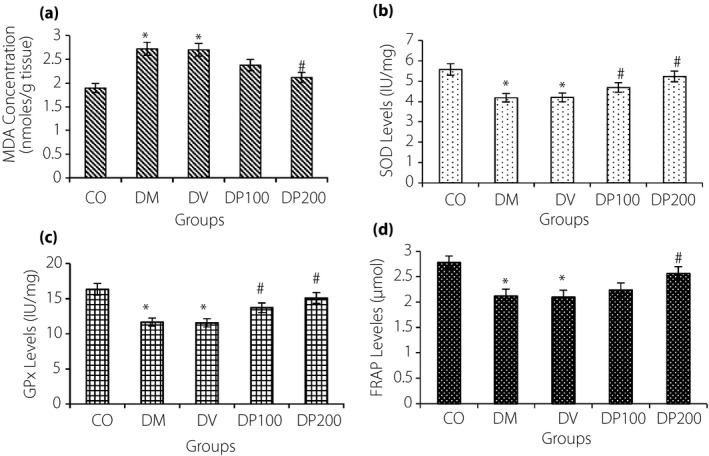
The unadjusted changes in (a) malondialdehyde levels (MDA), (b) superoxide dismutase (SOD), (c) glutathione peroxidase (GPx) and (d) ferric‐reducing anti‐oxidant power (FRAP) of renal tissue in control (CO), diabetes mellitus (DM) and diabetic vehicle (DV) groups, and diabetes mellitus‐treated rats with the ethanolic extract of propolis 100 and 200 mg/kg (DP100, DP200). The data are expressed as mean ± standard error of the mean. *Compared with the CO group. #Compared with the DM group and DV group. P < 0.05
Discussion
Hyperglycemia is considered a leading cause of diabetes, and strategies to improve glycemic control can reduce the incidence of its complications12, 13. In the present study, Iranian propolis suppressed the blood glucose levels in diabetes mellitus rats. Propolis is rich in anti‐oxidants, such as polyphones and flavonoids14, 15. The composition of propolis depends on the vegetation of the area from where it was collected. Colorimetric methods are convenient and appropriate for routine analysis of phenolics16. Anti‐oxidative activity has also been shown in propolis. It is proposed that strong anti‐oxidative activity occurs in propolis with high amounts of phenolic compounds, and weak activity occurs in low amounts17. The total polyphone content of ethanol extracts of this sample was 6.4 g/100 ± 0.03 g. According to other studies, the total polyphone content of this extract is more plentiful. It has been reported that flavonoids reduce blood glucose levels.
Hyperglycemia is an important factor for the intense oxidative stress in diabetes, and the toxicity induced by glucose autoxidation is likely to be one of the important sources of reactive oxygen species18. Additionally, lipid peroxidation plays an important role in the production of free radicals and oxidative stress in diabetes17. Several intra‐ and extracellular anti‐oxidant defense mechanisms counteract the destructive effects of free radicals by attenuating or omitting their activities6. However, in diabetes mellitus, the oxidative stress exceeds the body's anti‐oxidant defense mechanisms. Although oxidative stress and free radicals play a significant role in diabetic complications19, and treatment with anti‐oxidants reduces these complications20, recent studies have shown that propolis has hypoglycemic, hypolipidemic and antioxidant activity21, which can be used to prevent or postpone the incidence of diabetic complications. Its hypoglycemic activity has been attributed to inhibition of intestinal maltase activity, preventing the rise of blood glucose after carbohydrate intake. Propolis has also been reported to enhance the anti‐oxidant defense system10 and to protect pancreatic tissue21.
All diabetic rats in the present study experienced a significant reduction of lipid peroxidation levels nearing normal control values after treatment with propolis alone, which suggest that propolis prevents deterioration of β‐cell function. This finding confirms the earlier report that propolis causes partial restoration of β‐cell function16.
The glycemic control achieved by propolis treatment could be due to the stimulation of glucose uptake by peripheral tissues, inhibition of its release in circulation22 or reduced glucose absorption in the gut23.
The present results are in agreement with the findings reported by Fulianga et al 24. Studies suggest that propolis could prevent hyperglycemia by activating glucose uptake and insulin‐sensitive glucose transporter translocation in the skeletal muscle cells, as well as by inhibiting glucose release from the liver25.
The present data showed marked reduction in the total bodyweight, as well as an increase in the kidney weight of the diabetes mellitus group compared with that of the normal group. Propolis treatment showed a significant amelioration in both body and kidney weights. This improvement might be as a result of decreased glucose levels after treatment with propolis. Hyperglycemia causes accumulation of extracellular matrix proteins from mesangial cells, thickening of glomerular basement membrane, dilatation of the urinary space26, hypertrophy, hyperplasia and fibrosis of the medullary tube, and an increase in the kidney weight in diabetic rats27. It is likely that anti‐oxidants in the propolis extract, such as polyphenols and flavonoids, exert protection against kidney damage in diabetes mellitus rats by reducing blood glucose. Yajing et al.28 reported a similar observation earlier. In diabetic conditions, several pathways including polyol pathway, hexosamine pathway, production of advanced glycation end‐product pathway and protein kinase C pathway, are known to be activated29. Overexpression of SOD might block the mitochondrial electron‐transport chain resulting in a reduction of reactive oxygen species and deactivation of these pathways. In the present study, we observed increased renal MDA levels, and decreased FRAP, SOD and GPx activities in diabetes mellitus rats compared with the control group. Treatment of diabetes mellitus rats with propolis significantly improved these parameters. According to adjusting blood glucose and the significant correlation between blood glucose level, and each of anti‐oxidant parameters and differences between these parameters in experimental groups, we guess amelioration of these factors is as a result of reducing blood glucose; but it seems that other mechanisms have effects on the level of SOD. This is in agreement with the findings reported by Osama et al.30 Increased oxidative stress and reduction in anti‐oxidant levels have been reported in diabetes mellitus conditions27. MDA is one of the most common markers of lipid peroxidation, and an increase in MDA levels indicates impairment of non‐enzymatic and enzymatic anti‐oxidant defense mechanisms. Free radicals can react with unsaturated fatty acids and might cause lipid peroxidation31. Earlier studies have shown that in kidney tissue, MDA levels are significantly reduced in the presence of anti‐oxidants32. Propolis possesses strong anti‐oxidant activity, and thus can prevent lipid peroxidation and MDA levels in animal models and patients30. A previous study reported that the anti‐oxidative capacity of propolis is partly due to its high flavonoids and caffeic acid phenethyl ester, a flavonoid like compound28. Similar results were reported in another study that showed caffeic acid phenethyl ester as the major components of honeybee propolis, and showed its protective role in oxidative stress in the brain and cardiac tissues of diabetes mellitus rats33. Treatment with caffeic acid phenethyl ester in diabetes mellitus rats showed a marked increase in the activities of SOD, GPx and catalase, along with a reduction in MDA content in the cardiac tissue24.
To further investigate the mechanism of action of propolis, we measured the anti‐oxidative capacity and the activity of FRAP. We examined the total anti‐oxidant activity in the kidney tissue using FRAP as an indicator of the strength of non‐enzymatic anti‐oxidants. The results showed that in a diabetes mellitus rat kidney, there is a significant decrease in the anti‐oxidant activity (FRAP) in the DM group, and treatment with the propolis extract enhanced the anti‐oxidant capacity of the FRAP. SOD neutralizes the superoxide anions by converting them into hydrogen peroxide, and GPx reduces hydrogen peroxide to water; together, SOD and GPx serve as an anti‐oxidant defense mechanism34. In a diabetes mellitus rat kidney, both SOD and GPx levels were reduced, indicating an increase in superoxide anions and oxidative stress35. Restoration of the activities of SOD, GPx, FRAP and reduction of the content of MDA as a result of treatment with propolis show the protective effects by reduction of blood glucose and activation of anti‐oxidant factors.
In the present study, we noticed a significant damage to the glomeruli in the diabetes mellitus kidney. Compared with the normal kidney, a diabetes mellitus kidney has a significant increase in the glomerular area and glomerular basement membrane thickening. These findings are in agreement with those in the study by Lee et al.36 Accumulating evidence suggests that oxidative stress enhances the expression of several growth factors, such as transforming growth factor‐β, connective tissue growth factor and platelet‐derived growth factor, in glomerular endothelial cells, mesangial cells, proximal convoluted tubular epithelial cells, fibroblasts and macrophages37, 38. These factors contribute to an increase in the extracellular matrix. The glomerular basement membrane thickness occurs by increased expression of collagens type I, III, IV, V, VI, laminin and fibronectin38, 39. Consequently, this causes loss of elasticity of the glomerular capillaries, glomerular hypertension and increased glomerular filtration rate, eventually leading to destruction of the glomerular capillary38. Thus, the protective effect of ethanol extracts of propolis on the kidneys in diabetic rats could be as a result of a reduction of blood glucose levels. Additionally, hydroxyproline, a major component of collagen, was increased in the diabetes mellitus kidney29. Glomerular basement membrane thickening also increased in the diabetes mellitus kidney because of increased synthesis of collagen type 4, a component of the extracellular matrix in the kidney5. Studies have shown that specific matrix metalloproteinases (MMPs) are responsible for the degradation of the extracellular matrix. Furthermore, MMP2 and MMP9 are specifically responsible for the degradation and destruction of collagen. MMP2 and MMP9 activity was reduced in diabetes mellitus nephropathy, which could lead to a potential increase in extracellular matrix and basement membrane thickening40. It is likely that the propolis extract activates MMP2 and MMP9, prevents basement membrane thickening, mesangial matrix expansion, and protects against glomerular sclerosis and fibrosis of the kidney37. Although in the present study we did not measure blood pressure, it seems that clinic blood pressure was not elevated because of the aforementioned factors, but maybe the home blood pressure was elevated because of micro‐ and macrovascular complications after glomerular damage. Therefore, because of this damage there might be an albumin urea. According to the recovery of GBM thickness, we guess home blood pressure will be reduced.
In the present study, the renal glomerular area was increased in diabetes mellitus rats. However, the alcoholic extract of propolis (200 mg/kg) significantly decreased the glomerular area. In accordance with the previous finding, a potential mechanism for an increase of the glomerular area is the creation of new blood vessels. After these changes, the podocytes’ cytoplasmic processes also rise to encompass new vessels. Therefore, the size and area of the glomeruli and renal volume were increased. A decrease in the glomerular surface area after treatment with the propolis extract (200 mg/kg) in the present study is likely because of the reduced number of blood vessels, resulting in reduced thickness of the glomerular basement membrane and mesangial matrix. However, propolis extract (100 mg/kg) did not change the glomerular surface area. This would be as a result of the high activity of podocytes or the remaining blood vessels. Perhaps a longer time would be required for observing conclusive results.
In conclusion, the present study results confirm that treatment with the ethanolic extract of Iranian propolis significantly reduces blood glucose, and then suppresses the histopathological changes and oxidative stress in the kidneys of diabetes mellitus rats.
Disclosure
The authors declare no conflict of interest.
J Diabetes Investig 2016; 7: 506–513
References
- 1. Sforcin JM, Bankova V. Propolis: is there a potential for the development of new drugs? J Ethnopharmacol 2011; 133: 253–260. [DOI] [PubMed] [Google Scholar]
- 2. Bankova V, de Castro D, Marcucci M. Propolis: recent advances in research on chemistry and plant origin. Apidologie 2000; 31: 3–1. [Google Scholar]
- 3. Tao B, Pietropaolo M, Atkinson M, et al Estimating the cost of type 1 diabetes in the U.S.: a propensity score matching method. PLoS ONE 2010; 9: e511501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cooper ME. Pathogenesis, prevention, and treatment of diabetic nephropathy. Lancet 1998; 352: 213–219. [DOI] [PubMed] [Google Scholar]
- 5. Mohammadzadeh S, Hamedie M, Amanzadeh Y, et al Antioxidant power of Iranian propolis extract. Food Chem 2007; 103: 729–733. [Google Scholar]
- 6. Wei Zhu MC, Qiyang SH, Yinghua L, et al Biological activities of Chinese propolis and Brazilian propolis on streptozotocin induced type1 diabetes mellitus in rats. Evid Based Complement Alternat Med 2011; 20: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kukner A, Colakoglu N, Ozogul C, et al The effects of combined vitamin C and E in streptozotocin‐induced diabetic rat kidney. Res J Med Sci 2002; 3: 214–220. [Google Scholar]
- 8. Hui‐hui H, Dao‐quan T, Zhu‐min S, et al Protective effects of quercetin on streptozotocin induced diabetic nephropathy in rats. Phytother Res 2013; 27: 1580. [DOI] [PubMed] [Google Scholar]
- 9. Greenaway W, Scaysbrook T, Whatley FR. The composition and plant origins of propolis: a report of work at Oxford. Bee World 1990; 71: 107–118. [Google Scholar]
- 10. Spanos GA, Worlstad RE. Influence of processing and storage on the phenolic composition of Thompson seedless grape juice. J Agri Food Chem 1990; 38: 1565–1571. [Google Scholar]
- 11. Boutabet K, Kebsa W, Alyane M, et al Polyphenolic fraction of Algerian propolis protects rat kidney against acute oxidative stress induced by doxorubicin. Pharmacy Indian J Nephrol 2011; 21: 101–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Montilla P, Munoz M, Castaneda I, et al Red wine prevents brain oxidative stress and nephropathy in streptozotocin‐induced diabetic rats. J Biochem Mol Biol 2005; 38: 539–544. [DOI] [PubMed] [Google Scholar]
- 13. Benzie IF, Strain JJ. The Ferric Reducing Ability of Plasma (FRAP) as a measure of “Antioxidant Power”: the FRAP assay. Anal Biochem 1996; 239: 70–76. [DOI] [PubMed] [Google Scholar]
- 14. Vaya J, Aviram M. Nutritional antioxidants: mechanism of action, analyses of activities and medical applications. Endocr Metab Agents 2001; 1: 99–117. [Google Scholar]
- 15. Safari M, Sameni HR, Badban L, et al Protective effects of water extract of propolis on dopaminergic neurons, brain derived neurotrophic factor, and stress oxidative Factors in the rat model of Parkinson's disease. Int J Pharmacol 2015; 11: 300–308. [Google Scholar]
- 16. Noorafshan A, Esmail‐Zadeh B, Bahmanpour S, et al Early stereological changes in liver of Sprague–Dawley rats after streptozotocin injection. Indian J Gastroenterol 2005; 24: 104–107. [PubMed] [Google Scholar]
- 17. Halliwell B, Gutteridge JM. Lipid peroxidation, oxygen radicals, cell damage, and antioxidant therapy. Lancet 1994; 1: 1396–1397. [DOI] [PubMed] [Google Scholar]
- 18. Giugliano D, Ceriello A, Paolisso G. Oxidative stress and diabetic vascular complications. Diabetes Care 1996; 19: 257–267. [DOI] [PubMed] [Google Scholar]
- 19. Hamada Y, Fujii H, Kitazawa R, et al Thioredoxin‐1 overexpression in transgenic mice attenuates streptozotocin‐ induced diabetic osteopenia: a novel role of oxidative stress and therapeutic implications. Bone 2009; 44: 936–941. [DOI] [PubMed] [Google Scholar]
- 20. Yilmaz HR, Uz E, Yucel N, et al Protective effect of caffeic acid phenethyl ester (CAPE) on lipid peroxidation and antioxidant enzymes in diabetic rat liver. J Biochem Mol Toxicol 2004; 18: 234–238. [DOI] [PubMed] [Google Scholar]
- 21. El‐Sayed el‐ SM, Abo‐Salem OM, Aly HA, et al Potential antidiabetic and hypolipidemic effects of propolis extract in streptozotocin‐induced diabetic rats. Pak J Pharm Sci 2009; 22: 168–174. [PubMed] [Google Scholar]
- 22. Lee ES, Uhm KO, Lee YM, et al CAPE (caffeic acid phenethyl ester) stimulates glucose uptake through AMPK (AMP‐activated protein kinase) activation in skeletal muscle cells. Biochem Biophys Res Commun 2007; 361: 854–858. [DOI] [PubMed] [Google Scholar]
- 23. Matsui T, Ebuchi S, Fujise T, et al Strong antihyperglycemic effects of water‐soluble fraction of Brazilian propolis and its bioactive constituent, 3,4,5‐tri‐O‐caffeoylquinic acid. Biol Pharm Bull 2004; 27: 1797–1803. [DOI] [PubMed] [Google Scholar]
- 24. Fuliang HU, Hepburn HR, Xuan H, et al Effects of propolis on blood glucose, blood lipid and free radicals in rats with diabetes mellitus. Pharmacol Res 2005; 51: 147–152. [DOI] [PubMed] [Google Scholar]
- 25. Al‐Hariri M, Eldin TG, Abu‐Hozaifa B, et al Glycemic control and anti‐osteopathic effect of propolis in diabetic rats. Diabetes Metab Syndr Obes 2011; 4: 377–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yamanea T, Yamaguchib N, Yoshidaa Y, et al Regulation of extracellular matrix production and degradation of endothelial cells by shear stress”. Int Congr Ser 2006; 1262: 407–410. [Google Scholar]
- 27. Seyer‐Hansen K, Hansen J, Gundersen HJ. Renal hypertrophy in experimental diabetes. A morphometric study. Diabetologia 1980; 18: 501–505. [DOI] [PubMed] [Google Scholar]
- 28. Yajing L, Chen M, Xuan H, et al Effects of encapsulated propolis on blood glycemic control, lipid metabolism, and insulin resistance in type 2 diabetes mellitus rats. Evid Based Complement Alternat Med 2011; 2012: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Brownlee M. The pathobiology of diabetic complications: a unifying mechanism. Diabetes 2005; 54: 1615–1625. [DOI] [PubMed] [Google Scholar]
- 30. Osama M, Eldin M, Nermin H, et al Experimental diabetic nephropathy can be prevented by propolis: effect on metabolic disturbances and renal oxidative parameters. Pak J Pharm Sci 2009; 22: 205–210. [PubMed] [Google Scholar]
- 31. Valavanidis A, Vlahogianni T, Dassenakis M, et al Molecular biomarkers of oxidative stress in aquatic organisms in relation to toxic environmental pollutants. Ecotox Environ Safety 2006; 64: 178–189. [DOI] [PubMed] [Google Scholar]
- 32. Kedziora‐Kornatowska K, Szram S, Kornatowski T, et al Effect of vitamin E and vitamin C supplementation on antioxidative state and renal glomerular basement membrane thickness in diabetic kidney. Nephron Exp Nephrol 2003; 95: 134–143. [DOI] [PubMed] [Google Scholar]
- 33. Ozguner F, Oktem F, Ayata A, et al A novel antioxidant agent caffeic acid phenethyl ester prevents long‐term mobile phone exposure‐induced renal impairment in rat. Mol Cell Biochem 2005; 277: 73–80. [DOI] [PubMed] [Google Scholar]
- 34. Curtis SJ, Moritz M, Snodgrass PJ. Serum enzyme derived from liver cell fractions.The response of carbon tetrachloride intoxication in rats. Gastroenterology 1972; 62: 84–92. [PubMed] [Google Scholar]
- 35. Britton Chancea B, Greensteina DS, Roughton B. The mechanism of catalase action. 1. Steady‐state analysis. Arch Biochem Biophys 1952; 37: 301–321, 1952. [DOI] [PubMed] [Google Scholar]
- 36. Young Lee E, Young Lee M, Won Hong S, et al Blockade of oxidative stress by vitamin C ameliorates albuminuria and renal sclerosis in experimental diabetic rats. Yonsei Med J 2007; 48: 847–855, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sudhir VS, Baliga R, Rajapurkar M, et al Fonseca Oxidants in chronic kidney disease. J Am Soc Nephrol 2007; 18: 16–28. [DOI] [PubMed] [Google Scholar]
- 38. Roger M, Nadia AW. Extracellular matrix metabolism in diabetic Nephropathy. J Am Soc Nephrol 2003; 14: 1358–1373. [DOI] [PubMed] [Google Scholar]
- 39. Yamagishi S, Imaizumi T. Diabetic vascular complications: pathophysiology, biochemical basis and potential therapeutic strategy. Curr Pharm Des 2005; 11: 2279–2299. [DOI] [PubMed] [Google Scholar]
- 40. Sottile J. Regulation of angiogenesis by extracellular matrix. Biochim Biophys Acta 2004; 1654: 13–22. [DOI] [PubMed] [Google Scholar]


