Abstract
Aims/Introduction
This study was to assess the association between serum osteocalcin level and glucose metabolism in a Chinese male population.
Materials and Methods
We carried out a cross‐sectional study with a cohort of participants from the Fangchenggang Area Male Health and Examination Survey. The cross‐sectional study was carried out among 2,353 men, including 2,139 participants with normal glucose tolerance, 148 with impaired fasting glucose and 66 with type 2 diabetes. A subsample of 1,109 men with measurement of osteocalcin was observed in the cohort. After a 4‐year follow‐up period, 1,049 non‐diabetic and 983 participants with normal glucose tolerance who submitted the available information were enrolled in the cohort. Participants were divided into group‐H (≥23.33 ng/mL) and group‐L (<23.33 ng/mL) by osteocalcin level.
Results
In the cross‐sectional study, osteocalcin levels were highest in participants with normal glucose tolerance, followed by those with impaired fasting glucose and type 2 diabetes (P < 0.001). In partial correlation analysis adjusted for age, serum osteocalcin level was related to glucose level (r = −0.082, P < 0.001), insulin level (r = −0.079, P < 0.001) and insulin resistance (r = −0.065, P = 0.002). Compared with group‐H, group‐L was associated with an increased risk of type 2 diabetes (odds ratio 2.107, 95% confidence interval 1.123–3.955), impaired fasting glucose (odds ratio 2.106; 95% CI 1.528–2.902), and insulin resistance (odds ratio 1.359, 95% confidence interval 1.080–1.710) adjusted for age, education levels, cigarette smoking and lipid profiles. In the cohort study, the increased risk of impaired fasting glucose was significant in group‐L vs group‐H (3.3% vs 1.2%, P = 0.026).
Conclusions
Low serum osteocalcin level was a risk factor for impaired glucose metabolism and subsequent type 2 diabetes.
Keywords: Glucose metabolism, Insulin resistance, Osteocalcin
Introduction
Recently, more and more research has examined osteocalcin (OCN), which is reported to be central in the cross‐talk between bone metabolism and glucose metabolism1, 2, 3. OCN, a 49‐amino acid bone matrix non‐collagen protein, is secreted in the general circulation by osteoblastic cells. OCN consists of two forms, undercarboxylated osteocalcin (ucOCN) and carboxylated osteocalcin (cOCN). ucOCN is formed after the glutamic acid residues of OCN on the 17th, 21st and 24th sites are carboxylated by vitamin K‐dependent carboxylase. OCN is typically considered a biochemical marker of bone turnover and bone formation, and is involved in several physiological functions, such as maintaining normal bone mineralization, suppressing abnormal hydroxyapatite formation and slowing down growth cartilage mineralization4. However, a new function of OCN has been shown as a hormone regulating glucose metabolism. It is found to be responsible for some functions in type 2 diabetes, such as favoring β‐cell proliferation, insulin expression and secretion, as well as sensitivity to insulin in peripheral tissues5. Lack of the OCN gene in mice leads to insulin resistance and hyperglycemia6. Furthermore, administration of recombinant OCN into wild‐type mice enhances pancreatic β‐cell proliferation and insulin secretion, and protects them from obesity and type 2 diabetes7. Several cross‐sectional studies on Caucasian and Asian populations have shown that total OCN level is significantly negatively correlated with fasting blood glucose, fasting insulin and homeostasis model assessment of insulin resistance (HOMA‐IR)8, 9. A similar result was observed in a study on a Chinese population of 254 men and 246 women. That cross‐sectional study showed that total OCN level is closely associated with glucose metabolism10.
To date, the research investigating the relationship between OCN and glucose metabolism in the Chinese population has been carried out on a relatively small sample, and there is a lack of prospective study; thus, we carried out a cross‐sectional study with a cohort in the Fangchenggang male population to further explore the relationship.
Materials and Methods
Ethics statement
The study was approved by the medical ethics committee of Guangxi, China. All patients provided written informed consent.
Study design and population
The Fangchenggang Area Male Health and Examination Survey was a population‐based prospective cohort study carried out in Guangxi among Chinese males aged 17–88 years. A total of 4,043 subjects were investigated at the baseline survey from September to December 2009. Subjects with the following conditions were excluded from the study: (i) current coronary heart disease, stroke, hyperthyroidism, hypothyroidism, rheumatoid arthritis, hypercortisolism, chronic and/or acute kidney disease and cancer; (ii) liver dysfunction (alanine transaminase >3 times upper limit of normal); and (iii) current treatment with relative drug (vitamin D, bisphosphonate, calcitonin, estrogen, tamoxifen, denosumab, parathyroid hormone etc.). Finally, 2,353 men completed the OCN measurements and were enrolled in the cross‐sectional analysis. In the cohort, a relatively stable sample of 1,809 participants were included and followed up for 4 years, including 1,109 men with information on serum OCN. Finally, 1,049 non‐diabetic and 983 participants with normal glucose tolerance (NGT) who submitted the available information entered the cohort. All participants provided written informed consent. Participants were divided into two groups according to the median OCN level, group‐H (≥23.33 ng/mL) group‐L (<23.33 ng/mL).
Baseline examination
A complete physical examination was carried out. Information on personal sociodemographic characteristics (age, educational levels etc.), lifestyle (cigarette smoking, alcohol consumption, physical activity), medical history and medication use were also obtained by in‐person interviews. Education levels were categorized into three groups according to the number of years of education (0–6, 7–9 and ≥10 years). Cigarette smoking was defined as current smoker (still smoking or had smoked within the past 6 months), ex‐smoker (quit smoking more than 6 months previously) and non‐smoker (had never smoked). Frequency and total consumption of several forms of alcohol were recorded, including local spirits, branded spirits, wine, beer and so on. One drink was equal to 8 g of ethanol (260 mL of beer, 120 mL of wine and 30 mL of liquor etc.). Alcohol consumption was defined as more than one drink per week. Participants were prompted to report the frequency and duration of any physical activity with a minimum length of 10 consecutive minutes. Physical activity was considered as more than two times per week. Family history was considered as positive when at least one of the participant's parents or siblings had suffered from type 2 diabetes. Weight and height were measured by trained interviewers in the morning, requiring participants to wear light clothing and no shoes. Body mass index (BMI) was calculated as the weight in kilograms divided by the square of the height in meters (kg/m2).
Laboratory examination
All blood samples were drawn after an overnight fast of more than 12 h, and transported frozen to the testing center of the Department of Clinical Laboratory at the First Affiliated Hospital of Guangxi Medical University in Nanning, China, within 2 h; there they were centrifuged for 15–25 min and stored at −80°C until analysis. Fasting plasma glucose, insulin and lipid profiles, including total cholesterol, high‐density lipoprotein, low‐density lipoprotein and triglycerides, were determined by a Dimension‐RxL Chemistry Analyzer (Dade Behring, Newark, DE, USA) in the Department of Clinical Laboratory at the Fangchenggang First People's Hospital. Serum OCN was analyzed using a Cobas 6000 system E601 (Elecsys module) immunoassay analyzer (Roche Diagnostics, GmbH, Mannheim, Germany). The interassay coefficient of variation was 4.5%.
Definition of type 2 diabetes, impaired fasting glucose, NGT and insulin resistance
Type 2 diabetes was defined as the presence of one or more of the following: (i) fasting plasma glucose (FPG) level ≥7.0 mmol/L in two different measurements; (ii) use of antidiabetic medication; or (iii) self‐reported or physician diagnosis. Impaired fasting glucose (IFG) was defined as glucose levels of 6.1–7.0 mmol/L. Subjects were defined as having normal fasting glucose when the glucose level was less than 6.1 mmol/L. Insulin resistance was estimated by calculating HOMA‐IR. The formula was HOMA‐IR = (fasting plasma glucose [mmol/L] × fasting insulin [mcU/mL])/22.5. Insulin resistance was considered as HOMA‐IR greater than 2.611.
Statistical analysis
All statistical analyses were processed with SPSS 17.0 (SPSS, Chicago, IL, USA). Normal distributed data were expressed as the mean ± standard deviation, whereas variables with a skewed distribution were reported as the median (interquartile range 25–75%). Categorical variables were represented as frequency and percentage. The distribution of variables was analyzed by the Kolmogorov–Smirnov test. In the cross‐sectional study, a one‐way anova was used to compare numerical variables among participants with type 2 diabetes, IFG and NGT. Numerical data were analyzed by a non‐parametric test. Comparisons in categorical variables (cigarette smoking, alcohol consumption, family history, education levels) were analyzed by the χ2‐test with continuity correction or Fisher's exact test when required. Correlation coefficients between OCN level and BMI, lipid profiles, glucose level, HOMA‐IR and insulin level were calculated by partial correlation controlling for age. Potential confounding factors, including age, BMI, lipid profiles, cigarette smoking and education levels, were included in the regression models. Logistic regression analysis was used to determine the risk for type 2 diabetes, IFG and insulin resistance between two groups (group‐L and group‐H) adjusted for potential confounding factors. Group‐H acted as a reference, and the results were presented as odds ratios (OR) and 95% confidence interval (95% CI). In the cohort study, the incidences of type 2 diabetes and IFG were compared by the χ2‐test. For all comparisons, values were considered to be significant when P < 0.05.
Results
We describe the general characteristics of the cross‐sectional study participants in Table 1. In all, data are presented for 2,353 participants, divided into NGT (n = 2,139), IFG (n = 148) and type 2 diabetes (n = 66). The average age was 37.59 ± 10.936 years. Participants with type 2 diabetes had the lowest level of OCN, followed by those with IFG and NGT (18.58 ng/mL [15.75–22.59 ng/mL] vs 20.70 ng/mL [17.08–24.97 ng/mL] vs 23.6 ng/mL [19.24–29.23 ng/mL]; P = 0.001). Compared with participants with NGT, participants with IFG and type 2 diabetes were older, and showed higher insulin level, HOMA‐IR, BMI, cholesterol, triglycerides, lower education level and were more likely to have smoked.
Table 1.
General characteristics of the participants in the cross‐sectional study
| NGT | IFG | T2DM | P‐value | |
|---|---|---|---|---|
| n | 2,139 | 148 | 66 | |
| Age (years) | 37 ± 11 | 44 ± 12 | 46 ± 11 | <0.001** |
| BMI (kg/m2) | 23.02 ± 3.19 | 24.28 ± 3.63 | 24.24 ± 3.61 | <0.001** |
| Osteocalcin (ng/mL) | 23.6 (19.24–29.23) | 20.70 (17.08–24.97) | 18.58 (15.75–22.59) | <0.001** |
| Insulin (uU/mL) | 6.26 (4.19–9.24) | 8.64 (5.31–15.11) | 8.76 (4.7–19.85) | <0.001** |
| HOMA‐IR | 1.41 (0.94–2.15) | 2.47 (1.50–4.31) | 3.56 (1.98–7.78) | <0.001** |
| Total cholesterol (mmol/L) | 5.63 ± 1.00 | 6.10 ± 1.15 | 6.20 ± 1.31 | <0.001** |
| Triglyceride (mmol/L) | 1.10 (0.76–1.69) | 1.45 (0.90–2.63) | 1.96 (1.19–3.61) | <0.001** |
| HDL (mmol/L) | 1.40 ± 0.30 | 1.44 ± 0.38 | 1.67 ± 0.81 | 0.026* |
| LDL (mmol/L) | 2.93 ± 0.80 | 3.16 ± 0.88 | 3.11 ± 0.81 | 0.003* |
| Cigarette smoking, n (%) | ||||
| Non‐smoker | 997 (46.7%) | 63 (42.6%) | 29 (43.9%) | 0.003* |
| Ex‐smoker | 72 (3.4%) | 6 (4.1%) | 8 (21.1%) | |
| Current smoker | 1,067 (50.0%) | 79 (53.4%) | 29 (43.9%) | |
| Alcohol consumption (yes), n (%) | 858 (40.2%) | 70 (47.3%) | 33 (50.0%) | 0.073 |
| Physical activity (yes), n (%) | 500 (23.4%) | 34 (23.0%) | 16 (24.2%) | 0.980 |
| Family history for type 2 diabetes (yes), n (%) | 79 (3.7%) | 7 (4.7%) | 6 (9.1%) | 0.073 |
| Education levels (years) | ||||
| 0–6 | 52 (2.4%) | 13 (8.8%) | 10 (13.8%) | <0.001** |
| 7–10 | 1,359 (63.7%) | 101 (68.2%) | 41 (63.1%) | |
| >10 | 723 (33.9%) | 34 (23.0%) | 15 (23.1%) | |
Data are presented as mean ± standard deviation or n (%). Osteocalcin, insulin and homeostasis model assessment of insulin resistance (HOMA‐IR): the data were shown as median and interquartile range. The percentage may not sum to 100 because of rounding. *P < 0.05, **P < 0.001 (one‐way anova and non‐parametric test was used for numerical data, while the χ2‐test with continuity correction or Fisher's exact test was used for categorical variable). BMI, body mass index; HDL, high‐density lipoprotein; IGT, impaired glucose tolerance; LDL, low‐density lipoprotein; NGT, normal glucose tolerance; T2DM, type 2 diabetes mellitus.
Figure1 presents the distribution of OCN levels, and how the median level of OCN varied among participants with NGT, IFG and type 2 diabetes. Compared with participants with NGT, serum OCN level decreased significantly in those with IFG and type 2 diabetes.
Figure 1.
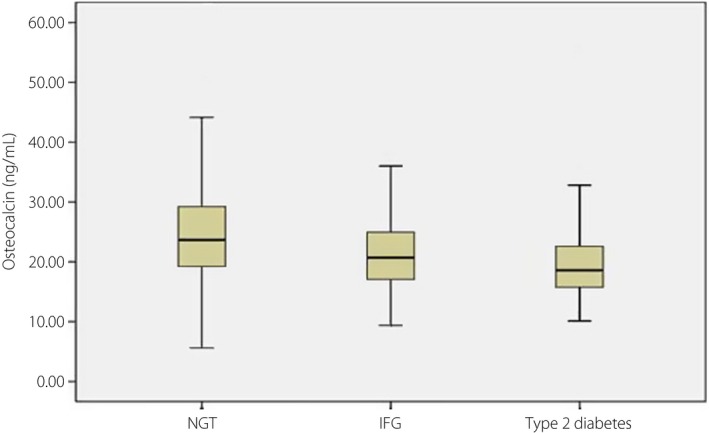
Distribution of serum osteocalcin level in subjects with normal glucose tolerance (NGT), impaired fasting glucose (IFG) and type 2 diabetes mellitus in the cross‐sectional study. The bars represented median, 25th, and 75th percentile of serum osteocalcin level. P for trend <0.001.
Partial correlations analyses adjusted for age were carried out between OCN levels and selected factors (Table 2). In the whole study population, serum OCN level was negatively correlated with glucose level (r = −0.082, P < 0.001), insulin level (r = −0.079, P < 0.01), low‐density lipoprotein (r = −0.094, P < 0.001), total cholesterol (r = −0.105, P < 0.001), triglycerides (r = −0.140, P < 0.001), BMI (r = −0.258, P < 0.001) and HOMA‐IR (r = −0.065, P = 0.002). OCN level, however, did not show a significant correlation with high‐density lipoprotein (P = 0.282).
Table 2.
Age‐adjusted partial correlation between osteocalcin and selected factors in the cross‐sectional study
| Correlation coefficient | P‐value | |
|---|---|---|
| BMI | −0.258 | <0.001** |
| Total cholesterol | −0.105 | <0.001** |
| Triglyceride | −0.140 | <0.001** |
| HDL | 0.022 | 0.282 |
| LDL | −0.094 | <0.001** |
| Glucose | −0.082 | <0.001** |
| HOMA‐IR | −0.065 | 0.002* |
| Insulin | −0.079 | <0.001** |
Data were analyzed by correlation coefficient and P‐value. *P < 0.05, **P < 0.001 (partial correlation between osteocalcin and selected factors adjusted for age). BMI, body mass index; HDL, high‐density lipoprotein; HOMA‐IR, homeostasis model assessment of insulin resistance; LDL, low‐density lipoprotein.
Logistic regression analysis was carried out using the presence of type 2 diabetes, IFG and insulin resistance as dependent variables, and Group‐H as a reference (Table 3). As the results (model 3) show, participants with low OCN level were significantly associated with type 2 diabetes (OR 2.107, 95% CI 1.123–3.955), IFG (OR 1.752, 95% CI 1.258–2.439) and insulin resistance (OR 1.359, 95% CI 1.080–1.710). After further adjustment for BMI (model 4), OR decreased slightly and remained significant for type 2 diabetes (OR 1.988, 95% CI 1.052–3.75) and IFG (OR 1.626, 95% CI 1.162–2.274), but not for insulin resistance (OR 1.034, 95% CI 0.807–1.326).
Table 3.
Logistic regression calculate the odds ratios for type 2 diabetes mellitus, impaired fasting glucose and insulin resistance in the cross‐sectional study
| Group‐L (n = 1,177) | Group‐H (n = 1,173) | |
|---|---|---|
| <23.33 ng/mL | ≥23.33 ng/mL | |
| T2DM | ||
| Unadjusted | 1 | 3.497 (1.955–6.255)** |
| Model 1 | 1 | 2.562 (1.416–4.635)* |
| Model 2 | 1 | 2.759 (1.495–5.089)* |
| Model 3 | 1 | 2.107 (1.123–3.955)* |
| Model 4 | 1 | 1.988 (1.052–3.758)* |
| IFG | ||
| Unadjusted | 1 | 2.657 (1.954–3.613)** |
| Model 1 | 1 | 2.000 (1.457–2.746)** |
| Model 2 | 1 | 2.106 (1.528–2.902)** |
| Model 3 | 1 | 1.752 (1.258–2.439)* |
| Model 4 | 1 | 1.626 (1.162–2.274)* |
| Insulin resistance | ||
| Unadjusted | 1 | 1.754 (1.426–2.159)** |
| Model 1 | 1 | 1.702 (1.372–2.112)** |
| Model 2 | 1 | 1.673 (1.346–2.078)** |
| Model 3 | 1 | 1.359 (1.080–1.710)* |
| Model 4 | 1 | 1.034 (0.807–1.326) |
Data were analyzed by odds ratios and 95% confidence intervals. *P < 0.05, **P < 0.001 (logistic regression was used to calculate the odds ratios for type 2 diabetes mellitis [T2DM], impaired fasting glucose [IFG] and insulin resistance). Model 1 was adjusted for age. Model 2 was further adjusted for cigarette smoking and education level. Model 3 was further adjusted for total cholesterol, triglyceride, high‐density lipoprotein and low‐density lipoprotein. Model 4 was further adjusted for body mass index. Group‐H, osteocalcin level ≥23.33 ng/mL; group‐L, osteocalcin level <23.33 ng/mL.
After the 4‐year follow up, no difference was observed between the two groups (group‐L 1.9%, group‐H 0.8%; P = 0.117). In contrast, low OCN level was associated with increased incidence of IFG (group‐L 3.3%, group‐H 1.2%; P = 0.026).
Discussion
Ever growing research explores the role of OCN in impaired glucose metabolism, and whether serum OCN level is independently associated with glucose metabolism is still under debate. In the current study, we assessed the association between OCN level and glucose metabolism by carrying out a cross‐sectional study within a cohort. As the results show, in the cross‐sectional study, we found a significantly higher OCN level in participants with NGT compared with those with IFG and type 2 diabetes. Furthermore, OCN level is negatively correlated with glucose level, insulin level and HOMA‐IR. Participants with a low OCN level showed an increased risk for type 2 diabetes, IFG and insulin resistance compared with those with a high OCN level. In the cohort, we also found increased incidences of type 2 diabetes and IFG in participants with a low OCN level. These results confirm the relationship that low OCN level is a risk factor for impaired glucose metabolism, in line with previous studies12, 13.
The mechanisms associated with OCN and glucose metabolism are explained in several ways. Animal experiments show that OCN mediates Gprc6a to promote the secretion of gut glucagon‐like peptide‐1, which mediates the stimulatory effect of OCN on insulin secretion14. Another researcher showed that OCN increases CyclinD2 and Cdk4 expression, two genes necessary for β‐cell proliferation in vivo, with a dose response similar to the one observed for insulin expression7, 15. Previous studies also suggest that OCN increases insulin sensitivity in the liver, muscle and adipose tissue by upregulation of adiponectin gene expression, which mediates through two adiponectin receptors (AdipoR1 and 2) to enhance insulin sensitivity and maintain a functional β‐cell mass6, 16. As the active form of OCN, the interaction of ucOCN with glucose metabolism has also been widely discussed. In animal models, the role of ucOCN has been shown to be a positive modulator of insulin secretion7. In human studies, a significant association of higher osteocalcin with a lower risk of diabetes is observed17. Meanwhile, previous studies suggest that total OCN level regulates glucose metabolism. A population‐based study of health in Pomerania reports a cross‐sectional association between high total OCN level and a lower prevalence of T2DM18. Pollock et al.19 reported a lower level of total OCN in prediabetes children. Furthermore, a studies of female patients reported that adiponectin is correlated with OCN20, and OCN might lie in the causal pathway between central adiposity and insulin resistance21. These observations, including ours, show that total OCN is associated with glucose metabolism.
Even though the association between OCN level and glucose metabolism has been confirmed by several cross‐sectional studies, little is known about whether OCN level plays a critical role in the pathogenesis or progression of type 2 diabetes and is able to predict the incidence of type 2 diabetes over time. A few studies have been carried out to determine the association between OCN level and type 2 diabetes using longitudinal follow up. In an older adult (approximately 65 years‐of‐age) population of 198 participants, exposure to a higher total OCN level predicted a significantly lower rise in fasting plasma glucose level in a 3‐year cohort22. Another cohort showed that baseline circulating total OCN level was an independent risk factor for the development of diabetes in 126 participants (approximately 47 years‐of‐age) during the 10‐year follow up23. These observations, however, are slightly different from the result in the present cohort. In the present study, low OCN level showed a higher incidence of IFG, but not of type 2 diabetes. The age of the population and length of the follow‐up period could be reasons to partially explain the discrepancy between these previous studies and the present findings. It was reported that diabetes prevalence increases with age, and men aged 40–49 years have a higher prevalence than younger men in an epidemiological study in 201024. The participants included in the present study, however, were almost all young adults (approximately 38 years‐of‐age), who tend to maintain better physical health and have a lower risk of developing type 2 diabetes compared with older people. Studies have shown that OCN is weakly correlated with insulin resistance and insulin level in children and adults25, whereas in old people, functions of OCN on glucose metabolism become evident26. In addition, as type 2 diabetes is a chronic illness, long‐term follow up might help to fully observe the occurrence and development of type 2 diabetes, and better understand the role of OCN in type 2 diabetes. So, in the future, we can test the association between OCN level and type 2 diabetes by expanding the timescale for the cohort.
The present study showed that OCN level has a negative and strong association with BMI. According to an observation in obese type 2 diabetic mice, daily OCN injections are reported to increase energy expenditure, and mice lacking OCN show increased body fat27. These results indicate that BMI, or fat homeostasis and energy expenditure, are affected by OCN level. Human studies showed similar results, where OCN level was shown to be negatively associated with obesity and fat mass,28 and moderate weight loss and regular exercise were proven to increase the level of serum OCN29. As obesity is also a risk factor for the development of insulin resistance and type 2 diabetes,30 further study is required to explore whether the function of OCN on obesity is related to the cross‐talk between OCN and impaired glucose metabolism.
To our knowledge, studies on the association between OCN and glucose metabolism in the Chinese population are limited; furthermore, the sample sizes included in studies are relatively small. In an attempt to fill this gap, we carried out a cross‐sectional study with our cohort. In this way, we can confirm not only the cross‐talk between OCN and glucose metabolism in a cross‐sectional study, but also test the role of OCN in impaired glucose metabolism after follow up.
Of course, limitations also exist. First, the study population only included male participants who visited hospital for a check‐up. Therefore, we do not know whether the association between OCN and glucose metabolism exists in the female population. Furthermore, we do not use the oral glucose tolerance test or glycated hemoglobin for the diagnosis of diabetes. Thus, the incidence of diabetes might be underestimated. Third, we describe the association between obesity and osteocalcin only according to BMI: more measurements are required in further research. What's more, we do not detect undercarboxylated osteocalcin, which might provide more insight to explain the mechanism. Despite these limitations, we were also able to provide initial evidence for a relationship between OCN and glucose metabolism.
The present study explored the association between OCN level and glucose metabolism. In a cross‐sectional study, the present results showed that decreased OCN serum level is significantly associated with increased fasting plasma glucose, insulin level and HOMA‐IR, and related to a high prevalence of type 2 diabetes, IFG and insulin resistance. Furthermore, in the cohort, participants with a decreased OCN level showed an increased risk of IFG after the 4‐year follow up. These observations suggest that low OCN level is a risk factor for impaired glucose metabolism. However, the underlying mechanism is still not identified, and further studies are necessary to clarify this point.
Disclosure
The authors declare no conflict of interest.
Acknowledgments
We thank all the participants of the Fangchenggang Area Male Health and Examination Survey for their enthusiastic collaboration, and cooperation of the staff in Fangchenggang First People Hospital. This work was supported by Guangxi Natural Science Foundation (2012GXNSFA053016, 2014GXNSFBA118150) and National Natural Science Foundation of China (81260130, 81460159).
J Diabetes Investig 2016; 7: 522–528
References
- 1. Lee NK, Karsenty G. Reciprocal regulation of bone and energy metabolism. Trends Endocrinol Metab 2008; 19: 161–166. [DOI] [PubMed] [Google Scholar]
- 2. Ducy P. The role of osteocalcin in the endocrine cross‐talk between bone remodelling and energy metabolism. Diabetologia 2011; 54: 1291–1297. [DOI] [PubMed] [Google Scholar]
- 3. Confavreux CB, Levine RL, Karsenty G. A paradigm of integrative physiology, the crosstalk between bone and energy metabolisms. Mol Cell Endocrinol 2009; 310: 21–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pearson DA. Bone health and osteoporosis: the role of vitamin K and potential antagonism by anticoagulants. Nutr Clin Pract 2007; 22: 517–544. [DOI] [PubMed] [Google Scholar]
- 5. Tan A, Gao Y, Yang X, et al Low serum osteocalcin level is a potential marker for metabolic syndrome: results from a Chinese male population survey. Metabolism 2011; 60: 1186–1192. [DOI] [PubMed] [Google Scholar]
- 6. Lee NK, Sowa H, Hinoi E, et al Endocrine regulation of energy metabolism by the skeleton. Cell 2007; 30: 456–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ferron M, Hinoi E, Karsenty G, et al Osteocalcin differentially regulates beta cell and adipocyte gene expression and affects the development of metabolic diseases in wild‐type mice. Proc Natl Acad Sci USA 2008; 105: 5266–7520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kindblom JM, Ohlsson C, Ljunggren O, et al Plasma osteocalcin is inversely related to fat mass and plasma glucose in elderly Swedish men. J Bone Miner Res 2009; 24: 785–791. [DOI] [PubMed] [Google Scholar]
- 9. Im JA, Yu BP, Jeon JY, et al Relationship between osteocalcin and glucose metabolism in postmenopausal women. Clin Chim Acta 2008; 396: 66–69. [DOI] [PubMed] [Google Scholar]
- 10. Zhou M, Ma X, Li H, et al Serum osteocalcin concentrations in relation to glucoseand lipid metabolism in Chinese individuals. Eur J Endocrinol 2009; 161: 723–729. [DOI] [PubMed] [Google Scholar]
- 11. Schwetz V, Lerchbaum E, Schweighofer N, et al Osteocalcin levels on oral glucose load in women being investigated for polycystic ovary syndrome. Endocr Pract 2014; 20: 5–14. [DOI] [PubMed] [Google Scholar]
- 12. Tang YJ, Sheu WH, Liu PH, et al Positive associations of bone mineral density with body mass index, physical activity, and blood triglyceride level in men over 70 years old: a TCVGHAGE study. J Bone Miner Metab 2007; 25: 54–59. [DOI] [PubMed] [Google Scholar]
- 13. Sarkar PD, Choudhury AB. Relationship of serum osteocalcin levels with blood glucose, insulin resistance and lipid profile in central Indian men with type 2 diabetes. Arch Physiol Biochem 2012; 118: 260–264. [DOI] [PubMed] [Google Scholar]
- 14. Mizokami A, Yasutake Y, Gao J, et al Osteocalcin induces release of glucagon‐like peptide‐1 and thereby stimulates insulin secretion in mice. PLoS One 2013; 8: e57375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kushner JA, Ciemerych MA, Sicinska E, et al Cyclins D2 and D1 are essential for postnatal pancreatic beta‐cell growth. Mol Cell Biol 2005; 25: 3752–3762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tao C, Sifuentes A, Holland WL. Regulation of glucose and lipid homeostasis by adiponectin: effects on hepatocytes, pancreatic cells and adipocytes. Best Pract Res Clin Endocrinol Metab 2014; 28: 43–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hwang YC, Jeong IK, Ahn KJ, et al The uncarboxylated form of osteocalcin is associated with improved glucose tolerance and enhanced beta‐cell function in middle‐aged male subjects. Diabetes Metab Res Rev 2009; 25: 768–772. [DOI] [PubMed] [Google Scholar]
- 18. Lerchbaum E, Schwetz V, Nauck M, et al Lower bone turnover markers in metabolic syndrome and diabetes: the population‐based Study of Health in Pomerania. Nutr Metab Cardiovasc Dis 2015; 25: 458–463. [DOI] [PubMed] [Google Scholar]
- 19. Pollock NK, Bernard PJ, Gower BA, et al Lower uncarboxylated osteocalcin concentrations in children with prediabetes is associated with beta‐cell function. J Clin Endocrinol Metab 2011; 96: 1092–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kanazawa I, Yamaguchi T, Yamauchi M, et al Serum undercarboxylated osteocalcin was inversely associated with plasma glucose level and fat mass in type 2 diabetes mellitus. Osteoporos Int 2011; 22: 187–194. [DOI] [PubMed] [Google Scholar]
- 21. Yeap BB, Chubb SA, Flicker L, et al Reduced serum total osteocalcin is associated with metabolic syndrome in older men via waist circumference, hyperglycemia, and triglyceride levels. Eur J Endocrinol 2010; 163: 265–272. [DOI] [PubMed] [Google Scholar]
- 22. Pittas AG, Harris SS, Eliades M, et al Association between serum osteocalcin and markers of metabolic phenotype. J Clin Endocrinol Metab 2009; 94: 827–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ngarmukos C, Chailurkit LO, Chanprasertyothin S, et al A reduced serum level of total osteocalcin in men predicts the development of diabetes in a long‐term follow‐up cohort. Clin Endocrinol (Oxf) 2012; 77: 42–46. [DOI] [PubMed] [Google Scholar]
- 24. Xu Y, Wang L, He J, et al Prevalence and control of diabetes in Chinese adults. JAMA 2013; 310: 948–959. [DOI] [PubMed] [Google Scholar]
- 25. Schwartz B, Jacobs DR Jr, Moran A, et al Measurement of insulin sensitivity in children: comparison between the euglycemic‐hyperinsulinemic clamp and surrogate measures. Diabetes Care 2008; 31: 783–788. [DOI] [PubMed] [Google Scholar]
- 26. Yeap BB, Chubb SA, Flicker L, et al Associations of total osteocalcin with all‐cause and cardiovascular mortality in older men. The Health In Men Study. Osteoporos Int 2012; 23: 599–606. [DOI] [PubMed] [Google Scholar]
- 27. Ferron M, McKee MD, Levine RL, et al Intermittent injections of osteocalcin improve glucose metabolism and prevent type 2 diabetes in mice. Bone 2012; 50: 568–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bao Y, Zhou M, Lu Z, et al Serum levels of osteocalcin are inversely associated with the metabolic syndrome and the severity of coronary artery disease in Chinese men. Clin Endocrinol (Oxf) 2011; 75: 196–201. [DOI] [PubMed] [Google Scholar]
- 29. Reinehr T, Roth CL. A new link between skeleton, obesity and insulin resistance: relationships between osteocalcin, leptin and insulin resistance in obese children before and after weight loss. Int J Obes (Lond) 2010; 34: 852–858. [DOI] [PubMed] [Google Scholar]
- 30. Rossger K, Charpin‐El‐Hamri G, Fussenegger M. A closed‐loop synthetic gene circuit for the treatment of diet‐induced obesity in mice. Nat Commun 2013; 4: 2825. [DOI] [PMC free article] [PubMed] [Google Scholar]


