Abstract
Aims/Introduction
‘The Standard Diabetes Manual’ has been developed by clinical researchers from multiple major institutions in Japan, such as the National Center for Global Health and Medicine, as a comprehensive disease management program, including collaboration between primary care physicians (PCPs) and specialist services. The present study evaluated the efficacy of the manual as a quality improvement strategy in diabetes care by PCPs.
Materials and Methods
A total of 42 PCPs in eight domestic districts of the Japan Medical Association were allocated to either the intervention group or the control group in a cluster‐randomized design. The PCPs in both groups were provided with a copy of the Diabetes Treatment Guide published by the Japan Diabetes Society, and the PCPs in the intervention group additionally received a copy of the manual and a 30‐min relevant seminar at the inception of the intervention. The primary end‐point was the adherence to the following performances as quality indicators: evaluation of retinopathy, and urinary albumin excretion measurements and serum creatinine measurements, as recommended by the Japan Medical Association.
Results
A total of 416 patients were enrolled by 36 PCPs. During the 1‐year follow‐up period, the proportion of PCPs who adhered to recommendation‐concordant measurements of urinary albumin excretion was significantly higher in the intervention group than in the control group (adherence: 17.9% vs 5.3%, P = 0.016). The other parameters were not statistically different between the two groups.
Conclusions
Implementation of ‘The Standard Diabetes Manual’ potentially leads to an improved quality of diabetes management by PCPs.
Keywords: Disease management, Quality indicator, Randomized trial
Introduction
There has been a growing body of evidence‐based clinical guidelines, and the implementation of such guidelines is expected to improve the quality and outcome of care. To make these decision aids feasible and practical in daily practice, clinical researchers from multiple major institutions in Japan, such as the National Center for Global Health and Medicine, designated as the national diabetes center, have developed ‘The Standard Diabetes Manual’ (‘The Manual’) as a comprehensive disease management program for primary care physicians (PCPs), including collaboration between PCPs and specialist services, which has been updated twice annually and is freely available to the public via the Internet1.
Whether clinical practice guidelines or disease management programs produce changes in actual clinical performance should be evaluated and measured2. However, there have been no clinical guidelines or disease management programs in Japan whose efficacy in the real world has been substantiated. We thus carried out the Study for the Efficacy Assessment of the Standard Diabetes Manual (SEAS‐DM), a cluster‐randomized exploratory trial to investigate the efficacy of ‘The Manual’ for improving the quality of diabetes care in several areas of Japan.
Materials and Methods
Study design
The SEAS‐DM was an open cluster‐randomized, two‐armed, exploratory trial carried out in major cities in Japan from September 2012 through March 2014. It was coordinated by the National Center for Global Health and Medicine in Japan. Ethical approval of this study was granted by the institutional review board at the National Center for Global Health and Medicine. Written informed consent was obtained from all the registered PCPs. The PCPs informed their participating patients of the study using posters, providing them with the opportunity to decline enrolment.
Study sites and participants
The present study was carried out in eight domestic districts of the Japan Medical Associations, in which more than three PCPs were able to participate in each district; each PCP was expected to enroll approximately 10 patients. The inclusion criteria for the participants were those who satisfied all the following conditions: type 2 diabetes mellitus, age between 20 and 75 years, and under the care of the registered PCP for more than 1 year.
Intervention
The PCPs in each district were randomly allocated to either an intervention or a control group, with each group as a cluster and each district as a stratum. The PCPs in both groups were provided with a copy of the Diabetes Treatment Guide, an excerpt of the evidence‐based clinical guideline3 published by the Japan Diabetes Society. The PCPs in the intervention group additionally received a copy of ‘The Manual.’ Furthermore, the intervention group received a 30‐min seminar regarding ‘The Manual’ at the start of the intervention. Updated copies were disseminated later, when they became available. The PCPs were not notified of the study end‐points at any point during the study period.
End‐points
The primary end‐point was the adherence to the following process measures, which were used as quality indicators for microvascular complication screening, during the 1‐year study period: evaluation of retinopathy by an ophthalmologist (once annually), measurement of urinary albumin excretion (every 6 months) and measurement of serum creatinine level (every 6 months), as recommended by the Japan Medical Association.
The secondary end‐points were the glycated hemoglobin (HbA1c) at the end of the 1‐year period and the adherence to the following recommendation‐concordant performances: measurement of HbA1c (every 3 months), blood pressure (every 3 months) and serum lipids (every 3 months).
Clinical research coordinators, who were not aware of the allocation of the PCPs, visited each clinic every 3 months and collected the pertinent data by reviewing the medical records.
Statistical analysis
The estimation of adherence has been described elsewhere4. Per‐protocol analyses of the performance were carried out at the individual PCP level and accounted for the clustering. The last observation carried forward method was used to impute the missing HbA1c variables. The differences in categorical and continuous parameters were evaluated using the chi‐square test and the Student's t‐test, respectively, with adjustments for the intracluster correlation coefficient (ICC), which was set at 0.1. Sensitivity analyses were also carried out for ICC levels of 0.05 and 0.15. A P‐value of less than 0.05 was deemed statistically significant. The analyses were carried out using Stata 12.0 software (StataCorp, College Station, TX, USA).
Results
A total of 42 PCPs in eight DMAs were randomly allocated to the two groups, and a total of 416 patients were recruited by 36 PCPs. The average interval between randomization and patient registration was 46.7 days in the intervention group and 47.3 days in the control group. During the 1‐year follow‐up period, five patients were lost to follow up: the follow‐up rate was 99.8% (Figure 1). The baseline characteristics and the process quality over the preceding 1‐year were similar between the intervention and the control groups (Table 1).
Figure 1.

Trial profile. PCP, primary care physician.
Table 1.
Baseline characteristics of participants
| Intervention group | Control group | P‐value* | |
|---|---|---|---|
| Physicians (n) | 22 | 20 | |
| Patients (n) | 234 | 182 | |
| Men (%) | 58.3 | 58.6 | 0.95 |
| Mean age, years (SD) | 62.2 (8.6) | 62.4 (9.2) | 0.95 |
| Mean HbA1c, % (SD) | 7.1 (0.1) | 7.0 (0.1) | 0.76 |
| Adherence (%) | |||
| Retinopathy screening | 8.7 | 7.2 | 0.44 |
| Urinary albumin measurement | 17.2 | 6.9 | 0.05 |
| Serum creatinine measurement | 82.4 | 78.7 | 0.56 |
| HbA1c measurement | 88.6 | 86.7 | 0.62 |
| Lipids measurement | 67.5 | 63.3 | 0.43 |
| Blood pressure measurement | 95.5 | 94.5 | 0.24 |
*Adjusted for intracluster correlation coefficient. HbA1c, glycated hemoglobin; SD, standard deviation.
Figure 2 shows the relative changes in adherence at the end of the study period. The adherence to the blood pressure measurements and blood tests remained relatively high, and there were no significant differences within or between the groups. In contrast, the adherence to the evaluation of retinopathy and albuminuria was relatively low. Both indicators tended to increase in the intervention group, whereas they declined in the control group. Although the changes within each group were not statistically significant, the proportion of PCPs who carried out recommendation‐concordant measurements of urinary albumin excretion was significantly higher in the intervention group than in the control group (adherence: 17.9% vs 5.3%, P = 0.016). This difference remained significant in the sensitivity analysis throughout the whole range of ICC (data not shown). The other quality parameters were not statistically different within or between the two groups. As an outcome variable, the HbA1c value at the end of the study period did not differ significantly between the intervention and control groups (mean ± standard error: 7.1 ± 0.1% vs 7.1 ± 0.1%, P = 0.90).
Figure 2.
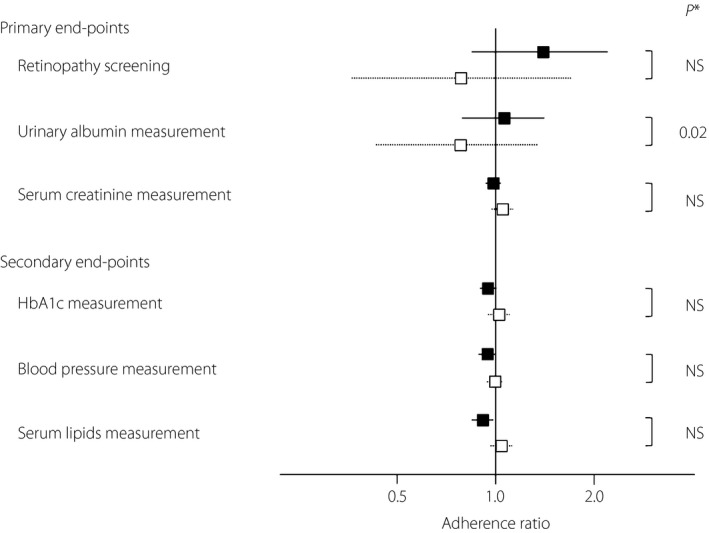
Adherence ratio for each performance. Relative changes in adherence (boxes) with the 95% confidence intervals (lines) are shown. Filled boxes and solid lines, intervention group; open boxes and dotted lines, control group. *The chi‐square test, adjusted for intracluster correlation coefficient. HbA1c, glycated hemoglobin; NS, not significant.
Discussion
‘The Manual’ has been developed mainly for PCPs to make the evidence‐based clinical guideline3 more practical and specific, with the intention of improving quality of care. Evidence‐based comprehensive disease management5, including collaboration between PCPs and specialist services, has been clinically shown to improve the quality and outcome of diabetes care in several other countries6, 7, 8, 9, 10, 11. In Japan, however, no studies have been published regarding quality improvement through the implementation of clinical practice guidelines or disease management, and this is the first randomized trial on this topic.
Our findings in the present pragmatic trial suggest a feasible improvement in daily clinical diabetes care by PCPs by implementing this practice manual. The strength of the present study was that it had a cluster‐randomized design to minimize bias and contamination. The extremely high follow‐up rate also made it highly valid. Although the allocation was not masked to the participants, the end‐points were not disclosed during the study period, which helped to minimize information bias. The findings of the sensitivity analysis based on the ICC were robust. In addition, the study provided actual facts regarding the current status of quality of care for diabetes by PCPs in Japan.
A recent meta‐analysis showed that disease management programs significantly increased the likelihood that patients receive screening for nephropathy (relative risk 1.28) and retinopathy (relative risk 1.22)7. In the current study, we found a grossly equivalent improvement in nephropathy monitoring based on microalbuminuria. The quality improvement strategies also significantly decreased the overall HbA1c level by 0.37%, but their effectiveness varied depending on the baseline HbA1c control: the change was non‐significant for those whose baseline HbA1c level was less than 8.0%7. There was no significant difference in the achieved HbA1c between the two groups in our trial, partly because of good glucose control. Although speculative, the lower adherence ratios in the measures of HbA1c, blood pressure and serum lipids observed in the intervention group might have reflected fewer clinic visits secondary to the improved outcomes for these factors.
Of note, although the adherence to blood pressure measurements and blood test‐related measures was relatively high, the adherence to blood test‐unrelated screenings for microvascular complications was lower in Japan than in other countries7. This fact points to the gap between ideal and actual care for patients with diabetes: despite high‐quality evidence showing improved clinical outcomes for patients with diabetes who receive various preventive and therapeutic interventions, many diabetic patients do not receive them in practical settings. The dissemination of ‘The Manual’ could potentially lead to an improvement in the quality of diabetes‐related vascular risk factor management in the real world.
Several limitations of our investigation should be noted. The present study was an exploratory pilot trial with a short follow‐up period, and the sample size was not prespecified. An investigation of the clinical outcomes would likely be premature, but process evaluation, rather than outcome measurement, is likely to be the best and fastest indicator of quality assurance10, 12, and the results would support validations of the efficacy of this manual. In light of the moderately long time‐lag between randomization and patient registration, the baseline adherence might have been affected, because performance changes during this interval were accounted for in the pre‐intervention adherence. However, the interval time and the baseline process measures were similar in both groups, and were unlikely to have caused bias in the final analysis. The lack of data regarding complications, lipid levels and blood pressure values might limit the external validity of this study.
Despite these limitations, our trial can provide clinicians with information regarding the potential quality improvement afforded by this disease management program, which might encourage more physician involvement in guideline development, and help to align clinical and economic incentives5, as positive consequences of behavior encourage the adoption of specific behaviors8.
In conclusion, our analysis of the quality indicators in this investigation suggests that ‘The Manual’ leads to an improved quality of diabetes management by PCPs. Larger trials are warranted to ascertain the potential long‐term impact on glucose control, the risk of diabetic complications and mortality.
Disclosure
The authors declare no conflict of interest.
Acknowledgments
This study was supported by Grants‐in‐Aid from the Japan Agency for Medical Research and Development (Grant: Practical Research Project for Life‐Style related Diseases including CVD and Diabetes), and from the Ministry of Health, Labour and Welfare, Japan (Grant number: Comprehensive Research on Life‐Style Related Diseases including CVD and Diabetes H25‐016). The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. The authors wish to acknowledge the following physicians for their dedicated participation in this study (those with consent are listed in alphabetical order): Mitsuru Amanuma, Tooru Arino, Ryuuji Asai, Kazutoshi Fujii, Hironobu Fujio, Kimiko Hayakawa, Takenori Hosokawa, Yuuji Isobe, Junko Kamijo, Kiminori Kimura, Fumio Kouchi, Naoko Kouno, Hirofumi Matsuoka, Yasuhiko Miyoshi, Shigeki Momose, Junichi Morita, Taeko Motoyama, Masato Nakamura, Mitsugi Nakamura, Kouichi Nakashima, Shizuko Narita, Hiroaki Okabe, Uran Onaka, Kaoru Ono, Fumiyo Oota, Shigehisa Oota, Yoko Pearce, Jun Shimizu, Hideaki Sudou, Tatsushi Sugiura, Atsuo Suzuki, Akira Takaki, Ken Takeda, Michihiko Takesue, Toyoaki Tanaka, Kouji Tanakihara, Akira Tanamura, Yoshiko Terai, Toshio Yajima, Fuyuki Yamada and Tetsuo Yokoyama.
J Diabetes Investig 2016; 7: 539–543
Clinical Trial Registry
UMIN Clinical Trials Registry
UMIN000009192
References
- 1. The Standard Diabetes Manual (Japaese). Available from: http://ncgm-dm.jp/center/diabetes_treatment_manual.pdf Accessed October 1, 2015.
- 2. Eccles M, Grimshaw J, Walker A, et al Changing the behavior of healthcare professionals: the use of theory in promoting the uptake of research findings. J Clin Epidemiol 2005; 58: 107–112. [DOI] [PubMed] [Google Scholar]
- 3. Tajima N, Noda M, Origasa H, et al Evidence‐based practice guideline for the treatment for diabetes in Japan 2013. Diabetol Int 2015; 6: 151–187. [Google Scholar]
- 4. Donner A, Klar N. Comparison of proportions Design and Analysis of Cluster Randomization Trials in Health Research. London: Wiley, 2010; 84–85. [Google Scholar]
- 5. Ellrodt G, Cook DJ, Lee J, et al Evidence‐based disease management. JAMA 1997; 278: 1687–1692. [PubMed] [Google Scholar]
- 6. Harris SB, Leiter LA, Webster‐Bogaert S, et al Teleconferenced educational detailing: Diabetes education for primary care physicians. J Contin Educ Health Prof 2005; 25: 87–97. [DOI] [PubMed] [Google Scholar]
- 7. Tricco AC, Ivers NM, Grimshaw JM, et al Effectiveness of quality improvement strategies on the management of diabetes: A systematic review and meta‐analysis. Lancet 2012; 379: 2252–2261. [DOI] [PubMed] [Google Scholar]
- 8. Carey M, Buchan H, Sanson‐Fisher R. The cycle of change: Implementing best‐evidence clinical practice. Int J Qual Health Care 2009; 21: 37–43. [DOI] [PubMed] [Google Scholar]
- 9. Pimouguet C, Le Goff M, Thiebaut R, et al Effectiveness of disease‐management programs for improving diabetes care: A meta‐analysis. CMAJ 2011; 183: E115–E127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Peterson KA, Radosevich DM, O'Connor PJ, et al Improving diabetes care in practice: Findings from the TRANSLATE trial. Diabetes Care 2008; 31: 2238–2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rothe U, Muller G, Schwarz PE, et al Evaluation of a diabetes management system based on practice guidelines, integrated care, and continuous quality management in a Federal State of Germany: A population‐based approach to health care research. Diabetes Care 2008; 31: 863–868. [DOI] [PubMed] [Google Scholar]
- 12. Lohr KN. (ed). Medicare: A Strategy for Quality Assurance. Volume I and II. National Academy Press: Washington, D.C., 1990. [PubMed] [Google Scholar]


