Abstract
Aims/Introduction
The influence of overweight/obesity on the clinical efficacy and safety of sodium‐glucose co‐transporter 2 inhibitors is unclear. We carried out a pooled analysis to examine the impact of body mass index on the efficacy and safety of ipragliflozin.
Materials and Methods
Patient‐level data were pooled for five Japanese double‐blind trials (NCT00621868, NCT01057628, NCT01135433, NCT01225081 and NCT01242215) in which patients were randomized to ipragliflozin or a placebo as monotherapy, or in combination with metformin, pioglitazone or a sulfonylurea. Outcomes included the changes in hemoglobin A1c, fasting plasma glucose, bodyweight and treatment‐emergent adverse events. Patients were divided into four body mass index categories.
Results
Hemoglobin A1c, fasting plasma glucose and bodyweight decreased significantly in the ipragliflozin group compared with the placebo group in all body mass index categories, and in the total cohort (all P < 0.001). Hemoglobin A1c did not improve in 11.2 and 69.2% of patients in the ipragliflozin and placebo groups, respectively. The change in hemoglobin A1c was weakly correlated with the change in bodyweight in all patients (r = 0.136, P = 0.002). Regarding laboratory variables, the placebo‐subtracted difference tended to be greater in patients with higher body mass index for aspartate aminotransferase, alanine aminotransferase, γ‐glutamyl transpeptidase and uric acid. The incidences of treatment‐emergent adverse events were similar between the ipragliflozin and placebo groups in all patients combined and in the four body mass index categories.
Conclusions
These results show that the efficacy and safety of ipragliflozin are not influenced by obesity/overweight in Japanese patients.
Keywords: Body mass index, Ipragliflozin, Sodium‐glucose co‐transporter 2
Introduction
Ipragliflozin was the first sodium‐glucose co‐transporter 2 (SGLT2) inhibitor to be approved in Japan. It was associated with significant improvements in glycemic control in terms of hemoglobin A1c (HbA1c) and fasting plasma glucose (FPG) when administered as monotherapy over 12 weeks1 or 16 weeks2, or for 24 weeks in combination with metformin3, pioglitazone4 or a sulfonylurea5. No studies have examined the impact of obesity on the efficacy and safety of SGLT2 inhibitors. To provide further insight into the clinical efficacy and safety of ipragliflozin, we carried out a pooled analysis of these five Japanese trials. We examined whether the clinical efficacy and safety of ipragliflozin are influenced by body mass index (BMI) by dividing the patients into categories based on their BMI at screening. To the best of our knowledge, no study has evaluated the efficacy or safety of SGLT2 inhibitors in groups of patients divided on the basis of a wide range of BMI.
Materials and methods
We pooled data from the following five randomized, confirmatory, placebo‐controlled, double‐blind clinical studies (study reference number and ClinicalTrials.gov identifier): a phase 2 dose‐finding trial (CL0103, NCT00621868)1; a phase 3 monotherapy trial (CL0105, NCT01057628)2; a phase 3 trial in combination with metformin (CL0106, NCT01135433)3; a phase 3 trial in combination with pioglitazone (CL0107, NCT01225081)4; and a phase 3 trial in combination with a sulfonylurea (CL0109, NCT01242215)5. Detailed descriptions of the design, outcomes and results of each trial can be found in their original reports, and a summary of each trial is provided in Tables S1 and S2. All of the trials were carried out in accordance with Good Clinical Practice, International Conference on Harmonization guidelines and applicable laws/regulations, and were approved by institutional review boards at all participating institutions. All patients provided written informed consent before enrolment.
The primary efficacy end‐point in each trial was the change in HbA1c from baseline to the end of the double‐blind treatment period (i.e., 12–24 weeks of treatment). HbA1c was measured according to the requirements of the Japan Diabetes Society, and units were converted to National Glycohemoglobin Standardization Program6 and International Federation of Clinical Chemistry values7. The Supporting Information (Appendix S1) lists the secondary and safety end‐points in the trials, the sample size calculations, and the statistical methods used in our pooled analysis.
Results
Patients
The pooled analysis comprised a total of 508 patients in the ipragliflozin group and 321 patients in the placebo group. The baseline characteristics of patients in the placebo and ipragliflozin groups are shown for all patients combined and after stratification into the four BMI categories in Table 1. The ipragliflozin and placebo groups were generally similar in each of the BMI categories, except for the proportions of patients on monotherapy or combination therapy, which differed significantly in all patients and in the BMI ≥28 kg/m2 category (P < 0.05), but not in the other BMI categories. However, this was to be expected, because patients were randomized to the ipragliflozin and placebo group, at a 1:1 ratio in the monotherapy trials and at a 2:1 ratio in the combination therapy trials. The mean BMI was also significantly different between the ipragliflozin and placebo groups in all patients, but was similar in both groups in the individual BMI categories.
Table 1.
Patient characteristics
| Variable | All patients | BMI <23 kg/m2 | BMI ≥23 to <25 kg/m2 | BMI ≥25 to <28 kg/m2 | BMI ≥28 kg/m2 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Placebo | Ipragliflozin | Placebo | Ipragliflozin | Placebo | Ipragliflozin | Placebo | Ipragliflozin | Placebo | Ipragliflozin | |
| n | 321 | 508 | 93 | 113 | 74 | 113 | 80 | 144 | 74 | 138 |
| Male (%) | 214 (66.7) | 337 (66.3) | 67 (72.0) | 76 (67.3) | 48 (64.9) | 76 (67.3) | 51 (63.8) | 104 (72.2) | 48 (64.9) | 81 (58.7) |
| Age (years) | 57.5 ± 10.0 | 57.8 ± 10.5 | 60.7 ± 8.5 | 61.0 ± 9.9 | 57.9 ± 9.8 | 59.9 ± 9.2 | 58.0 ± 9.6 | 57.7 ± 9.5 | 52.4 ± 10.7 | 53.7 ± 11.5 |
| BMI (kg/m2) | 25.4 ± 3.6 | 26.0 ± 3.8* | 21.6 ± 1.0 | 21.6 ± 0.9 | 23.9 ± 0.6 | 24.1 ± 0.5 | 26.5 ± 0.9 | 26.3 ± 0.9 | 30.5 ± 2.5 | 31.0 ± 2.8 |
| WC (cm) | 89.0 ± 9.5 | 90.0 ± 9.8 | 80.3 ± 5.0 | 79.2 ± 4.7 | 85.7 ± 4.8 | 86.7 ± 4.5 | 91.2 ± 5.3 | 90.7 ± 5.9 | 100 ± 7.7 | 101 ± 8.2 |
| Duration of DM ≥60 months (%) | 204 (63.8) | 310 (61.4) | 70 (76.1) | 83 (74.1) | 49 (66.2) | 76 (67.3) | 46 (57.5) | 86 (60.1) | 39 (52.7) | 65 (47.4) |
| No history of smoking (%) | 102 (40.5) | 181 (41.5) | 27 (37.0) | 46 (47.9) | 23 (41.1) | 35 (36.1) | 28 (45.2) | 43 (34.7) | 24 (39.3) | 57 (47.9) |
| Hypertension (%) | 156 (48.6) | 239 (47.0) | 36 (38.7) | 38 (33.6) | 30 (40.5) | 47 (41.6) | 43 (53.8) | 69 (47.9) | 47 (63.5) | 85 (61.6) |
| Monotherapy (%) | 136 (42.4) | 134 (26.4)* | 42 (45.2) | 34 (30.1) | 33 (44.6) | 30 (26.5) | 31 (38.8) | 38 (26.4) | 30 (40.5) | 32 (23.2)* |
| Concomitant therapy (%) | ||||||||||
| SU | 75 (23.4) | 165 (32.5)* | 28 (30.1) | 41 (36.3) | 20 (27.0) | 32 (28.3) | 18 (22.5) | 51 (35.4) | 9 (12.2) | 41 (29.7)* |
| BG | 56 (17.4) | 112 (22.0)* | 14 (15.1) | 28 (24.8) | 13 (17.6) | 31 (27.4) | 16 (20.0) | 22 (15.3) | 13 (17.6) | 31 (22.5)* |
| TZD | 54 (16.8) | 97 (19.1)* | 9 (9.7) | 10 (8.8) | 8 (10.8) | 20 (17.7) | 15 (18.8) | 33 (22.9) | 22 (29.7) | 34 (24.6)* |
Values are presented as n (%) or mean ± standard deviation. *Significantly different at P < 0.05. Values were compared between the ipragliflozin and placebo groups within each body mass index (BMI) category using Fisher's exact test for categorical variables or independent‐samples t‐tests for continuous variables. BG, biguanide; DM, diabetes mellitus; SU, sulfonylurea; TZD, thiazolidinedione; WC, waist circumference.
Efficacy
Glycemic control
The changes in HbA1c from baseline to the end of treatment are shown in Table 2. HbA1c decreased significantly in the ipragliflozin group, but not in the placebo group in all patients combined with a placebo‐adjusted mean change of −1.17% (P < 0.001). Consistent with the change in HbA1c in all patients, the placebo‐adjusted mean change in HbA1c was also statistically significant in each BMI category (all P < 0.001), with values of −1.10, −1.25, −1.12, and −1.24% for the <23 kg/m2, ≥23 to <25 kg/m2, ≥25 to <28 kg/m2 and ≥28 kg/m2 BMI categories, respectively (Table 2). Overall, 18.3 and 2.5% of patients in the ipragliflozin and placebo groups, respectively, achieved the target HbA1c of <7.0%, with similar proportions in each BMI category (ipragliflozin: 15.9–20.4%; placebo: 1.3–4.3%). In all patients combined, there was a significant negative correlation (r = −0.412, P < 0.001) between baseline HbA1c and the change in HbA1c from baseline to the end of treatment in the ipragliflozin group, but not in the placebo group. Overall, 11.2 and 69.2% of patients in the ipragliflozin and placebo groups, respectively, did not show any reduction of HbA1c levels (Figure 1).
Table 2.
Baseline and end‐of‐treatment values for hemoglobin A1c and fasting plasma glucose
| Variable | All patients | BMI <23 kg/m2 | BMI ≥23 to <25 kg/m2 | BMI ≥25 to <28 kg/m2 | BMI ≥28 kg/m2 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Placebo | Ipragliflozin | Placebo | Ipragliflozin | Placebo | Ipragliflozin | Placebo | Ipragliflozin | Placebo | Ipragliflozin | |
| HbA1c (%) | ||||||||||
| n | 321 | 508 | 93 | 113 | 74 | 113 | 80 | 144 | 74 | 138 |
| Baseline | 8.34 ± 0.716 | 8.32 ± 0.716 | 8.32 ± 0.679 | 8.26 ± 0.732 | 8.40 ± 0.855 | 8.35 ± 0.699 | 8.31 ± 0.677 | 8.28 ± 0.716 | 8.35 ± 0.661 | 8.39 ± 0.716 |
| EOT | 8.74 ± 1.164 | 7.53 ± 0.734 | 8.72 ± 12.83 | 7.53 ± 0.694 | 8.81 ± 1.151 | 7.52 ± 0.698 | 8.66 ± 1.105 | 7.54 ± 0.805 | 8.78 ± 1.099 | 7.53 ± 0.725 |
| Change | 0.40 ± 0.919 | −0.79 ± 0.664 | 0.40 ± 1.041 | −0.73 ± 0.627 | 0.41 ± 0.817 | −0.82 ± 0.649 | 0.36 ± 0.859 | −0.74 ± 0.694 | 0.43 ± 0.931 | −0.85 ± 0.674 |
| Difference (95% CI) | −1.17 (−1.281, −1.067) | −1.10 (−1.331, −0.869) | −1.25 (−1.462, −1.039) | −1.12 (−1.330, −0.912) | −1.24 (−1.456, −1.020) | |||||
| P‐value | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |||||
| FPG (mg/dL) | ||||||||||
| n | 321 | 508 | 93 | 113 | 74 | 113 | 80 | 144 | 74 | 138 |
| Baseline† | 176.6 ± 34.85 | 173.1 ± 34.98 | 174.1 ± 36.08 | 171.8 ± 34.67 | 183.4 ± 37.67 | 174.1 ± 31.92 | 172.8 ± 32.23 | 174.9 ± 35.81 | 177.1 ± 32.72 | 171.4 ± 36.93 |
| EOT | 182.3 ± 41.38 | 138.4 ± 24.30 | 176.5 ± 45.79 | 137.8 ± 22.26 | 192.7 ± 44.75 | 140.3 ± 26.51 | 177.5 ± 34.63 | 137.8 ± 23.92 | 184.5 ± 37.08 | 138.1 ± 24.59 |
| Change | 6.1 ± 31.84 | −34.6 ± 31.27 | 2.4 ± 32.07 | −34.0 ± 31.67 | 9.4 ± 33.18 | −33.8 ± 30.37 | 4.8 ± 31.24 | −37.1 ± 29.61 | 8.8 ± 30.86 | −33.3 ± 33.45 |
| Difference (95% CI)† | −41.8 (−45.56, −38.09) | −36.9 (−44.73, −29.08) | −47.4 (−55.85, −38.97) | −39.7 (−46.21, −33.14) | −44.2 (−51.80, −36.64) | |||||
| P‐value | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |||||
Values are presented as mean ± standard deviation. Baseline values were compared between the ipragliflozin and placebo groups within each body mass index (BMI) category using independent‐samples t‐tests (P > 0.05 for all baseline variables). †Adjusted mean difference between groups. CI, confidence interval; EOT, end‐of‐treatment; FPG, fasting plasma glucose; HbA1c, hemoglobin A1c.
Figure 1.
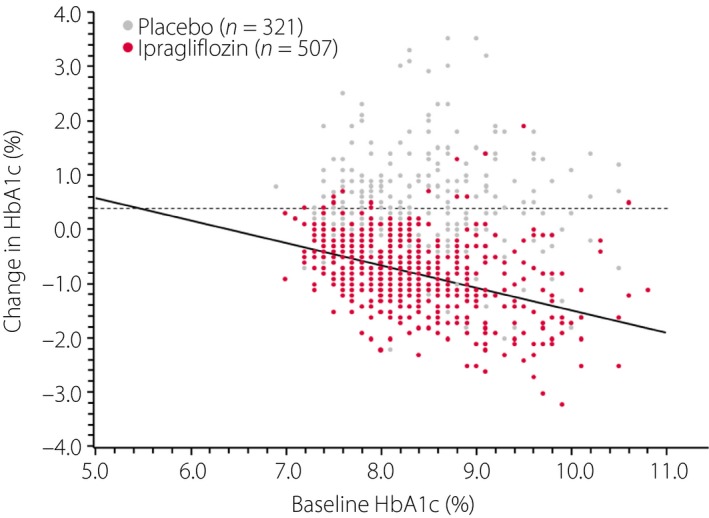
Scatter plots for the relationship between baseline hemoglobin A1c (HbA1c) and the change in HbA1c from baseline to the end of treatment in the placebo (n = 321, r = 0.004, P = 0.942) and ipragliflozin (n = 507, r = −0.438, P < 0.001) groups.
As shown in Table 2, FPG decreased significantly in the ipragliflozin group, but not in the placebo group, with a placebo‐adjusted mean change of −41.8 mg/dL in all patients combined (P < 0.001). The placebo‐adjusted mean change in FPG was also significant in each BMI category, with values of −36.9, −47.4, −39.7, and −44.2 mg/dL for those in the <23, ≥23 to <25, ≥25 to <28 and ≥28 kg/m2 BMI categories, respectively (all P < 0.001).
Bodyweight and waist circumference
In all patients combined, the mean reduction in bodyweight was −2.2 and −0.5 kg in the ipragliflozin and placebo groups, respectively, corresponding to a placebo‐adjusted change of −1.7 kg (P < 0.001; Table 3). Although the placebo‐adjusted change in bodyweight tended to be greater in the highest BMI categories, the placebo‐adjusted mean percent change of baseline value was comparable in each of the four BMI categories, with values between −2.00 and −2.95% (Table 3). Bodyweight in the ipragliflozin group shifted to lower values than that in the placebo group. Overall, 23.2% of patients in the ipragliflozin group and 5.0% of patients in the placebo group showed a reduction in bodyweight of ≥5% (P < 0.001; Figure 2). As shown in Figure 3, the change in bodyweight was only weakly correlated with the change in HbA1c in the ipragliflozin group (r = 0.136, P = 0.002), but not in the placebo group (r = −0.013, P = 0.818). The placebo‐adjusted reductions in waist circumference from baseline to the end of the double‐blind treatment period were also significant (P ≤ 0.01) for all categories except for the <23 kg/m2 BMI category (Table 3).
Table 3.
Baseline and end‐of‐treatment values for bodyweight and waist circumference
| Variable | All patients | BMI <23 kg/m2 | BMI ≥23 to <25 kg/m2 | BMI ≥25 to <28 kg/m2 | BMI ≥28 kg/m2 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Placebo | Ipragliflozin | Placebo | Ipragliflozin | Placebo | Ipragliflozin | Placebo | Ipragliflozin | Placebo | Ipragliflozin | |
| Bodyweight (kg) | ||||||||||
| n | 321 | 508 | 93 | 113 | 74 | 113 | 80 | 144 | 74 | 138 |
| Baseline | 67.7 ± 12.59 | 69.0 ± 12.87 | 57.1 ± 6.57 | 56.8 ± 6.79 | 63.4 ± 7.04 | 63.8 ± 7.13 | 70.2 ± 7.07 | 70.3 ± 7.50 | 82.7 ± 11.83 | 81.9 ± 12.60 |
| EOT | 67.2 ± 12.70 | 66.7 ± 12.69 | 56.5 ± 6.83 | 54.8 ± 7.01 | 62.7 ± 6.91 | 61.7 ± 7.04 | 69.9 ± 7.00 | 67.9 ± 7.66 | 82.2 ± 12.08 | 79.4 ± 12.35 |
| Change | −0.5 ± 1.88 | −2.2 ± 1.93 | −0.5 ± 1.44 | −2.0 ± 1.51 | −0.7 ± 1.84 | −2.1 ± 1.64 | −0.4 ± 2.11 | −2.3 ± 1.94 | −0.5 ± 2.16 | −2.5 ± 2.36 |
| Change difference (95% CI)† | −1.7 (−1.96, −1.43) | −1.4 (−1.80, −0.98) | −1.4 (−1.76, −0.74) | −2.0 (−2.57, −1.46) | −2.1 (−2.76, −1.43) | |||||
| P‐value | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |||||
| Percent change (%) | −0.8 ± 2.75 | −3.3 ± 2.69 | 1.0 ± 2.53 | −3.5 ± 2.80 | −1.1 ± 2.93 | −3.2 ± 2.55 | −0.5 ± 3.01 | −3.3 ± 2.74 | −0.6 ± 2.55 | −3.1 ± 2.66 |
| Percent change difference, (95% CI)† | −2.5 (−2.88, −2.11) | −2.5 (−3.22, −1.75) | −2.0 (−2.81, −1.18) | −3.0 (−3.74, −2.16) | −2.5 (−3.28, −1.75) | |||||
| P‐value | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |||||
| WC (cm) | ||||||||||
| n | 252 | 436 | 73 | 96 | 56 | 97 | 62 | 124 | 61 | 119 |
| Baseline | 89.02 ± 9.460 | 89.98 ± 9.845 | 80.32 ± 5.045 | 79.16 ± 4.710 | 85.71 ± 4.751 | 86.69 ± 4.468 | 91.19 ± 5.248 | 90.65 ± 5.889 | 100.26 ± 7.717 | 100.68 ± 8.189 |
| EOT | 88.61 ± 9.849 | 88.13 ± 9.910 | 79.16 ± 5.355 | 77.50 ± 5.056 | 85.91 ± 4.615 | 84.87 ± 4.864 | 90.63 ± 5.278 | 88.69 ± 6.071 | 100.30 ± 7.981 | 98.75 ± 8.177 |
| Change | −0.44 ± 3.438 | −1.86 ± 3.459 | −1.07 ± 2.865 | −1.69 ± 3.569 | 0.11 ± 3.196 | −1.79 ± 3.231 | −0.66 ± 3.230 | −1.99 ± 3.606 | 0.05 ± 4.310 | −1.91 ± 3.430 |
| Change difference (95% CI)† | −1.35 (−1.896, −0.807) | −0.83 (−1.837, 0.184) | −1.76 (−2.819, −0.706) | −1.40 (−2.466, −0.343) | −1.98 (−3.183, −0.770) | |||||
| P‐value | <0.001 | 0.108 | 0.001 | 0.010 | 0.001 | |||||
Values are presented as mean ± standard deviation. Baseline values were compared between the ipragliflozin and placebo groups within each body mass index (BMI) category using independent‐samples t‐tests (P > 0.05 for all baseline variables). †Adjusted mean difference between groups. CI, confidence interval; EOT, end‐of‐treatment; WC, waist circumference.
Figure 2.
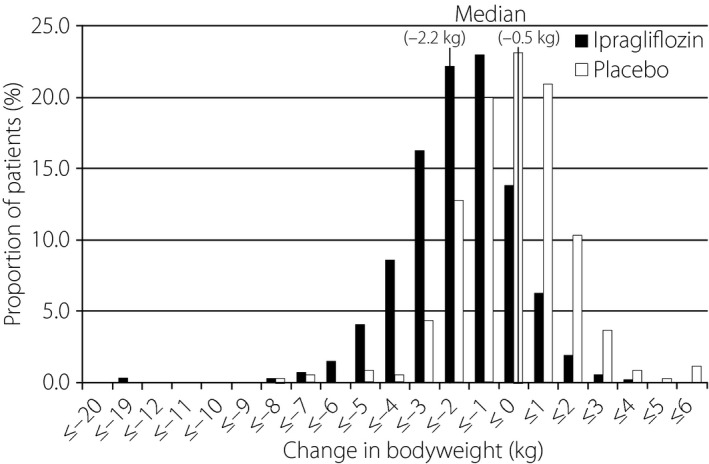
Distribution of changes in bodyweight from baseline to the end of treatment in the placebo (n = 321) and ipragliflozin (n = 508) groups. The median change in bodyweight was −0.5 and −2.2 kg in the placebo and ipragliflozin groups, respectively. A reduction in bodyweight of ≥5% from the baseline value was observed in 5.0 and 23.2% of patients in the placebo and ipragliflozin groups, respectively (P < 0.001).
Figure 3.
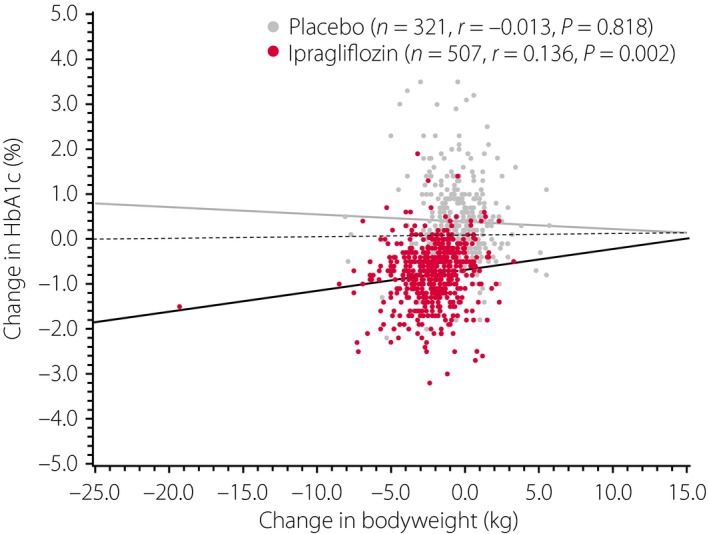
Scatter plots for the relationship between the changes in bodyweight and hemoglobin A1c from baseline to the end of treatment in the placebo (n = 321, r = −0.013, P = 0.818) and ipragliflozin (n = 507, r = 0.136, P = 0.002) groups. HbA1c, hemoglobin A1c.
Blood pressure
The changes in systolic blood pressure (SBP) and diastolic blood pressure (DBP) are presented in Table 4. The reductions in SBP were significantly greater in the ipragliflozin group than in the placebo group in all patients (P < 0.001), and in the ≥23 to <25 kg/m2 (P < 0.05) and ≥25 to <28 kg/m2 BMI categories (P < 0.001), but not in the other BMI categories. The reductions in DBP were significantly greater in the ipragliflozin group than in the placebo group in all patients (P < 0.001), and those in the ≥23 to <25, ≥25 to <28 and ≥28 kg/m2 BMI categories (all P < 0.05), but not in those in the <23 kg/m2 BMI category. In all ipragliflozin‐treated patients combined, the reduction in SBP from baseline to the end of treatment was greater in patients with a reduction in bodyweight of ≥5% (−7.0 ± 14.95 mmHg; n = 118) than in patients with a reduction in bodyweight of <5% (−3.1 ± 12.49 mmHg, n = 390). By contrast, the reduction in DBP was similar in these two subgroups (−3.1 ± 9.85 and −2.5 ± 8.41 mmHg, respectively).
Table 4.
Changes in systolic and diastolic blood pressure
| Variable | All patients | BMI <23 kg/m2 | BMI ≥23 to <25 kg/m2 | BMI ≥25 to <28 kg/m2 | BMI ≥28 kg/m2 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Placebo | Ipragliflozin | Placebo | Ipragliflozin | Placebo | Ipragliflozin | Placebo | Ipragliflozin | Placebo | Ipragliflozin | |
| SBP | ||||||||||
| n | 321 | 508 | 94 | 114 | 74 | 113 | 80 | 144 | 74 | 138 |
| Baseline | 128.0 ± 13.61 | 129.3 ± 13.84 | 127.1 ± 14.31 | 125.3 ± 13.89 | 127.6 ± 14.08 | 128.3 ± 13.77 | 127.1 ± 11.87 | 131.1 ± 14.38* | 130.4 ± 13.96 | 131.4 ± 12.56 |
| EOT | 127.6 ± 13.16 | 125.2 ± 13.96 | 126.0 ± 13.13 | 122.4 ± 15.13 | 127.4 ± 14.42 | 124.3 ± 11.10 | 128.4 ± 11.52 | 126.2 ± 13.19 | 128.9 ± 13.57 | 127.2 ± 15.45 |
| Change | −0.4 ± 13.18 | −4.0 ± 13.19 | −1.4 ± 13.46 | −2.8 ± 14.68 | −0.2 ± 13.91 | −4.0 ± 12.66 | 1.3 ± 12.09 | −4.9 ± 13.23 | −1.5 ± 13.25 | −4.2 ± 12.31 |
| Difference (95% CI) | −3.5 (−5.35, −1.62) | −1.9 (−5.66, 1.95) | −4.1 (−8.07, −0.17) | −6.5 (−10.14, −2.95) | −2.0 (−5.73, 1.71) | |||||
| P‐value‡ | <0.001 | 0.337 | 0.041 | <0.001 | 0.288 | |||||
| DBP | ||||||||||
| n | 321 | 508 | 94 | 114 | 74 | 113 | 80 | 144 | 74 | 138 |
| Baseline | 76.5 ± 9.81 | 77.4 ± 9.99 | 75.4 ± 9.57 | 75.0 ± 9.66 | 75.0 ± 9.65 | 75.6 ± 10.63 | 77.0 ± 9.84 | 78.5 ± 9.7 | 78.8 ± 9.97 | 79.9 ± 9.36 |
| EOT | 76.2 ± 9.95 | 74.8 ± 10.23 | 73.5 ± 8.92 | 72.6 ± 9.89 | 75.3 ± 9.86 | 73.6 ± 9.96 | 76.2 ± 10.36 | 75.6 ± 8.95 | 80.4 ± 9.62 | 76.8 ± 11.51 |
| Change | −0.3 ± 9.05 | −2.6 ± 8.76 | −1.9 ± 9.26 | −2.3 ± 8.44 | 0.3 ± 8.92 | −2.0 ± 8.79 | −0.8 ± 8.21 | −2.8 ± 8.11 | 1.6 ± 9.55 | −3.1 ± 9.67 |
| Difference (95% CI)† | −2.4 (−3.67, −1.15) | −0.4 (−2.88, 2.07) | −2.7 (−5.36, −0.05) | −2.4 (−4.63, −0.12) | −4.7 (−7.56, −1.88) | |||||
| P‐value‡ | <0.001 | 0.750 | 0.046 | 0.039 | 0.001 | |||||
Values are presented as mean ± standard deviation. *Significantly different at P < 0.05. Baseline values were compared between the ipragliflozin and placebo groups within each body mass index (BMI) category using independent‐samples t‐tests. †Adjusted mean difference between groups. ‡Analysis of variance with treatment group and clinical trial as fixed effects. CI, confidence interval; DBP, diastolic blood pressure; EOT, end‐of‐treatment; SBP, systolic blood pressure.
Laboratory variables
Tables S3–S5 show the values at baseline and the changes in laboratory variables from baseline to the end of treatment. There were significant differences between the ipragliflozin and placebo groups for the baseline fasting serum insulin and leptin in all patients combined, and in fasting serum insulin and low‐density lipoprotein cholesterol (LDL‐C) in the BMI ≥28 kg/m2 category.
Significant reductions in fasting serum insulin were observed in the ipragliflozin group compared with the placebo group in all patients, and those in all BMI categories (all P < 0.05), except for the ≥23 to <25 kg/m2 category.
Reductions in leptin concentrations were significantly greater in the ipragliflozin groups in all BMI categories (all P < 0.05), except in the ≥28 kg/m2 BMI category. Adiponectin increased significantly in the ipragliflozin groups in all patients (P < 0.001), and in the <23 kg/m2 (P < 0.05) and ≥23 to <25 kg/m2 (P < 0.01) BMI categories.
The reduction in triglycerides was significantly different between the ipragliflozin and placebo groups in all patients (P < 0.01), and in the <23 kg/m2 (P < 0.05) and ≥28 kg/m2 (P < 0.01) BMI categories. There were no consistent changes in LDL‐C across the BMI categories of study groups, and the changes were not significantly different between the ipragliflozin and placebo groups. By contrast, high‐density lipoprotein cholesterol (HDL‐C) increased significantly in the ipragliflozin groups relative to the placebo groups in all patients (P < 0.001) and in all BMI categories (all P < 0.05), except for the ≥28 kg/m2 BMI category.
Safety
Laboratory variables
At baseline, aspartate aminotransferase (AST: all patients), uric acid (BMI <23 kg/m2 and BMI ≥28 kg/m2), and estimated glomerular filtration rate (BMI 23–25 kg/m2) were significantly different between the ipragliflozin and placebo groups.
Liver enzymes (AST, alanine aminotransferase [ALT] and γ‐glutamyl transpeptidase [γ‐GTP]) decreased in the ipragliflozin groups compared with the placebo groups in all patients (P < 0.001 for all enzymes), and in the ≥23 to <25 kg/m2 (P < 0.01 for ALT and P < 0.001 for γ‐GTP), ≥25 to <28 kg/m2 (P < 0.001 for AST and ALT, P < 0.01 for γ‐GTP) and ≥28 kg/m2 (P < 0.001 for all enzymes) BMI categories. Baseline BMI was weakly, but significantly, correlated with the changes in AST (n = 508, r = 0.149, P < 0.001) and ALT (n = 508, r = 0.208, P < 0.001), but not with γ‐GTP from baseline to the end of treatment in the ipragliflozin group. The change in bodyweight from baseline to the end of treatment was also correlated with the changes in AST (n = 508, r = 0.129, P = 0.004) and ALT (n = 508, r = 0.131, P = 0.003), but not with γ‐GTP in the ipragliflozin group. Baseline BMI and the change in bodyweight were not correlated with changes in liver enzymes in the placebo group, except for the change in bodyweight with the change in ALT (n = 321, r = 0.169, P = 0.003) and the change in bodyweight with the change in γ‐GTP (n = 321, r = 0.176, P = 0.002).
Hematocrit and blood urea nitrogen increased in all patients and in all BMI categories in the ipragliflozin groups compared with the placebo groups (all P < 0.01). The β2‐microglobulin/creatinine ratio increased significantly in the ipragliflozin groups compared with the placebo groups in all patients (P < 0.05) and in the ≥28 kg/m2 BMI category (P < 0.05). By contrast, there were no significant differences between the ipragliflozin groups and placebo groups in terms of the changes in the urinary N‐acetyl‐β‐D‐glucosaminidase/creatinine ratio.
There were significant decreases in the estimated glomerular filtration rate from baseline to the end of treatment between the ipragliflozin group and the placebo group in all patients combined (P < 0.05) and in patients in the <23 kg/m2 BMI category (P < 0.01).
Adverse events
The incidence rates of treatment‐emergent adverse events (TEAEs), serious TEAEs, TEAEs leading to study discontinuation and TEAEs of special interest (hypoglycemia, urinary tract/genital infections, and polyuria/pollakiuria) are shown in Table 5 for all patients combined, and in Table S6 for patients divided into the four BMI categories. Overall, there were no marked differences in the incidences of TEAEs among the four BMI categories or between the ipragliflozin and placebo groups, with the exceptions of genital infection, polyuria/pollakiuria and thirst, which were more common in the ipragliflozin groups. Genital infection‐related and urinary tract infection‐related TEAEs were more commonly observed in females than in males.
Table 5.
Treatment‐emergent adverse events in all patients
| Variable | Placebo (n = 322; 214 men, 108 women) | Ipragliflozin (n = 509; 338 men, 171 women) |
|---|---|---|
| TEAEs | 216 (67.1) | 361 (70.9) |
| TEAEs resulting in discontinuation | 38 (11.8) | 17 (3.3) |
| Serious TEAEs | 11 (3.4) | 8 (1.6) |
| TEAEs of special interest | ||
| Hypoglycemia | 3 (0.9) | 5 (1.0) |
| Genital tract infection | ||
| Males | 1 (0.5) | 3 (0.9) |
| Females | 2 (1.9) | 9 (5.3) |
| Urinary tract infection | ||
| Males | 1 (0.5) | 2 (0.6) |
| Females | 7 (6.5) | 8 (4.7) |
| Increased urinary volume | 0 (0.0) | 1 (0.2) |
| Pollakiuria | 6 (1.9) | 39 (7.7) |
| Polyuria | 1 (0.3) | 10 (2.0) |
| Thirst | 5 (1.6) | 22 (4.3) |
| Blood pressure decreased | 0 (0.0) | 1 (0.2) |
Values are presented as n (%). Treatment‐emergent adverse events (TEAEs) in patients divided into the four body mass index categories are presented in Table S6.
Discussion
In the present pooled analysis of five randomized confirmatory, placebo‐controlled, double‐blind clinical studies, we found that ipragliflozin significantly reduced both HbA1c and FPG as compared with placebo in all patients combined and in each BMI category. The pooled analyses also showed significantly greater reductions in bodyweight from baseline to the end of treatment in the ipragliflozin group (by a mean of 2.5%) than in the placebo group in all four BMI categories. Notably, the reduction in HbA1c in the ipragliflozin group was essentially independent of the change in bodyweight owing to the correlation coefficient of 0.136. Furthermore, when we carried out stepwise multiple regression to identify variables associated with the change in HbA1c from baseline to the end of treatment, the final model did not include BMI as an explanatory variable, which suggests that baseline BMI was not associated with the change in HbA1c (data not shown). The analyses also showed that the safety profile of ipragliflozin was mostly unaffected by BMI, except for the changes in some laboratory variables, as the reductions in liver enzymes tended to be greater in the higher BMI categories, whereas the increase in HDL‐C tended to be greater in the lower BMI categories.
To our knowledge, this is the first pooled analysis to evaluate the potential impact of BMI on the efficacy and safety of an SGLT2 inhibitor. However, several pooled analyses and meta‐analyses of clinical trials have documented the efficacy and safety of other SGLT2 inhibitors8, 9, 10. In a meta‐analysis of 10 empagliflozin trials (6,203 patients)8, the mean placebo‐subtracted changes in HbA1c were −0.62% (95% confidence interval [CI] −0.68 to −0.57) and −0.66% (95% CI −0.76 to −0.57) for 10 and 25 mg empagliflozin, respectively. Unfortunately, the study by Liakos et al.8 did not examine the effects of empagliflozin on FPG. A significant placebo‐subtracted reduction in bodyweight was also found (−1.84 kg, 95% CI −2.30 to −1.38 kg). Although 25 mg empagliflozin did not increase the incidence of hypoglycemia (odds ratio [OR] 1.10, 95% CI 0.87 to 1.39), it did increase the incidence of genital tract infections compared with placebo (OR 3.31, 95% CI 1.55 to 7.09).
Similar findings were also reported in two meta‐analyses of dapagliflozin9, 10. In the first meta‐analysis of 12 trials9, the placebo‐subtracted reductions in HbA1c, FPG, and bodyweight were −0.52% (95% CI −0.60 to −0.45%), −1.13 mmol/L (95% CI −1.33 to −0.93 mmol/L), and −2.10 kg (95% CI −2.32 to −1.88 kg), respectively. In a meta‐analysis involving ten trials10, the placebo‐subtracted reductions in HbA1c, FPG, and bodyweight were −0.53% (95% CI −0.58 to −0.47%), −1.06 mmol/L (−1.20 to −0.92 mmol/L) and −1.63 kg (95% CI −1.83 to −1.43 kg), respectively.
In a meta‐analysis of four randomized, placebo‐controlled 26‐week studies of 100 or 300 mg canagliflozin11, the placebo‐subtracted mean changes in HbA1c in patients aged <65 years were −0.7% (95% CI −0.8 to −0.6%) and −0.9% (95% CI −1.0 to −0.8%) for 100 and 300 mg canagliflozin, respectively. In the 100 and 300 mg canagliflozin groups, the mean placebo‐subtracted changes in FPG were −1.7 mmol/L (95% CI −2.0 to −1.5 mmol/L) and −2.2 mmol/L (95% CI −2.5 to −2.0 mmol/L), respectively, whereas the changes in bodyweight were −2.2% (95% CI −2.6 to −1.8%; −2.0 kg) and −2.8% (95% CI −3.3 to −2.4%; −2.5 kg), respectively.
In the present pooled analysis, the placebo‐adjusted changes in HbA1c, FPG and bodyweight were −1.17%, −41.8 mg/dL (−2.32 mmol/L) and −1.7 kg (−2.5%), respectively, for all patients combined. These results show that there are fairly consistent effects of SGLT2 inhibitors on HbA1c, FPG, and bodyweight with minor differences in the changes in those parameters, as compared with the other clinical trials using different SGLT2 inhibitors9, 10, 11.
Considering that the present analyses were based on Japanese studies, which included very lean participants with type 2 diabetes, it is important to compare the present results with studies of ipragliflozin in Western patients, who typically have greater BMI than Japanese patients. So far, only one study of ipragliflozin in Western patients has been published12. In that 12‐week study, patients were randomized to 12.5, 50, 150, or 300 mg ipragliflozin or placebo in combination with metformin. Over 12 weeks, 12.5, 50, 150 or 300 mg ipragliflozin significantly reduced HbA1c (placebo‐adjusted mean changes: −0.22, −0.34, −0.40 and −0.48%, respectively), FPG (−0.41, −0.73, −1.29 and −1.48 mmol/L, respectively) and bodyweight (−0.44, −1.62, −1.51 and −1.73 kg, respectively). Further studies are necessary to provide additional information on the efficacy of ipragliflozin in Western patients, and allow more detailed comparisons between Japanese and Western patients.
In the present pooled analysis, there was a significant negative correlation (r = −0.438, P < 0.001) between the baseline HbA1c and the reduction in HbA1c from baseline to the end of treatment in the ipragliflozin group. In other words, patients with poor glycemic control showed a greater reduction in HbA1c during treatment with ipragliflozin. However, as shown in Figure 1, 11.2% of patients in the ipragliflozin group did not show a reduction in HbA1c. Owing to the design of these analyses, we could not identify the factors that might be related to the lack of a response to ipragliflozin, even in some patients with high baseline HbA1c levels.
Our pooled analyses showed that ipragliflozin reduced triglycerides and increased HDL‐C levels, whereas LDL‐C levels were unchanged, and were not different between the ipragliflozin and placebo groups. Reductions in triglycerides and increases in HDL‐C and LDL‐C were reported in a clinical trial11 and in a pooled analysis13 of canagliflozin. In the pooled analysis of 100 and 300 mg canagliflozin13, the placebo‐subtracted mean changes in triglycerides, HDL‐C, and LDL‐C were −5.2% (95% CI −10.0 to −0.3), 5.4% (95% CI 3.6 to 7.2) and 4.5% (95% CI 1.4 to 7.6), respectively, for 100 mg canagliflozin. The placebo‐subtracted mean changes in triglycerides, HDL‐C, and LDL‐C were −7.6% (95% CI −12.5 to −2.8), 6.3% (4.5 to 8.2) and 8.0% (4.9 to 11.1), respectively, for 300 mg canagliflozin. In a pooled analysis of dapagliflozin studies14, the mean percent changes in triglycerides, HDL‐C, and LDL‐C ranged from −3.2 to −5.4%, from 3.8 to 6.5% and from 0.6 to 2.7%, respectively, in the dapagliflozin groups, compared with −0.7, 3.8 and −1.9%, respectively, in the placebo group. These findings suggest that SGLT2 inhibitors influence lipid metabolism and might increase triglyceride degradation, possibly to counteract the reduced glucose availability. The effects of SGLT2 inhibitors on LDL‐C levels, in particular, warrant further evaluation to understand the potential clinical relevance, although ipragliflozin did not increase LDL‐C levels based on the present pooled analysis.
Another clinically relevant metabolite, uric acid, showed greater placebo‐subtracted reductions in patients with higher BMI in the present study. In three placebo‐controlled studies of dapagliflozin, the placebo‐corrected mean changes ranged from −0.5 to −0.8 mg/dL15. In a meta‐analysis of ten dapagliflozin studies, dapagliflozin reduced uric acid compared with placebo (mean difference −36.17 mmol/L [−0.61 mg/dL], 95% CI −40.99 to −31.36 mmol/L [−0.69 to −0.53 mg/dL])10. For ipragliflozin‐treated patients, the mean change in uric acid was −0.2 mg/dL, which was statistically significant (P < 0.001).
Several mechanisms could be involved in the reduction in bodyweight, including an increase in lipid metabolism in order to offset the reduction in glucose availability16, 17 and changes in plasma volume18. The increase in urinary glucose excretion has an osmotic effect, causing an increase in water loss (osmotic diuresis), as reflected by the moderate incidence of polyuria/pollakiuria‐related TEAEs. This fluid loss might reduce plasma volume and induce hemoconcentration, as shown by the increases in hematocrit and blood urea nitrogen.
The present analyses also showed significant reductions in SBP and DBP, especially in the ≥23 to <25 and ≥25 to <28 kg/m2 BMI categories. Similar results were reported for other SGLT2 inhibitors, and it was speculated that the reductions are mediated by osmotic diuresis19. In the present analyses, although we observed increases in hematocrit and blood urea nitrogen indicative of a reduction in fluid volume, only the change in hematocrit was very weakly correlated with the change in DBP from baseline to the end of treatment (n = 507, r = 0.095, P = 0.034).
Several pooled analyses have examined the overall safety and rare adverse events (AEs) associated with other SGLT2 inhibitors, including dapagliflozin14 and canagliflozin13. In a pooled analysis of 12 randomized controlled trials14, hypoglycemia (dapagliflozin vs placebo; 11.8 vs 7.0%), urinary tract infections (4.8 vs 3.7%), vulvovaginitis/balanitis and related infections (5.1 vs 0.9%), and non‐serious volume‐related AEs (0.8 vs 0.4%) were more common in the dapagliflozin group. Usiskin et al.13 carried out a pooled analysis of patient‐level data from four clinical trials involving 2,313 patients treated with 100 or 300 mg canagliflozin, or placebo. They noted that canagliflozin increased the incidence of hypoglycemia in a dose‐dependent manner, and was associated with higher incidences of genital infections and osmotic diuresis‐related AEs.
Genital and urinary tract infections15, 20, 21, 22 are among the most common TEAEs in patients treated with SGLT2 inhibitors, and are generally more common in women than in men.
Some limitations of this pooled analysis should be mentioned, including the differences in the treatments used in each study (monotherapy or combination with metformin, sulfonylurea, or pioglitazone), and the varying numbers of patients between each trial. These factors could introduce some bias into the analyses, although we tried to overcome this by including trial as a fixed effect in the analyses. Despite these limitations, pooled analyses of patient‐level data might be more informative than meta‐analyses, which calculate weighted mean differences, and might not fully address the relationship between the baseline value and the change from baseline for clinical variables or the distributions of values.
We originally intended on using the following BMI cut‐off values, as set by the Japanese Society for the Study of Obesity: 20–22, 22–25, 25–30 and ≥30 kg/m2. However, this resulted in a very small number of patients in the highest BMI category, which reduced the reliability of the statistical analyses. This is perhaps unsurprising given that just 3.8% of men and 3.2% of women in Japan had a BMI of ≥30 kg/m2 in a recent epidemiological study23. To improve the reliability of the analyses, we used alternative BMI cut‐off values, which provided similar distributions of patients across the four BMI categories.
In conclusion, these pooled analyses showed consistent effects of ipragliflozin on the reductions in HbA1c, FPG and bodyweight in Japanese patients stratified into four BMI categories. Of note, the reductions in HbA1c were weakly correlated with the change in bodyweight. Ipragliflozin was also associated with favorable changes in blood pressure, triglycerides, HDL‐C, uric acid, liver enzymes (AST, ALT and γ‐GTP), adiponectin and leptin. Finally, the safety/tolerability of ipragliflozin was unaffected by BMI, although the incidence of genital infection‐related AEs was higher in women than men in the ipragliflozin group. Patients and clinicians should be aware of the risk of genital/urinary tract infections and polyuria/pollakiuria‐related TEAEs, when prescribing ipragliflozin.
Disclosure
AK has acted as a consultant for Astellas Pharma Inc. and has received consulting fees/honoraria from Astellas Pharma Inc. SY, IN, K Kazuta, EU, HT, HS, YK and K Kawamuki are employees of Astellas Pharma Inc., Japan. The study sponsor contributed to the study design; the collection, analysis and interpretation of data; the writing of the report; and the decision to submit the paper for publication.
Supporting information
Table S1¦ The study design of the five randomized, double‐blind, placebo‐controlled studies.
Table S2¦ Baseline characteristics, changes in efficacy end‐points and the incidence of treatment‐emergent adverse events in the five randomized, double‐blind, placebo‐controlled studies.
Table S3¦ Baseline values, end‐of‐treatment values and changes from baseline in laboratory variables 1.
Table S4¦ Baseline values, end‐of‐treatment values and changes from baseline in laboratory variables 2.
Table S5¦ Baseline values, end‐of‐treatment values and changes from baseline in laboratory variables 3.
Table S6¦ Treatment‐emergent adverse events.
Appendix S1¦ Trial design and treatments, secondary and safety end‐points, sample size calculations in individual trials, and statistical analyses.
Acknowledgments
The authors thank all of the investigators involved in each trial. Medical writing and editorial support was funded by Astellas and provided by Dr Nicholas D Smith (Edanz Group Ltd) and Elsevier/ELMCOM™. This study was sponsored by Astellas Pharma Inc., Japan.
J Diabetes Investig 2016; 7: 544–554
References
- 1. Kashiwagi A, Kazuta K, Yoshida S, et al Randomized, placebo‐controlled, double‐blind glycemic control trial of novel sodium‐dependent glucose cotransporter 2 inhibitor ipragliflozin in Japanese patients with type 2 diabetes mellitus. J Diabetes Investig 2014; 5: 382–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kashiwagi A, Kazuta K, Takinami Y, et al Ipragliflozin improves glycemic control in Japanese patients with type 2 diabetes mellitus: the BRIGHTEN study. Diabetol Int 2015; 6: 8–18. [Google Scholar]
- 3. Kashiwagi A, Kazuta K, Goto K, et al Ipragliflozin in combination with metformin for the treatment of Japanese patients with type 2 diabetes: ILLUMINATE, a randomized, double‐blind, placebo‐controlled study. Diabetes Obes Metab 2015; 17: 304–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kashiwagi A, Shiga T, Akiyama N, et al Efficacy and safety of ipragliflozin as an add‐on to pioglitazone in Japanese patients with inadequately controlled type 2 diabetes: a randomized, double‐blind, placebo‐controlled study (the SPOTLIGHT study). Diabetol Int 2015; 6: 104–116. [Google Scholar]
- 5. Kashiwagi A, Akiyama N, Shiga T, et al Efficacy and safety of ipragliflozin as an add‐on to a sulfonylurea in Japanese patients with inadequately controlled type 2 diabetes: results of the randomized, placebo‐controlled, double‐blind, phase III EMIT study. Diabetol Int 2015; 6: 125–138. [Google Scholar]
- 6. Kashiwagi A, Kasuga M, Araki E, et al International clinical harmonization of glycated hemoglobin in Japan: from Japan Diabetes Society to National Glycohemoglobin Standardization Program values. J Diabetes Investig 2012; 3: 39–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. International Federation of Clinical Chemistry (IFCC) . Harmonizing Hemoglobin A1c Testing: Standardization of HbA1c. Available from: http://www.ngsp.org/docs/IFCCstd.pdf. Accessed November 3, 2014.
- 8. Liakos A, Karagiannis T, Athanasiadou E, et al Efficacy and safety of empagliflozin for type 2 diabetes: a systematic review and meta‐analysis. Diabetes Obes Metab 2014; 16: 984–993. [DOI] [PubMed] [Google Scholar]
- 9. Sun YN, Zhou Y, Chen X, et al The efficacy of dapagliflozin combined with hypoglycaemic drugs in treating type 2 diabetes mellitus: meta‐analysis of randomised controlled trials. BMJ Open 2014; 4: e004619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhang M, Zhang L, Wu B, et al Dapagliflozin treatment for type 2 diabetes: a systematic review and meta‐analysis of randomized controlled trials. Diabetes Metab Res Rev 2014; 30: 204–221. [DOI] [PubMed] [Google Scholar]
- 11. Sinclair A, Bode B, Harris S, et al Efficacy and safety of canagliflozin compared with placebo in older patients with type 2 diabetes mellitus: a pooled analysis of clinical studies. BMC Endocr Disord 2014; 14: 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wilding JP, Ferrannini E, Fonseca VA, et al Efficacy and safety of ipragliflozin in patients with type 2 diabetes inadequately controlled on metformin: a dose‐finding study. Diabetes Obes Metab 2013; 15: 403–409. [DOI] [PubMed] [Google Scholar]
- 13. Usiskin K, Kline I, Fung A, et al Safety and tolerability of canagliflozin in patients with type 2 diabetes mellitus: pooled analysis of phase 3 study results. Postgrad Med 2014; 126: 16–34. [DOI] [PubMed] [Google Scholar]
- 14. Ptaszynska A, Johnsson KM, Parikh SJ, et al Safety profile of dapagliflozin for type 2 diabetes: pooled analysis of clinical studies for overall safety and rare events. Drug Saf 2014; 37: 815–829. [DOI] [PubMed] [Google Scholar]
- 15. Nicolle LE, Capuano G, Fung A, et al Urinary tract infection in randomized phase III studies of canagliflozin, a sodium glucose co‐transporter 2 inhibitor. Postgrad Med 2014; 126: 7–17. [DOI] [PubMed] [Google Scholar]
- 16. Bolinder J, Ljunggren Ö, Kullberg J, et al Effects of dapagliflozin on body weight, total fat mass, and regional adipose tissue distribution in patients with type 2 diabetes mellitus with inadequate glycemic control on metformin. J Clin Endocrinol Metab 2012; 97: 1020–1031. [DOI] [PubMed] [Google Scholar]
- 17. Yokono M, Takasu T, Hayashizaki Y, et al SGLT2 selective inhibitor ipragliflozin reduces body fat mass by increasing fatty acid oxidation in high‐fat diet‐induced obese rats. Eur J Pharmacol 2014; 727: 66–74. [DOI] [PubMed] [Google Scholar]
- 18. Sha S, Polidori D, Heise T, et al Effect of the sodium glucose co‐transporter 2 inhibitor canagliflozin on plasma volume in patients with type 2 diabetes mellitus. Diabetes Obes Metab 2014; 16: 1087–1095. [DOI] [PubMed] [Google Scholar]
- 19. Oliva RV, Bakris GL. Blood pressure effects of sodium‐glucose co‐transport 2 (SGLT2) inhibitors. J Am Soc Hypertens 2014; 8: 330–339. [DOI] [PubMed] [Google Scholar]
- 20. Nicolle LE, Capuano G, Ways K, et al Effect of canagliflozin, a sodium glucose co‐transporter 2 (SGLT2) inhibitor, on bacteriuria and urinary tract infection in subjects with type 2 diabetes enrolled in a 12‐week, phase 2 study. Curr Med Res Opin 2012; 28: 1167–1171. [DOI] [PubMed] [Google Scholar]
- 21. Nyirjesy P, Zhao Y, Ways K, et al Evaluation of vulvovaginal symptoms and Candida colonization in women with type 2 diabetes mellitus treated with canagliflozin, a sodium glucose co‐transporter 2 inhibitor. Curr Med Res Opin 2012; 28: 1173–1178. [DOI] [PubMed] [Google Scholar]
- 22. Johnsson KM, Ptaszynska A, Schmitz B, et al Vulvovaginitis and balanitis in patients with diabetes treated with dapagliflozin. J Diabetes Complications 2013; 27: 479–484. [DOI] [PubMed] [Google Scholar]
- 23. Yoshiike N, Miyoshi M. [Epidemiological aspects of overweight and obesity in Japan–international comparisons]. Nihon Rinsho 2013; 71: 207–216. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1¦ The study design of the five randomized, double‐blind, placebo‐controlled studies.
Table S2¦ Baseline characteristics, changes in efficacy end‐points and the incidence of treatment‐emergent adverse events in the five randomized, double‐blind, placebo‐controlled studies.
Table S3¦ Baseline values, end‐of‐treatment values and changes from baseline in laboratory variables 1.
Table S4¦ Baseline values, end‐of‐treatment values and changes from baseline in laboratory variables 2.
Table S5¦ Baseline values, end‐of‐treatment values and changes from baseline in laboratory variables 3.
Table S6¦ Treatment‐emergent adverse events.
Appendix S1¦ Trial design and treatments, secondary and safety end‐points, sample size calculations in individual trials, and statistical analyses.


