Abstract
Aims/Introduction
To assess efficacy and safety of liraglutide in combination with insulin compared with insulin monotherapy in Japanese patients with type 2 diabetes.
Materials and methods
This was a 36‐week, multicenter, double‐blind, parallel‐group trial, where patients on stable insulin therapy (basal/premixed/basal–bolus) were randomized 1:1 to additional liraglutide 0.9 mg/day (n = 127) or placebo (n = 130). The insulin dose was fixed for 16 weeks, and titrated based on self‐measured plasma glucose thereafter. The primary end‐point was change in glycosylated hemoglobin after 16 weeks.
Results
Superiority of liraglutide plus insulin versus insulin monotherapy was confirmed based on estimated mean difference in glycosylated hemoglobin after 16 weeks of −1.30% (−14 mmol/mol; 95% confidence interval −1.47 to −1.13 [−16, −12]; P < 0.0001). Statistical significance was maintained to week 36. More patients on liraglutide achieved a glycosylated hemoglobin target of <7.0% (<53 mmol/mol) at week 16 (estimated odds ratio 50.57; 95% confidence interval 16.59 to 154.16; P < 0.0001). Improvements in seven‐point self‐measured plasma glucose and fasting plasma glucose were significantly greater with liraglutide than the placebo at week 16. Insulin dose after 36 weeks was lower with liraglutide than the placebo (estimated treatment ratio: 0.82 [95% confidence interval 0.76–0.90; P < 0.0001]). Occurrence of adverse events was similar in the two groups (85.8 and 81.5%, respectively); most were mild in severity. There were no significant differences in the number of hypoglycemic episodes during the 36 weeks.
Conclusions
Adding liraglutide to insulin results in superior glycemic control compared with insulin alone in Japanese patients with type 2 diabetes, and is generally well tolerated.
Keywords: Insulin, Liraglutide, Type 2 diabetes mellitus
Introduction
Type 2 diabetes is a progressive metabolic disorder, in which combination therapy often becomes necessary, involving multiple oral antidiabetic drugs, glucagon‐like peptide 1 (GLP‐1) receptor agonists (GLP‐1RAs) and eventually insulin1, 2. Insulin initiation normally begins with a basal dose; although intensified as required, this is often associated with hypoglycemia and weight gain1. Addition of a GLP‐1RA to basal insulin provides an intensification option that minimizes these risks. Recent studies suggest that GLP‐1RAs exert greater glycosylated hemoglobin (HbA1c)‐lowering effects in Japanese patients with type 2 diabetes3. Possible factors include lower body mass index (BMI)3 and impaired postprandial insulin4. Differences in body composition between Caucasian and Asian patients explain most differences in insulin sensitivity and β‐cell responses between these populations5, although further elucidation is required.
The GLP‐1RA liraglutide is an analog of human GLP‐1 with 97% sequence homology to the endogenous protein6. Endogenous GLP‐1 is an incretin hormone that stimulates glucose‐dependent insulin secretion and suppresses glucagon secretion, but has a short half‐life (1.5 min), limiting therapeutic potential7, 8. Liraglutide has a half‐life of 13 h, with a pharmacokinetic profile suitable for once‐daily dosing9. The efficacy and safety of liraglutide were evaluated in a clinical development program including seven large, randomized, phase 3 trials in adults with type 2 diabetes10, 11, 12, 13, 14, 15, 16.
In Japan, liraglutide was approved by the Ministry of Health, Labor and Welfare in 2010. The first phase 3 trial carried out in Japan investigated liraglutide 0.9 mg/day as monotherapy, showing superior control of HbA1c after 24 weeks versus glibenclamide 2.5 mg, with benefits maintained for 1 year (treatment difference at 1 year [%; mmol/mol] −0.49; −5 [95% confidence interval (CI) −0.71 to −0.27])17. Liraglutide had additional benefits regarding weight loss and hypoglycemia18. The second study, comparing liraglutide (0.6 or 0.9 mg/day) versus placebo, as an add‐on to sulfonylurea18, showed greater HbA1c reduction with liraglutide, maintained for 1 year. Treatment differences versus placebo at 1 year (%; mmol/mol) were −0.96; −10 (95% CI −1.25 to −0.67; −14 to −7) and −1.33; −15 (95% CI −1.62 to −1.04; −18 to −11), with liraglutide 0.6 and 0.9 mg, respectively18, 19. The safety and efficacy of liraglutide in Japanese patients have been further investigated in clinical practice. Evidence suggests that liraglutide's glucose‐lowering effect depends on the remaining pancreatic β‐cells20, implying the necessity of insulin + liraglutide combination in patients with long duration of type 2 diabetes and limited β‐cell function20.
Pharmacokinetic and pharmacodynamic data suggest co‐administration of liraglutide and basal insulin analog insulin detemir produces additive glucose‐lowering effects in patients with type 2 diabetes without affecting pharmacokinetic profiles of either drug21. Data from phase 3 studies have shown the efficacy of combination therapy with liraglutide + insulin22, 23, 24. One trial showed the superiority of adding insulin detemir to liraglutide over liraglutide alone (all with metformin) in reducing HbA1c (treatment difference at 1 year [%; mmol/mol] −0.51; −6 [95% CI −0.70 to −0.31; −8 to −3])22, 23. Another trial showed that the addition of liraglutide to insulin degludec and metformin decreased HbA1c by 0.74% (8 mmol/mol) over 26 weeks, a significantly greater change than with the addition of bolus insulin aspart (treatment difference [%; mmol/mol] −0.32; −4 [95% CI −0.53 to −0.12; −6 to −1])24. In another trial, the addition of liraglutide versus placebo in patients uncontrolled on basal insulin ± metformin resulted in superior HbA1c reductions (treatment difference [%, mmol/mol] −1.19; −13 [95% CI −1.39 to −0.99; −15 to −11])25. In these studies, the combination also had positive effects on bodyweight and/or hypoglycemia, and was associated with reduced insulin dose requirements25, 26, 27, 28.
Until now, no trial has examined the efficacy/safety of liraglutide combined with pretrial insulin therapy in Japanese patients with type 2 diabetes. This trial was designed to confirm the superiority of adding liraglutide (0.9 mg/day) to fixed‐dose insulin versus continuing on fixed‐dose insulin monotherapy, with respect to glycemic control, after 16 weeks in Japanese patients with type 2 diabetes.
Materials and Methods
Trial Design and Interventions
The present 36‐week, randomized, multicenter, double‐blind, parallel‐group trial investigated the efficacy and safety of liraglutide combined with insulin versus insulin monotherapy in Japanese patients with type 2 diabetes. It was carried out at 23 sites in Japan from April 2012 to March 2013.
Patients were randomized 1:1 to liraglutide or placebo, using an Interactive Voice‐/Web‐Responsive Service. Trial treatment was given according to approved dosage/administration of liraglutide in Japan. Patients subcutaneously self‐injected once‐daily at approximately the same time each day. The starting liraglutide dose was 0.3 mg/day, then 0.6 mg/day after 1 week and 0.9 mg/day after a further week. The dose was maintained until study completion.
Patients continued pretrial insulin therapy. Until week 16, insulin dosage could not be changed, except when unacceptable hypoglycemia or adverse events (AEs) occurred. During the subsequent 20 weeks, insulin titration was allowed based on self‐measured plasma glucose (SMPG) values and a predefined algorithm: SMPG values were converted from self‐monitored blood glucose. At randomization, patients were stratified according to pretrial insulin type.
Participants
The participants were male or female, aged ≥20 years, with type 2 diabetes for ≥6 months, HbA1c 7.5–11.0% (59–97 mmol/; inclusive) and BMI <45.0 kg/m2. All patients received stable insulin therapy in addition to diet and exercise for ≥12 weeks before screening. ‘Insulin therapy’ was defined as basal insulin, premixed insulin or basal–bolus regimen. The insulin dose was required to be stable (daily fluctuation ±20%) for ≥12 weeks before screening and current dose ≥10 (I)U/day. Exclusion criteria are listed in Doc S1.
Informed consent was obtained before trial‐related activities. The protocol was reviewed by the Japanese authority according to local regulations, and reviewed and approved by institutional review boards at each site. The trial is registered with clinicaltrials.gov (NCT01572740) and Japanese Clinical Trials Registry (JapicCTI‐121802), and carried out in accordance with the Declaration of Helsinki29 and International Conference on Harmonisation Good Clinical Practice30, 31, 32, 33.
End‐Points and Assessments
The primary end‐point was HbA1c change from baseline after 16 weeks. Secondary efficacy end‐points (assessed at 16 and 36 weeks unless stated) included: change from baseline in HbA1c (36 weeks); fasting plasma glucose (FPG); patients achieving HbA1c <7.0% (<53 mmol/mol) and ≤6.5% (≤48 mmol/mol); patients achieving HbA1c <7.0 and ≤6.5% without weight gain; patients achieving HbA1c <7.0 and ≤6.5% with no severe/minor hypoglycemia during the last 4 weeks; change from baseline in insulin dose (36 weeks) and bodyweight; and seven‐point SMPG profiles.
Secondary safety end‐points assessed over 36 weeks included the number of AEs and hypoglycemic episodes, and change from baseline in blood pressure and pulse. The nature and severity of AEs were recorded. Treatment‐emergent AEs were defined as those with onset on/after first day of treatment exposure and no later than 7 days after the last day of treatment. Hypoglycemia was classified by American Diabetes Association definitions (severe; documented symptomatic; asymptomatic; probable symptomatic; relative)31 and the additional ‘minor’ category (defined as an episode with symptoms consistent with hypoglycemia, confirmed by plasma glucose <56 mg/dL [3.1 mmol/L] or full blood glucose <50 mg/dL [2.8 mmol/L], and handled by the patients themselves; or any asymptomatic plasma glucose value <56 mg/dL [3.1 mmol/L] or full blood glucose value <50 mg/dL [2.8 mmol/L]). The pool of severe/minor episodes was referred to as ‘confirmed’ hypoglycemic episodes.
Statistical Analysis
Sample size was determined to show the superiority of liraglutide combined with fixed‐dose insulin therapy versus fixed‐dose insulin monotherapy, regarding change from baseline in HbA1c to 16 weeks, using a significance level of 5% and a two‐sided test. A mean difference in HbA1c change of 0.4% with liraglutide + insulin versus insulin monotherapy (standard deviation 1.0%) was assumed. Using this assumption, sample size was set to 114/arm to achieve 85% power. Assuming a 10% dropout rate, the randomization target was 254 participants.
Full analysis set included randomized patients receiving at least one dose of the trial product. Statistical evaluation of the full analysis set followed the intention‐to‐treat principle, and participants contributed ‘as randomized.’ Safety analysis set included participants receiving at least one dose, and participants contributed ‘as treated.’ All randomized participants were included in the full analysis set and safety analysis set. A last observation carried forward approach was used for participants with at least one valid post‐baseline measurement.
Statistical tests used a significance level of 5% and two‐sided tests; estimates are reported with 95% CI. Baseline demographics and observed end‐of‐treatment values are expressed as mean ± standard deviation.
The primary end‐point was analyzed using analysis of covariance (ancova) with treatment and pretrial insulin therapy as fixed effects and baseline HbA1c as the covariate. Superiority of liraglutide combined with fixed‐dose insulin versus fixed‐dose insulin monotherapy at week 16 was confirmed if the P‐value for comparison was <5% and the estimated difference favored liraglutide.
Secondary efficacy end‐points (except for participants achieving HbA1c targets) were analyzed using a model similar to that used for the primary end‐point, where baseline HbA1c value was replaced by the corresponding baseline values. Owing to the skewed distribution of insulin dose, a post‐hoc analysis was carried out; the log‐transformed actual daily dose after 36 weeks was analyzed using ancova with treatment and pretrial insulin at screening as fixed effects, and the log‐transformed baseline dose as the covariate. Participants achieving target HbA1c, and participants achieving the target with no severe/minor hypoglycemia during the last 4 weeks after 16/36 weeks’ treatment were analyzed by logistic regression with treatment and pretrial insulin therapy as fixed factors, and baseline HbA1c as the covariate; the results include 95% CI for odds ratio (OR; liraglutide over placebo). For analysis of participants achieving target HbA1c without increased bodyweight, the baseline weight was included as the covariate.
Treatment‐emergent AE data are shown as number/percentage of participants with at least one event, and rate/100 patient‐years of exposure (PYE). Treatment‐emergent hypoglycemia was analyzed using negative binomial regression with a log‐link function and logarithm of the time in which an episode was considered treatment‐emergent as offset. The model included treatment and type of pretrial insulin as fixed factors.
Results
Demographics
In total, 296 participants were screened, 257 randomized and exposed to trial products, and 246 (95.7%) completed the trial (Figure 1). Two participants (one/group) withdrew as a result of AEs.
Figure 1.
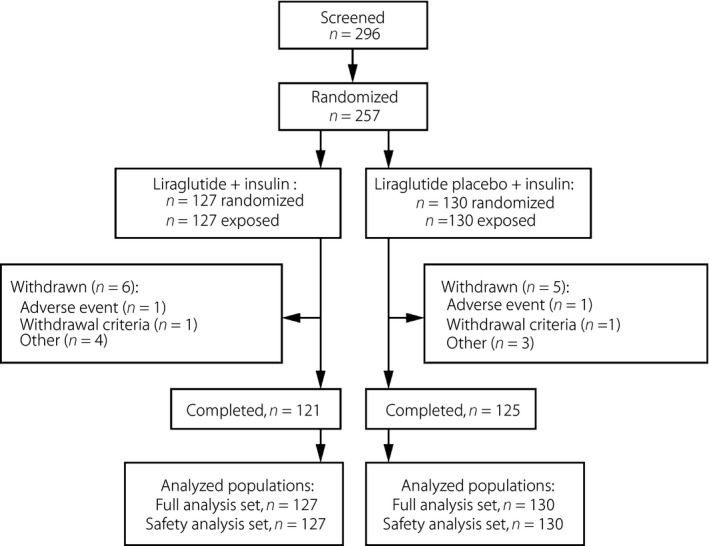
Participant flow and demographics. Participant flow during the trial.
The proportions randomized to each treatment were similar regarding the type of pretrial insulin (approximately 2:2:1 basal/premix/basal‐bolus, respectively). Baseline parameters were well balanced between groups (Table 1). The population was 56.0% men, mean age 60.5 years, mean diabetes duration 14.5 years, mean HbA1c 8.8% (73 mmol/mol), mean FPG 156 mg/dL (8.7 mmol/L) and mean BMI 25.6 kg/m2. All participants were Japanese.
Table 1.
Participants’ baseline characteristics
| Liraglutide + insulin n = 127 | Liraglutide placebo + insulin n = 130 | Total n = 257 | |
|---|---|---|---|
| Male, n (%) | 69 (54.3) | 75 (57.7) | 144 (56.0) |
| Age (years) | 61.3 (11.0) | 59.8 (11.3) | 60.5 (11.2) |
| Bodyweight (kg) | 67.7 (15.2) | 65.9 (13.0) | 66.8 (14.1) |
| BMI (kg/m2) | 26.2 (4.9) | 25.2 (4.0) | 25.6 (4.5) |
| Duration of diabetes (years) | 14.32 (8.89) | 14.69 (8.60) | 14.51 (8.73) |
| FPG, mg/dL (mmol/L) | 153 (44); 8.5 (2.4) | 158 (45); 8.8 (2.5) | 156 (44); 8.7 (2.4) |
| HbA1c, % (mmol/mol) | 8.8 (0.9); 73 (10) | 8.8 (0.9); 73 (10) | 8.8 (0.9); 73 (10) |
| Pretrial insulin, n (%) | |||
| Basal | 50 (39.4) | 50 (38.5) | 100 (38.9) |
| Basal–bolus | 27 (21.3) | 28 (21.5) | 55 (21.4) |
| Premix | 50 (39.4) | 52 (40.0) | 102 (39.7) |
| Pretrial daily insulin dose (U) | 30 (14) | 29 (17) | 29 (16) |
Data are mean (standard deviation) unless otherwise stated. BMI, body mass index; FPG, fasting plasma glucose; HbA1c, glycosylated hemoglobin; SD, standard deviation.
Efficacy
After 16 weeks of treatment with liraglutide or placebo added to insulin therapy, the observed mean (standard deviation) HbA1c levels (%; mmol/mol) were 7.1; 54 (0.9; 9.8) and 8.4; 68 (0.9; 9.8), respectively (last observation carried forward). The observed mean change from baseline in HbA1c (%; mmol/mol) was −1.73; −19 with liraglutide and −0.43; −5 with placebo. The mean estimated treatment difference (ETD) between groups (liraglutide minus placebo) was −1.30; −14 (95% CI −1.47 to −1.13; −16 to −12; P < 0.0001; Figure 2). Statistical significance of this difference was maintained to week 36 (mean ETD −0.81; −9; 95% CI −0.99 to −0.63; −11 to −7; P < 0.0001).
Figure 2.
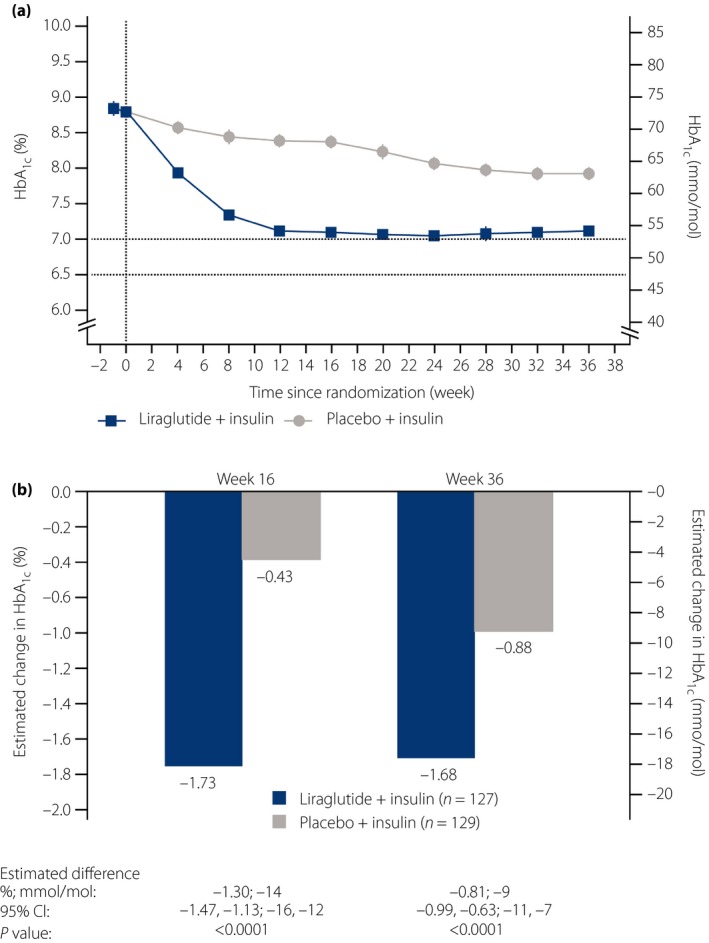
Effect of treatment on glycosylated hemoglobin (HbA1c) levels. (a) Mean HbA1c by treatment week (full analysis set). Last observation carried forward imputed data. Error bars: ±standard error (mean). (b) Mean change from baseline in HbA1c at weeks 16 and 36. Analyzed using an analysis of covariance method. CI, confidence interval.
The estimated proportion of participants achieving HbA1c <7.0% (<53 mmol/mol) at week 16 was 52.83% with liraglutide and 2.17% with the placebo (estimated OR 50.57, 95% CI 16.59–154.16; P < 0.0001). At week 36, the estimated proportions of participants achieving HbA1c <7.0% (<53 mmol/mol) were 58.04 and 6.04%, respectively (estimated OR 21.51, 95% CI 9.43–49.11; P < 0.0001). The proportion of participants achieving HbA1c ≤6.5% (≤48 mmol/mol) was also significantly higher with liraglutide than the placebo at 16 and 36 weeks (P < 0.0001 for both; Table S1).
After 16 weeks, the estimated proportions of participants achieving HbA1c <7.0% (<53 mmol/mol) without weight gain in the liraglutide and placebo groups were 33.22 and 1.99%, respectively (estimated OR 24.51, 95% CI 8.08, 74.29; P < 0.0001); after 36 weeks, the proportions were 27.47 and 3.40%, respectively (estimated OR 10.75; 95% CI 4.20–27.53; P < 0.0001). The proportions achieving HbA1c ≤6.5% (≤48 mmol/mol) without weight gain were also higher with liraglutide than the placebo (P = 0.0002 at week 16, and P = 0.0008 at 36 weeks; Table S1).
The proportion of participants achieving HbA1c <7.0% (<53 mmol/mol) with no severe/minor hypoglycemia during the last 4 weeks was assessed after 16 and 36 weeks: the estimated proportions after 16 weeks with liraglutide and placebo were 47.91 and 1.71%, respectively (estimated OR 52.80, 95% CI 15.37–181.38; P < 0.0001); after 36 weeks, the proportions were 52.35 and 5.69%, respectively (estimated OR 18.22; 95% CI 8.10–41.02; P < 0.0001). The proportion achieving HbA1c ≤6.5% (≤48 mmol/mol) without severe/minor hypoglycemia during the previous 4 weeks after 16/36 weeks was also higher with liraglutide than placebo (P < 0.0001; Table S1).
At week 16, significantly greater FPG reduction was observed with liraglutide (estimated change from baseline [mg/dL; mmol/L] −23.6; −1.3) versus placebo (−8.7; −0.5); mean ETD [mg/dL; mmol/L] was −14.9; −0.8 (95% CI −23.4 to −6.4; −1.3, −0.4; P = 0.0006). By week 36, there was no significant between‐group difference in FPG (estimated mean change [mg/dL; mmol/L] −27.9; −1.5 and −23.3; −1.3 with liraglutide and the placebo, respectively; ETD [mg/dL; mmol/L] −4.6; −0.3 [95% CI −12.5 to 3.3; −0.7, 0.2; P = 0.2511]).
Figure S1 shows the mean seven‐point SMPG profiles for liraglutide and the placebo at baseline, week 16 and 36. At week 16, significantly greater reductions in mean plasma glucose were observed with liraglutide than placebo (estimated mean change [mg/dL; mmol/L] −43.4; −2.4 and −9.6; −0.5, respectively; mean ETD [mg/dL; mmol/L] −33.8; −1.9 [95% CI −42.7 to −24.9; −2.4, −1.4; P < 0.0001]). Statistical significance was maintained at week 36 (estimated mean change [mg/dL; mmol/L] −47.7; −2.6 and −24.6; −1.4, respectively; mean ETD [mg/dL; mmol/L] −23.1; −1.3 [95% CI −31.2 to −14.9; −1.7, −0.8; P < 0.0001]). Estimated changes in mean prandial plasma glucose increment from baseline (mg/dL; mmol/L) were −24.1; −1.3 with liraglutide at week 16 and 36, and −10.9; −0.6 and −17.0; −0.9 with the placebo at weeks 16 and 36, respectively. ETD (mg/dL; mmol/L) in mean prandial plasma glucose increment from baseline to week 16 (liraglutide minus placebo) was −13.2; −0.7 (95% CI −21.7 to −4.8; −1.2, −0.3; P = 0.0023). By week 36, this difference was no longer significant (ETD [mg/dL; mmol/L] −7.1; −0.4 [95% CI −17.4 to 3.3; −1.0, 0.2; P = 0.1787]).
Mean bodyweight was stable in both groups: at weeks 16 and 36, the mean bodyweight change was −0.42 kg and +0.17 kg with liraglutide, and −0.28 kg and +0.52 kg with the placebo, respectively (estimated), relative to baseline. There was no significant difference in bodyweight change between groups at week 16 (ETD −0.14 kg [95% CI −0.54 to 0.25; P = 0.4806]) or week 36 (ETD −0.35 kg [95% CI −0.91 to 0.20; P = 0.2074]).
The observed mean actual daily insulin doses at baseline and week 36 were 30 and 36 U with liraglutide, and 29 and 40 U with the placebo (Figure S2). The observed mean changes in actual daily insulin dose at week 36 were 6.0 and 11.1 U with liraglutide and the placebo, respectively. In a post‐hoc analysis using a log‐scale, the actual insulin dose after 36 weeks was significantly lower with liraglutide than the placebo (geometric mean doses 28.49 and 34.55 U, respectively; the estimated treatment ratio 0.82 [95% CI 0.76–0.90; P < 0.0001]).
Safety (36‐Week Data)
The overall proportion of participants reporting AEs was similar between groups (85.8% liraglutide; 81.5% placebo), whereas the rate of AEs was higher with liraglutide than the placebo (449 and 350 events/100 PYE, respectively; Table 2). Most AEs were mild. The most frequently reported AE in both groups was nasopharyngitis. Gastrointestinal (GI) disorders were more common with liraglutide than the placebo (52.0 and 20.0%; 116 and 43 events/100 PYE, respectively). Although more participants reported GI AEs with liraglutide than the placebo during the first 4 weeks of the study, fewer GI AEs were reported during the remaining treatment period, when AEs were comparable between groups.
Table 2.
Summary of treatment‐emergent adverse events (safety analysis set)
| Liraglutide + insulin (n = 127) | Liraglutide placebo + insulin (n = 130) | |||||
|---|---|---|---|---|---|---|
| n (%) | No. events | Event rate per 100 PYE | n (%) | No. events | Event rate per 100 PYE | |
| All AEs | 109 (85.8) | 379 | 449 | 106 (81.5) | 302 | 350 |
| Serious AEs | 6 (4.7) | 7 | 8 | 4 (3.1) | 4 | 5 |
| Severity | ||||||
| Severe | 4 (3.1) | 4 | 5 | 1 (0.8) | 1 | 1 |
| Moderate | 5 (3.9) | 7 | 8 | 10 (7.7) | 12 | 14 |
| Mild | 109 (85.8) | 368 | 436 | 104 (80.0) | 289 | 335 |
| Treatment‐emergent adverse events occurring with a frequency ≥5% | ||||||
| Nasopharyngitis | 55 (43.3) | 74 | 88 | 40 (30.8) | 60 | 70 |
| Gastroenteritis | 4 (3.1) | 7 | 8 | 7 (5.4) | 7 | 8 |
| GI disorders | ||||||
| Nausea | 14 (11.0) | 16 | 19 | 7 (5.4) | 9 | 10 |
| Diarrhea | 15 (11.8) | 17 | 20 | 4 (3.1) | 4 | 5 |
| Constipation | 15 (11.8) | 16 | 19 | 2 (1.5) | 2 | 2 |
| Dyspepsia | 7 (5.5) | 9 | 11 | 0 (0) | 0 | 0 |
| Diabetic retinopathy | 9 (7.1) | 9 | 11 | 13 (10.0) | 16 | 19 |
| Headache | 8 (6.3) | 10 | 12 | 6 (4.6) | 6 | 7 |
| Hypertension | 8 (6.3) | 8 | 9 | 6 (4.6) | 6 | 7 |
| Back pain | 6 (4.7) | 6 | 7 | 7 (5.4) | 7 | 8 |
AE, adverse event; GI, gastrointestinal; PYE, patient years of exposure.
The proportion and rates of participants with AEs possibly/probably related to trial products were higher with liraglutide than the placebo (44.9 and 19.2%, and 102 and 42 events/100 PYE, respectively). The incidence of serious AEs was low and comparable between groups (4.7 and 3.1%, and 8 and 5 events/100 PYE, with liraglutide and placebo, respectively). Two serious AEs (brainstem thrombosis and angina pectoris) with liraglutide were evaluated as possibly/probably related to the trial product by the investigator.
The number of AEs leading to withdrawal was very low (1/group). No deaths were reported during the trial.
There was a small increase in median lipase levels from screening to week 36 with liraglutide, compared with a small decrease with the placebo; small increases in median amylase levels were observed in both groups (Table S2). Median amylase and lipase values at baseline and 36 weeks were within reference ranges, and no participants had values >3 times the upper normal range during the trial. No notable changes in calcitonin were observed, and no events of pancreatitis, suspicion of pancreatitis or thyroid disease were reported.
The proportion of participants with confirmed hypoglycemic episodes with liraglutide was 33.1% (146 episodes/100 PYE), and 27.7% with the placebo (187 episodes/100 PYE). There was no significant difference in the number of confirmed hypoglycemic episodes during the 36‐week treatment period between groups (estimated treatment ratio [liraglutide/placebo] 0.94, 95% CI 0.52, 1.70; P = 0.8328). Overall, 10 nocturnal confirmed hypoglycemic episodes were reported by six participants with liraglutide (4.7%; 12 episodes/100 PYE), and 28 episodes reported by 11 participants with the placebo (8.5%; 32 episodes/100 PYE). No severe hypoglycemia was recorded. At week 36, there was a decrease in systolic blood pressure with liraglutide, and a small increase with the placebo; there was also an increase in pulse with liraglutide (Table S3).
Discussion
The present trial met its primary end‐point, showing the superiority of liraglutide plus insulin therapy over insulin alone regarding HbA1c reduction at week 16. The difference in HbA1c levels was sustained up to 36 weeks. This supports data from a recent international phase 3 trial, showing that the addition of liraglutide to patients inadequately controlled on basal insulin ± metformin decreased HbA1c by 1.3% (14 mmol/mol) after 26 weeks25. It also aligns with another international phase 3 study, showing that the addition of liraglutide to insulin degludec and metformin decreased HbA1c by 0.74% (8 mmol/mol) over 26 weeks24. In both studies, liraglutide was added to basal insulin, whereas the present study included patients on basal, premixed or basal–bolus regimens. HbA1c reductions in the present study were achieved with a lower liraglutide dose (0.9 mg/day) than the international studies (1.8 mg/day).
When treated with liraglutide as an add‐on to existing insulin therapy in the present study, 58% of patients achieved HbA1c <7.0% (<53 mmol/mol) after 36 weeks, similar to findings from international studies24, 25. FPG and SMPG profiles at week 16 also suggest that combination therapy with liraglutide + insulin would be an effective option for treatment intensification.
A meta‐analysis of studies examining GLP‐1RAs (exenatide, liraglutide, lixisenatide) use as add‐on to insulin suggested that combination therapy offers additional HbA1c lowering versus insulin alone32.
In the present study, the insulin dose was fixed during the first 16 weeks, but could subsequently be adjusted. During the adjustment period, combination liraglutide + insulin was associated with significant reductions in insulin dose relative to insulin therapy alone. This effect was observed in previous studies27, 28.
Insulin initiation is often associated with dose‐dependent weight gain and increased hypoglycemia risk33, 34. Many oral agents that might be combined with insulin, including sulfonylureas and thiazolidinediones, are associated with weight gain and hypoglycemia, whereas dipeptidyl peptidase‐4 inhibitors and α‐glucosidase inhibitors are weight‐neutral, but have less pronounced glucose‐lowering effects than other agents16, 35. By contrast, liraglutide 1.2 and 1.8 mg/day consistently showed beneficial effects on bodyweight in international phase 3 trials, with significant weight loss of up to 2.8 kg across the Liraglutide Effect and Action in Diabetes (LEAD) 1‐6 trials, whereas treatment with active comparators led to weight gains of 1.0–2.1 kg36. Hence, weight loss associated with liraglutide could offset weight gain typically seen with insulin. In previous Japanese trials, liraglutide doses of 0.6 and 0.9 mg/day were associated with weight loss up to 0.9 kg17. In the present study, the addition of liraglutide 0.9 mg/day to insulin provided superior glycemic control to insulin alone without weight gain or increased hypoglycemia incidence. No major changes in bodyweight were observed in either group, possibly because BMI at baseline (25.6 kg/m2) was not far above the normal range (18.5–24.9 kg/m2).
Adding liraglutide (0.9 mg/day) to insulin was generally well tolerated, and the safety profile was consistent with previous findings from international trials10, 11, 12, 13, 14, 15, 22, including those carried out in Japan17, 19. The overall proportion of participants reporting AEs was similar between groups, although the rate of AEs was higher with liraglutide than the placebo. This difference was mainly driven by GI AEs, which are commonly reported during treatment with GLP‐1RAs and occur predominantly during the first 4 weeks36. All GI AEs with liraglutide were mild in severity, except for constipation (moderate). Stepwise dose escalation with liraglutide might help mitigate GI events.
The present study had certain limitations. The study lasted 36 weeks, therefore the durability of results beyond that time is unknown. There might be selection bias in patients volunteering for a study, particularly when involving injectables. Finally, data cannot be extrapolated to younger patients (18–19 years‐of‐age) who were excluded from the trial.
Overall, adding liraglutide to existing insulin therapy results in superior glycemic control compared with insulin alone in Japanese patients with type 2 diabetes, and is generally well tolerated.
Disclosure
K Nishijima is a Novo Nordisk Pharma Ltd employee. H Bosch‐Traberg is a Novo Nordisk A/S employee. Y Seino is a medical advisor to Astellas, BD, Boehringer Ingelheim, Eli Lilly, GSK, Johnson & Johnson, Novo Nordisk, Otsuka, Sanofi, Taisho Pharmaceutical Co., Ltd and Takeda. S Kaneko has conflicts of interest in respect to Takeda, Lilly, Novartis, Novo Nordisk, Astellas, Boehringer Ingelheim, AstraZeneca, Kowa, Mitsubishi Tanabe Pharma, Sanwa Kagaku Kogyo, Sanofi, Kyowa Hakko Kirin, Daiichi Sankyo, Pfizer, Sumitomo Dainippon Pharma, Ono, Teijin Pharma and Kissei. T Osonoi received honoraria for lectures from Novo Nordisk, Astellas, Takeda, Sanwa Kagaku Kohyo, Tanabe‐Mitshubishi and Kowa Pharmaceuticals, and for manuscripts from Novo Nordisk and Sanwa Kagaku Kogyo K.K. Receipt of clinical commissioned/joint research grants from Novo Nordisk, Sanwa Kagaku Kogyo, Tanabe‐Mitshubishi, Fuji Firm Pharma, Eli Lilly, AbbVie, Kowa Pharmaceuticals, Taisho Pharmaceuticals, GSK and Dainippon‐Sumitomo. T Shiraiwa received honoraria for lectures from Sanofi, Shionogi & Co., Ltd, Astellas, Takeda and Eli Lilly; and research grants from Novo Nordisk, Fuji Firm Pharma, Astellas, Sanofi, GSK and MSD. K Kaku is an advisor to Novo Nordisk, Sanwa Kagaku Kogyo, Takeda and Taisho Pharmaceutical Co., Ltd; received honoraria for lectures from MSD, Kowa, Sumitomo Dainippon Pharma, Novartis, Novo Nordisk, Takeda and Mitsubishi Tanabe Pharma; and received scholarship grants from AstraZeneca, Nippon Boehringer Ingelheim Co., Ltd, Chugai, Daiichi Sankyo, MSD, Novartis, Novo Nordisk, Sanofi and Takeda. S Fukuda declares no conflict of interest. Writing support was provided by Watermeadow Medical, Witney, UK, funded by Novo Nordisk.
Supporting information
Doc S1 ¦ Exclusion criteria.
Table S1 ¦ Estimated proportions of participants achieving glycosylated hemoglobin ≤6.5% (≤48 mmol/mol) at weeks 16 and 36, and associated composite end‐points.
Figure S1 ¦ Mean seven‐point self‐measured plasma glucose (SMPG) profiles. SMPG (full analysis set). Last observation carried forward imputed data. Error bars: ±standard error (mean).
Figure S2 ¦ Actual daily insulin dose by treatment week. Data are from the full analysis set. Last observation carried forward imputed data. Error bars: ±standard error (mean).
Table S2 ¦ Median amylase and lipase levels.
Table S3 ¦ Mean changes in vital signs from baseline to week 36.
J Diabetes Investig 2016; 7: 565–573
References
- 1. Inzucchi SE, Bergenstal RM, Buse JB, et al Management of hyperglycemia in type 2 diabetes: a patient‐centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2012; 35: 1364–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Japan Diabetes Society . Treatment Guide for Diabetes. 2012–2013. Available from: http://www.jds.or.jp/common/fckeditor/editor/filemanager/connectors/php/transfer.php?file=/uid000025_54726561746D656E745F47756964655F666F725F44696162657465735F323031322D323031332E706466. Accessed March 2014.
- 3. Kim YG, Hahn S, Oh TJ, et al Differences in the HbA1c‐lowering efficacy of glucagon‐like peptide‐1 analogues between Asians and non‐Asians: a systematic review and meta‐analysis. Diabetes Obes Metab 2014; 16: 900–909. [DOI] [PubMed] [Google Scholar]
- 4. Yabe D, Kuroe A, Watanabe K, et al Early phase glucagon and insulin secretory abnormalities, but not incretin secretion, are similarly responsible for hyperglycemia after ingestion of nutrients. J Diabetes Complications 2015; 29: 413–421. [DOI] [PubMed] [Google Scholar]
- 5. Møller JB, Pedersen M, Tanaka H, et al Body composition is the main determinant for the difference in type 2 diabetes pathophysiology between Japanese and Caucasians. Diabetes Care 2014; 37: 796–804. [DOI] [PubMed] [Google Scholar]
- 6. Yabe D, Seino Y. Liraglutide in adults with type 2 diabetes: global perspective on safety, efficacy and patient preference. Clin Med Insights Endocrinol Diabetes 2011; 4: 47–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nauck MA, Kleine N, Orskov C, et al Normalization of fasting hyperglycaemia by exogenous glucagon‐like peptide 1 (7‐36 amide) in type 2 (non‐insulin‐dependent) diabetic patients. Diabetologia 1993; 36: 741–744. [DOI] [PubMed] [Google Scholar]
- 8. Knudsen LB. Glucagon‐like peptide‐1: the basis of a new class of treatment for type 2 diabetes. J Med Chem 2004; 47: 4128–4134. [DOI] [PubMed] [Google Scholar]
- 9. Agerso H, Jensen LB, Elbrond B, et al The pharmacokinetics, pharmacodynamics, safety and tolerability of NN2211, a new long‐acting GLP‐1 derivative, in healthy men. Diabetologia 2002; 45: 195–202. [DOI] [PubMed] [Google Scholar]
- 10. Marre M, Shaw J, Brändle M, et al Liraglutide, a once‐daily human GLP‐1 analogue, added to a sulphonylurea over 26 weeks produces greater improvements in glycaemic and weight control compared with adding rosiglitazone or placebo in subjects with type 2 diabetes (LEAD‐1 SU). Diabet Med 2009; 26: 268–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nauck M, Frid A, Hermansen K, et al Efficacy and safety comparison of liraglutide, glimepiride, and placebo, all in combination with metformin, in type 2 diabetes: the LEAD (liraglutide effect and action in diabetes)‐2 study. Diabetes Care 2009; 32: 84–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Garber A, Henry R, Ratner R, et al Liraglutide versus glimepiride monotherapy for type 2 diabetes (LEAD‐3 Mono): a randomised, 52‐week, phase III, double‐blind, parallel‐treatment trial. Lancet 2009; 373: 473–481. [DOI] [PubMed] [Google Scholar]
- 13. Zinman B, Gerich J, Buse JB, et al Efficacy and safety of the human glucagon‐like peptide‐1 analog liraglutide in combination with metformin and thiazolidinedione in patients with type 2 diabetes (LEAD‐4 Met+TZD). Diabetes Care 2009; 32: 1224–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Russell‐Jones D, Vaag A, Schmitz O, et al Liraglutide vs insulin glargine and placebo in combination with metformin and sulfonylurea therapy in type 2 diabetes mellitus (LEAD‐5 met+SU): a randomised controlled trial. Diabetologia 2009; 52: 2046–2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Buse JB, Rosenstock J, Sesti G, et al Liraglutide once a day versus exenatide twice a day for type 2 diabetes: a 26‐week randomised, parallel‐group, multinational, open‐label trial (LEAD‐6). Lancet 2009; 374: 39–47. [DOI] [PubMed] [Google Scholar]
- 16. Pratley RE, Nauck M, Bailey T, et al Liraglutide versus sitagliptin for patients with type 2 diabetes who did not have adequate glycaemic control with metformin: a 26‐week, randomised, parallel‐group, open label trial. Lancet 2010; 375: 1447–1456. [DOI] [PubMed] [Google Scholar]
- 17. Kaku K, Rasmussen MF, Nishida T, et al Fifty‐two‐week, randomized, multicenter trial to compare the safety and efficacy of the novel glucagon‐like peptide‐1 analog liraglutide vs glibenclamide in patients with type 2 diabetes. J Diabetes Investig 2011; 2: 441–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kaku K, Rasmussen MF, Clauson P, et al Improved glycaemic control with minimal hypoglycaemia and no weight change with the once‐daily human glucagon‐like peptide‐1 analogue liraglutide as add‐on to sulphonylurea in Japanese patients with type 2 diabetes. Diabetes Obes Metab 2010; 12: 341–347. [DOI] [PubMed] [Google Scholar]
- 19. Seino Y, Rasmussen MF, Nishida T, et al Glucagon‐like peptide‐1 analog liraglutide in combination with sulfonylurea safely improves blood glucose measures vs sulfonylurea monotherapy in Japanese patients with type 2 diabetes: Results of a 52‐week, randomized, multicenter trial. J Diabetes Investig 2011; 2: 280–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Usui R, Yabe D, Kuwata H, et al Retrospective analysis of safety and efficacy of insulin‐to‐liraglutide switch in Japanese type 2 diabetes: a caution against inappropriate use in patients with reduced β‐cell function. J Diabetes Investig 2013; 4: 585–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Morrow L, Hompesch M, Guthrie H, et al Co‐administration of liraglutide with insulin detemir demonstrates additive pharmacodynamic effects with no pharmacokinetic interaction. Diabetes Obes Metab 2011; 13: 75–80. [DOI] [PubMed] [Google Scholar]
- 22. DeVries JH, Bain SC, Rodbard HW, et al Sequential intensification of metformin treatment in type 2 diabetes with liraglutide followed by randomized addition of basal insulin prompted by A1C targets. Diabetes Care 2012; 35: 1446–1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rosenstock J, Rodbard HW, Bain SC, et al One‐year sustained glycemic control and weight reduction in type 2 diabetes after addition of liraglutide to metformin followed by insulin detemir according to HbA1c target. J Diabetes Complications 2013; 27: 492–500. [DOI] [PubMed] [Google Scholar]
- 24. Mathieu C, Rodbard HW, Cariou B, et al A comparison of adding liraglutide versus a single daily dose of insulin aspart to insulin degludec in subjects with type 2 diabetes (BEGIN: VICTOZA ADD‐ON). Diabetes Obes Metab 2014; 16: 636–644. [DOI] [PubMed] [Google Scholar]
- 25. Ahmann AJ, Rodbard HW, Rosenstock J, et al Efficacy and safety of liraglutide versus placebo added to basal insulin analogues (with or without metformin) in patients with type 2 diabetes: a randomized, placebo‐controlled trial. Diabetes Obes Metab 2015; 17: 1056–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lane W, Weinrib S, Rappaport J. The effect of liraglutide added to U‐500 insulin in patients with type 2 diabetes and high insulin requirements. Diabetes Technol Ther 2011; 13: 592–595. [DOI] [PubMed] [Google Scholar]
- 27. Li CJ, Li J, Zhang QM, et al Efficacy and safety comparison between liraglutide as add‐on therapy to insulin and insulin dose‐increase in Chinese subjects with poorly controlled type 2 diabetes and abdominal obesity. Cardiovasc Diabetol 2012; 11: 142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lind M, Jendle J, Torffvit O, et al Glucagon‐like peptide 1 (GLP‐1) analogue combined with insulin reduces HbA1c and weight with low risk of hypoglycemia and high treatment satisfaction. Prim Care Diabetes 2012; 6: 41–46. [DOI] [PubMed] [Google Scholar]
- 29. World Medical Association Declaration of Helsinki . Ethical principles for medical research involving human subjects – Last amended by the 59th WMA General Assembly, Seoul; 2008. [Google Scholar]
- 30. ICH Harmonised Tripartite Guideline . Guideline for Good Clinical Practice E6 (R1), Step 4. 10 June 1996.
- 31. Workgroup on hypoglycemia, American Diabetes Association . Defining and reporting hypoglycemia in diabetes: a report from the American Diabetes Association Workgroup on Hypoglycemia. Diabetes Care 2005; 28: 1245–1249. [DOI] [PubMed] [Google Scholar]
- 32. Berlie H, Hurren KM, Pinelli NR. Glucagon‐like peptide‐1 receptor agonists as add‐on therapy to basal insulin in patients with type 2 diabetes: a systematic review. Diabetes Metab Syndr Obes 2012; 5: 165–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Dailey G, Admane K, Mercier F, et al Relationship of insulin dose, A1c lowering, and weight in type 2 diabetes: comparing insulin glargine and insulin detemir. Diabetes Technol Ther 2010; 12: 1019–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Barnett AH. Complementing insulin therapy to achieve glycemic control. Adv Ther 2013; 30: 557–576. [DOI] [PubMed] [Google Scholar]
- 35. Nathan DM, Buse JB, Davidson MB, et al Medical management of hyperglycaemia in type 2 diabetes mellitus: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement from the American Diabetes Association and the European Association for the Study of Diabetes. Diabetologia 2009; 52: 17–30. [DOI] [PubMed] [Google Scholar]
- 36. Davies MJ, Kela R, Khunti K. Liraglutide – overview of the preclinical and clinical data and its role in the treatment of type 2 diabetes. Diabetes Obes Metab 2011; 13: 207–220. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Doc S1 ¦ Exclusion criteria.
Table S1 ¦ Estimated proportions of participants achieving glycosylated hemoglobin ≤6.5% (≤48 mmol/mol) at weeks 16 and 36, and associated composite end‐points.
Figure S1 ¦ Mean seven‐point self‐measured plasma glucose (SMPG) profiles. SMPG (full analysis set). Last observation carried forward imputed data. Error bars: ±standard error (mean).
Figure S2 ¦ Actual daily insulin dose by treatment week. Data are from the full analysis set. Last observation carried forward imputed data. Error bars: ±standard error (mean).
Table S2 ¦ Median amylase and lipase levels.
Table S3 ¦ Mean changes in vital signs from baseline to week 36.


