Abstract
Aims/Introduction
Obesity plays a central role in metabolic syndrome. Obesity indexes are important in clinical work. In the present study, we sought to determine the relationships between obesity indexes and metabolic risk factors.
Materials and Methods
We studied 11,568 participants over 35 years. Body mass index, waist circumference (WC), waist‐to‐height ratio (WHtR) and waist‐to‐hip ratio were measured and calculated. To compare the predictive ability of the obesity indexes in diagnosing multiple metabolic risk factors, the areas under receiver operating characteristic curves were calculated, and cut‐off values were determined. A partial correlation coefficient was used to assess the intercorrelations between the obesity indexes, and to evaluate the correlations between each index and each metabolic risk factor.
Results
The partial correlation coefficient for WHtR and WC was 0.947. In diagnosing multiple metabolic risk factors, the WHtR areas under receiver operating characteristic curves was greater than that for the other obesity indexes in both sexes. The cut‐off point for the WHtR was 0.50 in men and 0.52 in women. The cut‐off point for WC was 85 cm in men and 80 cm in women.
Conclusions
WHtR strongly correlates with WC. The WHtR might show the same predictive ability as the WC in diagnosing multiple metabolic risk factors.
Keywords: Metabolic risk factors, Waist circumference, Waist‐to‐height ratio
Introduction
Obesity plays a central role in metabolic syndrome (MetS), is closely related to many cardiovascular diseases, and has become an increasing concern for both developed and many developing countries. The traditional obesity indexes, body mass index (BMI) and waist circumference (WC), have always been used to diagnose MetS.
The World Health Organization recommends using BMI to determine whether an individual is overweight, but the International Diabetes Federation and National Cholesterol Education Program use WC as a criterion for MetS1, 2, 3. However, the BMI cannot reflect the distribution of fat on one's body; therefore, it cannot be used to determine central obesity. WC estimates visceral fat and central obesity, but it does not account for height, and it differs between races and sexes4, 5. Recent studies found that, in contrast to BMI and WC, one's waist‐to‐height ratio (WHtR) and waist‐to‐hip ratio (WHpR) might also be associated with cardiovascular diseases6, 7, 8. However, studies in different countries have yielded different conclusions on whether these four obesity indexes can be used to diagnose MetS, and how they are associated with MetS risk factors9, 10, 11.
We carried out a cross‐sectional study in a rural area of northern China with a large sample size. We attempted to determine how these four obesity indexes correlated with each other, and how they were associated with metabolic risk factors. We also attempted to evaluate the best cut‐off values for obesity indexes in diagnosing multiple metabolic risk factors for this population.
Methods
Participants
Liaoning Province is located in northeast China. From January 2012 to August 2013, a representative sample of participants at aged ≥35 years was used to characterize the prevalence, incidence and natural history of cardiovascular risk factors in rural areas of Liaoning Province. We used a multistage, stratified, random‐cluster sampling scheme.
Participants who were pregnant, or with malignant tumors or mental disorders were excluded from the study. All permanent residents from each village aged ≥35 years were invited to participate in the study (14,016 participants). Of the participants, 11,956 participants agreed to and completed the study, which yielded an 85.3% response rate. The study was approved by the ethics committee of China Medical University (Shenyang, China).
All procedures were consistent with ethical standards. Written consent was obtained from all participants after they were informed of the objectives, benefits and medical items, as well as a confidentiality agreement regarding their personal information. For the present study, we only used data from participants whose relevant study data were complete and credible, which provided a final sample size of 11,568 (5,353 men and 6,215 women).
Data collection
The weights and heights were measured to the nearest 0.1 kg and 0.1 cm, respectively, with the participants wearing lightweight clothing and without shoes. The WCs were measured at the umbilicus using non‐elastic tape (to the nearest 0.1 cm) at the end of normal expiration and with the participants standing. The hip circumferences were measured at the maximal gluteal protrusion. The BMIs were calculated as the participant's weight in kilograms divided by the square root of the height in meters. The WHtRs and WHpRs were determined as the participant's WC in centimeters divided by the height and hip circumference in centimeters, respectively.
Based on the American Heart Association protocol, blood pressure was measured three times in 2‐min intervals after at least 5 min of rest using a standardized automatic electronic sphygmomanometer (HEM‐907; Omron, Dalian, China), which was validated using the British Hypertension Society protocol. The participants were advised to avoid caffeinated beverages and exercise for at least 30 min before the measurement. During the measurement, the participants were seated with their arms supported at heart level. The mean of three blood pressure measurements was calculated and used.
Fasting blood samples were collected in the morning after at least 12 h of fasting. Blood samples were obtained from an antecubital vein, and added to vacutainer tubes containing ethylenediaminetetraacetic acid. Fasting plasma glucose (FPG), high‐density lipoprotein cholesterol, triglyceride and other routine blood indexes were enzymatically analyzed using an autoanalyzer. All laboratory equipment was calibrated, and blinded duplicate samples were used for these analyses.
The participants’ medical history and lifestyle, including smoking habits and drinking habits, were collected through a face‐to‐face interview using a standard questionnaire.
Definition
To evaluate the four obesity indexes with metabolic risk factors, we roughly adopted the revised criteria from the Adult Treatment Panel report. Participants with two or more of the following items were considered to have multiple metabolic risk factors in the present study: (i) triglyceride levels ≥150 mg/dL (1.7 mmol/L); (ii) high‐density lipoprotein cholesterol levels <40 mg/dL (<1.04 mmol/L) in men or <50 mg/dL (<1.30 mmol/L) in women; (iii) systolic blood pressure ≥130 mmHg or diastolic blood pressure ≥85 mmHg, or taking antihypertensive medications; and (iv) FPG ≥100 mg/dL (5.6 mmol/L) or taking diabetes medications.
Statistical analysis
We calculated descriptive statistics for all variables. The continuous variables were reported as the mean values and standard deviations between the study groups using Student's t‐test. The significant differences in prevalence between the normal and multiple risk factors groups for both sexes were tested using a χ 2‐test. We generated receiver operator characteristic curves, and identified optimal cut‐off values with the maximum Youden index (sensitivity plus specificity‐1) for obesity indexes to diagnose multiple metabolic risk factors. The areas under receiver operator characteristic curve (AUC) were calculated to compare the effectiveness of the different indexes. Pearson's partial correlation coefficients were calculated to reflect the relationships between four obesity indexes, and to characterize how these indexes correlated with metabolic risk factors adjusted for age, sex, smoking habits and drinking habits. A two‐sided analysis with SPSS version 17.0 (SPSS, Chicago, IL, USA) was used for the data, and a P‐value <0.05 was considered significant.
Results
Table 1 shows the baseline characteristics of participants with or without multiple metabolic risk factors according to sex. The participants comprised 5,353 men and 6,215 women. The individual metabolic risk factors were significantly different between the multiple metabolic risk factors and normal groups for both sexes. Except for height in men, the anthropometry indexes, including bodyweight, WC, BMI, WHtR and WHpR, were also significantly different between the two groups for both men and women. In the multiple risk factors group, hypertension accounted for 91.54 and 83.46% in men and women, respectively. A high FPG accounted for 78.48 and 68.23% in men and women, respectively. The difference in drinking habits was not significant between the normal and multiple risk factors groups for both sexes. The differences in smoking habits were significant for men.
Table 1.
Baseline characteristics of study participants
| Men (n = 5,353) | Women (n = 6,215) | |||||
|---|---|---|---|---|---|---|
| Normal | Multiple risk factors | P‐value | Normal | Multiple risk factors | P‐value | |
| n (%) | 2,361 (44.1%) | 2,992 (55.9%) | 2,617 (43.0%) | 3,598 (57.0%) | ||
| Age (years) | 53.2 ± 10.9 | 55.3 ± 10.7 | <0.001 | 50.2 ± 9.8 | 55.7 ± 10.1 | <0.001 |
| Height (cm) | 166.34 ± 6.31 | 166.48 ± 6.38 | 0.416 | 156.02 ± 6.04 | 155.32 ± 6.08 | <0.001 |
| Bodyweight (kg) | 65.38 ± 9.83 | 71.12 ± 11.40 | <0.001 | 57.78 ± 9.46 | 62.01 ± 10.21 | <0.001 |
| WC (cm) | 80.35 ± 8.79 | 86.48 ± 9.63 | <0.001 | 77.75 ± 8.85 | 83.82 ± 9.55 | <0.001 |
| BMI (kg/m2) | 23.61 ± 3.24 | 25.61 ± 3.51 | <0.001 | 23.71 ± 3.51 | 25.70 ± 3.73 | <0.001 |
| WHtR | 0.48 ± 0.05 | 0.52 ± 0.06 | <0.001 | 0.50 ± 0.06 | 0.54 ± 0.06 | <0.001 |
| WHpR | 0.69 ± 0.08 | 0.73 ± 0.09 | <0.001 | 0.61 ± 0.08 | 0.64 ± 0.07 | <0.001 |
| TG (mmol/L) | 1.07 ± 0.63 | 2.12 ± 2.03 | <0.001 | 1.03 ± 0.43 | 2.04 ± 1.58 | <0.001 |
| HDL (mmol/L) | 1.52 ± 0.42 | 1.32 ± 0.40 | <0.001 | 1.56 ± 0.32 | 1.30 ± 0.32 | <0.001 |
| SBP (mmHg) | 135.10 ± 21.50 | 150.39 ± 21.12 | <0.001 | 129.87 ± 21.08 | 147.64 ± 23.28 | <0.001 |
| DBP (mmHg) | 79.23 ± 10.82 | 87.33 ± 11.31 | <0.001 | 76.35 ± 10.39 | 83.65 ± 11.31 | <0.001 |
| FPG (mmol/L) | 5.34 ± 0.82 | 6.44 ± 1.98 | <0.001 | 5.23 ± 0.63 | 6.33 ± 1.90 | <0.001 |
| Prevalence (%) | ||||||
| High TG | 5.34 | 50.10 | <0.001 | 3.29 | 51.06 | <0.001 |
| Low HDL | 4.32 | 27.41 | <0.001 | 16.77 | 60.62 | <0.001 |
| Hypertension | 47.69 | 91.54 | <0.001 | 37.87 | 83.46 | <0.001 |
| High FPG | 15.46 | 78.48 | <0.001 | 11.31 | 68.23 | <0.001 |
| Smoking | 61.03 | 53.91 | <0.001 | 16.43 | 16.65 | 0.82 |
| Drinking | 44.77 | 45.96 | 0.669 | 3.06 | 2.86 | 0.655 |
BMI, body mass index; DBP, diastolic blood pressure; FPG, fasting plasma glucose; HDL‐C, high‐to‐density lipoprotein cholesterol; LDL‐C, low‐density lipoprotein cholesterol; SBP, systolic blood pressure; TC, total cholesterol; TG, triglyceride; WC, waist circumference; WHtR, waist‐to‐height ratio; WHpR, waist‐to‐hip ratio.
The partial correlation coefficients for the obesity indexes are shown in Table 2. All correlations reached statistical significance at P < 0.001. The greatest correlation was observed between WHtR and WC, with a coefficient of 0.947.
Table 2.
Relationship between obesity indices adjusted by age, sex, smoking habits and drinking habits
| BMI (kg/m2) | WC (cm) | WHtR | WHpR | |
|---|---|---|---|---|
| BMI (kg/m2) | 1 | 0.800 | 0.826 | 0.724 |
| WC (cm) | 1 | 0.947 | 0.637 | |
| WHtR | 1 | 0.506 | ||
| WHpR | 1 |
P < 0 .001. BMI, body mass index; WC, waist circumference; WHtR, waist‐to‐height ratio; WHpR, waist‐to‐hip ratio.
To compare the predictive ability of the obesity indexes to diagnose multiple metabolic risk factors, the AUC were calculated, and the cut‐off values were determined. As Figure 1 shows, receiver operator characteristic curves were generated according to sex for WC, BMI, WHtR and WHpR to identify multiple metabolic risk factors. The WHtR AUC was similar to the WC AUC. However, WHtR produced the greatest AUC for both sexes, with 0.689 (range 0.675–0.703) for men and 0.696 (range 0.683–0.709) for women. The optimal WHtR cut‐off value for diagnosing multiple metabolic risk factors in men was 0.5, with a 65.3 sensitivity and 63.7 specificity. The cut‐off value was 0.52 for women, with a 61.8 sensitivity and 68.2 specificity. The optimal WC cut‐off value for diagnosing multiple metabolic risk factors in men was 84.38 cm, with a 58.5 sensitivity and 69.8 specificity; the cut‐off value was 80.02 cm for women, with a 65.5 sensitivity and 63.8 specificity (Table 3).
Figure 1.
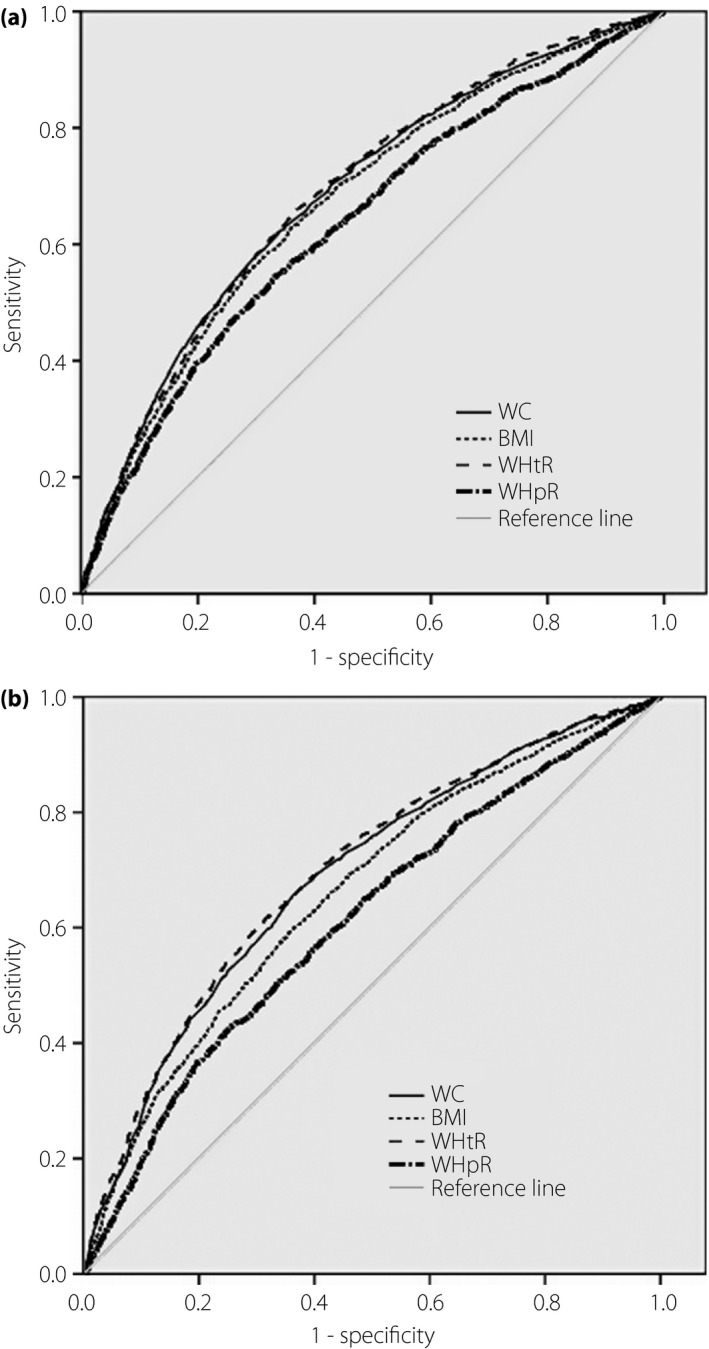
Receiver operating characteristic curves of obesity indices to predict ≥2 metabolic risk factors according to sex. (a) Men. (b) Women. BMI, body mass index; WC, waist circumference, WHpR, waist‐to‐hip ratio; WHtR, waist‐to‐height ratio.
Table 3.
Area under the receiver operator characteristic curves, optimal cut‐off values, sensitivity and specificity of obesity indices to predict ≥2 metabolic risk factors according to sex
| AUC (95% CI) | Cut‐off value | Sensitivity (%) | Specificity (%) | Youden index | |
|---|---|---|---|---|---|
| Men | |||||
| BMI | 0.674 (0.660–0.689) | 24.35 | 62.6 | 63.6 | 0.27 |
| WC | 0.687 (0.673–0.701) | 84.38 | 58.5 | 69.8 | 0.28 |
| WHtR | 0.689 (0.675–0.703) | 0.50 | 65.3 | 63.7 | 0.29 |
| WHpR | 0.636 (0.621–0.651) | 0.72 | 54.4 | 66.8 | 0.21 |
| Women | |||||
| BMI | 0.660 (0.646–0.673) | 24.65 | 59.8 | 36.4 | 0.23 |
| WC | 0.688 (0.674–0.701) | 80.02 | 65.5 | 63.8 | 0.29 |
| WHtR | 0.696 (0.683–0.709) | 0.52 | 61.8 | 68.2 | 0.3 |
| WHpR | 0.612 (0.598–0.626) | 0.65 | 41.9 | 75.4 | 0.17 |
BMI, body mass index; CI, confidence interval; ROC, receiver operating characteristic; WC, waist circumference; WHtR, waist‐to‐height ratio; WHpR, waist‐to‐hip ratio.
Table 4 shows the correlation coefficients for the obesity indexes with each metabolic risk factor. All correlations reached statistical significance (P < 0.001), but the coefficients were small. The WC, WHtR and BMI similarly correlated with the individual risk factors. The WHpR weakly correlated with the risk factors, except for diastolic blood pressure (r = 0.108). Among the metabolic risk factors, the coefficients for FPG with each index were less than 0.10.
Table 4.
Partial correlation coefficients between obesity indices and metabolic risk factors adjusted by age, sex, smoking habits, and drinking habits
| TG (mmol/L) | HDL (mmol/L) | SBP (mmHg) | DBP (mmHg) | FPG (mmHg) | |
|---|---|---|---|---|---|
| WC (cm) | 0.155 | –0.216 | 0.191 | 0.086 | 0.074 |
| BMI (kg/m2) | 0.126 | –0.212 | 0.213 | 0.08 | 0.048 |
| WHtR | 0.153 | –0.191 | 0.22 | 0.054 | 0.069 |
| WHpR | 0.098 | –0.193 | 0.073 | 0.108 | 0.048 |
All P < 0.001. BMI, body mass index; DBP, diastolic blood pressure; FPG, fasting plasma glucose; HDL, high‐to‐density lipoprotein cholesterol; SBP, systolic blood pressure; TG, triglyceride; WC, waist circumference; WHtR, waist‐to‐height ratio; WHpR, waist‐to‐hip ratio.
Discussion
The present study compares the associations of four obesity indexes with metabolic risk factors. We confirm the strong associations between WHtR and WC, as well as WHtR and metabolic risk factors. We also estimated the optimal cut‐off values for the four indexes to diagnose multiple metabolic risk factors.
The present study found that, among the four indexes, WHtR strongly correlated with WC (r = 0.947). Both NCEP and FDA regard WC as an important index for diagnosing MetS; thus, WHtR can be a new obesity index for diagnosing MetS.
In fact, many previous studies have found a close relationship between WHtR and metabolic risk factors. A meta‐analysis carried out by Aswell confirmed that WHtR is better at screening metabolic risk factors than WC and BMI8. A study in Spain showed a close relationship between WHtR and cardiovascular risk factors in elderly individuals with a high cardiovascular risk2. In the present study, the WHtR correlated best with multiple metabolic risk factors. Different studies have produced different conclusions on the relationship between obesity indexes with each metabolic component12, 13. In the present study, the WHtR and WC show a stronger correlation with each metabolic risk factor than the other indexes. However, we did not detect an obvious difference between the WHtR and WC. The different conclusions among different studies might be as a result of different populations, statistical methods and MetS definitions. In summary, recent studies, including the present study, found that the order of the indexes related to metabolic risk factors is WHtR ≥ WC > BMI > WHpR7, 11, 14.
Although it reflects the fat distribution on one's body, in the present study, WHpR showed the weakest relationship with the metabolic risk factors, except for diastolic blood pressure. This observation is roughly consistent with the study by Knowles et al.6, which also determined that this relationship was weak, except with respect to elevated blood pressure in men. The WC likely reflects both subcutaneous fat and visceral fat, and the hip circumference likely only reflects the subcutaneous fat at the gluteofemoral position. The WC is divided by the hip circumference, which might eliminate a certain level of influence by the subcutaneous adipose. This approach could be the basis for the weak relationship between the WHpR and metabolic risk factors.
The cut‐off values for WHtR in the present study were 0.50 for men and 0.52 for women, with a higher sensitivity than WC. These data are consistent with a Taiwanese study that included 36,642 Taiwanese adults, and that found a WHtR greater than 0.5 is a simple, but effective, indicator of centralized obesity associated with metabolic risk7. Ashwell also suggested that a 0.5 WHtR threshold was appropriate for identifying metabolic risk regardless of sex and race15. A report from China showed that the best cut‐off values for men and women are 0.51 and 0.53, respectively16. The aforementioned evidence further confirms the universal use of WHtR across different populations and sexes.
The WHtR measurements are convenient in clinical work because of their simplicity. Due to the close relationship between WHtR and WC, as well as WHtR and metabolic risk factors, it might play a central role in diagnosing MetS. Accounting for height makes up for the lack of WC, and renders WHtR universal across race and sex. The aforementioned confirm that WHtR might be a better predictor for MetS than WC.
The International Diabetes Federation proposed that different races used different WC criteria to diagnose MetS. Asian populations still use different optimal cut‐off values to diagnose MetS in different countries. A Korean study reported that the optimal WC cut‐offs were 90 cm for men and 85 cm for women, which was also recommended by a Chinese adult dyslipidemia prevention guide17. However, considering the different anthropometry between the northern and southern China populations, the cut‐off values differ even in the same country. A report on the Beijing population estimated an optimal cut‐off value of 87 cm for men and 80 cm for women18. A report from several northeastern city populations calculated a WC cut‐off value of 91.3 cm for men and 87.1 cm for women16. In the present study, the optimal cut‐off values were 85 cm for men and 80 cm for women. The population in the present study is from the northeastern rural area, where labor work is the major lifestyle and might yield a slimmer rural population compared with an urban population, which engages in office work. This phenomenon could be the basis for our lower cut‐off values.
Although obesity correlates highly with diabetes, among the metabolic risk factors, we found the weakest correlation between FPG and the obesity indexes. Fahim Abbasi also reported this finding, with a correlation coefficient between FPG and obesity indexes under 0.2013.
Although smoking and drinking habits are considered related to cardiovascular diseases, the only significant difference between the normal and multiple risk factors groups that we found was for smoking habits in men. To avoid this influence, we adjusted for drinking and smoking habits in the present study.
Our study was limited. As our research was a cross‐sectional study, an interpretation of the observed associations might be restricted with regard to cause and effect. In addition, although the anthropometric indexes were measured by trained researchers, the measurements carried out in a single visit might have produced errors.
In conclusion, the four obesity indexes, WC, BMI, WHtR and WHpR, were related to metabolic risk factors. The WC and WHtR were strongly associated. The WHtR was most associated with multiple metabolic risk factors, and the cut‐off values were approximately 0.50 for both sexes. The WC cut‐off value was 85 cm in men and 80 cm in women.
Disclosure
The authors declare no conflict of interest.
Acknowledgments
The authors thank Dr Zhao Li, who has provided great support for completing this project. The “Twelfth Five‐Year” project funds (National Science and Technology Support Programme of China, project number: 2012BAJ18B02) that Pro Yingxian Sun is responsible for enabled completion of the project.
J Diabetes Investig 2016; 7: 601–606
References
- 1. Expert Panel on the Identification . Evaluation, and Treatment of Overweight in Adults. Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults: executive summary. Am J Clin Nutr 1998; 68: 899–917. [DOI] [PubMed] [Google Scholar]
- 2. Grundy SM, Cleeman JI, Daniels SR, et al Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation 2005; 112: 2735–2752. [DOI] [PubMed] [Google Scholar]
- 3. Alberti KG, Zimmet P, Shaw J. The metabolic syndrome—a new worldwide definition. Lancet 2005; 366: 1059–1062. [DOI] [PubMed] [Google Scholar]
- 4. Misra A, Wasir JS, Vikram NK. Waist circumference criteria for the diagnosis of abdominal obesity are not applicable uniformly to all populations and ethnic groups. Nutrition 2005; 21: 969–976. [DOI] [PubMed] [Google Scholar]
- 5. Hsu CH, Lin JD, Hsieh CH, et al Adiposity measurements in association with metabolic syndrome in older men have different clinical implications. Nutr Res 2014; 34: 219–225. [DOI] [PubMed] [Google Scholar]
- 6. Knowles KM, Paiva LL, Sanchez SE, et al Waist Circumference, Body Mass Index, and Other Measures of Adiposity in Predicting Cardiovascular Disease Risk Factors among Peruvian Adults. Int J Hypertens 2011; 2011: 931402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Li WC, Chen IC, Chang YC, et al Waist‐to‐height ratio, waist circumference, and body mass index as indices of cardiometabolic risk among 36,642 Taiwanese adults. Eur J Nutr 2013; 52: 57–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ashwell M, Gunn P, Gibson S. Waist‐to‐height ratio is a better screening tool than waist circumference and BMI for adult cardiometabolic risk factors: systematic review and meta‐analysis. Obes Rev 2012; 13: 275–286. [DOI] [PubMed] [Google Scholar]
- 9. Bener A, Yousafzai MT, Darwish S, et al Obesity Index That Better Predict Metabolic Syndrome:body Mass Index, Waist Circumference, Waist Hip Ratio, or Waist Height Ratio. Hindawi Publishing Corporation. J Obes 2013; 2013: 269038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ko KP, Oh DK, Min H, et al Prospective study of optimal obesity index cutoffs for predicting development of multiple metabolic risk factors: the Korean genome and epidemiology study. J Epidemiol 2012; 22: 433–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gharipour M, Sarrafzadegan N, Sadeghi M, et al Predictors of metabolic syndrome in the Iranian population: waist circumference, body mass index, or waist to hip ratio?. Cholestrol 2013; 2013: 198384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Guasch‐Ferré M, Bulló M, Martínez‐González MÁ, et al Waist‐to‐Height Ratio and Cardiovascular Risk Factors in Elderly Individuals at High Cardiovascular Risk. PLoS ONE 2012; 7: e43275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Abbasi F, Malhotra D, Mathur A, , etal. Body mass index waist circumference associate to a comparable degree with insulin resistance related metabolic abnormalities in South Asian women, men. Diab Vasc Dis Res 2000; 9: 296–300. [DOI] [PubMed] [Google Scholar]
- 14. Herrera VM, Casas JP, Miranda JJ, et al Interethnic differences in the accuracy of anthropometric indicators of obesity in screening for high risk of coronary heart disease. Int J Obes (Lond) 2009; 33: 568–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ashwell M, Hsieh SD. Six reasons why the waist‐to‐height ratio is a rapid and effective global indicator for health risks of obesity and how its use could simplify the international public health message on obesity. Int J Food Sci Nutr 2005; 56: 303–307. [DOI] [PubMed] [Google Scholar]
- 16. Liu Y, Tong G, Tong W, et al Can body mass index, waist circumference, waist‐hip ratio and waist‐height ratio predict the presence of multiple metabolic risk factors in Chinese subjects? BMC Public Health 2011; 11: 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lee SY, Park HS, Kim DJ, et al Appropriate waist circumference cutoff points for central obesity in Korean adults. Diabetes Res Clin Pract 2007; 75: 72–80. [DOI] [PubMed] [Google Scholar]
- 18. Wang W, Luo Y, Liu Y, et al Prevalence of metabolic syndrome and optimal waist circumference cut‐off points for adults in Beijing. Diabetes Res Clin Pract 2010; 88: 209–216. [DOI] [PubMed] [Google Scholar]


