Abstract
Aims/Introduction
There is still no obvious evidence proving that androgen deprivation therapy (ADT) would increase the risk of diabetes. To determine if ADT is associated with diabetes in men with prostate cancer, we carried out the present study.
Materials and Methods
We systematically searched Medline, Embase and the Cochrane Library Central Register through 2014. Studies comparing ADT vs control aimed at treating prostate cancer reporting diabetes as outcome were included. Data were extracted independently by two reviewers. This meta‐analysis was reported based on the Preferred Reporting Items for Systematic reviews and Meta‐Analyses checklist. Observational studies were evaluated through the Meta‐analysis Of Observational Studies in Epidemiology checklist.
Results
Eight studies were identified with 65,695 ADT users and 91,893 non‐ADT users. The pooled incidence of diabetes was 39% higher in ADT groups. A significant association was observed in the overall analysis (risk ratio [RR] 1.39, 95% confidence interval [CI] 1.27–1.53; P < 0.001). In subgroup analyses, diabetes was found to be significantly associated with gonadotropin‐releasing hormone (GnRH) alone (RR 1.45, 95% CI 1.36–1.54; P < 0.001), GnRH plus oral antiandrogen (RR 1.40, 95% CI 1.01–1.93; P = 0.04) and orchiectomy (RR 1.34, 95% CI 1.20–1.50; P < 0.001), but not with antiandrogen alone (RR 1.33, 95% CI 0.75–2.36; P = 0.33). Diabetes was strongly related to long duration of ADT (RR 1.43, 95% CI 1.22–1.68; P < 0.001), and was slightly associated with short duration of ADT (RR 1.29, 95% CI 1.12–1.49; P = 0.0004).
Conclusions
ADT, especially long duration (>6 months) of this treatment, GnRH alone, GnRH plus antiandrogen and orchiectomy can increase the incidence of diabetes.
Keywords: Androgen deprivation therapy, Diabetes, Meta‐analysis
Introduction
Prostate cancer (PCa) is one of the most common malignancies in the USA, and the second leading cause of cancer‐related mortality worldwide1. Because the development and growth of PCa cells are dependent on androgens2, androgen deprivation therapy (ADT) plays an important role in the treatment of PCa, and has been increasingly used in the past decade, both as primary and neoadjuvant therapy, with approximately one‐third of the estimated 2 million PCa patients in the USA3, 4.
ADT results in a rapid decrease in serum concentrations of testosterone. Epidemiological studies have shown that low testosterone levels independently predict the development of insulin resistance, type 2 diabetes, and metabolic syndrome5, 6. Additionally, one population‐based cohort study7 involving 38,158 PCa patients showed that ADT is related to a higher incidence of diabetes (adjusted hazard ratio [HR] 1.24, 95% confidence interval [CI] 1.15–1.35). However, there is still no obvious evidence proving that ADT would increase the risk of diabetes. In addition, some short‐term prospective studies (with duration of ADT ≤6 months)8, 9 showed that fasting glucose levels did not change compared with non‐ADT users. However, many population‐based cohort studies7, 10 showed a significant association between long duration of ADT (>6 months) and diabetes morbidity.
Based on the current situation of this clinical issue, our research group carried out a meta‐analysis and systemic review to determine if ADT is associated with an increased risk of diabetes.
Materials and methods
Search strategy and study selection
A literature search was carried out using MEDLINE, EMBASE and the Cochrane Library database up to 30 December 2014. The search strategy consisted of combining keywords and subject headings with all possible combinations. Full search terms can be found in Appendix S1. No publication year, language or other restrictions were used. A hand search was also carried out of the references of all included studies.
Studies were included if they fulfilled the criteria as follows: (i) patients diagnosed with PCa; (ii) the treatment in intervention groups is ADT or ADT combined with other therapy; (iii) patients in control groups never received ADT; (iv) studies must either report risk estimates with 95% confidence intervals (CIs), or report sufficient data to estimate these; and (v) included studies had to provide comparative data. The most recent or complete study was chosen, if more than one article were identified from the same population database.
Data extraction and quality assessment
Two reviewers (Wang and Sun) independently extracted the data from eligible and potentially relevant publications, with differences resolved by the third reviewer (J Zhao) when necessary. For each included publication, the following information was considered: publication year and medical center, study design, study population (number of participants, median age), follow‐up period, treatment in both groups, types and duration of ADT, definition of diabetes, hazard ratios (HRs) or risk ratios (RRs) and corresponding 95% CIs of estimates in each comparison, or the data available to calculate them. The definition of diabetes was consistent with what the authors described in their studies.
The quality of the included trial was assessed by Jadad Score11, and was identified to be of high quality if it achieved more than four scores. We assessed the quality of selected cohort studies according to the Newcastle–Ottawa quality assessment scale12, and considered to be of high quality with more than six stars. The Agency for Healthcare Research and Quality assessment13 was used to assess the cross‐sectional studies. Two reviewers independently assessed and discussed discrepancies until agreement was reached. Additionally, the level of evidence of all included articles was assessed according to Phillips' classifications.14 The present study was carried out based on the Preferred Reporting Items for Systematic reviews and Meta‐Analyses guidelines (Appendix S2). Observational studies were evaluated through the Meta‐analysis Of Observational Studies in Epidemiology checklist (Appendix S3).
Subgroups analyses
As a mainstream therapy for PCa, ADT was divided into long duration (more than 6 months) and short duration (6 months or less) in many studies15, 16. According to this cut‐off level, analyses of ADT with long duration (>6 months) and short duration (≤6 months) were particularly carried out. Additional subgroup‐analyses for various types of ADT vs non‐ADT were also carried out to minimize the heterogeneity in overall analysis.
Statistical analysis
As described in our previous study17, different methods were used to estimate the HRs or RRs according to the data provided in the studies. When two or more types of ADT were respectively compared with the same control group, random effects meta‐analyses were used to combine these results.
Interstudy heterogeneity was evaluated using the Cochrane's Q statistic18. In addition, inconsistency was quantified by the I 2 statistic (100% × [(Q – d.f.) / Q]), with a lower value denoting minor heterogeneity19. The assumption of homogeneity was considered invalid for P < 0.05. Using the DerSimonian and Laird method, we chose random effects models throughout this analysis no matter whether heterogeneity existed or not.
We used Begg's adjusted rank correlation test and Egger's linear regression test to evaluate publication bias. All analyses were carried out with Review Manages (version 5.3; The Cochrane Collaboration, Oxford, USA) and stata (version 11.0; College Station, TX, USA). Two‐tailed P < 0.05 was considered to be statistically significant.
Results
Literature search and characteristics of the included studies
The initial database searches produced 462 articles, of which 181 duplicates were removed. Of the remaining articles, 254 were then excluded through titles and abstracts. Through the full text screening, nine studies were further removed, because they did not mention diabetes as an end‐point. Three studies with ADT also used in a control group and three20, 21, 22 with duplicated data were also excluded. Additionally, the last four studies were excluded for failing to provide a control group. Eight studies7, 10, 23, 24, 25, 26, 27, 28 were finally included in the present study (Figure 1). Table S1 showed the excluded reasons of full‐text articles. No further studies were evaluated through the search of the references listed in reviews.
Figure 1.
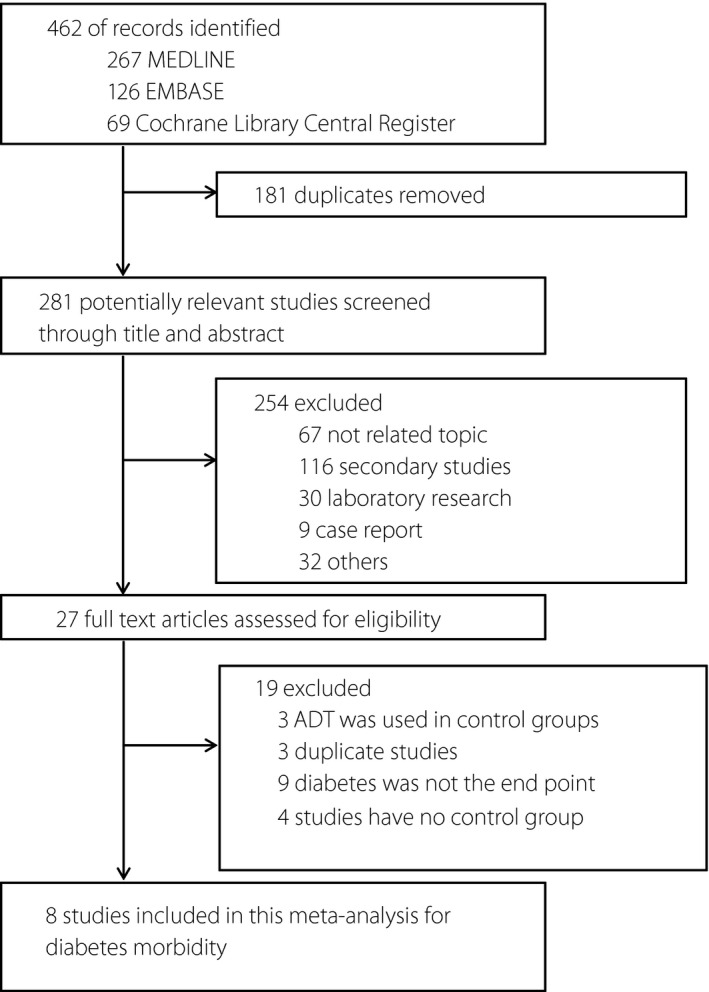
Flow diagram of search strategy and study selection. ADT, androgen deprivation therapy.
The characteristics of included publications for diabetes are listed in Table 1. The results of quality assessment according to the Newcastle–Ottawa quality assessment scale for cohort studies and Agency for Healthcare Research and Quality assessment for cross‐sectional studies are presented in Tables S2 and S3. All eligible cohort studies were of high quality based on the Newcastle–Ottawa quality assessment scale, with scores ranging from seven to nine stars. Level of evidence of included cohort studies were all 2a, and the cross‐sectional studies were all 3a.
Table 1.
Characteristics of studies investigating diabetes related to androgen deprivation therapy
| First author Year (country) | Design, LOE | Database source (duration) | Definition of diabetes | Types of ADT | Treatments of control | No. of ADT/control | Age year† (SD)/no. patients | Duration of ADT (months†) | Follow up (year†) | RRs (95% CI) | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lage et al.27, 2007 (England) | Cohort, 2a | The i3 Magnifi LabRx Database (2000–2005) | Diabetes (ICD‐9 codes 250.XX) | ADT | Non‐ADT | 1,231 | 7,250 | ADT: 65.93 ± 9.51 non‐ADT: 64.48 ± 9.63 | ≥12 | 1 | 1.36 (1.07–1.74)‡ | |
| 1.5 | ||||||||||||
| Keating et al.10, 2006 (US) | Cohort, 2a | SEER (1992–1999) | Diabetes (ICD‐9 codes 250.XX) | GnRH | WW/AS | 26,570 | 41,575 | 74.2 ± 5.8 | GnRH (1–4; 5–12; 13–24; ≥25) | 4.55 | 1.44 (1.34–1.55)‡ | 1.41 (1.32–1.50)§ |
| Orchiectomy | 5,050 | 1.34 (1.20–1.50)‡ | ||||||||||
| Alibhai et al.7, 2009 (Canada) | Cohort, 2a | ICES (1995–2005) | Diabetes (ICD‐9‐CM codes 250) | ADT | Non‐ADT | 19,079/19,079 | 75.0 ± 6.3 | ≥6 | 6.47 | 1.24 (1.15–1.35)‡ | ||
| Keating et al.26, 2010 (USA) | Cohort, 2a | Veterans Healthcare Administration (2001–2004) | Diabetes (ICD‐9 codes 250.XX) | GnRH | WW/AS | 13,065 | 23,823 | 66.9 ± 8.6 | NA | 2.6 | 1.48 (1.31–1.67)‡ | 1.46 (1.31–1.63)§ |
| AA | 1,230 | 1.33 (0.75–2.36)‡ | ||||||||||
| GnRH+AA | 1,829 | 1.40 (1.01–1.93)‡ | ||||||||||
| Orchiectomy | 268 | 1.36 (0.79–2.31)‡ | ||||||||||
| Cleffi et al.25, 2011 (Germany) | Cross‐sectional, 3a | NA | Glucose levels≥110 mg/dL | ADT | Non‐ADT | 54 | 25 | 73.28 ± 7.71 | 15.37 ± 2.48 | / | 2.16 (0.68–6.85) | |
| Basaria et al.27, 2006 (USA | Cross‐sectional, 3a | NA | Fasting glucose≥126 mg/dL | ADT | Non‐ADT | 18 | 17 | ADT: 70.2 ± 1.8 non‐ADT: 65.9 ± 2.5 | ≥12 | / | 3.78 (0.93–15.33) | |
| Braga‐Basaria et al.23, 2006 (USA) | Cross‐sectional, 3a | NA | Glucose levels≥110 mg/dL | GnRH | Non‐ADT | 17 | 18 | ADT: 69.9 ± 7.8 non‐ADT: 66.2 ± 10.0 | 45 (12–101) | / | 3.90 (1.32–11.51) | |
| Orchiectomy | 3 | |||||||||||
| Morote et al.28, 2014 (Spain) | Cross‐sectional, 3a | NA | Glucose levels≥110 mg/dL | GnRH | Non‐ADT | 53 | 106 | ADT: 72 ± 11 non‐ADT: 71 ± 9 | ≥12 | / | 1.80 (0.90–3.59) | |
†Mean or median. ‡Hazard ratio was directly given in the publication. §Combined estimates from all types of ADT with random effect meta‐analysis. AA, oral antiandrogens; ADT, androgen deprivation therapy; GnRH, gonadotropin‐releasing hormone (leuteinizing hormone releasing hormone); ICES, Institute for Clinical Evaluative Sciences; LOE, level of evidence; NA, not applicable; RRs, risk ratios; SD, standard deviation; SEER, Surveillance, Epidemiology and End Results; WW/AS, watchful waiting/active surveillance.
Meta‐analysis results
Eight studies7, 10, 23, 24, 25, 26, 27, 28 involving 157,588 participants were identified to investigate the relationship between ADT and diabetes. Among 65,695 ADT users, 7,136 patients (approximately 10.9%) developed diabetes compared with 6,987 events (approximately 7.6%) in 91,893 non‐ADT users (RR 1.39; 95% CI 1.27–1.53; P < 0.001; Figure 2a), indicating that ADT is strongly associated with an increased risk of diabetes morbidity.
Figure 2.
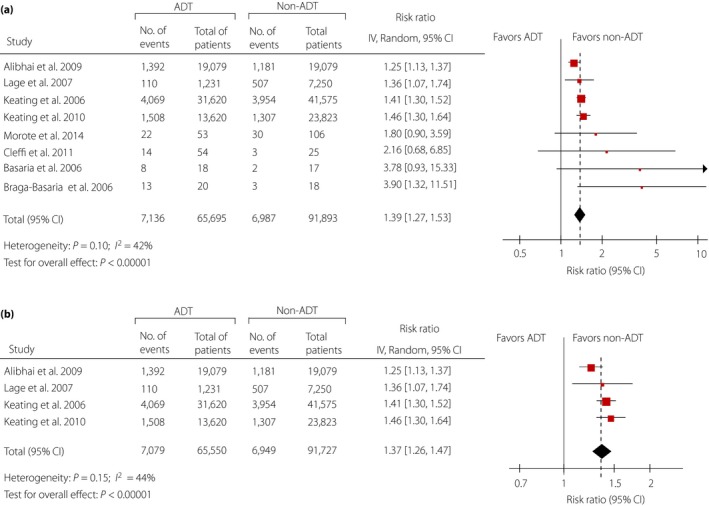
(a) Overall relative risks of diabetes related to androgen deprivation therapy (ADT). (b) Relative risks of diabetes related to ADT for sensitivity analysis. CI, confidence interval.
Data from two studies10, 26 were available for subgroup analyses comparing different types of ADT with non‐ADT: one study26 was available for antiandrogen (AA) alone and gonadotropin‐releasing hormone (GnRH) plus AA, two studies10, 26 were available for GnRH only and orchiectomy. As shown in Figure 3a, subgroup analyses for different types of ADT showed that diabetes was strongly related with GnRH alone (RR 1.45, 95% CI 1.36–1.54; P < 0.001), GnRH plus AA (RR 1.40, 95% CI 1.01–1.93; P = 0.04) and orchiectomy (RR 1.34, 95% CI 1.20–1.50; P < 0.001), but not with AA alone (RR 1.33, 95% CI 0.75–2.36; P = 0.33). Details of meta‐analyses for each type of ADT are presented in Figure S1.
Figure 3.
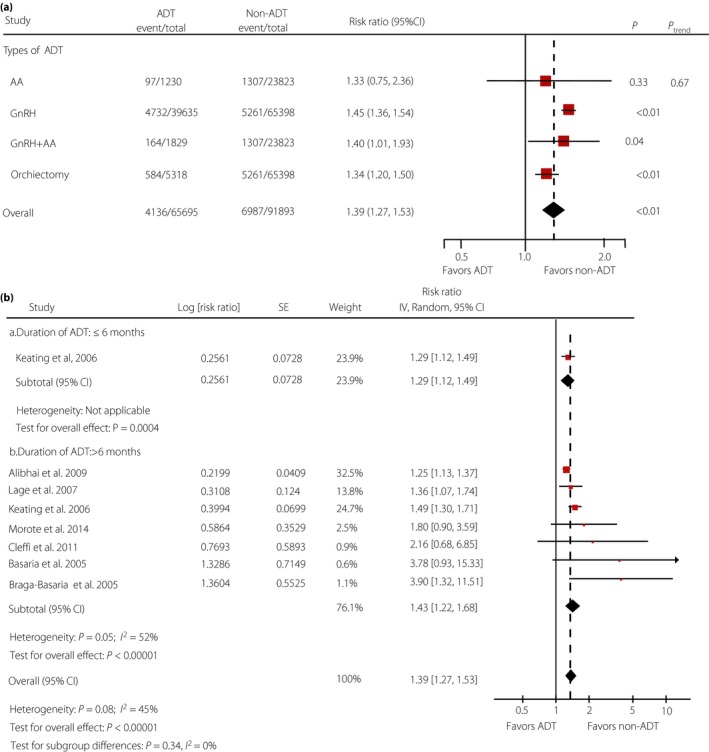
(a) Relative risks of subgroup analyses for diabetes related to different types of androgen deprivation therapy (ADT). (b) Relative risks of diabetes related to long and short durations of ADT. AA, antiandrogen; CI, confidence interval; GnRH, gonadotropin‐releasing hormone.
Seven studies7, 10, 23, 24, 25, 27, 28 were included for subgroup analysis of ADT with long duration (>6 months) and short duration (≤6 months). Only one study10 was available to investigate the effect of short duration (≤6 months) of ADT, and seven7, 10, 23, 24, 25, 27, 28 were available for long duration(>6 months). As presented in Figure 3b, diabetes was strongly related to long duration of ADT (RR 1.43, 95% CI 1.22–1.68; P < 0.001), and was slightly associated with short duration (RR 1.29, 95% CI 1.12–1.49; P = 0.0004), compared with overall‐analysis (RR 1.39, 95% CI 1.27–1.53; P < 0.001).
Sensitivity analysis and publication bias
Sensitivity analyses for the association between ADT and diabetes were carried out to evaluate the reliability of this meta‐analysis. After excluding all these cross‐sectional studies23, 24, 25, 28, the analysis result was similar to the overall analysis and achieved the reduction of heterogeneity (RR 1.37, 95% CI 1.26–1.47; P < 0.001; Figure 2b). In our assessment of publication bias, funnel plots showed balance, with points distributing around the verticals, indicating no obvious publication bias (Figure S2). Additionally, actualized data from Begg's and Egger's tests also supported no exhibited publication bias (Table S4).
Discussion
The present study carried out a meta‐analysis involving eight observational studies with a total of 157,588 PCa patients, and showed that ADT was associated with diabetes. The direct evidence was proved by Keating et al.10, showing a significantly increased risk of diabetes over a median follow‐up period of 4.55 years in GnRH users with PCa (HR 1.44, P < 0.001). One cross‐sectional study24 also found that men on ADT had a higher prevalence of abdominal hyperglycemia.
ADT is considered to be effective when serum testosterone has declined to the recommended levels of 50 ng/dL, according to the 2012 National Comprehensive Cancer Network guidelines29. However, low serum testosterone levels might decrease lean body mass and increase fat mass30, and might also reduce insulin sensitivity31, resulting in insulin resistance. Furthermore, obesity and insulin resistance are strongly associated with type 2 diabetes mellitus32. Taken together, all of those aforementioned supported our finding that ADT is a risk factor for diabetes.
Among included publications, four studies7, 10, 26, 27 were large‐scale cohort studies, and all the others23, 24, 25, 28 were cross‐sectional studies. No trials met the inclusion criteria of our meta‐analysis. To ensure the quality of the included studies, we carried out a sensitivity analysis only including cohort studies. When cross‐sectional studies were ruled out of consideration, RRs were obtained, and the analysis result was similar to the overall analysis and achieved the reduction of heterogeneity.
Because varied types of ADT were reported in two eligible studies10, 26, bias might exist in the results of overall analyses. In order to reduce this heterogeneity, subgroup analyses stratified by ADT type were carried out. A significantly increased risk of diabetes was associated with GnRH, GnRH plus AA and orchiectomy, but not with AA monotherapy. Treatment with antiandrogen monotherapy is not approved for PCa in the USA, so few men likely received such therapy.22 GnRH agonist could be responsible for diabetes toxicity through indirect mechanism, in which hypogonadism plays a critical role in the onset of metabolic syndrome33. The direct evidence proved by Keating et al.26, evaluating the relationship between GnRH agonist and diabetes events over a median follow‐up period of 2.6 years in men with PCa, was in accordance with our findings that GnRH agonist could significantly increase the risk of diabetes morbidity (adjusted HR 1.48, 95% CI 1.31–1.67). However, the present meta‐analysis had limitations that should be acknowledged. First, two or more types of ADT groups from two articles10, 26 were respectively compared with the same control group. Random effects meta‐analysis was used to combine these data together for compositing overall RRs. Second, all eligible publications were retrospective observational studies, which could introduce recall limitation so that the integrity of the records weakened the reliability of the results. However, as one adverse effect of ADT, diabetes is not the main end‐point randomized controlled trials always focus on, and the strict inclusion in randomized controlled trials might lead to the limitations of external validity as a result34. For the purpose of investigating adverse drug reactions, it is more credible to carry out a large‐scale observational study with long duration of follow up, high quality of design and implementation. Third, there was not an adequate number of studies available for the subgroup analysis of short duration of ADT. We tried to add the short duration to our subgroup analysis only for comparison with long duration of ADT. As to the different types of ADT, there was the same problem that only one study was available for AA and GnRH plus AA. We are aware of this limitation that the analysis result of AA and GnRH plus AA might be not credible; these results were only for comparison with other therapies. Furthermore, the basic characteristics of patients (e.g., age, the stage of PCa, comorbidities) with or without ADT might be different, and these could affect the incidence of diabetes. However, the HRs directly given in all of our included cohort studies7, 10, 26, 27 were already adjusted for the baseline characteristics of patients. Therefore, the influence of mixing basic characteristics of populations on our meta‐analysis would be minimized. Finally, the present study only included articles with binary variables reporting diabetes morbidity as the end‐point, but studies with continuous variables (e.g., fasting blood glucose or fasting serum insulin) were ruled out. However, this potential bias is likely to have been minimal, because the result of this meta‐analysis was similar to the studies35, 36 only reporting continuous variables that ADT users had a significantly higher glucose level compared with controls.
In conclusion, this meta‐analysis proves that ADT is associated with diabetes. Subgroup analyses show that GnRH, GnRH plus AA and orchidectomy can significantly increase the risk of diabetes. Additionally, diabetes is significantly related to long duration of ADT (≤6 months), and is slightly associated with short duration (>6 months). The present findings might help clinicians be conscious of the potential risks of ADT and ensure medical safety. Additionally, randomized controlled trials are required to further investigate the relationship between ADT and diabetes.
Disclosure
The authors declare no conflict of interest.
Supporting information
Appendix S1 │ Literature search strategy.
Appendix S2 │ The Preferred Reporting Items for Systematic reviews and Meta‐Analyses checklist for identification of systematic reviews and meta‐analysis.
Appendix S3 │ Meta‐analyses checklist. Observational studies were evaluated through the Meta‐analysis Of Observational Studies in Epidemiology Checklist.
Table S1 │ List of excluded full‐text articles with reasons for exclusions
Table S2 │ Newcastle–Ottawa Scale quality assessment of cohort studies
Table S3 │ Agency for Healthcare Research and Quality assessment of cross‐sectional studies
Table S4 │ Pooled results and publication bias for all comparisons
Figure S1 │ Details of subgroup analyses for diabetes related to different types of androgen deprivation therapy
Figure S2 │ Funnel plots for all meta‐analyses. (a) Funnel plots for overall meta‐analyses. (b) Funnel plots for sensitivity analysis
J Diabetes Investig 2016; 7: 629–636
Wang H, Sun X and Zhao L contributed equally to this article.
References
- 1. Grossmann M, Wittert G. Androgens, diabetes and prostate cancer. Endocr Relat Cancer Oct 2012; 19: F47–F62. [DOI] [PubMed] [Google Scholar]
- 2. Huggins C, Hodges CV. Studies on prostatic cancer. I. The effect of castration, of estrogen and androgen injection on serum phosphatases in metastatic carcinoma of the prostate. CA Cancer J Clin 1972; 22: 232–240. [DOI] [PubMed] [Google Scholar]
- 3. Barry MJ, Delorenzo MA, Walker‐Corkery ES, et al The rising prevalence of androgen deprivation among older American men since the advent of prostate‐specific antigen testing: a population‐based cohort study. BJU Int Nov 2006; 98: 973–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Shahinian VB, Kuo YF, Freeman JL, et al Increasing use of gonadotropin‐releasing hormone agonists for the treatment of localized prostate carcinoma. Cancer 2005; 103: 1615–1624. [DOI] [PubMed] [Google Scholar]
- 5. Haffner SM, Valdez RA, Mykkanen L, et al Decreased testosterone and dehydroepiandrosterone sulfate concentrations are associated with increased insulin and glucose concentrations in nondiabetic men. Metabolism 1994; 43: 599–603. [DOI] [PubMed] [Google Scholar]
- 6. Laaksonen DE, Niskanen L, Punnonen K, et al Testosterone and sex hormone‐binding globulin predict the metabolic syndrome and diabetes in middle‐aged men. Diabetes Care 2004; 27: 1036–1041. [DOI] [PubMed] [Google Scholar]
- 7. Alibhai SM, Duong‐Hua M, Sutradhar R, et al Impact of androgen deprivation therapy on cardiovascular disease and diabetes. J Clin Oncol 2009; 27: 3452–3458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dockery F, Bulpitt CJ, Agarwal S, et al Testosterone suppression in men with prostate cancer leads to an increase in arterial stiffness and hyperinsulinaemia. Clin Sci (Lond) 2003; 104: 195–201. [DOI] [PubMed] [Google Scholar]
- 9. Smith MR, Lee H, Nathan DM. Insulin sensitivity during combined androgen blockade for prostate cancer. J Clin Endocrinol Metab 2006; 91: 1305–1308. [DOI] [PubMed] [Google Scholar]
- 10. Keating NL, O'Malley AJ, Smith MR. Diabetes and cardiovascular disease during androgen deprivation therapy for prostate cancer. J Clin Oncol 2006; 24: 4448–4456. [DOI] [PubMed] [Google Scholar]
- 11. Moher D, Pham B, Jones A, et al Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta‐analyses? Lancet 1998; 352: 609–613. [DOI] [PubMed] [Google Scholar]
- 12. Wells GSB, O' Connell D, Peterson J, et al The Newcastle‐Ottawa Scale (NOS) for assessing the quality of non‐randomised studies in meta‐analyses. Ottawa Hospital Research Institute Web site.
- 13. Smetana GW, Umscheid CA, Chang S, et al Methods guide for authors of systematic reviews of medical tests: a collaboration between the Agency for Healthcare Research and Quality (AHRQ) and the Journal of General Internal Medicine. J Gen Intern Med 2012; 27(Suppl 1): S1–S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Phillips B. GRADE: levels of evidence and grades of recommendation. Arch Dis Child 2004; 89: 489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kim J, Vaid M, Tyldesley S, et al Population‐based study of cardiovascular mortality among patients with prostate cancer treated with radical external beam radiation therapy with and without adjuvant androgen deprivation therapy at the British Columbia Cancer Agency. Int J Radiat Oncol Biol Phys 2011; 80: 742–750. [DOI] [PubMed] [Google Scholar]
- 16. Merrick GS, Butler WM, Wallner KE, et al Androgen deprivation therapy does not impact cause‐specific or overall survival in high‐risk prostate cancer managed with brachytherapy and supplemental external beam. Int J Radiat Oncol Biol Phys 2007; 68: 34–40. [DOI] [PubMed] [Google Scholar]
- 17. Zhu S, Zhang H, Tang Y, et al Polymorphisms in XPD and hOGG1 and prostate cancer risk: a meta‐analysis. Urol Int 2012; 89: 233–240. [DOI] [PubMed] [Google Scholar]
- 18. Handoll HH. Systematic reviews on rehabilitation interventions. Arch Phys Med Rehabil 2006; 87: 875. [DOI] [PubMed] [Google Scholar]
- 19. Higgins JP, Thompson SG, Deeks JJ, et al Measuring inconsistency in meta‐analyses. BMJ 2003; 327: 557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hajdenberg J. ACP Journal Club . Continuous androgen‐deprivation therapy increased risk for diabetes and fragility fractures in older men with prostate cancer. Ann Intern Med. 2009;151:JC6–JC14. [DOI] [PubMed] [Google Scholar]
- 21. Keating NL, Liu PH, O'Malley AJ, et al Androgen‐deprivation therapy and diabetes control among diabetic men with prostate cancer. Eur Urol 2014; 65: 816–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Keating NL, O'Malley AJ, Freedland SJ, et al Does comorbidity influence the risk of myocardial infarction or diabetes during androgen‐deprivation therapy for prostate cancer? Eur Urol 2013; 64: 159–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Basaria S, Muller DC, Carducci MA, et al Hyperglycemia and insulin resistance in men with prostate carcinoma who receive androgen‐deprivation therapy. Cancer 2006; 106: 581–588. [DOI] [PubMed] [Google Scholar]
- 24. Braga‐Basaria M, Dobs AS, Muller DC, et al Metabolic syndrome in men with prostate cancer undergoing long‐term androgen‐deprivation therapy. J Clin Oncol 2006; 24: 3979–3983. [DOI] [PubMed] [Google Scholar]
- 25. Cleffi S, Neto AS, Reis LO, et al Androgen deprivation therapy and morbid obesity: do they share cardiovascular risk through metabolic syndrome? Actas Urol Esp 2011; 35: 259–265. [DOI] [PubMed] [Google Scholar]
- 26. Keating NL, O'Malley AJ, Freedland SJ, et al Diabetes and cardiovascular disease during androgen deprivation therapy: observational study of veterans with prostate cancer. J Natl Cancer Inst 2010; 102: 39–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lage MJ, Barber BL, Markus RA. Association between androgen‐deprivation therapy and incidence of diabetes among males with prostate cancer. Urology 2007; 70: 1104–1108. [DOI] [PubMed] [Google Scholar]
- 28. Morote J, Ropero J, Planas J, et al Metabolic syndrome in patients with prostate cancer undergoing androgen suppression. Actas Urol Esp 2014; 38: 285–289. [DOI] [PubMed] [Google Scholar]
- 29. Adolfsson J. Words of wisdom. Re: Parenteral estrogen versus combined androgen deprivation in the treatment of metastatic prostatic cancer: part 2. Final evaluation of the Scandinavian Prostatic Cancer Group (SPCG) Study No. 5. Eur Urol 2009; 55: 525. [DOI] [PubMed] [Google Scholar]
- 30. Smith MR. Changes in fat and lean body mass during androgen‐deprivation therapy for prostate cancer. Urology 2004; 63: 742–745. [DOI] [PubMed] [Google Scholar]
- 31. Vettor R, De Pergola G, Pagano C, et al Gender differences in serum leptin in obese people: relationships with testosterone, body fat distribution and insulin sensitivity. Eur J Clin Invest 1997; 27: 1016–1024. [DOI] [PubMed] [Google Scholar]
- 32. Diagnosis and classification of diabetes mellitus. Diabetes Care 2008; 31(Suppl 1): S55–S60. [DOI] [PubMed] [Google Scholar]
- 33. Conteduca V, Di Lorenzo G, Tartarone A, et al The cardiovascular risk of gonadotropin releasing hormone agonists in men with prostate cancer: an unresolved controversy. Crit Rev Oncol Hematol 2013; 86: 42–51. [DOI] [PubMed] [Google Scholar]
- 34. Zumsteg ZS, Zelefsky MJ. Short‐term androgen deprivation therapy for patients with intermediate‐risk prostate cancer undergoing dose‐escalated radiotherapy: the standard of care? Lancet Oncol 2012; 13: e259–e269. [DOI] [PubMed] [Google Scholar]
- 35. Bo JJ, Zhang C, Zhang LH, et al Androgen deprivation therapy through bilateral orchiectomy: increased metabolic risks. Asian J Androl 2011; 13: 833–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mohamedali HZ, Breunis H, Timilshina N, et al Changes in blood glucose and cholesterol levels due to androgen deprivation therapy in men with non‐metastatic prostate cancer. Can Urol Assoc J 2011; 5: 28–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1 │ Literature search strategy.
Appendix S2 │ The Preferred Reporting Items for Systematic reviews and Meta‐Analyses checklist for identification of systematic reviews and meta‐analysis.
Appendix S3 │ Meta‐analyses checklist. Observational studies were evaluated through the Meta‐analysis Of Observational Studies in Epidemiology Checklist.
Table S1 │ List of excluded full‐text articles with reasons for exclusions
Table S2 │ Newcastle–Ottawa Scale quality assessment of cohort studies
Table S3 │ Agency for Healthcare Research and Quality assessment of cross‐sectional studies
Table S4 │ Pooled results and publication bias for all comparisons
Figure S1 │ Details of subgroup analyses for diabetes related to different types of androgen deprivation therapy
Figure S2 │ Funnel plots for all meta‐analyses. (a) Funnel plots for overall meta‐analyses. (b) Funnel plots for sensitivity analysis


