Abstract
The social network structure of animal populations has major implications for survival, reproductive success, sexual selection and pathogen transmission of individuals. But as of yet, no general theory of social network structure exists that can explain the diversity of social networks observed in nature, and serve as a null model for detecting species and population-specific factors. Here we propose a simple and generally applicable model of social network structure. We consider the emergence of network structure as a result of social inheritance, in which newborns are likely to bond with maternal contacts, and via forming bonds randomly. We compare model output with data from several species, showing that it can generate networks with properties such as those observed in real social systems. Our model demonstrates that important observed properties of social networks, including heritability of network position or assortative associations, can be understood as consequences of social inheritance.
 Social networks have important implications to a variety of ecological and evolutionary processes. Here, Ilany and Akçay develop a social network model and show that inheritance of social contacts leads to networks with properties observed in species such as sleepy lizards and spotted hyenas.
Social networks have important implications to a variety of ecological and evolutionary processes. Here, Ilany and Akçay develop a social network model and show that inheritance of social contacts leads to networks with properties observed in species such as sleepy lizards and spotted hyenas.
The transition to sociality is one of the major shifts in evolution, and social structure is an important and ever-present selective factor, affecting both reproductive success1 and survival2,3. Sociality affects individual health, ecological dynamics and evolutionary fitness via multiple mechanisms in humans and other animals, such as pathogen transmission4,5, and promoting or hindering of particular social behaviours6,7,8. The social structure of a population summarizes the social bonds of its members9. Hence, understanding the processes generating variation in social structure across populations and species is crucial to uncovering the impacts of sociality.
Recent years have seen a surge in the study of the causes and consequences of social structure in human and animal societies, based on theoretical and computational advances in social network analysis10,11,12,13,14,15,16. The new interdisciplinary network science provides many tools to construct, visualize, quantify and compare social structures, facilitating advanced understanding of social phenomena. Researchers studying a variety of species, from insects to humans, have used these tools to gain insights into the factors determining social structure13,17,18,19. Using social network analysis provided evidence for the effects of social structure on a range of phenomena, such as sexual selection20 structure and cultural transmission21,22.
At the same time, most applications of social network analysis to non-human animals have been at a descriptive level, using various computational methods to quantify features of social structure and individuals' position in it. These methods, combined with increasingly detailed data ‘reality mining'23 about social interactions in nature, provided valuable insights about the complex effects of social interaction on individual behaviours and fitness outcomes. Yet, we still lack a comprehensive theory that can explain the generation and diversity of social structures observed within and between species. There have been only a few efforts to model animal social network structure. Notably, Seyfarth24 used a generative model of grooming networks based on individual preferences for giving and receiving grooming, and showed that a few simple rules can account for complex social structure. This model and related approaches, for example ref. 25, have been very influential in the study of social structure and continue to drive empirical research. At the same time, they mostly focused on primates and were geared towards specific questions such as the effects of relatedness, social ranks or ecological factors in determining social structure.
Independently, a large body of theoretical work in network science aims to explain the general properties of human social networks through simple models of how networks form. Yet these models tend to focus either on networks with a fixed set of agents26, or on boundlessly growing networks27, with few exceptions28,29. These network formation models therefore have limited applicability to animal (and many human) social groups where individuals both join (through birth of immigration) and leave (through death or emigration) the network. Furthermore, most work in network science concentrates on the distribution of number of connections individuals have (the degree distribution). Models that fit the degree distribution of real-world networks tend to be a poor fit to other important properties, notably the tendency of social connections to be clustered27,30, that is, two individuals to be connected with each other if they are both connected to a third individual. Real-world human and animal networks exhibit significantly more clustering than random or preferential attachment models predict13,27.
Simple generative models of complex systems have been highly useful in other fields, such as metabolic networks31 and food webs32, but there has been little effort to build such models applicable to animal social networks. In this paper, we provide a widely applicable network formation model based on simple demographic and social processes. Our model assumes a simple neutral demography and focuses on a central social process that is in operation in many social species: the ‘inheritance' of social connections from parents. This central component of our model is based on the observation that in many species with stable social groups, individuals interact with the social circle of their parents. This is essentially the case in all mammals, where newborns stay close to their mothers until weaning, but also found in many other taxa, such as birds33, fish34 and arthropods35. After positively interacting with the parents' social contacts, young individuals are likely to form social bonds with these conspecifics, as was found in African elephants, Loxodonta africana36.
We demonstrate that this simple social inheritance process can result in networks that match both the degree and local clustering distributions of real-world animal social networks, as well as their modularity (which measures the strength of division of a network into modules or subgroups). We also show that social heritability of connections can result in the appearance of genetic heritability of individual social network traits, as well as assortativity in the absence of explicit preference for homophily. Our approach highlights commonalities among groups, populations and species, and uncovers a general process that underlies variation in social structure.
Results
Model description
Our departure point is the model by Jackson and Rogers27, in which ‘role models' in a network introduce their new contact to their other contacts. This model can reproduce many attributes of large-scale human social networks. Similar models reconstruct the structure of other systems, such as protein interaction networks37 and the World Wide Web38. However, the model of Jackson and Rogers27 (like most other models in this family) is based on a constantly growing network with no death or emigration of agents and their results hold asymptotically for very large networks. Since we are interested in small-scale animal networks that do not grow unboundedly, we model a population where existing individuals die and get replaced at an equal rate with newborn individuals28 (see Methods and Supplementary Figs 1–3 for results for slowly growing and shrinking networks). We model binary and undirected networks, so implicitly assume social bonds are neutral or cooperative, but our model can be extended to weighted networks that describe the strength of each social bond, and to directed ones, such as agonistic networks.
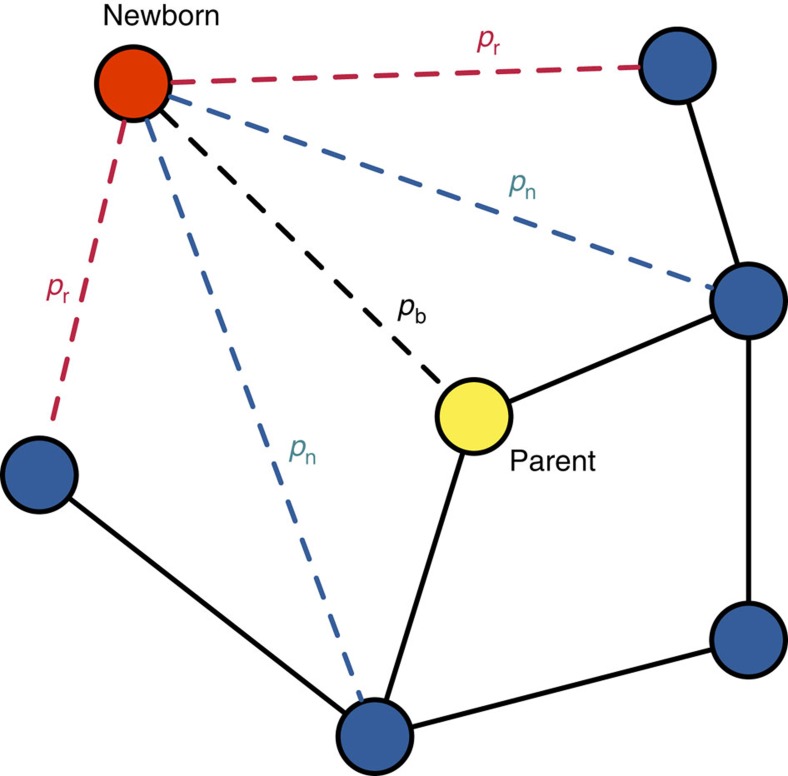
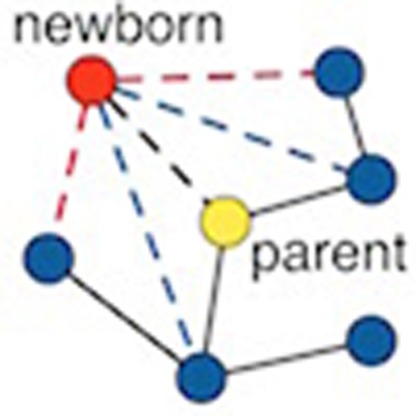
Consider a social group of size N. Suppose that each time step an individual is born to a random mother and one individual is selected to die at random. With probability pb, the newborn will meet and connect to its mother (generally, pb will be close to 1, but can be low or 0 in species such as some insects, where individuals might not meet their mothers). A crucial component of our model is the general assumption that the likelihood of a newborn A connecting with another individual B depends on the relationship between A's mother and B: the probability A will connect to B is given by pn if A's mother is connected to B, and pr if not (n and r stand for neighbour and random node, respectively; Fig. 1). Hence, pn is the probability an offspring ‘inherits' a given connection of its parent. Here we focus on the case where A always connects to its mother (pb=1), but the model can be extended to include a lower probability to connect to the contacts of A's mother if A does not connect to its mother, when pb<1. If pn>pr, the population exhibits a tendency for clustering, a well-established and general phenomenon in social networks13,39. In the Methods section, we present an extension of this basic model to account for two sexes, where only females reproduce. We show that if newborns are likely to copy only their mothers, the resulting social network is similar.
Figure 1. Graphical illustration of the model.
A newborn individual is connected to its parent with probability pb, to its parent's connections with probability pn and to individuals not directly connected to its parent with probability pr.
Network statistics
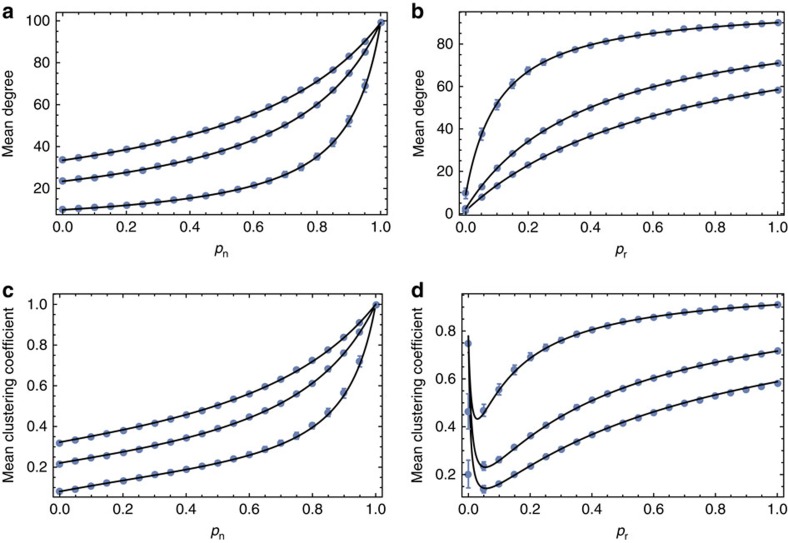
We simulate social network dynamics to test how social inheritance and stochastic social bonding affect network structure, heritability and assortativity (see Methods for simulation details and the Supplementary Data 1 for the code). We also provide analytical expressions for the degree distribution, and approximations for mean degree and mean local clustering coefficient in the Methods section. For all of our numerical results, we assume pb=1. As expected, the network density (the number of edges out of all possible edges) depends on pn and pr. The mean clustering coefficient, a measure of the extent to which nodes tend to cluster together, also depends on these parameters, but not monotonically; high levels of clustering were observed in simulations with low or high pr, but not at intermediate levels (Fig. 2). We also test how changes in network size, caused by increased or decreased probabilities of death during the simulations, affected its properties. These tests do not provide a general conclusion, but suggest that the network structure might be moderately influenced by whether the network is growing or not (see Methods).
Figure 2. Mean degree and clustering coefficient as a function of model parameters.
The dependency of mean degree (a,b) and clustering coefficient (c,d) on social inheritance, pn (a,c), and probability of random bonding, pr (b,d). In each panel, the black curves depict our analytical approximation while the blue dots with error bars are mean and s.d. of 50 replicate runs. For the two panels on the left, the curves correspond to, from top to bottom, pr=0.5, 0.3 and 0.1; for the two panels on the right, from top to bottom, pn=0.9, 0.6 and 0.3. For all panels, network size N=100; the simulations were initiated with random networks and run for 2,000 time steps.
Social inheritance recreates the structure of real networks
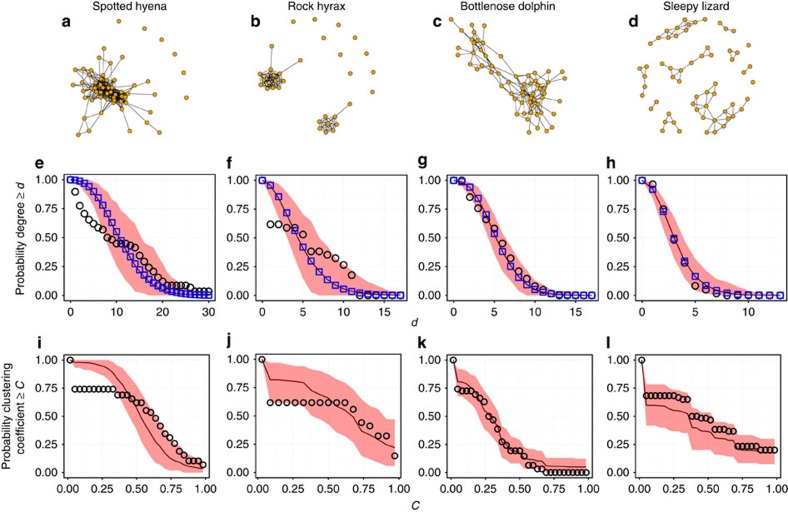
Next, we compare the output of our model with observed animal social networks of four different species, namely spotted hyena (Crocuta crocuta13), rock hyrax (Procavia capensis40), bottlenose dolphin (Tursiops spp41) and sleepy lizard (Tiliqua rugosa42). We use two independent ways to estimate model parameters using data from each of the four species: a computational dimensionality reduction approach (partial least squares regression, PLS) and analytical approximations for the mean degree and local clustering coefficients (see Methods and Supplementary Figs 4–7). When we run our model using pn and pr estimated from the data using either method, we recapture the distributions of degree and local clustering coefficient, as well as the network modularity. Figure 3 illustrates that our model of social inheritance can produce networks with realistic social structure (see Methods, and Supplementary Fig. 8 and Supplementary Table 1 for fitting the two-sex model to observed networks). Our model's good match of local clustering distributions distinguishes it from other network formation models based on assortative or generalized social preferences, as well as the preferential attachment models that are popular in network science27 (see also Methods and Supplementary Figs 9–11). Furthermore, our model generates networks with realistic modularity values (see Methods and Supplementary Fig. 12). The values we find suggest that social inheritance is stronger in hyena and hyrax than in dolphins and sleepy lizards (Table 1).
Figure 3. Social inheritance captures essential properties of animal social networks in the wild.
This figure shows that our model can account for the degree and clustering coefficient distributions of observed networks in four species. (a–d): observed networks. (e–h): cumulative degree distributions of observed and simulated networks (d stands for degree). (i–l): Cumulative local clustering coefficient distributions of observed and simulated networks (C stands for clustering coefficient). Black circles represent observed values. Blue squares in the middle row depict mean-field estimation for the degree distribution. The red curve denotes mean distribution for 500 simulated networks (2,000 simulation steps) with the species-specific pn and pr values estimated using partial least squares regression (values given in Table 1; see Methods for more on the estimation procedure), whereas light red area depicts 95% confidence intervals.
Table 1. Fitted parameter values.
| Species |
PLS |
Analytical |
||
|---|---|---|---|---|
| pn | pr | pn | pr | |
| Spotted hyena13 | 0.85 | 0.017 | 0.83 | 0.018 |
| Rock hyrax40 | 0.75 | 0.007 | 0.66 | 0.026 |
| Bottlenose dolphin39 | 0.50 | 0.028 | 0.43 | 0.036 |
| Sleepy lizard42 | 0.51 | 0.007 | 0.38 | 0.012 |
PLS, partial least squares.
Predicted parameter values used in the simulations for each species (predicted by PLS regression; and predicted using analytical approximation of the mean degree and clustering coefficients). PLS values are used in Fig. 3. Supplementary Figure 7 plots the same using the estimates from the analytical approximations. A more detailed description of the PLS regression procedure and analytic approximation is in the Methods.
Social inheritance causes apparent genetic heritability
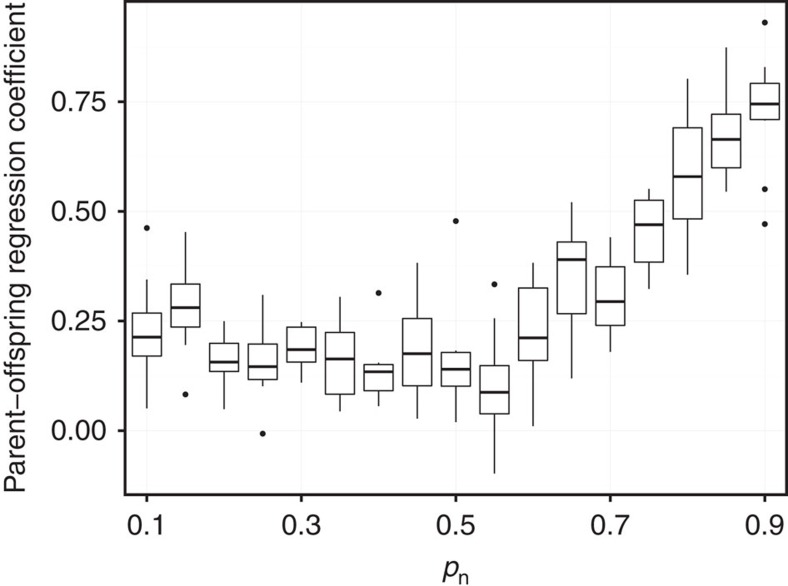
Next, we test if social inheritance can result in heritability of indirect network traits in social networks. Direct network traits (individual network traits that depend only on direct association with others, that is, on the immediate social environment), such as degree, will by definition be heritable when pn is high and pr is low. To see if this also holds for indirect network traits (traits that may depend also on associations between other individuals), we measure the correlation between parent and offspring betweenness centrality (which quantifies the number of times a node acts as a bridge along the shortest path between two other nodes; see Methods) for a set of social inheritance (pn) values. As Fig. 4 shows, high probabilities of social inheritance result in a pattern of heritability. In other words, when individuals are likely to copy their parents in forming social associations, the resulting network will exhibit heritability of centrality traits, although the only heritability programmed into the model is that of social inheritance and stochastic bonding. Similar patterns obtain for local clustering coefficient and eigenvalue centrality (Supplementary Figs 13 and 14).
Figure 4. Heritability as a consequence of social inheritance.
The regression of betweenness centrality among parents and their offspring as a function of the strength of social inheritance (pn). The bottom and top of the box mark the first and third quartiles, respectively. The upper whisker extends from the hinge to the highest value that is within 1.5 × IQR of the hinge, where IQR is the inter-quartile range, or distance between the first and third quartiles. The lower whisker extends from the hinge to the lowest value within 1.5 × IQR of the hinge. Data beyond the end of the whiskers are outliers and plotted as points. Ten replications were run for each pn value. Parameter values: simulation steps=2,000, N=100, pr=0.01.
Social inheritance causes assortativity
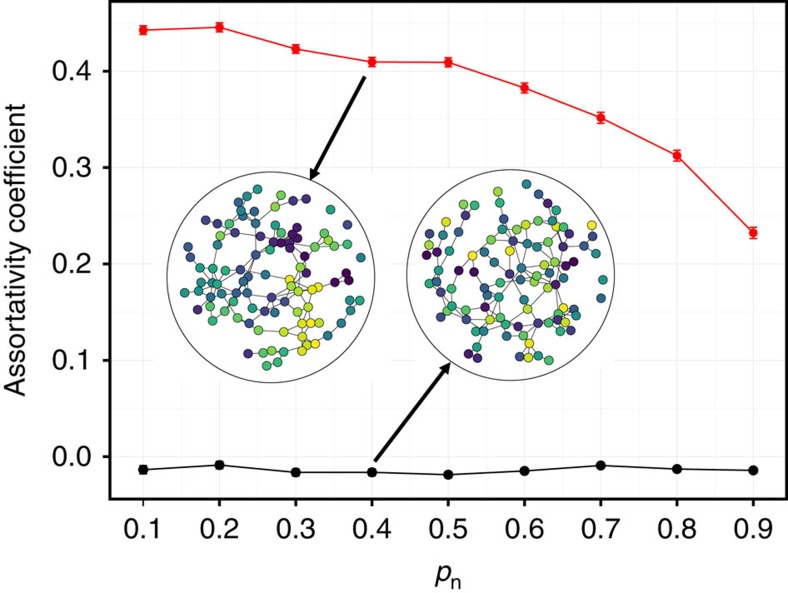
Finally, we test the effect of social inheritance on assortativity, that is, the preference of individuals to bond with others with similar traits. We simulate networks where each individual had one trait with an arbitrary value between 0 and 1. Newborns inherit their mother's trait with probability 1−μ, where μ is the rate of large mutations. If a large mutation happens, the newborn has a random uniformly distributed trait value; otherwise, its trait is randomly picked from a Gaussian distribution around the mother's trait, with variance σ2. Individuals follow the same rules of the basic model when forming social bonds. Hence, individuals do not explicitly prefer to bond with others with the same trait value. Nevertheless, we observe that the assortativity coefficient is significantly higher than in random networks, in which the trait values were re-assigned randomly (Fig. 5).
Figure 5. Assortativity as a consequence of social inheritance.
Illustration of assortativity without explicit assortative preference. Dots and notches note assortativity coefficients and standard errors, respectively, for model networks (red) and shuffled networks, where trait values were re-assigned randomly (black). Inset networks illustrate examples from the two groups. Circle colours represent arbitrary continuous trait values. Lines represent social bonds between individuals. Parameter values are the same as in Fig. 4, with mutation probability μ=0.05.
As an alternative model generating assortativity, we consider an explicit assortativity model, in which newborns explicitly prefer bonding with those with similar traits. Although this model (unsurprisingly) generates networks with high assortativity (mean assortativity coefficient±s.e.m.: 0.53±0.006 compared with −0.01±0.002 in networks with randomly shuffled trait values), it fails to recover the high clustering and modularity observed in networks generated by social inheritance and in the data (Supplementary Figs 9 and 10). This result further suggests that assortativity might be a byproduct of social inheritance rather than a driving force of social network structure. A more generalized preferential attachment model, described in the Methods section, shows the converse is not true (Supplementary Fig. 11), that is, the network patterns generated by social inheritance do not arise as a byproduct of genetically inherited traits and association preferences (see Discussion for more).
Discussion
Our model provides a step towards a general theory of social structure in animals that is grounded in social and demographic processes. Our approach is to use dynamic generative models based on simple processes to predict network-scale patterns that those processes are expected to produce, and compare them with observed networks. Such an approach has been widely and productively used in network theory and social sciences27,43,44, as well as other subfields of ecology31,32 but not in animal social networks. Our work addresses this gap. Our main result is that the combination of neutral demography and social inheritance can replicate important properties of animal social networks in the wild.
In particular, we show that our model can capture essential features of social networks of four different species in the wild, including not just the degree distribution and modularity, but also the clustering coefficient distribution, in contrast to most studies of social network formation. Clustering is an important feature of social networks, distinguishing them from other types of networks, such as transportation networks and the internet30. Theory predicts that clustered networks are more conducive to cooperation45, and empirical studies document a tendency to close triads13,40, suggesting that it might be a generally adaptive feature of social structure. Nevertheless, many previous models of sociality and network formation fail to account for the high clustering observed. For example, while preferential attachment can reconstruct the degree distribution of social networks, it fails to capture their high degree of clustering27. The social inheritance process is crucial to the formation of cohesive clusters in social networks because it biases newly formed connections to those that close triads of relationships.
Social inheritance requires a behavioural mechanism that facilitates introduction of newborns to their mother's social partners. As in many species, young individuals tend to follow their mothers, it is easy to think about such a passive mechanism: young individuals are introduced to other individuals by spending time with their mother's partners. This process is consistent with the long-held view that mother–offspring units are fundamental to social structure46. Direct evidence for social inheritance comes from the study by Goldenberg et al.36, who documented the tendency of female African elephants to ‘inherit' the social bonds of their mothers, driving network resilience. Moreover, in many species group members show active interest in newborns47, promoting the initiation of a social bond between newborns and their mother's partners. Further work can test if initial interest in newborns later translates to stronger social bonds with individuals reaching adulthood. We note that social inheritance does not necessarily require an active process of ‘introductions' but can also happen passively, for example, as a result of spatial fidelity among group members. Our model is agnostic with regard to the mechanism of social inheritance. That being said, the fitted model parameters for the four networks vary in ways that are suggestive for socio-ecological factors: for hyenas and hyraxes, we find high pn values, which may reflect the strong philopatry in these societies. In contrast, the relatively low fitted value of pn in dolphins may reflect their multi-level society featuring mother–son avoidance48.
We make a number of simplifying assumptions, such as no individual heterogeneity, or age- or stage-structure in our demography. Models of this type have a long and distinguished history in ecology and evolution49, and in the same spirit, we do not believe that nature is actually as simple as we model it. Nonetheless, the fact that this very simple model (but not other simple models, for example, the explicit assortativity and generalized preference models) can reproduce important aspects of real networks suggests that the social inheritance of connections is likely to be important in structuring social networks. Even though the details will no doubt vary across species and contexts, this simple, quantifiable process can explain observed variation in social networks. For example, our model does not treat sex-specific dispersal, a mechanism that results in different social environments for the two sexes. Nevertheless, there is evidence for social bonding with familiar individuals after dispersal50. This suggests that even after dispersal, individuals may ‘inherit' the social bonds of certain conspecifics serving as role models. Another use of simple models such as ours is to serve as a base model to test the effect of additional factors. For instance, after fitting the model to an observed social network, one could test whether personality can explain the variance not explained by social inheritance and stochasticity. This can be attained by adding personality to the agent-based model as a factor that influences individual bonding decisions.
Our model has implications for how the inheritance of positions in social networks, which has important implications for social dynamics, is to be interpreted. For example, Fowler et al.51 found that in humans, network traits such as degree and transitivity were heritable. In rhesus macaques, Brent et al.52 found that indirect network traits such as betweenness are more heritable than direct ones. In contrast, a study of yellow-bellied marmots, Marmota flaviventris, presented evidence for heritability of social network measures based on direct interactions53, but not indirect interactions. Taken together, these studies show that network position can be heritable, but have not been able to elucidate the mechanism of inheritance. It is not unlikely that some social network traits are genetically inherited; for example, individuals might genetically inherit social preferences from their parents that lead them to connect to the same individuals. With the generalized preference model, we show that such a mechanism is unlikely to account for the observed levels of clustering. Therefore, our work suggests that at least some of the heritability of network traits might be social (as opposed to genetic), from individuals copying their parents. This prediction is borne out by recent studies in elephants36. Importantly, while these previous studies attempt to control for effects of the social environment at the group or lineage level using quantitative genetics methods, for example ref. 54, they were not designed to distinguish social inheritance at the individual level from genetic inheritance. Studying the dynamics of social bond acquisition can be a way to separate genetic and social inheritance.
Another robust finding in network science and animal behaviour is that individuals tend to connect to others with traits similar to themselves (for example, refs 55, 56, 57). This assortativity is crucial for social evolutionary theory, as the costs and benefits of social interactions depend on partner phenotypes. Recent work has found that assortative mating can arise without assortative preferences, as a result of dynamic processes in a closed system58. Our results show that social inheritance can lead to high assortativity in the absence of explicitly assortative preferences for social bonding. Indeed, an alternative model based on explicit assortativity failed to reconstruct topological features of observed networks. Empirically, our results call for a careful assessment of networks with apparent phenotypic assortment and controlling for social inheritance. This will be difficult to do with only static network data, but will be feasible for species with long-term data on the network dynamics.
Our work points to several interesting avenues to be explored in future research. First, we used binary networks to describe the strength of social bonds that are inherently on a continuous scale11,59. Weighted networks that can describe the delicate differences in the strength of social bonds between individuals would be more relevant in some cases. Future generative models can consider varying bond strength by coupling a weighted network model with a model of behavioural dynamics of social bond formation for pairs of individuals. Second, even though our model is extremely simplistic, most of its mathematical properties (including probability distributions over network measures such as the degree distribution) are analytically intractable, which makes model-fitting a challenge. Methods such as approximate bayesian computation60, coupled with dimensionality reduction techniques61 can be used to develop algorithms for estimating parameters of the model and also incorporate more information about individual variation and environmental effects (See Methods for more). In addition, long-term data sets on social network dynamics can allow estimation of the social inheritance and random bonding parameters pn and pr directly. Last, our model does not consider changes in social bonds after these were established. Although this assumption is supported by empirical findings concerning bond stability in some species12,13, future models in which this assumption is relaxed should be developed. We also assume a single type of bond between individuals, whereas in nature different social networks exist for different kinds of interactions (for example, affiliative, agonistic and so on). Such ‘multilayer networks'62 represent an important future direction.
In conclusion, the theory we present here is based on the idea that social networks should be regarded and analysed as the result of a dynamic process63 that depend on environmental, individual and structural effects13. Our work represents a first step in developing a theory for the structure of social networks and highlights the potential of generative models of social and demographic processes in reaching this goal.
Methods
Expected mean degree
We can approximate some important aspects of our model analytically. First, we write a simple approximation of the expected mean degree, 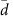 , of a network changing according to our model at stationarity. To do that, we note that at stationarity, killing an individual at random is expected to remove
, of a network changing according to our model at stationarity. To do that, we note that at stationarity, killing an individual at random is expected to remove 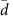 connections from the network. After this individual is removed, the average degree of the network becomes:
connections from the network. After this individual is removed, the average degree of the network becomes: 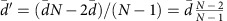 . The expected degree of the connections made by the newborn is then:
. The expected degree of the connections made by the newborn is then: 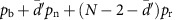 . At stationarity, the links destroyed and added need to be the same on average, so we can write:
. At stationarity, the links destroyed and added need to be the same on average, so we can write:
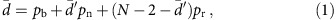 |
and solve for 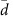 to obtain:
to obtain:
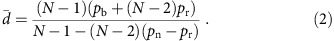 |
This approximation gives an excellent fit to simulated networks across all ranges of mean degree (Fig. 2).
Expected mean local clustering coefficient
Similar to the mean degree, we use a stationarity argument to calculate an approximation for the mean local clustering degree of a network, by equating the expected clustering coefficient (CC) of a randomly killed individual with the expected change in the clustering coefficients of all remaining individuals with the birth of the newborn plus the expected clustering of the newborn itself:
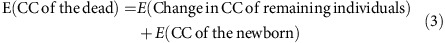 |
The expected clustering coefficient of an individual randomly selected to die is equal to 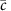 , the mean clustering coefficient. When an individual is killed, the clustering coefficient of its connections will in principle change, but one can show that the ‘typical' connection (that is, one with degree
, the mean clustering coefficient. When an individual is killed, the clustering coefficient of its connections will in principle change, but one can show that the ‘typical' connection (that is, one with degree 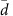 and clustering
and clustering 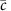 ) will not experience a change in its clustering coefficient. This can be seen by calculating the new clustering coefficient after death,
) will not experience a change in its clustering coefficient. This can be seen by calculating the new clustering coefficient after death,
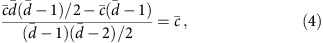 |
where the first term in the numerator is the expected number of closed triangles a typical connection of the dead individual had before death, the second term the number of triangles that were removed by death, and the denominator is the number of all potential triangles after death.
The birth of a new individual changes the total of the clustering coefficients in two ways: (i) by changing the clustering coefficients of individuals connected to the newborn, and (ii) by adding the newborn with the newborn's clustering coefficient. Let us calculate the first effect: the clustering coefficient of an individual with initial degree d and clustering coefficient c that becomes connected to the newborn is going to change as follows:
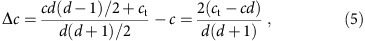 |
where the first term in the numerator of the middle part is the number of closed triangles among the focal individual's connections before getting connected to the newborn, and ct is the expected number of closed triangles among the focal individual's connections established by the newborn. The denominator is the total number of triangles after the focal individual gets connected to the newborn. To calculate ct, we need to consider the three kinds of connections of the newborns separately: its parent (with probability pb), its parent's connections (with probability pn) and individuals not connected to its parent (with probability pr).
For the parent, the expected number of closed triangles generated by the newborn is simply
 |
where dp is the degree of the parent. For a parent's connection, each has on average cp(dp−1) connections to other connections of the parent, which in turn have a probability of pn of getting connected to the newborn. Further, on average parent's connections will have 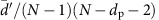 connections to non-connections of the parent (where
connections to non-connections of the parent (where 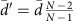 , the expected degree of individuals after a death occurs), each of which have probability pr of getting connected to the newborn. Thus, for parent's connections, we have
, the expected degree of individuals after a death occurs), each of which have probability pr of getting connected to the newborn. Thus, for parent's connections, we have
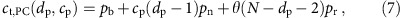 |
where 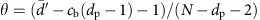 is the probability a given non-connection of the parent is connected to a parent's connection.
is the probability a given non-connection of the parent is connected to a parent's connection.
By a similar argument, one can write for non-connections of the parent:
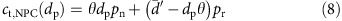 |
Thus, substituting ct,p, ct,PC and ct,NPC into equation (5), we can write for the expected total change in the clustering coefficient of existing individuals with the birth of the newborn, when the parent has degree dp:
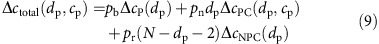 |
Next we need to calculate the expected clustering coefficient of the newborn, given the parent's degree dp and clustering coefficient cp: E(cNB|dp,cp). This number is the ratio of two random variables: Tc, the number of closed triangles that have the newborn as a vertex, and Tt, the total number of pairs connected to the newborn, that is,
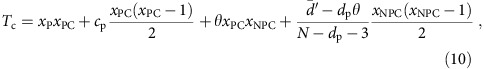 |
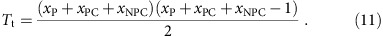 |
Here 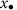 denotes the number of connections of the newborn to each class of individual (P for parent; PC for parent's connections; and NPC for individuals not connected to the parent). Thus, xp is distributed according to a Bernoulli distribution with probability pb; xPC a binomial with parameters dp and pn; and xNPC a binomial with parameters N−dp−2 and pr. The fractions in the third and fourth term in Tc give the expected density of connections between a parent's connection and non-connection, and among the non-connections, respectively. The expectation of the ratio of two random variables Tc and Tt can be approximated by their moments as follows:
denotes the number of connections of the newborn to each class of individual (P for parent; PC for parent's connections; and NPC for individuals not connected to the parent). Thus, xp is distributed according to a Bernoulli distribution with probability pb; xPC a binomial with parameters dp and pn; and xNPC a binomial with parameters N−dp−2 and pr. The fractions in the third and fourth term in Tc give the expected density of connections between a parent's connection and non-connection, and among the non-connections, respectively. The expectation of the ratio of two random variables Tc and Tt can be approximated by their moments as follows:
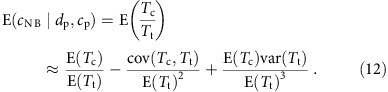 |
Using the distributions of Tc and Tt, computing equation (12) is a straightforward if tedious calculation.
For the final step in our computation, we assume that the parent is chosen at random from the population, so has expected degree 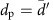 , and clustering coefficient
, and clustering coefficient 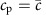 . Thus, our stationarity condition can be written as:
. Thus, our stationarity condition can be written as:
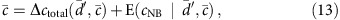 |
which can be solved for 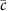 analytically and
analytically and 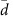 substituted from equation (2) to obtain an expression for
substituted from equation (2) to obtain an expression for 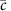 as a function of model parameters. We carried out our calculations in Mathematica 10 (Wolfram Research Inc.). As Fig. 2 shows, our approximation for the mean local clustering coefficient gives an excellent fit to simulated networks, except for low pn and very low pr.
as a function of model parameters. We carried out our calculations in Mathematica 10 (Wolfram Research Inc.). As Fig. 2 shows, our approximation for the mean local clustering coefficient gives an excellent fit to simulated networks, except for low pn and very low pr.
Expected degree distribution
Finally, we characterize the expected degree distribution in our networks using a mean-field model. We denote the degree distribution by φd for 0≤d≤N−1. In other words, φd is the probability that a randomly selected individual in the population has degree d.
Consider a focal individual that has degree d at time period t. In period t+1, the probability that this individual increases its degree by 1, 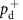 , is:
, is:
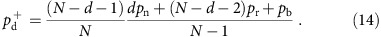 |
The first fraction in equation (14) is the probability that the individual selected to die is not connected to the focal individual, while the second fraction is the expected probability that the newborn individual born to one of the remaining N−1 individuals becomes connected to the focal individual.
The probability of a focal individual's degree d (>0) going down by 1, 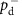 , is likewise given by
, is likewise given by
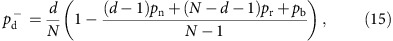 |
which is simply the probability that the individual selected to die is connected to the focal individual, multiplied by the probability that the newborn individual does not connect to the focal individual.
Next, we need the probability that a newborn is born with d connections, denoted by bd. To compute this probability, we assume pb=1 (the extension to pb<1 is trivial), so that the newborn always connects to its parent, then bd(φ) is given by (for d≥1; b0=0 in that case):
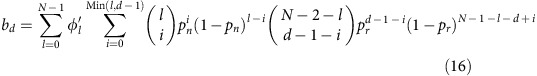 |
where the inner sum is the probability that an offspring of a parent of degree l is born with degree d, and the outer sum takes the expectation over 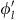 , the expected degree distribution after the death of a random individual, which for 0≤l≤N−1 is given by:
, the expected degree distribution after the death of a random individual, which for 0≤l≤N−1 is given by:
 |
reflecting the facts that the death of a random individual does not change the expected frequency of individuals that had degree d before the death, but with each death, an individual with degree d has a probability d/N of becoming degree d−1.
Putting everything together, we can write the rate equation for the mean-field dynamics of the degree distribution28:
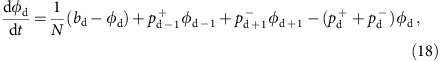 |
where the first term is the rate of change in the frequency of degree d caused by the replacement of individuals of degree d by death and birth, and the rest of the terms give rates of degree changes due to losing and gaining connections.
Setting equation (18) equal to 0 for all d and solving the resulting N equations, we can obtain the stationary degree distribution. We were unable to obtain closed-form solutions to the stationary distribution, but numerical solutions display excellent agreement with simulation results (see Fig. 3). It is worth noting that although the 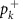 and
and 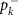 terms are similar to models of preferential attachment with constant network size, for example ref. 28, these models assume that each new addition to the network has exactly the same degree, whereas in our model the number of links of a newborn is distributed according to equation (16). Furthermore, the degree distribution does not capture the clustering behaviour of preferential attachment models, which generate much less clustering than our model for a similar mean degree (results not shown), consistent with results in growing networks27.
terms are similar to models of preferential attachment with constant network size, for example ref. 28, these models assume that each new addition to the network has exactly the same degree, whereas in our model the number of links of a newborn is distributed according to equation (16). Furthermore, the degree distribution does not capture the clustering behaviour of preferential attachment models, which generate much less clustering than our model for a similar mean degree (results not shown), consistent with results in growing networks27.
Simulation process
We initialized networks as random graphs, and ran them long enough to converge to steady state, which we evaluated by the mean degree distribution of ensembles matching the expected degree distribution, mean degree and clustering values derived analytically. The time to convergence to steady state depends on the network size, pn and pr: we found as a rule of thumb that 10 times the network size (that is, 10 complete population turnovers on average) is enough for networks to come to stationarity, hence our choosing of 2,000 steps for network size of 100. The only exception is with pn close to 1 (and to a lesser extent, pr very close to 0), where we find that convergence can take significantly longer. Code for running the simulations is provided in the Supplementary Data 1.
Fitting the model using partial least squares regression
To obtain estimates of parameter values pn and pr from observed networks, we used two methods: (i) a computational approach using dimensionality reduction on the degree and local clustering distributions of simulated networks, and (ii) an analytical approach using approximations of the mean degree and local clustering coefficients. In this subsection, we describe the dimensionality reduction approach. For each empirically observed network, we ran the model with 10,000 random values of pn and pr between 0 and 1, and the network size was set to match the observed network. We then used PLS regression, using the R package pls (version 2.4-3), to obtain a regression of the network degree and clustering coefficient distributions on pn and pr. Based on the regression formula, we predicted the values of pn and pr. The values predicted by the regression were sufficient to simulate networks that were close in their degree and clustering coefficient distributions to the observed networks. The values given in Table 1 are the result of the PLS fit. They are meant to demonstrate the ability of the model to generate realistic looking networks.
Figure 3 shows that an objective procedure using PLS regression can statistically identify values of pn and pr that will generate networks similar to the observed networks.
To validate PLS regression approach, we simulated networks using known parameter values and tested the predictions of PLS regression. Specifically, we simulated 10,000 networks from our basic model over 2,000 time steps, using random pn and pr values. We then used PLS regression to fit the degrees and clustering coefficients to parameter values. We then simulated sets of 100 networks each using a given set of parameter values (pn=0.6–0.9, pr=0.014) and checked whether the PLS regression fit could predict those values. For example, in Supplementary Fig. 4 we plot the distribution of predicted pn and pr values compared with the real values used to simulate the networks. Supplementary Figure 5 shows the distribution of predictions for 10 different values of pn, whereas pr was fixed at 0.014.
Fitting models using analytical approximations
We also use equation (2) and (13) to estimate the parameters pn and pr (assuming pb=1) from the mean degree and clustering coefficient of a given network. In simulated networks, this method works well to estimate parameters (Supplementary Fig. 6) except for high pn and moderately high pr values, where it tends to underestimate especially the pr values, and for low pn and very low pr, where it overestimates pr. Three of the four real-life networks we apply our model to fall comfortably in the region where the method yields reasonable accurate estimates (with pr values of the order of 0.01), with only the sleepy lizard network seemingly in a region where our estimate of pr somewhat inflated. Table 1 gives the values calculated for the four species, which produce networks that are similar to observed ones (Supplementary Fig. 7) for hyenas, hyraxes and dolphins, but somewhat underpredicts clustering coefficients for the sleepy lizard network relative to the PLS method. The difference between the estimates for pr obtained from PLS and analytical approximation is consistent with the bias in the analytical estimators in simulated networks for low pn and pr.
To validate our estimation of model parameters using the analytical approximation, we generate 1,000 pairs of pn and pr values randomly drawn from a uniform distribution (on [0,0.95] for pn, [0,0.2] for pr). For each pair, we simulate a network (with N=100) for 2,000 time steps, and use equation (2) and (13) to estimate pn and pr values from the final network at the end of the simulation. Supplementary Figure 6 plots the parameters estimated using the analytical method against the inputed ones.
Data
We compared the output of our model with observed animal social networks of four different species. For this analysis, we used data from published studies of spotted hyena (C. crocuta13), rock hyrax (P. capensis40), bottlenose dolphin (Tursiops spp.41) and sleepy lizard (T. rugosa42).
The hyena social network was obtained from one of the binary networks analysed by Ilany et al.13, where details on social network construction can be found. Briefly, the network is derived from association indexes based on social proximity in a spotted hyena clan in Maasai Mara Natural Reserve, Kenya, over 1 full year (1997). The binary network was created using a threshold retaining only the upper quartile of the association index values. Similarly, the hyrax network was described by Ilany et al.40, and is based on affiliative interactions in a rock hyrax population in the Ein Gedi Nature Reserve, Israel, during a 5-month field season (2009). The same upper quartile threshold on the association indices was used to generate a binary network. The dolphin network was published in ref. 41, and is based on spatial proximity of bottlenose dolphins observed over 12 months in Doubtful Sound, Fiordland, New Zealand. ‘Preferred companionships' in the dolphin network represent associations that were more likely than by chance, after comparing the observed association index to that in 20,000 permutations. The lizard social network was published by Bull et al.42, and is also based on spatial proximity, measured using GPS collars. To get a binary network, we filtered this network to retain only social bonds with association index above the 75% quartile.
The effect of varying network size
Population size might influence social structure in unknown ways. To test how changes in population size affect the resulting network, we simulated networks that grow or shrink in size. We then compared measures of the networks with those of stable networks, where the network size was kept constant. In a shrinking network model, we started the simulation with 200 individuals and ran it for the first 1,000 time steps as a constant size network (1 born and 1 dead at each time step). After 1,000 steps, we set the probability of each individual to die at any time step at 0.05, corresponding to an expected mortality of 10 individuals per time step initially. We kept the number of individuals born at each time step at 1. We kept running the simulation until population size fell to 100 individuals, and compared network characteristics to a parallel simulation where the population size started out with N=100 and held constant throughout. Similarly, in a growth model we started with 100 individuals for the first 1,000 steps, and then changed the probability of each individual to die at a given time step to 0.001 (instead of 0.01 in a stable network size). We stopped the simulation when the network size increased to 200. Again, we compared these networks with networks that started out with N=200 were kept constant throughout. We present results for a series of 15 parameter sets, where pn varied between 0.5 and 0.9 (5 values) and pr was one of 0.01, 0.05 and 0.1. For each parameter set, we ran 100 replicate pairs of shrinking (or growing) and constant size networks. Supplementary Figures 1–3 compare the network measures of stable with shrinking and growing networks, for the tested parameter sets.
The effect of shrinking the network size was not consistent for all parameter sets. Nevertheless, shrinking networks tended to be denser in ties and less modular than networks of constant size for low pr. In a similar manner, the effect of growing network size was not consistent for all parameter sets.
We conclude that the effect of changes in population size on network structure is unpredictable, and depends on the bonding probabilities. Future work should explore many interesting questions about the interaction of population size and social structure.
Two-sex models
In the main text, we presented the simplest model, in which the population was asexual. The basic model allows a newborn to choose any present individual as a role model to copy social associations. Here we show a version of the basic model for a sexual population. At birth, newborns are uniformly assigned a sex, and only females reproduce. Newborns copy only their mother's associations. Thus males may form social associations when they are born, and also if a newborn connects to them, but they are not being copied by any newborn in terms of social associations.
Fitting the two-sex model to data shows similar results to the basic model (Supplementary Table 1). This suggests that sexual reproduction is theoretically not a major determinant of social structure. Note that this does not mean that males and females play similar social roles in a population, but rather that if newborns tend to copy only one sex the resulting social structure is not very different.
We then tested two more models with sexual populations, in which the newborn may copy both parents with probability pn. In the first of these models, a newborn would copy any randomly chosen male and female as parents. In the second model, a newborn can be born only to connected pairs. Thus, in each iteration a pair of connected male–female was chosen as parents. Both these models generated networks that were not clustered, and could not be fit to observed data. This suggests that in natural populations individuals follow one role model, leading to the observed high levels of clustering. Theoretically, it is easy to see that if an individual follows multiple role models that is more similar to random connectivity, deviating from the structured observed networks of natural populations.
An alternative assortativity model
We constructed an alternative model of social network dynamics, focused on preference to form social bonds with other individuals with similar traits. The purpose of this model is to test the notion that explicit assortativity is the main factor determining network structure, as suggested empirically in various species. In this alternative model, newborns still bond their mother with probability pb, but then form bonds with all others with probability proportional to the similarity of an arbitrary trait value. The trait is inherited from the mother in the same manner as in the main model. Specifically, the probability of a newborn to connect with any other individual was defined as 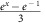 , where x is the absolute difference in trait values of the newborn and a candidate individual. This term ensures the connection probability to be in a realistic range, resulting in networks with similar density to the mean density of the four observed networks (0.123, see main text).
, where x is the absolute difference in trait values of the newborn and a candidate individual. This term ensures the connection probability to be in a realistic range, resulting in networks with similar density to the mean density of the four observed networks (0.123, see main text).
Unsurprisingly, simulations of the explicit assortativity model (2,000 time steps, 100 individuals, 500 replications) resulted in networks with high assortativity (Supplementary Fig. 9). However, the resulting networks failed to reconstruct other important topological features of the observed networks, namely the global clustering coefficient and modularity (Supplementary Fig. 10). The only exception was the spotted hyena, where modularity values, but not global clustering coefficient, matched levels of the explicit assortativity model.
To conclude, a model of social structure where individuals base their social bonding almost exclusively on assortativity fails to reconstruct the topological features of observed networks in the tested species.
A generalized association preference model
A potential alternative interpretation of social inheritance is that it might arise as an epiphenomenon from genetically inherited association preferences (that may or may not be assortative): if individuals inherit their preferences for associating with certain types of individuals from their parents, they would be expected to be associated with their parents' connections more than unconnected individuals.
In this section, we address this possibility by constructing a model to explore whether a more generalized model of co-inherited association preferences and traits might mimic the process of social inheritance. To generalize the assortative preferences model, we now assume each individual carries two traits, one describing a real-valued attribute (as in the assortment model above; we call this the ‘display trait'), and the other the preference for that trait (the preference trait'). For example, if a focal individual has display and preference trait values (0.1,0.5), it is being preferred most by others with preference trait 0.1 but the focal individual prefers to associate with those having trait value 0.5. We assume both trait values are on a circle and normalize them to be between 0 and 1. We let both traits to be inherited from the parent when an individual is born, with (independent) deviations in each trait from parental values distributed according to N(0,σ). When an offspring j is born, it makes a connection to each existing individual i in the population with probability 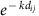 , where k is a positive constant and dij is the shortest distance on the circle between the offspring j's preference trait and the individual i's display trait. Individuals are selected to die and give birth at random as in the basic model.
, where k is a positive constant and dij is the shortest distance on the circle between the offspring j's preference trait and the individual i's display trait. Individuals are selected to die and give birth at random as in the basic model.
Supplementary Figure 11 illustrates the results from this alternative model. It shows that although model parameters exist that generate realistic looking degree distributions, these generate networks that are far less clustered than the real-life networks. The reason is that when individuals connect to others purely based on their inherited display and preference traits, they tend to connect to both partners of their parents as well as others with similar traits that are not connected to their parents. The latter connections do not close triads, and hence the resulting network is much less clustered. Thus, purely genetic inheritance of association preferences (independent of parental connections) is insufficient to generate the process of social inheritance as a byproduct.
Network measures
To study the networks produced by our model and compare them with observed networks, we used a number of commonly used network measures. Network density is defined as 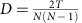 where T is the number of ties (edges) and N the number of nodes. The global clustering coefficient is based on triplets of nodes. A triplet includes three nodes that are connected by either two (open triplet) or three (closed triplet) undirected ties. Measuring the clustering in the whole network, the global clustering coefficient is defined as
where T is the number of ties (edges) and N the number of nodes. The global clustering coefficient is based on triplets of nodes. A triplet includes three nodes that are connected by either two (open triplet) or three (closed triplet) undirected ties. Measuring the clustering in the whole network, the global clustering coefficient is defined as
 |
The local clustering coefficient measures the clustering of each node:
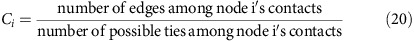 |
The betweenness centrality of a node v is given by
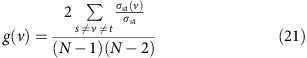 |
where σst is the total number of shortest paths from node s to node t and σst(v) is the number of those paths that pass through v.
We detected network modules (also known as communities or groups) using the walktrap community detection method64. We used the maximal network modularity across all partitions for a given network. The modularity measures the strength of a division of the network into modules. The modularity of a given partition to c modules in an undirected network is
 |
where eii is the fraction of edges connecting nodes inside module i, and 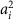 is the fraction of edges with at least one edge in module i.
is the fraction of edges with at least one edge in module i.
Finally, we used the assortativity coefficient to measure how likely are individuals to be connected to those with a similar trait value65. For an undirected network, this coefficient is given by:
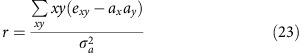 |
where exy is the fraction of all edges in the network that connect nodes with traits x and y, ax is defined as 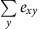 and
and 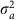 is the variance of the distribution ax.
is the variance of the distribution ax.
Modularity of model networks
Social networks feature higher modularity than random networks. That is, social networks can usually be partitioned into subgroups of individuals (communities in network jargon), more densely connected within than between those subgroups. To test another aspect of our model, we calculated the modularity of simulated networks after identifying the community (subgroup) structure. Modularity measures the strength of division into communities, where high modularity indicates dense connection between individuals within communities and sparse connections between individuals across communities. We used the Walktrap community finding algorithm, based on the idea that short random walks on a network tend to stay in the same community64. In all four tested networks (see main text), the modularity of the observed network was not an outlier in the distribution of modularity values of simulated networks. Thus, we could not reject the null hypothesis that the observed network belongs to the family of simulated networks, when considering their modularity (Supplementary Fig. 12).
Data availability
The network data for bottlenose dolphin network41 is available publicly at http://konect.uni-koblenz.de/networks/. The data for the sleepy lizard network42 is available publicly at http://datadryad.org/resource/doi:10.5061/dryad.jk87h. The data for the spotted hyena13 and rock hyrax networks40 are available from the authors upon request.
Additional information
How to cite this article: Ilany, A. & Akçay, E. Social inheritance can explain the structure of animal social networks. Nat. Commun. 7:12084 doi: 10.1038/ncomms12084 (2016).
Supplementary Material
Supplementary Figures 1-14 and Supplementary Table 1
Code for simulating the basic social inheritance model
Acknowledgments
We are thankful to Kay E. Holekamp and Eli Geffen for sharing data, and to Robert Seyfarth, Elliot Aguilar, Cağlar Akcay, Jeremy Van Cleve, Slimane Dridi and Tim Linksvayer for valuable comments. This study was supported by the University of Pennsylvania and NSF Grant EF-1137894.
Footnotes
Author contributions AI and EA designed the study, performed the analysis and wrote the manuscript.
References
- Silk J. B., Alberts S. C. & Altmann J. Social bonds of female baboons enhance infant survival. Science 302, 1231–1234 (2003). [DOI] [PubMed] [Google Scholar]
- Silk J. B. et al. Strong and consistent social bonds enhance the longevity of female baboons. Curr. Biol. 20, 1359–1361 (2010). [DOI] [PubMed] [Google Scholar]
- Barocas A., Ilany A., Koren L., Kam M. & Geffen E. Variance in centrality within rock hyrax social networks predicts adult longevity. PLoS ONE 6, e22375 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamede R. K., Bashford J., McCallum H. & Jones M. Contact networks in a wild Tasmanian devil (Sarcophilus harrisii) population: using social network analysis to reveal seasonal variability in social behaviour and its implications for transmission of devil facial tumour disease. Ecol. Lett. 12, 1147–1157 (2009). [DOI] [PubMed] [Google Scholar]
- Mossong J. et al. Social contacts and mixing patterns relevant to the spread of infectious diseases. PLoS Med. 5, e74 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtsuki H., Hauert C., Lieberman E. & Nowak M. A. A simple rule for the evolution of cooperation on graphs and social networks. Nature 441, 502–505 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos F. C., Santos M. D. & Pacheco J. M. Social diversity promotes the emergence of cooperation in public goods games. Nature 454, 213–216 (2008). [DOI] [PubMed] [Google Scholar]
- Rand D. G., Nowak M. A., Fowler J. H. & Christakis N. A. Static network structure can stabilize human cooperation. Proc. Natl Acad. Sci. USA 111, 17093–17098 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinde R. A. Interactions, relationships and social structure. Man 11, 1–17 (1976). [Google Scholar]
- Wey T., Blumstein D. T., Shen W. & Jordãn F. Social network analysis of animal behaviour: a promising tool for the study of sociality. Anim. Behav. 75, 333–344 (2008). [Google Scholar]
- Croft D. P., Madden J. R., Franks D. W. & James R. Hypothesis testing in animal social networks. Trends Ecol. Evol. 26, 502–507 (2011). [DOI] [PubMed] [Google Scholar]
- Shizuka D. et al. Across-year social stability shapes network structure in wintering migrant sparrows. Ecol. Lett. 17, 998–1007 (2014). [DOI] [PubMed] [Google Scholar]
- Ilany A., Booms A. S. & Holekamp K. E. Topological effects of network structure on long-term social network dynamics in a wild mammal. Ecol. Lett. 18, 687–695 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehead H. Analyzing animal societies: quantitative methods for vertebrate social analysis Univ. of Chicago Press (2008). [Google Scholar]
- Tung J. et al. Social networks predict gut microbiome composition in wild baboons. eLife 4, e05224 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause J., Croft D. & James R. Social network theory in the behavioural sciences: potential applications. Behav. Ecol. Sociobiol. 62, 15–27 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fewell J. H. Social insect networks. Science 301, 1867–1870 (2003). [DOI] [PubMed] [Google Scholar]
- Lewis K., Gonzalez M. & Kaufman J. Social selection and peer influence in an online social network. Proc. Natl Acad. Sci. USA 109, 68–72 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aplin L. et al. Individual personalities predict social behaviour in wild networks of great tits (parus major). Ecol. Lett. 16, 1365–1372 (2013). [DOI] [PubMed] [Google Scholar]
- Oh K. P. & Badyaev A. V. Structure of social networks in a passerine bird: consequences for sexual selection and the evolution of mating strategies. Am. Nat. 176, E80–E89 (2010). [DOI] [PubMed] [Google Scholar]
- Aplin L. M. et al. Experimentally induced innovations lead to persistent culture via conformity in wild birds. Nature 518, 538–541 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen J., Weinrich M., Hoppitt W. & Rendell L. Network-based diffusion analysis reveals cultural transmission of lobtail feeding in humpback whales. Science 340, 485–488 (2013). [DOI] [PubMed] [Google Scholar]
- Krause J. et al. Reality mining of animal social systems. Trends Ecol. Evol. 28, 541–551 (2013). [DOI] [PubMed] [Google Scholar]
- Seyfarth R. M. A model of social grooming among adult female monkeys. J. Theor. Biol. 65, 671–698 (1977). [DOI] [PubMed] [Google Scholar]
- Sterck E. H. M., Watts D. P. & Schaik C. P. v. The evolution of female social relationships in nonhuman primates. Behav. Ecol. Sociobiol. 41, 291–309 (1997). [Google Scholar]
- Skyrms B. & Pemantle R. A dynamic model of social network formation. Proc. Natl Acad. Sci. USA 97, 9340–9346 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson M. O. & Rogers B. W. Meeting strangers and friends of friends: how random are social networks? Am. Econ. Rev. 97, 890–915 (2007). [Google Scholar]
- Moore C., Ghoshal G. & Newman M. E. J. Exact solutions for models of evolving networks with addition and deletion of nodes. Phys. Rev. E 74, 036121 (2006). [DOI] [PubMed] [Google Scholar]
- Ghoshal G., Chi L. & Barabãsi A.-L. Uncovering the role of elementary processes in network evolution. Sci. Rep. 3, 2920 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman M. E. J. & Park J. Why social networks are different from other types of networks. Phys. Rev. E 68, 036122 (2003). [DOI] [PubMed] [Google Scholar]
- Ravasz E., Somera A. L., Mongru D. A., Oltvai Z. N. & Barabãsi A.-L. Hierarchical organization of modularity in metabolic networks. Science 297, 1551–1555 (2002). [DOI] [PubMed] [Google Scholar]
- Williams R. J. & Martinez N. D. Simple rules yield complex food webs. Nature 404, 180–183 (2000). [DOI] [PubMed] [Google Scholar]
- Brown E. D. & Farabaugh S. M. in Social Influences on Vocal Development 98–127Cambridge Univ. Press (1997). [Google Scholar]
- Ward A. J. & Hart P. J. The effects of kin and familiarity on interactions between fish. Fish Fish. 4, 348–358 (2003). [Google Scholar]
- Whitehouse M. E. & Lubin Y. The functions of societies and the evolution of group living: spider societies as a test case. Biol. Rev. 80, 347–361 (2005). [DOI] [PubMed] [Google Scholar]
- Goldenberg S. Z., Douglas-Hamilton I. & Wittemyer G. Vertical transmission of social roles drives resilience to poaching in elephant networks. Curr. Biol. 26, 75–79 (2016). [DOI] [PubMed] [Google Scholar]
- Pastor-Satorras R., Smith E. & Solã R. V. Evolving protein interaction networks through gene duplication. J. Theor. Biol. 222, 199–210 (2003). [DOI] [PubMed] [Google Scholar]
- Kumar R. et al. in Proceedings of the 41st Annual Symposium on Foundations of Computer Science, 57-65 (IEEE Computer Society Washington, DC, USA (2000).
- Lusseau D. The emergent properties of a dolphin social network. Proc. R. Soc. Lond. B 270, S186–S188 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilany A., Barocas A., Koren L., Kam M. & Geffen E. Structural balance in the social networks of a wild mammal. Anim. Behav. 85, 1397–1405 (2013). [Google Scholar]
- Lusseau D. et al. The bottlenose dolphin community of doubtful sound features a large proportion of long-lasting associations. Behav. Ecol. Sociobiol. 54, 396–405 (2003). [Google Scholar]
- Bull C., Godfrey S. & Gordon D. Social networks and the spread of salmonella in a sleepy lizard population. Mol. Ecol. 21, 4386–4392 (2012). [DOI] [PubMed] [Google Scholar]
- Newman M. E. J., Watts D. J. & Strogatz S. H. Random graph models of social networks. Proc. Natl Acad. Sci. USA 99, 2566–2572 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vãzquez A. Growing network with local rules: preferential attachment, clustering hierarchy, and degree correlations. Phys. Rev. E 67, 056104 (2003). [DOI] [PubMed] [Google Scholar]
- Cavaliere M., Sedwards S., Tarnita C. E. & Nowak M. A. Prosperity is associated with instability in dynamical networks. J. Theor. Biol. 299, 126–138 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kummer H. Primate Societies: Group Techniques of Ecological Adaptation Aldine-Atherton (1971). [Google Scholar]
- Kinnaird M. F. Pregnancy, gestation and parturition in free-ranging tana river crested mangabeys (cercocebus galeritus galeritus). Am. J. Primatol. 22, 285–289 (1990). [DOI] [PubMed] [Google Scholar]
- Tsai Y.-J. J. & Mann J. Dispersal, philopatry, and the role of fission-fusion dynamics in bottlenose dolphins. Mar. Mam. Sci. 29, 261–279 (2013). [Google Scholar]
- Servedio M. R. et al. Not just a theory the utility of mathematical models in evolutionary biology. PLoS Biol. 12, e1002017 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheney D. L. & Seyfarth R. M. Nonrandom dispersal in free-ranging vervet monkeys: social and genetic consequences. Am. Nat. 122, 392–412 (1983). [Google Scholar]
- Fowler J. H., Dawes C. T. & Christakis N. A. Model of genetic variation in human social networks. Proc. Natl Acad. Sci. USA 106, 1720–1724 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brent L. J. N. et al. Genetic origins of social networks in rhesus macaques. Sci. Rep. 3, 1042 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lea A. J., Blumstein D. T., Wey T. W. & Martin J. G. Heritable victimization and the benefits of agonistic relationships. Proc. Natl Acad. Sci. USA 107, 21587–21592 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruuk L. & Hadfield J. How to separate genetic and environmental causes of similarity between relatives. J. Evol. Biol. 20, 1890–1903 (2007). [DOI] [PubMed] [Google Scholar]
- Croft D. P. et al. Assortative interactions and social networks in fish. Oecologia 143, 211–219 (2005). [DOI] [PubMed] [Google Scholar]
- Lusseau D. & Newman M. E. J. Identifying the role that animals play in their social networks. Proc. R. Soc. Lond. B 271, S477–S481 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf J. B. W., Mawdsley D., Trillmich F. & James R. Social structure in a colonial mammal: unravelling hidden structural layers and their foundations by network analysis. Anim. Behav. 74, 1293–1302 (2007). [Google Scholar]
- Xie Y., Cheng S. & Zhou X. Assortative mating without assortative preference. Proc. Natl Acad. Sci. USA 112, 5974–5978 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farine D. R. Measuring phenotypic assortment in animal social networks: weighted associations are more robust than binary edges. Anim. Behav. 89, 141–153 (2014). [Google Scholar]
- Marjoram P., Molitor J., Plagnol V. & Tavare S. Markov chain monte carlo without likelihoods. Proc. Natl Acad. Sci. USA 100, 15324–15328 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegmann D., Leuenberger C. & Excoffier L. Efficient approximate bayesian computation coupled with markov chain monte carlo without likelihood. Genetics 182, 1207–1218 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kivelã M. et al. Multilayer networks. J. Comp. Netw. 2, 203–271 (2014). [Google Scholar]
- Pinter-Wollman N. et al. The dynamics of animal social networks: analytical, conceptual, and theoretical advances. Behav. Ecol. 25, 242–255 (2014). [Google Scholar]
- Pons P. & Latapy M. in Computer and Information Sciences-ISCIS 2005 284–293Springer (2005). [Google Scholar]
- Newman M. E. Mixing patterns in networks. Phys. Rev. E 67, 026126 (2003). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figures 1-14 and Supplementary Table 1
Code for simulating the basic social inheritance model
Data Availability Statement
The network data for bottlenose dolphin network41 is available publicly at http://konect.uni-koblenz.de/networks/. The data for the sleepy lizard network42 is available publicly at http://datadryad.org/resource/doi:10.5061/dryad.jk87h. The data for the spotted hyena13 and rock hyrax networks40 are available from the authors upon request.