Abstract
Background:
Immunoglobulin G4-related disease (IgG4-RD) is a newly recognized systemic disease that can involve multiple organs and various clinical phenotypes. The purpose of this study was to analyze different types of organ involvement in IgG4-RD patients in China.
Methods:
We conducted a prospective cohort study on IgG4-RD patients to analyze the clinical manifestations and rare features of IgG4-RD. Patients were grouped into different types according to organ involvement regarding organ number and organ site. The constituent ratio in different types was also analyzed.
Results:
A total of 200 IgG4-RD patients, with a male:female ratio of 2.08:1, were grouped into different types. Cases having involvement of two or three organs were the most common whereas the fewest number of patients had multi-organ (≥4) involvement. Serum IgG4 and IgE levels, IgG4/IgG ratio, and percentage of eosinophils increased as the number of involved organs increased. In addition, constituent ratio analysis revealed that patients with salivary gland/lacrimal gland swelling, who also constituted the largest number of IgG4-RD patients, had higher serum IgG4 concentrations and IgG4/IgG values, had higher percentage of Eos, and were more likely to have had a history of allergies relative to patients with internal organ involvement.
Conclusions:
The characteristic feature of IgG4-RD is multiple organ involvement with various clinical manifestations and different types. Although serum IgG4 levels increased with the number of involved organs, serum IgG4 levels were higher for those patients with salivary gland/lacrimal gland swelling compared with those with internal organ involvement. Thus, valuable clues to the differential diagnosis of IgG4-RD could be obtained by examining the clinical patterns of organ involvement.
Keywords: Immunoglobulin G4, Immunoglobulin G4-related Disease, Organ Involvement, Prospective Cohort
INTRODUCTION
Immunoglobulin G4-related disease (IgG4-RD) is a newly recognized immune-mediated fibro-inflammatory condition that is characterized by enlargement of tissues or organs, abundant IgG4+ plasma cell infiltration in damaged organs, and elevated serum IgG4 levels.[1,2] IgG4-RD was first characterized in 2001 as sclerosing pancreatitis, and then was referred to as type I IgG4-related autoimmune pancreatitis.[3] However, within just a few years a variety of extrapancreatic organ involvement linked by unique histopathological features led to the recognition of IgG4-RD as a systemic disease.[4] The comprehensive diagnostic criteria of IgG4-RD was first determined in a 2012 study by Umehara et al., which normalized the diagnosis of IgG4-RD internationally.[5] Due to its relative novelty, the prevalence of IgG4-RD remains unclear, as does the exact mechanism of pathogenesis. We previously reported that IgG4-RD patients showed disturbed B-cell subsets and dysfunction of regulatory B-cells,[6] whereas Wallace et al. described elevated levels of circulating IgG4+ plasmablasts, and regarded the levels of such cells as a diagnostic biomarker and reliable indicator of disease activity in IgG4-RD patients.[7] These findings indicated that B-cell subsets may play a crucial role in IgG4-RD. The clinical features of IgG4-RD manifest as single or multiple organ swellings or masses that occur in various sites, including lacrimal glands, salivary glands, pancreas, bile ducts, retroperitoneal tissues, lung, kidney, prostate, pituitary gland, thyroid, and uterus.[2,8,9] To date, most studies focused on the clinical and laboratory features of IgG4-RD patients, including one of our prospective cohort studies that concerned the clinical characteristics of IgG4-RD in 118 Chinese patients, which contributed to a more comprehensive understanding of this disease.[10,11,12,13] However, few studies have focused on the correlation between organ involvement and serum IgG4 level.[14] The 2015 international consensus guidance statement on the management and treatment of IgG4-RD highlighted the necessity of a large cohort study to define the patient's clinical phenotype and clarify the natural history of IgG4-RD.[1] Based on previous studies, we conducted a detailed analysis concerning the organ involvement in 200 IgG4-RD patients in China that emphasized the types and numbers of organs involved in IgG4-RD patients.
METHODS
Cohort overview
A multidisciplinary collaborative prospective cohort study of IgG4-RD patients was conducted at the Peking Union Medical College Hospital (PUMCH, Beijing, China) from January 2011 to January 2015. Newly diagnosed IgG4-RD patients who fulfilled the 2011 comprehensive diagnostic criteria for definite, probable, or possible IgG4-RD[5] were consecutively enrolled. Patients with malignancies or other autoimmune diseases were excluded. Detailed clinical and laboratory data were recorded, including demographic data, initial symptoms, disease duration, history of allergies, physical examination, laboratory assessments, imaging studies, and pathological biopsy. This study was approved by the Medical Ethics Committee of PUMCH and all patients provided written informed consent.
Laboratory assessments, imaging studies, and histological examination
All the patients were tested for complete blood count, liver and renal function tests, erythrocyte sedimentation rate (ESR), hyper-sensitivity C-reactive protein (CRP), serum IgG/IgA/IgM levels, total IgE level, serum IgG subclasses, complement, and autoantibodies, including rheumatoid factor (RF), and antinuclear antibodies (ANAs). Serum IgG subclasses were measured by immunonephelometry using Siemens N IgG1-2 and IgG3-4 (formerly Dade Behring, Marburg, Germany) on a Siemens BNTMII specific protein analyzer.
All the patients underwent imaging examinations, including ultrasonography, computed tomography (CT), or magnetic resonance imaging, and some patients underwent 18F-fluorodeoxyglucose positron emission tomography/CT.
Tissue biopsies were performed on 115 patients, and samples were analyzed using previously described pathology methods.[10,15]
Assessment of organ involvement
Organ involvement was assessed by reviewing the patient's symptoms, past medical history, physical examination findings, laboratory studies, imaging examinations, and/or tissue biopsies.
Statistical analysis
All statistical analyses were performed using SPSS version 20.0 (SPSS Inc., Chicago, IL, USA). Continuous, normally distributed data were shown as mean ± standard deviation (SD) and analyzed by Student's t-test; all continuous nonnormally distributed data were shown as median (Q1, Q3) and evaluated using nonparametric tests. Categorical variables were assessed by Chi-square or Fisher's exact tests. Correlations between variables were assessed using Pearson's rank test (normally distributed) or Spearman's rank correlation test (nonnormally distributed). The value of P < 0.05 was considered statistically significant.
RESULTS
Patient demographics and clinical manifestations
The male to female ratio of the patient cohort was 2.08:1, and the average age at diagnosis was 52.5 ± 14.0 years (range: 16–80 years). The majority (91%) of patients were northern Chinese. The mean disease duration was 2.18 ± 2.97 years.
According to the 2011 comprehensive diagnostic criteria for definite, probable, and possible IgG4-RD, the number of corresponding cases was 115 (57.5%), 7 (3.5%), and 78 (39.0%), respectively.
The main clinical manifestations were summarized, with the most common being submandibular gland swelling (102, 51.0%), lacrimal gland swelling (84, 42.0%), superficial lymph node enlargement (74, 37.0%), abdominal pain (70, 35.0%), parotid gland swelling (48, 24.0%), or nasal congestion (43, 21.5%). Less common manifestations included jaundice (28, 14.0%), pruritus (27, 13.5%), cough (27, 13.5%), low back pain (27, 13.5%), dysuresia (26, 13.0%), dry mouth and/or dry eye (25, 12.5%), or nausea and/or vomiting (24, 12.0%). A minority of patients experienced fever (18, 9.0%), edema (17, 8.5%), exophthalmos (11, 5.5%), arthralgia (8, 4.0%), or thyroid enlargement (5, 2.5%). Very few patients experienced chest pain, hoarseness, limb numbness, visual changes, subcutaneous nodules, headache, or hearing loss as initial symptoms, whereas two patients were asymptomatic until a regular medical check-up. Notably, 118 (59.0%) patients in this cohort had a history of allergy.
Laboratory studies
The majority of IgG4-RD patients had normal white blood cell counts, as well as platelet and hemoglobin tests (87.5%, 88.5%, and 95.5%, respectively). ESR and CRP were elevated in 57.7% and 50.6% of patients, and RF was positive in 23.4% of patients. Only 13.5% of patients were positive for ANAs with a titer range from 1:80 to 1:320 among different immunofluorescence types. No patient was positive for anti-extractable nuclear antibodies. Hypocomplementemia (low concentration of C3 and/or C4) occurred in 31.1% of patients. In immunoglobulin tests, 67.0% of patients had an elevated IgG serum level, while most patients had normal serum IgM and IgA levels (92.4% and 94.0%, respectively). With respect to serum IgG subclasses, IgG1, IgG2, IgG3, and IgG4 were above normal levels in 19.7%, 34.8%, 23.2%, and 96.5% of patients, respectively. Only 7 (3.5%) cases had a normal IgG4 level.
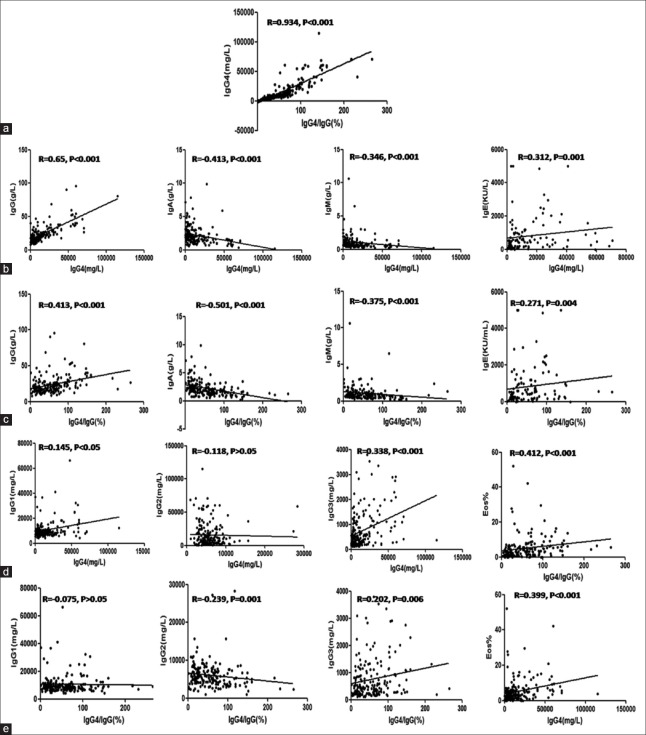
The relationship between serum IgG4 level and IgG4/IgG (%) in this cohort was remarkable (r = 0.934, P < 0.001) [Figure 1a]. Moreover, the serum IgG4 level and IgG4/IgG ratio were positively correlated with IgG values (r = 0.65, P < 0.001 and r = 0.413, P < 0.001), and were negatively correlated with IgA and IgM values (IgA: r = −0.413, P < 0.001 and r = −0.501, P < 0.001; IgM: r = −0.346, P < 0.001 and r = −0.375, P < 0.001) [Figure 1b and 1c]. IgG1 levels were positively correlated with serum IgG4 value (r = 0.145, P = 0.042) and IgG3 showed remarkable positive correlations with both serum IgG4 level and IgG4/IgG ratio (r = 0.338, P < 0.001 and r = 0.202, P < 0.01, respectively), while only IgG2 showed negative correlations with the IgG4/IgG ratio (r = −0.239, P = 0.001) [Figure 1d and 1e]. However, neither serum IgG4 level nor IgG4/IgG ratio showed statistical correlation with age at disease diagnosis, disease duration, ESR, or CRP (data not shown).
Figure 1.
Correlation analysis of IgG4-RD patient laboratory findings. Correlations between serum IgG4 level and IgG4/IgG value (a); correlations between serum levels of Ig G/A/M/E/G1/G2/G3/eosinophil percentage and serum IgG4 level or IgG4/IgG (%) (b-e). Values are shown as r, which was determined by Spearman's rank correlation test. Ig: Immunoglobulin; IgG4-RD: Immunoglobulin G4-related disease.
Notably, eosinophil (Eos) levels were elevated in 33.7% of cases, and the IgE level was increased in 83.6% of IgG4-RD patients. Both peripheral Eos% and serum IgE level showed remarkable positive correlations with serum IgG4 level and IgG4/IgG value (Eos%: r = 0.412, P < 0.001, r = 0.399, P < 0.001; IgE: r = 0.312, P = 0.001; r = 0.271, P = 0.004) [Figure 1]. Interestingly, relative to patients with or without allergic history, there was no statistical difference in serum IgE level and Eos% in the patient cohort (data not shown).
Analysis of organ involvement in immunoglobulin G4-related disease
Various sites of the body were involved in IgG4-RD patients, including: lymph nodes (113 cases, 56.5%), submandibular gland (102, 51.0%), lacrimal gland (84, 42.0%), pancreas (77, 38.5%), lung (including parenchyma, airway and pleura) (64, 32.0%), parotid gland (48,24.0%), sinus (43, 21.5%), bile ducts (38, 19.0%), retroperitoneal tissue (36, 18.0%), prostate (26, 13.0%), kidney (20,10.0%), artery (14, 7.0%), gallbladder (11, 5.5%), skin (11, 5.5%), inflammatory pseudotumor (10, 5.0%), liver (6, 3.0%), and others (including thyroid gland, mediastinum, pituitary gland, spinal cord, endocranium, spleen, ureter, bladder, and gastrointestinal tract). The mean number of organs involved was 3.0 ± 1.6 (range: 1–10).
Immunoglobulin G4-related disease patient grouping according to the number of involved organs
We divided the IgG4-RD patients into four groups according to the number of organs involved as follows (lymph nodes were not counted except for those patients who had lymphadenopathy as the main clinical manifestation, e.g., Castleman disease): only one organ involved (G1), 2 or 3 organs involved (G2), 4 or 5 organs involved (G3), and more than 5 organs involved (G4). G2 was the most common categorization in this cohort (117 cases, 58.5%), followed by G3 (43, 21.5%) and G1 (26, 13.0%). G4 was the least common (14, 7.0%).
Comparisons of clinical and laboratory findings among the different groups are shown in Figure 2a–2j. Among each group, there was no significant difference in age at disease diagnosis, disease duration, ESR, and CRP (data not shown), as well as in IgG1, IgG2, and IgG3. However, serum IgG levels were highest for the G4 group. Importantly, there were significant differences among each group in serum IgG4 level, IgG4/IgG ratio, and percentage of Eos, all of which increased concurrently with an increase in the number of involved organs. Serum IgE levels also showed the same trend except for the G4 group; this discrepancy may be due to the small number of patients in this group.
Figure 2.
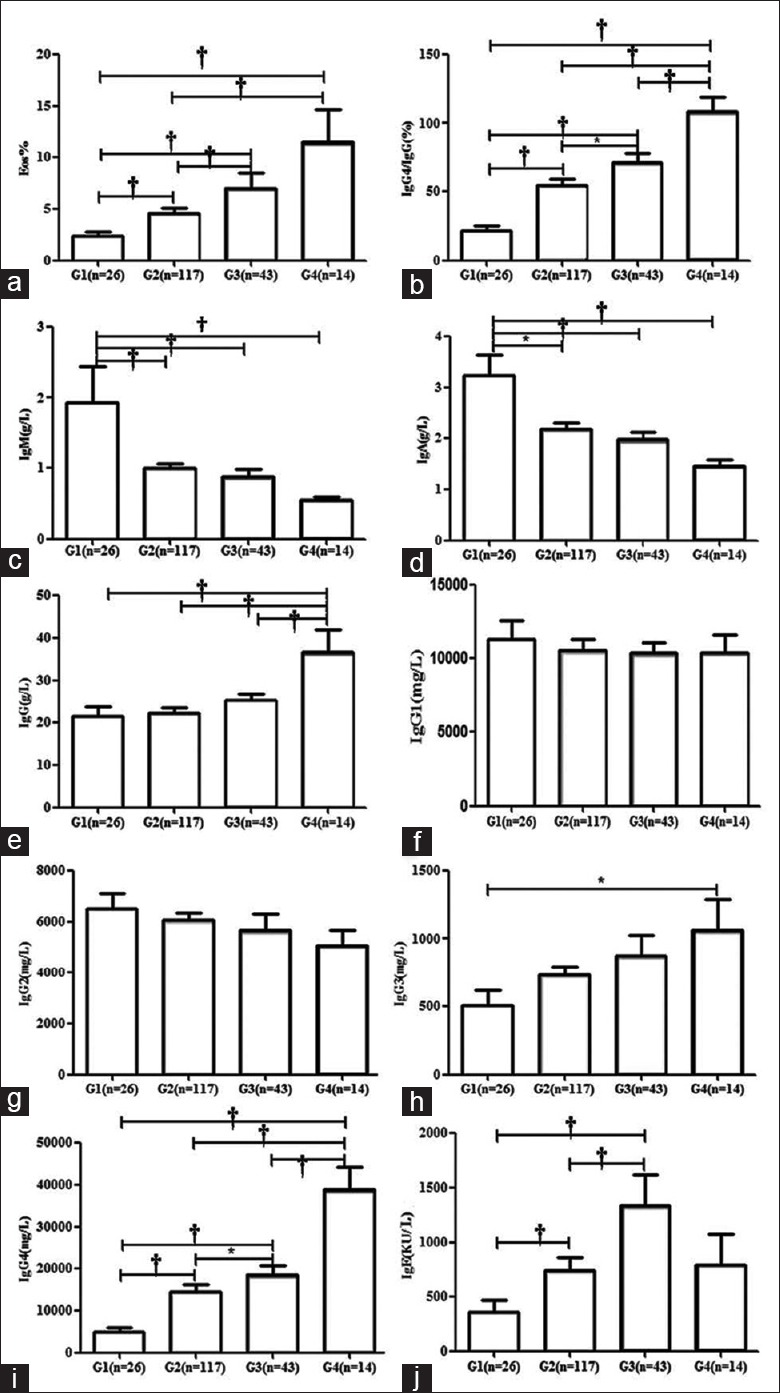
Comparisons of laboratory findings among IgG4-RD patients with different numbers of involved organs. Patients were divided into four groups according to the number of organs involved as follows: only one organ involved (G1), 2 or 3 organs involved (G2), 4 or 5 organs involved (G3), and more than 5 organs involved (G4). Comparisons of eosinophil percentage, IgG4/IgG(%), IgM, IgA, IgG, IgG1, IgG2, IgG3, IgG4, and IgE among different groups were analyzed (a-j). Parametric or nonparametric tests were performed to determine the statistical difference among different groups, *P < 0.05, and †P < 0.001. Ig: Immunoglobulin; IgG4-RD: Immunoglobulin G4-related disease.
Types of organ involvement and constituent ratio in immunoglobulin G4-related disease patients
The types of organs involved were classified according to their different anatomic sites: salivary/lacrimal glands (Mikulicz's disease [MD]), retroperitoneal/mediastinal fibrosis, pancreatic-hepatobiliary-splenic involvement, urinary system (kidney/ureter/bladder/prostate) involvement (n.b., ureterostenosis and/or hydronephrosis caused by retroperitoneal fibrosis were excluded), respiratory system (sinus/lung parenchymal/airway/pleura) involvement, regional and/or systemic lymphadenopathy, central nervous system (pituitary gland/spinal cord/endocranium/brain) involvement, and gastrointestinal (stomach/intestinal tract) involvement.
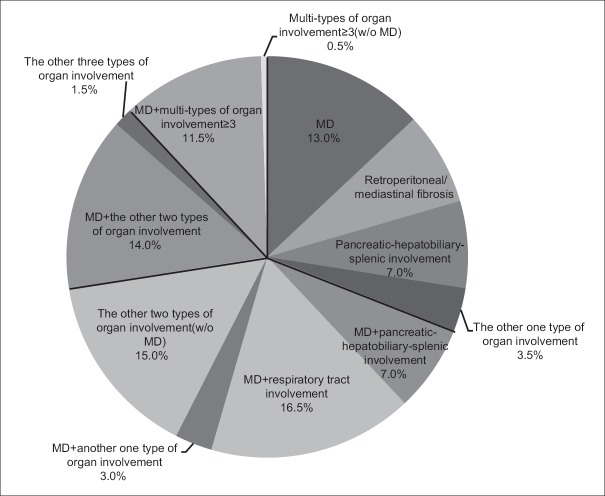
We also analyzed the discrete constituent ratio of organ involvement types described above [Figure 3]. The majority of patients with only one type of organ involved suffered from MD, and accounted for up to 13.0% of patients, followed by retroperitoneal/mediastinal fibrosis (7.5%), and pancreatic-hepatobiliary-splenic system involvement (7.0%). Meanwhile, patients who had only lung or kidney involvement were rare. Among patients with two types of involved organs, MD combined with another organ type was more frequent (26.5%), and of these, the respiratory system was most commonly involved (16.5%), followed by the pancreatic-hepatobiliary-splenic system (7.0%). Combinations of two types of involved organs without MD were less common, with each accounting for <3.0% of cases. In addition, a combination of MD with two other types of involved organs represented 14.0% of cases, of which MD combined with pancreatic-hepatobiliary-splenic and respiratory system involvement accounted for 6.5%, followed by MD combined with the pancreatic-hepatobiliary-splenic system and retroperitoneal/mediastinal fibrosis. However, patients with three types of involved organs without MD accounted for only 1.5% of cases. Moreover, the constituent ratio of patients with multi-type (≥3) organ involvement was 12.0%, and most of these patients also suffered from MD. We thus evaluated the level of confidence for IgG4-RD indication as strong, moderate, or weak according to the proportion of these types of organ involvement and our experience in diagnosing IgG4-RD, which might yield insights into IgG4-RD indications [Table 1].
Figure 3.
Constituent ratio of organ involvement in IgG4-RD patients. Organ involvements were classified according to different anatomic sites: superficial gland swelling, retroperitoneum/mediastinum involvement, hepatobiliary-pancreatic involvement, urinary system involvement, central nervous system involvement, respiratory system involvement, gastrointestinal involvement, and regional and/or systemic lymphadenopathy. The constituent ratio of different types of organ involvement in IgG4-RD patients is summarized in a pie chart. IgG4-RD: Immunoglobulin G4-related disease.
Table 1.
Confidence level for IgG4-RD indication according to type of organ involved
| Types of organ involved | Confidence for indication of IgG4-RD |
|---|---|
| Only one type of involved organ | |
| Salivary glands/lacrimal glands (MD) | Strong |
| Hepatobiliary-pancreatic-splenic | Moderate |
| Retroperitoneal/mediastinal fibrosis | Moderate |
| Regional or systemic lymphadenopathy | Weak |
| Respiratory system (sinus/lung parenchymal/airway/pleura) | Weak |
| Urinary system (kidney/ureter/bladder/prostate) | Weak |
| Two types of involved organs | |
| MD + respiratory system | Strong |
| MD + pancreatic-hepatobiliary-splenic | Strong |
| MD + one other type of organ | Moderate |
| Retroperitoneal/mediastinal fibrosis + pancreatic-hepatobiliary-splenic | Moderate |
| Two other types of organs | Weak |
| Three types of involved organs | |
| MD + pancreatic-hepatobiliary-splenic + respiratory system | Strong |
| MD + pancreatic-hepatobiliary-splenic + retroperitoneal/mediastinal fibrosis | Strong |
| MD + pancreatic-hepatobiliary-splenic + urinary | Moderate |
| MD + two other organ types involved | Moderate |
| Three other organ types involved | Weak |
| Multi-type organ involvement | |
| MD + greater than or equal to three types of organs involved | Strong |
| Other multi-type organ involvement | Moderate |
MD: Mikulicz's disease; IgG4-RD: Immunoglobulin G4-related disease.
Comparison of clinical and laboratory findings in different groups
Comparison analysis revealed obvious differences between patients with and without superficial gland involvement. Table 2 lists parameters for patients with and without superficial gland involvement in patients with one or two types of involved organs (n.b., patients with more than two types of involved organs were not included because of the small number of patients without MD). The average age at diagnosis showed no difference among the groups. When compared with patients with and without MD, patients with MD were more likely to be female, have longer disease duration, have higher percentage of Eos, and have had a history of allergy (64.6% vs. 43.9%, P < 0.001). Notably, both serum IgG4 levels and IgG4/IgG ratios were significantly higher in patients with MD, whereas IgA levels were lower. In addition, patients with salivary/lacrimal gland swelling had lower CRP levels than patients without swelling. Among serum levels of IgG, IgM, IgG1, IgG2, IgG3, and IgE showed no significant difference between patients with and without MD.
Table 2.
Demographic and laboratory findings of patients with different types of organ involvement
| Items | (A) Only one type of organ involved | (B) Two types of organs involved | A + B | |||
|---|---|---|---|---|---|---|
| MD (n = 26) | Non-MD (n = 36) | With MD (n = 53) | Without MD (n = 30) | With MD (n = 79) | Without MD (n = 66) | |
| Duration (years) | 1.31 (0.85, 4.40)* | 0.56 (0.30, 0.96)* | 2.00 (1.00, 4.00)* | 0.38 (0.08, 1.12)* | 1.65 (0.88, 4.25)* | 0.50 (0.17, 1.00)* |
| Male:female | 12:14* | 27:8* | 26:27* | 26:4* | 38:41* | 53:12* |
| Age (years) | 47.6 ± 13.1 | 50.7 ± 15.1 | 53.7 ± 13.6 | 53.3 ± 16.5 | 51.7 ± 13.6 | 51.9 ± 15.7 |
| Allergy history, n/N (%) | 14/26 (53.8) | 15/36 (41.7) | 39/53 (73.6)* | 14/30 (46.7)* | 53/79 (67.1)* | 29/66 (43.9)* |
| Eosinophil (%) | 4.36 (1.93, 6.23) | 2.30 (1.15, 5.05) | 4.40 (2.30, 6.65)* | 1.20 (0.57, 3.07)* | 4.37 (2.15, 6.32)* | 2.15 (0.93, 4.33)* |
| ESR | 15.0 (4.2, 28.0)* | 35.0 (12.7, 70.7)* | 17.5 (6.2, 63.7) | 38.5 (11.5, 80.7) | 17.2 (6.2, 56.0) | 36.5 (12.7, 73.5) |
| CRP (mg/L) | 2.03 (0.85, 7.36) | 5.01 (1.96, 13.51) | 1.45 (0.42, 4.28)* | 8.45 (2.37, 38.58)* | 1.50 (0.64, 5.46)* | 7.52 (2.52, 24.81)* |
| IgG (g/L) | 22.2 ± 10.3 | 19.0 ± 7.7 | 23.5 ± 12.6 | 21.9 ± 15.0 | 23.5 ± 12.6 | 21.9 ± 15.0 |
| IgA (g/L) | 1.6 ± 0.7* | 2.9 ± 1.6* | 1.8 ± 0.9* | 2.5 ± 1.5* | 1.8 ± 0.8* | 2.7 ± 1.5* |
| IgM (g/L) | 1.2 ± 1.3 | 1.54 ± 1.85 | 0.85 ± 0.5 | 0.9 ± 0.5 | 0.95 ± 0.8 | 1.2 ± 1.4 |
| IgG1 (g/L) | 0.98 ± 0.50 | 1.08 ± 0.59 | 0.96 ± 0.48 | 1.25 ± 1.15 | 0.96 ± 0.48 | 1.16 ± 0.89 |
| IgG2 (g/L) | 0.68 ± 0.48 | 0.63 ± 0.29 | 0.54 ± 0.23 | 0.63 ± 0.30 | 0.59 ± 0.34 | 0.63 ± 0.29 |
| IgG3 (g/L) | 0.54 (0.37, 1.16)* | 0.31 (0.21, 0.53)* | 0.44 (0.24, 0.94) | 0.59 (0.23, 1.25) | 0.53 (0.30, 0.97) | 0.42 (0.22, 0.78) |
| IgG4 (g/L) | 11.25 (3.78, 16.83)* | 3.26 (1.74, 6.66)* | 10.80 (4.73, 21.20)* | 4.02 (2.61, 9.92)* | 11.00 (4.63, 18.22)* | 3.71 (2.02, 7.53)* |
| IgE (kU/L) | 163.5 (36.9, 462.0) | 180.0 (88.7, 509.2) | 537.0 (203.0, 1130.0) | 377.0 (128.5, 906.0) | 526.3 (207.5, 921.5) | 262.0 (118.2, 845.7) |
| IgG4/IgG (%) | 57.86 (25.74, 70.29)* | 17.55 (10.84, 30.62)* | 61.63 (30.11, 81.37)* | 24.62 (15.89, 44.26)* | 61.35 (29.57, 80.60)* | 21.07 (12.82, 36.44)* |
*P<0.05. P value was determined by Fisher's exact test (categorical), Student's t-test (parametric) and nonparametric tests (nonparametric); parametric test values were shown as mean ± SD, and nonparametric test values were shown as median (percentiles 25, percentiles 75); MD: Mikulicz's disease; ESR: Erythrocyte sedimentation rate; CRP: C-reactive protein; Ig: Immunoglobulin.
DISCUSSION
The clinical, laboratory, imaging, and histopathological features of IgG4-RD patients have been discussed by many studies and case reports.[10,12,13,15,16] Based on previous cohort studies, we further expanded the number of IgG4-RD patients in the cohort examined here so as to supplement the clinical features and laboratory findings, such as rarely involved organs. To further our understanding of IgG4-RD, we focused on an analysis of clinical and laboratory features among patients with different numbers and types of involved organs. On the whole, the patient demographics and clinical manifestations of our cohort were consistent with previous studies. The disease predominantly occurred in middle-aged to elderly men and patients with involvement of two or three organs were the most common case types. Salivary/lacrimal gland swelling, which is manifested as MD, was the most common among IgG4-RD patients, followed by involvement of the pancreatic gland, respiratory system, retroperitoneal/mediastinum, and urinary system. Meanwhile, the thyroid gland, pituitary gland, central nervous system, and spleen were rarely affected in IgG4-RD patients. Thus, except for typical characteristics that include salivary and/or lacrimal gland swelling, the presence of lymph node enlargement, abdominal pain, jaundice, nasal congestion, cough, or low back pain, which might indicate the involvement of salivary/lacrimal glands, abdominal organs, the respiratory system, and retroperitoneal fibrosis, respectively, as well as some rare clinical symptoms, such as those associated with the central nervous system, should also be taken into account when diagnosing IgG4-RD. Lymphadenopathy was very common in IgG4-RD patients, whereas lymphadenopathy manifested as Castleman disease was present in only a few patients. The prevalence of organ involvement differed slightly from Japanese or western patients.[12,14,17] Although this study involved multidisciplinary cooperation, selection bias might have occurred in part because the majority of our patients were enrolled from rheumatology unit.
Elevated serum IgG and IgG4 levels were the most important laboratory features of IgG4-RD, and the serum IgG4 level and IgG4/IgG ratio increased with an increasing number of involved organs. Patients with hypocomplementemia were not uncommon in our cohort, which is consistent with previous reports, and this situation was more remarkable in patients with kidney disease.[12,18,19] Given that the IgG4 molecule lacks complement-binding activity, Wallace et al. speculated that an alternate IgG subtype might be responsible for the low complement levels in IgG4-RD patients.[12] However, we found no relationship between ESR and either serum IgG4 or CRP levels, which indicated that inflammatory factors may not play crucial roles in IgG4-RD progression. Indeed, the majority of our patients had normal serum IgA and IgM levels. Although serum IgG3 levels were normal in most patients of our cohort, its value increased with IgG4 level, although the mechanistic explanation of this observation is unclear.
Elevated Eos numbers and total serum IgE levels were also observed in 33.7% and 83.6% of IgG4-RD patients, respectively, which is consistent with a previous study.[20] In addition, both the Eos percentage and serum IgE level were significantly correlated to serum IgG4 level and IgG4/IgG value and increased as the number of involved organs increased. These findings support the view that IgG4-RD itself rather than atopy contributes to the elevation in Eos percentages and IgE level.[20] Interestingly, although nearly 60% of our IgG4-RD patients had allergic history, there was no significant difference in Eos numbers and serum IgE levels relative to patients without allergic disease.
The presentation of various manifestations and organ involvement in IgG4-RD patients highlights the potential interest of classifying clinical phenotypes. We believe that the characteristics of the different organ types that are involved in this disease are not only useful for indicating IgG4-RD rather than malignancies or other benign diseases, but also for understanding the nature of the disease. In addition, the first international consensus guidance statement on the management and treatment of IgG4-RD, which proposed research priorities to achieve advances in the management and treatment of IgG4-RD, emphasized the necessity of defining the patient's clinical phenotype.[1] In reviewing the literature, we found only one recent study that analyzed organ involvement correlations in 132 IgG4-RD patients, although this correlation was restricted to serum IgG4 level and organ involvement.[14] In this study, patients were classified into different types according to the numbers and anatomic sites of involved organs, with two main types of organ involvement being the most common while patients with involvement of multiple (≥4) organs were the rarest. Notably, in patients with one type of involved organ, salivary/lacrimal gland swelling was the most common clinical feature, followed by retroperitoneal/mediastinal fibrosis and pancreatic-hepatobiliary-splenic system involvement. In patients with two types of organ involvement, salivary/lacrimal gland swelling along with respiratory system involvement was the most common pattern. In addition, salivary/lacrimal gland swelling combined with hepatobiliary-pancreatic and respiratory system involvement was the most common in patients who had three types of organs involved. Patients who had only one type of internal organ involved accounted for only a small portion of this cohort. The clinical types of organ involvement could thus provide valuable clues for the diagnosis and differential diagnosis of IgG4-RD.
In analyzing differences between patients with salivary/lacrimal gland and internal organ involvement, we found no difference in gender distribution, while elderly male patients were more inclined to have internal organ involvement. Consistent with studies by Hamano et al. and Koizumi et al., which reported that patients with lacrimal and/or salivary gland lesions had higher serum IgG4 levels than patients who did not,[14,17] our data revealed that patients with salivary/lacrimal gland swelling had higher serum IgG4 concentrations and IgG4/IgG values than those with internal organ involvement. Higher percentages of Eos and allergic history was also more frequent in patients with salivary/lacrimal gland involvement. Together these findings suggested that lacrimal and salivary gland involvement in IgG4-RD translated into different disease manifestations relative to cases that did not involve these glands. This outcome might thus provide clues about IgG4-RD pathogenesis although additional study will be needed.
There are some limitations to this study. First, this is a single-center prospective study, and most of our patients were enrolled from the rheumatology unit. As such, a multicenter prospective study is needed to evaluate more broadly the clinical and laboratory features of IgG4-RD. The number of patients who underwent tissue biopsy was relatively small, and represented only 60% of cases. Furthermore, we encountered some difficulties in classifying involvement of various organs, such as lymph nodes, as well as those patients with rare disease manifestations.
In conclusion, our data provided new insights into the characteristics of IgG4-RD. Patients with different types of organ involvement revealed distinct clinical and laboratory features in some important ways, which might be helpful for confirming the presence of IgG4-RD rather than malignancies or other benign diseases. Our findings would also be useful for understanding the nature of IgG4-RD while the prognosis and outcome of IgG4-RD patients with different types of organ involvement deserves further long-term follow-up study.
Financial support and sponsorship
This work was supported by grants from the National Natural Science Foundation of China (Nos. 81373190, 81571587).
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Li-Shao Guo
REFERENCES
- 1.Khosroshahi A, Wallace ZS, Crowe JL, Akamizu T, Azumi A, Carruthers MN, et al. International consensus guidance statement on the management and treatment of IgG4-related disease. Arthritis Rheumatol. 2015;67:1688–99. doi: 10.1002/art.39132. doi: 10.1002/art.39132. [DOI] [PubMed] [Google Scholar]
- 2.Stone JH, Zen Y, Deshpande V. IgG4-related disease. N Engl J Med. 2012;366:539–51. doi: 10.1056/NEJMra1104650. doi: 10.1056/NEJMra1104650. [DOI] [PubMed] [Google Scholar]
- 3.Hamano H, Kawa S, Horiuchi A, Unno H, Furuya N, Akamatsu T, et al. High serum IgG4 concentrations in patients with sclerosing pancreatitis. N Engl J Med. 2001;344:732–8. doi: 10.1056/NEJM200103083441005. doi: 10.1056/NEJM200103083441005. [DOI] [PubMed] [Google Scholar]
- 4.Della-Torre E, Lanzillotta M, Doglioni C. Immunology of IgG4-related disease. Clin Exp Immunol. 2015;181:191–206. doi: 10.1111/cei.12641. doi: 10.1111/cei.12641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Umehara H, Okazaki K, Masaki Y, Kawano M, Yamamoto M, Saeki T, et al. Comprehensive diagnostic criteria for IgG4-related disease (IgG4-RD). 2011. Mod Rheumatol. 2012;22:21–30. doi: 10.1007/s10165-011-0571-z. doi: 10.3109/s10165-011-0571-z. [DOI] [PubMed] [Google Scholar]
- 6.Lin W, Jin L, Chen H, Wu Q, Fei Y, Zheng W, et al. Bcell subsets and dysfunction of regulatory B cells in IgG4-related diseases and primary Sjögren's syndrome:The similarities and differences. Arthritis Res Ther. 2014;16:R118. doi: 10.1186/ar4571. doi:10.1186/ar4571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wallace ZS, Mattoo H, Carruthers M, Mahajan VS, Della Torre E, Lee H, et al. Plasmablasts as a biomarker for IgG4-related disease, independent of serum IgG4 concentrations. Ann Rheum Dis. 2015;74:190–5. doi: 10.1136/annrheumdis-2014-205233. doi: 10.1136/annrheumdis-2014-205233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kamisawa T, Zen Y, Pillai S, Stone JH. IgG4-related disease. Lancet. 2015;385:1460–71. doi: 10.1016/S0140-6736(14)60720-0. doi: 10.1016/S0140-6736(14)60720-0. [DOI] [PubMed] [Google Scholar]
- 9.Guma M, Firestein GS. IgG4-related diseases. Best Pract Res Clin Rheumatol. 2012;26:425–38. doi: 10.1016/j.berh.2012.07.001. doi: 10.1016/j.berh.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 10.Chen H, Lin W, Wang Q, Wu Q, Wang L, Fei Y, et al. IgG4-related disease in a Chinese cohort: A prospective study. Scand J Rheumatol. 2014;43:70–4. doi: 10.3109/03009742.2013.822094. doi: 10.3109/03009742.2013.822094. [DOI] [PubMed] [Google Scholar]
- 11.Zhang J, Chen H, Ma Y, Xiao Y, Niu N, Lin W, et al. Characterizing IgG4-related disease with 18F-FDG PET/CT:A prospective cohort study. Eur J Nucl Med Mol Imaging. 2014;41:1624–34. doi: 10.1007/s00259-014-2729-3. doi: 10.1007/s00259-014-2729-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wallace ZS, Deshpande V, Mattoo H, Mahajan VS, Kulikova M, Pillai S, et al. IgG4-related disease:Clinical and laboratory features in one hundred twenty-five patients. Arthritis Rheumatol. 2015;67:2466–75. doi: 10.1002/art.39205. doi: 10.1002/art.39205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lin W, Lu S, Chen H, Wu Q, Fei Y, Li M, et al. Clinical characteristics of immunoglobulin G4-related disease:A prospective study of 118 Chinese patients. Rheumatology (Oxford) 2015;54:1982–90. doi: 10.1093/rheumatology/kev203. doi: 10.1093/rheumatology/kev203. [DOI] [PubMed] [Google Scholar]
- 14.Koizumi S, Kamisawa T, Kuruma S, Tabata T, Chiba K, Iwasaki S, et al. Organ correlation in IgG4-related diseases. J Korean Med Sci. 2015;30:743–8. doi: 10.3346/jkms.2015.30.6.743. doi: 10.3346/jkms.2015.30.6.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zen Y, Nakanuma Y. IgG4-related disease:A cross-sectional study of 114 cases. Am J Surg Pathol. 2010;34:1812–9. doi: 10.1097/PAS.0b013e3181f7266b. doi: 10.1097/PAS.0b013e3181f7266b. [DOI] [PubMed] [Google Scholar]
- 16.Ebbo M, Daniel L, Pavic M, Sève P, Hamidou M, Andres E, et al. IgG4-related systemic disease:Features and treatment response in a French cohort:Results of a multicenter registry. Medicine (Baltimore) 2012;91:49–56. doi: 10.1097/MD.0b013e3182433d77. doi: 10.1097/MD.0b013e3182433d77. [DOI] [PubMed] [Google Scholar]
- 17.Hamano H, Arakura N, Muraki T, Ozaki Y, Kiyosawa K, Kawa S. Prevalence and distribution of extrapancreatic lesions complicating autoimmune pancreatitis. J Gastroenterol. 2006;41:1197–205. doi: 10.1007/s00535-006-1908-9. doi: 10.1007/s00535-006-1908-9. [DOI] [PubMed] [Google Scholar]
- 18.Saeki T, Nishi S, Imai N, Ito T, Yamazaki H, Kawano M, et al. Clinicopathological characteristics of patients with IgG4-related tubulointerstitial nephritis. Kidney Int. 2010;78:1016–23. doi: 10.1038/ki.2010.271. doi: 10.1038/ki.2010.271. [DOI] [PubMed] [Google Scholar]
- 19.Muraki T, Hamano H, Ochi Y, Komatsu K, Komiyama Y, Arakura N, et al. Autoimmune pancreatitis and complement activation system. Pancreas. 2006;32:16–21. doi: 10.1097/01.mpa.0000188308.75043.e4. doi: 10.1097/01.mpa.0000188308.75043e4. [DOI] [PubMed] [Google Scholar]
- 20.Della Torre E, Mattoo H, Mahajan VS, Carruthers M, Pillai S, Stone JH. Prevalence of atopy, eosinophilia, and IgE elevation in IgG4-related disease. Allergy. 2014;69:269–72. doi: 10.1111/all.12320. doi: 10.1111/all.12320. [DOI] [PMC free article] [PubMed] [Google Scholar]