Abstract
Background:
Accurately, characterizing plaques is critical for selecting the optimal intervention strategy for the left main coronary artery (LMCA) bifurcation. Coronary angiography cannot precisely assess the location or nature of plaques in bifurcation lesions. Few intravascular ultrasound (IVUS) classification scheme has been reported for angiographic imaging of true bifurcation lesions of the unprotected LMCA thus far. In addition, the plaque composition at the bifurcation has not been elucidated. This study aimed to detect plaque composition at LMCA bifurcation lesions by IVUS.
Methods:
Fifty-eight patients were recruited. The location, concentricity or eccentricity, site of maximum thickness, and composition of plaques of the distal LMCA, ostial left anterior descending (LAD) coronary artery and, left circumflex (LCX) coronary artery were assessed using IVUS and described using illustrative diagrams.
Results:
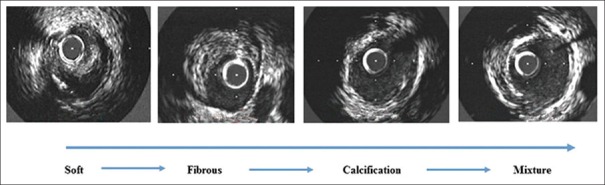
True bifurcation lesions of the unprotected LMCA were classified into four types: Type A, with continuous involvement from the distal LMCA to the ostial LAD and the ostial LCX with eccentric plaques; Type B, with concentric plaques at the distal LMCA, eccentric plaques at the ostial LAD, and no plaques at the LCX; Type C, with continuous involvement from the distal LMCA to the ostial LCX, with eccentric plaques, and to the ostial LAD, with eccentric plaques; and Type D, with continuous involvement from the distal LMCA to the ostial LAD, with eccentric plaques, and to the ostial LCX, with concentric plaques. The carina was involved in only 3.5% of the plaques. A total of 51.7% of the plaques at the ostium of the LAD were soft, while 44.8% and 44.6% were fibrous in the distal LMCA and in the ostial LCX, respectively.
Conclusions:
We classified LMCA true bifurcation lesions into four types. The carina was always free from disease. Plaques at the ostial LAD tended to be soft, whereas those at the ostial LCX and the distal LMCA tended to be fibrous.
Keywords: Intravascular Ultrasound, Left Main True Bifurcation Lesion, Plaque
INTRODUCTION
Compared with other lesion sites, vessel bifurcations are at a higher risk of atherosclerotic plaque accumulation.[1] Approximately, 15–20% of percutaneous coronary interventions (PCIs) involve bifurcation lesions. Coronary bifurcation lesions represent a technical challenge for PCI and always include a relatively high rate of restenosis, risk of myocardial infarction, and risk of stent thrombosis, even in the era of drug-eluting stents. Accurately, characterizing plaques is critical for selecting the optimal intervention strategy for the left main coronary artery (LMCA) bifurcation. An angiographic classification scheme for bifurcation lesions was previously suggested based on the features of plaque distribution. This classification is helpful clinically for intervention strategies and discussion among colleagues;[2,3] however, coronary angiography (CAG) cannot precisely assess the location or nature of plaques in bifurcation lesions.[4,5] Therefore, the proposed CAG classification of plaque distribution is likely to be inaccurate. Sometimes, plaque accumulation that can be observed by intravascular ultrasound (IVUS) cannot be detected by CAG.[6] A medina classification scheme based on angiography for bifurcation lesions has been generally validated but it is still unreliable for the assessment of plaque characteristics, particularly for LMCA bifurcation lesions.[7] Oviedo et al. proposed an IVUS classification scheme for distal LMCA bifurcation lesions. However, only 33.5% of true bifurcation lesions were reported in his study.[5] Thus, we aimed to analyze the features of plaque distribution in unprotected LMCA true bifurcation lesions by IVUS in order to develop a new IVUS classification.
A limited number of studies have examined plaque composition, particularly that of left main bifurcation lesions, thus far. Atherosclerosis begins with fatty streak formation in an atherogenic milieu that is created by the interplay of systemic cardiovascular risk factors, vascular biology, and local hemodynamic forces, including wall shear stress (WSS). According to Parham's findings,[8] segments within bifurcations are more likely to have low WSS, and low WSS is associated with the necrotic core and calcium content of plaques. For LMCA bifurcations, the ostium of the left anterior descending (LAD) coronary artery has been demonstrated to have lower WSS. The purpose of this study was to detect plaque composition at LMCA bifurcation lesions by IVUS.
METHODS
Study population
We enrolled 58 patients with LMCA true bifurcation lesions as documented by CAG from Guangzhou Red Cross Hospital and Gansu Provincial People's Hospital between 2012 and 2015. Among these 58 patients, there were twenty patients with a Medina classification score of 1, 1, 1; 14 with 1, 0, 1; 15 with 1, 1, 0; and 9 with 0, 1, 1. Patients with acute myocardial infarctions, in-stent restenosis, giant ramus intermedius coronary artery, or thrombosis were excluded from this study. There were 46 males and 12 females in this study, and the average age was 69.4 ± 13.1 years.
Intravascular ultrasound and analysis
A 0.014 inch guide wire was inserted into the distal LAD and the left circumflex (LCX) coronary artery through a 6–7 F left coronary artery guide catheter. Before coronary intervention, intracoronary nitroglycerin (200 μg) was administered to prevent coronary artery spasm. Commercially available IVUS systems (2.7 F and 40-MHz IVUS catheter [Boston Scientific Corp., CA, USA] and iLab [Boston Scientific Corp., USA]) and an IVUS detection meter were used along with Sonos Intravascular off-line image analysis software (Hewlett-Packard Development Company, USA). IVUS assessments focused primarily on plaque distributions at the ostial LAD and LCX and the distal LMCA via manual pullback at a constant speed. All of the IVUS images were saved on 0.5-ins-VHS tapes and CD-Rs for off-line analyses, which were performed by three independent observers according to the criteria of the American College of Cardiology Clinical Expert Consensus Document on Standards for Acquisition, Measurement and Reporting of Intravascular Ultrasound Studies.[9] The measured parameters included the maximum and minimum thickness of the plaque; the site of major plaque on the vessel cross-section, such as laterally, toward the pericardium, or toward the myocardium; and involvement of the carina.
Definitions
Eccentric plaque was defined as a ratio of major plaque thickness to minor plaque thickness of >3. If the ration was <3, the plaque was defined as concentric. Soft plaque was defined as a no echo or low echo area in comparison with the adventitia, whereas fibrotic plaque was described as a strong echo without an acoustic shadow. Mixed plaque was defined as more than one echo image, and calcium plaque was defined as a strong echo with an acoustic shadow (patients with a calcium arch of >90° were excluded).
Coronary angiography and analysis
CAG was performed on all of the patients before IVUS. Several angiographic projections were required to show the bifurcation lesions. Three independent intervention cardiologists utilized the Medina classification system for the qualitative analysis of the LMCA bifurcation lesions. In the Medina system, 0 indicates the absence of stenosis and 1 indicates the presence of stenosis in three segments separated by commas (LMCA, LAD, and LCX).
Illustrative diagrams
After IVUS examination along the distal LAD and LCX to the LMCA, the plaque location and spatial position on cross-sections were visualized by creating simulated drawings of the distal LM, ostial LAD, and ostial LCX separately according to the IVUS findings. The plaque location was classified, a longitudinal simulated divider was established to represent an LMCA bifurcation, and the plaque location was drawn at the bifurcation area.
Statistical analysis
Categorical variables are described as counts and proportions. SAS 9.0 (SAS Institute Inc., Cary, NC, USA) was used to analyze the plaque composition data. Because the 25% lattice frequency was <5, categorical data were compared by Fisher's exact probability test. P < 0.05 was considered statistically significant.
RESULTS
Features of ostial left anterior descending and left circumflex plaque distribution detected by intravascular ultrasound
The incidence rate of stenotic lesions was 100% (58/58) at the ostial LAD with eccentric plaques; 96.6% (56/58) at the ostial LCX, with 96.4% (54/56) eccentric plaque; 93.1% (54/58) at both the LAD and LCX with eccentric plaques [Figure 1a and 1b]; and 3.5% (2/58) at the LAD with eccentric plaques and at the LCX with concentric plaques [Figure 1c]. In total, 5.6% (3/54) of the plaque maximum thickness at the ostial LCX was toward the myocardium with eccentric plaque located at the lateral wall of the LAD [Figure 1d], while 3.7% (2/54) of the plaque maximum thickness at both the ostial LAD and LCX was toward the myocardium [Figure 1e].
Figure 1.
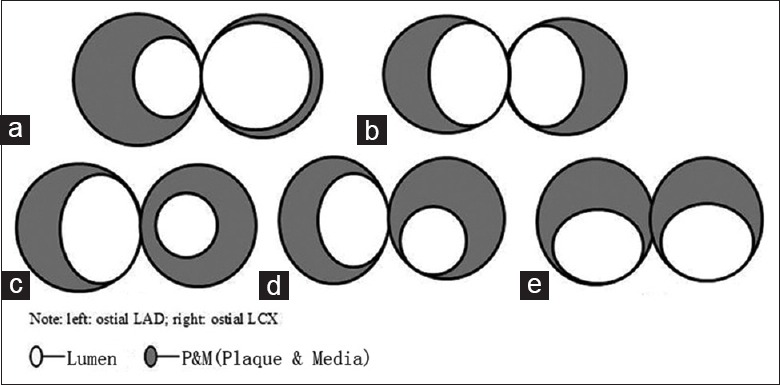
Schematic diagram of ostial LAD and LCX plaque distribution (a-e). Left: Ostial LAD; right: Ostial LCX. LAD: Left anterior descending; LCX: Left circumflex.
Features of the distal left main coronary artery plaque distribution detected by intravascular ultrasound
There were five cases with concentric plaques (8.6%) [Figure 2a], 49 with eccentric plaques oriented toward the ostial LAD (84.5%) [Figure 2b], and four with eccentric plaques oriented toward the ostial LCX (6.3%) [Figure 2c].
Figure 2.
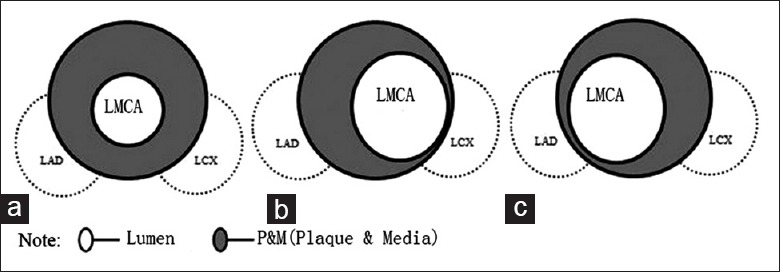
Schematic diagram of distal LMCA plaque distribution (a-c). LMCA: Left main coronary artery. LAD: Left anterior descending; LCX: Left circumflex.
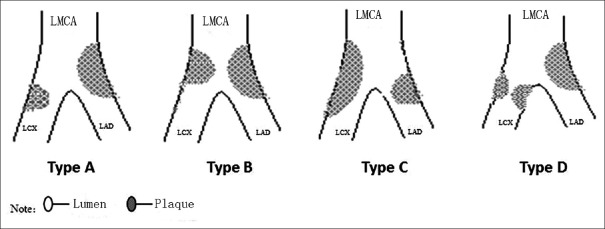
Intravascular ultrasound classification of the left main coronary artery true bifurcation lesions
The LMCA true bifurcation lesions were classified into four types [Figure 3]: Type A (50/58; 86.2%), with continuous plaque from the distal LM to the ostial LAD with eccentric plaque oriented laterally and to the ostial LCX with eccentric plaque oriented laterally; Type B (4/58; 6.9%), with concentric plaques at the distal LMCA, eccentric plaques oriented laterally at the ostial LAD, and no plaques at the ostial LCX; Type C (2/58; 3.5%), with continuous involvement from the distal LMCA to the ostial LCX, with eccentric plaques oriented laterally, and to the ostial LAD, with eccentric plaques oriented laterally; and Type D (2/58; 3.5%), with continuous lesions from the distal LMCA to the ostial LAD, with eccentric plaques oriented laterally, and to the ostial LCX, with concentric plaques.
Figure 3.
Schematic diagram of the IVUS classification for LMCA true bifurcation lesions. LMCA: Left main coronary artery; IVUS: Intravascular ultrasound. LAD: Left anterior descending; LCX: Left circumflex.
Plaque composition of the distal left main coronary artery and ostial left anterior descending and left circumflex
The four categories of plaque composition significantly differed between the LMCA, LAD, and LCX (P = 0.0004). A total of 51.7% of the plaques at the ostial LAD were soft, which was significantly higher than the percentage of soft plaques in the LMCA and LCX. In total, 44.8% and 44.6% of the plaques were fibrous in the distal LMCA and ostial LCX, respectively, and were significantly more common than those in the ostial LAD [Table 1]. Illustrations of the categories of plaque composition detected by IVUS are shown in Figure 4.
Table 1.
Plaque composition at the distal LMCA and at the ostial LAD and LCX, n (%)
| Plaque | LMCA (n = 58) | LAD (n = 58) | LCX (n = 56) |
|---|---|---|---|
| Soft | 16 (27.6) | 30 (51.7) | 17 (30.4) |
| Fibrous | 26 (44.8) | 11 (19.0) | 25 (44.6) |
| Calcification | 16 (27.6) | 19 (32.8) | 11 (19.6) |
| Mixture | 0 | 8 (13.8) | 3 (5.4) |
LMCA: Left main coronary artery; LAD: Left anterior descending; LCX: Left circumflex.
Figure 4.
Plaque composition at the distal LMCA and ostial LAD and LCX as detected by IVUS. LMCA: Left main coronary artery; IVUS: Intravascular ultrasound; LAD: Left anterior descending; LCX: Left circumflex.
DISCUSSION
In this study, we designed a new IVUS classification for LMCA true bifurcation lesions according to plaque features. LMCA true bifurcation lesions were classified into four types: Type A, with continuous plaques from the distal left main (LM) to the ostial LAD, with eccentric plaques oriented laterally, and to the ostial LCX, with eccentric plaques oriented laterally; Type B, with concentric plaques at the distal LMCA, eccentric plaques oriented laterally at the ostial LAD, and no plaques at the ostial LCX; Type C, with continuous involvement from the distal LMCA to the ostial LCX, with eccentric plaques oriented laterally, and to the ostial LAD, with eccentric plaques oriented laterally; and Type D, with continuous lesions from the distal LM to the ostial LAD, with eccentric plaques oriented laterally, and to the ostial LCX, with concentric plaques. In this study group, 86.2% of LMCA true bifurcation lesions were Type A, 6.8% were Type B, 3.4% were Type C, and 3.4% were Type D.
The rates of major adverse cardiac events are higher in LMCA bifurcation lesions compared to non-LMCA bifurcation lesions (20–30% vs. 10–20%). In addition, restenosis rates for the ostial LCX are higher with LMCA bifurcation lesions; this phenomenon is related to the distinct peculiarity of plaque distributions in LMCA and non-LMCA bifurcation lesions.[10] The LMCA diameter is approximately 6.97 ± 0.47 mm, as measured by IVUS, which is broader than other coronary arteries.[11] Moreover, LMCA bifurcation varies from non-LMCA bifurcation in its angle of LAD and LCX. The usual angle of LAD and LCX is >90°, whereas the angle between the main branch and the side branch in a non-LMCA bifurcation lesion is <90°.[12,13,14] Thus, homeostasis and shear stress in bifurcation lesions differ from those of LMCA bifurcations in contrast to non-LMCA bifurcations.[15] Previous IVUS studies have demonstrated differences between plaque features and locations in LMCA bifurcation lesions and LAD/diagonal branches.[7] Therefore, it is crucial to study plaque features in LMCA true bifurcation lesions. Oviedo et al. proposed an IVUS classification scheme for distal LMCA bifurcation lesions. However, only 33.5% of true bifurcation lesions were reported in their study, and their classification was unreliable because two-third of the bifurcation lesions in the sample were nontrue.[5]
The four standard angiographic classifications used for the bifurcation lesions include the Lefèvre classification, the Sanborn classification, the Duke classification, and the Safian classification. Recently, the Medina classification has become commonly used; however, the Medina classification has some limitations compared to other IVUS classification schemes. Bifurcation carina lesions were demonstrated in most of these angiographic classifications. By contrast, IVUS studies of non-LMCA bifurcation lesions revealed that most of the bifurcation lesions presented laterally were not bifurcation carina lesions, which further demonstrates the inexactness of angiographic classification.[16] In fact, the carina is seldom affected by plaque accumulation, which is in line with our present findings.
Our results showed that only 6.8% of concentric plaques were present in distal LMCA lesions and that most lesions were eccentric plaques oriented toward the ostial LAD. There were four cases with distal LM eccentric plaques oriented toward the ostial LCX in this study. In addition, eccentric plaques opposite the flow divider were more common than concentric plaques in the ostial LAD and ostial LCX with a spared flow divider. The angle was approximately 180° between the ostial LAD lesions and the ostial LCX lesions. Ostial LAD lesions and ostial LCX lesions were rarely located in the same direction (pericardium) or in a diagonal opposite direction with an angle <180° (ostial LCX plaque oriented toward the pericardium). There was only one case with an ostial LCX concentric plaque.
Local hemodynamics of vessel bifurcation are an essential determinant of atherosclerosis formation.[17] According to physics and fluid mechanics theory, there are variations in blood flow velocity, tension forces and shear stresses between vessel bifurcations and other segments. These factors increase the susceptibility of vessel bifurcations to plaque accumulation.
Prior studies utilizing reconstructive technology have shown that atherosclerotic plaques are present on the opposite sides of flow dividers in analog living vessels with bifurcation.[18] In this study, only 3.4% involvement of the carina had plaques. An IVUS study of LAD lesion distribution revealed that 94% of plaques were located close to the LMCA bifurcation and that most plaques were distributed opposite of the ostial LCX.[19] Perktold et al. showed that regardless of which method is used to measure the ostial velocity, the results will differ when blood flows from the LMCA to the ostial LAD and LCX.[20] Although the axial velocity does not change much, this flow change will still cause a variation in shear stress between the lateral wall and the inside wall. The ratio of shear stress of the inside wall to that of the lateral wall could be as high as 1.4–1.7. Lateral walls with lower shear stress are at higher risk for injuries and plaque accumulation. In addition, vessel wall vibrations caused by blood flow pulsation are related to plaque distribution. He and Ku modeled the pulsatile hemodynamics of LMCA bifurcation in vivo under anatomical and physiological environments utilizing the spectral element technique[21] and concluded that arterial WSS was markedly lower in the bifurcation area and the side walls compared to the nonbifurcation area. The highest oscillatory nature was confined to the outer wall of the LCX, and the highly restricted distributions of flow and oscillatory shear stress along the walls firmly corresponded with the principal locations of atheroma in the left coronary artery; hence, the rates of atherosclerosis in LCX are, in theory, higher. This study, along with some previous studies, demonstrated a higher incidence of ostial LAD lesions. One possible explanation could be that the hemodynamics differ between LMCA bifurcations and non-LMCA bifurcations. Previous studies have shown that the three-dimensional wall pressure gradient (WPG) is lower in proximal left coronary artery regions, where atherosclerosis often develops, in contrast to distal segments. Low WPG exists at bifurcations in areas opposite the apexes, which are susceptible to plaque formation.[22] Indeed, the anatomical region with the least WPG in the bifurcation region is the outer wall of the side branch below the ostia, close to the main branch in non-LMCA bifurcation, and the outer wall of the ostial LAD and LCX in LMCA bifurcation. In addition, the angle between the side branch and the main branch is another essential aspect that influences plaque distribution. Plaques are always deposited across the side branch, which vertically separates from the main branch. Plaques in the main branch or in nonvertically separated side branches usually occur at the opposite of the side branch oriented toward the acute angle side and away from the obtuse angle side.[23] This finding strongly indicates that LMCA bifurcation carinas are always free from lesions as presented in this study and other previous studies.
To date, limited data about the plaque composition of LMCA bifurcation lesions have been reported. Plaque composition has been suggested to be associated with WSS. Low WSS is associated with the necrotic core and calcium content of plaques,[8] which suggests a link between WSS and coronary plaque phenotypes. For LMCA bifurcations, the LAD has been demonstrated to have lower WSS and thus has always been considered to be the main branch. A significant body of experimental literature supports that low WSS results in alterations in endothelial cell signaling, low-density lipoprotein uptake, and proinflammatory gene expression, all of which lead to an activated endothelial phenotype.[24,25,26] Although these studies do not precisely refer to LMCA bifurcations, our finding of soft plaques mainly located at the ostial LAD is partially coincident with previous studies. The ostial LAD, an area with low WSS, tends to develop plaques with a more lipid core. Another finding of this study was that fibrous plaques mainly located at the ostial LCX were demonstrated to have higher WSS compared to those in the ostial LAD. We hypothesized that relatively high WSS was associated with fibrous plaque development, but we prefer the hypothesis that there must be multiple factors that can influence the plaque composition of LMCA bifurcations.
The major limitation of this study was its small sample size. In addition, our preferred method of IVUS cannot accurately observe the angle of the side branch and the main branch, which should be considered by the intervention cardiologist. We described plaque composition only by IVUS findings but did not combine other detection methods or analyze other potentially influential factors, including blood flow velocity, tension forces, and wall stress, among others. Other instruments and methods, for example, optical coherence tomography, can be used to describe plaque components more accurately.
Our IVUS classification scheme for LMCA true bifurcation lesions differs from conventional angiographic classifications. The primary features of our IVUS classification include lower frequencies of lesions in the bifurcation carina and more eccentric plaques in the lateral wall of the ostial LAD and the LCX. Second, distal LMCA plaques were eccentric and oriented toward the ostial LAD. Plaques at the ostial LAD tended to be soft, whereas those at the LCX and LMCA tended to be fibrous.
The selection of interventions for unprotected LMCA bifurcation lesions is still difficult, and angiographic classifications are prone to errors in assessing plaque distribution for this condition.[27] IVUS classification is generally accepted because it provides more information about plaques. In this study, we described detailed plaque information for unprotected LMCA true bifurcation lesions, and we proposed a new IVUS classification for these lesions. This new classification scheme may aid in managing this condition.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Li-Min Chen
REFERENCES
- 1.Asakura T, Karino T. Flow patterns and spatial distribution of atherosclerotic lesions in human coronary arteries. Circ Res. 1990;66:1045–66. doi: 10.1161/01.res.66.4.1045. doi: 10.1161/01.RES.66.4.1045. [DOI] [PubMed] [Google Scholar]
- 2.Kurt M, Tanboga IH, Karakas MF, Büyükkaya E, Akçay AB, Sen N, et al. Clinical and morphological evaluation of coronary bifurcation lesions. Turk Kardiyol Dern Ars. 2013;41:207–11. doi: 10.5543/tkda.2013.38073. doi: 10.1016/S0167-5273(12)70386-8. [DOI] [PubMed] [Google Scholar]
- 3.Thompson CA, Sidhu MS, Brown JR, Sabir SA, Floyd KC, DE Vries JT, et al. Classification and atherosclerosis distribution in patients with left main coronary disease. J Interv Cardiol. 2009;22:431–6. doi: 10.1111/j.1540-8183.2009.00490.x. doi: 10.1111/j.1540-8183.2009.00490.x. [DOI] [PubMed] [Google Scholar]
- 4.Papadopoulou SL, Girasis C, Gijsen FJ, Rossi A, Ottema J, van der Giessen AG, et al. A CT-based Medina classification in coronary bifurcations:Does the lumen assessment provide sufficient information?Catheter Cardiovasc Interv. 2014;84:445–52. doi: 10.1002/ccd.25496. doi:10.1002/ccd.25496. [DOI] [PubMed] [Google Scholar]
- 5.Oviedo C, Maehara A, Mintz GS, Araki H, Choi SY, Tsujita K, et al. Intravascular ultrasound classification of plaque distribution in left main coronary artery bifurcations:Where is the plaque really located?Circ Cardiovasc Interv. 2010;3:105–12. doi: 10.1161/CIRCINTERVENTIONS.109.906016. doi: 10.1161/CIRCINTERVENTIONS.109.906016. [DOI] [PubMed] [Google Scholar]
- 6.Hermiller JB, Buller CE, Tenaglia AN, Kisslo KB, Phillips HR, Bashore TM, et al. Unrecognized left main coronary artery disease in patients undergoing interventional procedures. Am J Cardiol. 1993;71:173–6. doi: 10.1016/0002-9149(93)90734-t. doi: 10.1016/0002-9149(93)90734-T. [DOI] [PubMed] [Google Scholar]
- 7.Yakushiji T, Maehara A, Mintz GS, Saito S, Araki H, Oviedo C, et al. An intravascular ultrasound comparison of left anterior descending artery/first diagonal branch versus distal left main coronary artery bifurcation lesions. EuroIntervention. 2013;8:1040–6. doi: 10.4244/EIJV8I9A160. doi: 10.4244/EIJV8I9A160. [DOI] [PubMed] [Google Scholar]
- 8.Eshtehardi P, McDaniel MC, Suo J, Dhawan SS, Timmins LH, Binongo JN, et al. Association of coronary wall shear stress with atherosclerotic plaque burden, composition, and distribution in patients with coronary artery disease. J Am Heart Assoc. 2012;1:e002543. doi: 10.1161/JAHA.112.002543. doi:10.1161/JAHA.112.002543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mintz GS, Nissen SE, Anderson WD, Bailey SR, Erbel R, Fitzgerald PJ, et al. American College of Cardiology Clinical Expert Consensus Document on Standards for Acquisition, Measurement and Reporting of Intravascular Ultrasound Studies (IVUS). A report of the American College of Cardiology Task Force on Clinical Expert Consensus Documents. J Am Coll Cardiol. 2001;37:1478–92. doi: 10.1016/s0735-1097(01)01175-5. doi: 10.1016/S0735-1097(01)01175-5. [DOI] [PubMed] [Google Scholar]
- 10.Kim WJ, Kim YH, Park DW, Yun SC, Lee JY, Kang SJ, et al. Comparison of single- versus two-stent techniques in treatment of unprotected left main coronary bifurcation disease. Catheter Cardiovasc Interv. 2011;77:775–82. doi: 10.1002/ccd.22915. doi: 10.1002/ccd.22915. [DOI] [PubMed] [Google Scholar]
- 11.Dodge JT, Jr, Brown BG, Bolson EL, Dodge HT. Lumen diameter of normal human coronary arteries. Influence of age, sex, anatomic variation, and left ventricular hypertrophy or dilation. Circulation. 1992;86:232–46. doi: 10.1161/01.cir.86.1.232. doi: 10.1161/01.CIR.86.1.232. [DOI] [PubMed] [Google Scholar]
- 12.Han SH, Puma J, García-García HM, Nasu K, Margolis P, Leon MB, et al. Tissue characterisation of atherosclerotic plaque in coronary artery bifurcations:An intravascular ultrasound radiofrequency data analysis in humans. EuroIntervention. 2010;6:313–20. doi: 10.4244/EIJV6I3A53. doi: 10.4244/EIJV6I3A53. [DOI] [PubMed] [Google Scholar]
- 13.Kang SJ, Kim WJ, Yun SC, Park DW, Lee SW, Kim YH, et al. Vascular remodeling at both branch ostia in bifurcation disease assessed by intravascular ultrasound. Catheter Cardiovasc Interv. 2013;81:1150–5. doi: 10.1002/ccd.24390. doi: 10.1002/ccd.24390. [DOI] [PubMed] [Google Scholar]
- 14.Dong J, Sun Z, Inthavong K, Tu J. Fluid-structure interaction analysis of the left coronary artery with variable angulation. Comput Methods Biomech Biomed Engin. 2015;18:1500–8. doi: 10.1080/10255842.2014.921682. doi: 10.1080/10255842.2014.921682. [DOI] [PubMed] [Google Scholar]
- 15.Soulis J, Fytanidis D, Seralidou K, Giannoglou G. Wall shear stress oscillation and its gradient in the normal left coronary artery tree bifurcations. Hippokratia. 2014;18:12–6. [PMC free article] [PubMed] [Google Scholar]
- 16.Kimura BJ, Russo RJ, Bhargava V, McDaniel MB, Peterson KL, DeMaria AN. Atheroma morphology and distribution in proximal left anterior descending coronary artery In vivo observations. J Am Coll Cardiol. 1996;27:825–31. doi: 10.1016/0735-1097(95)00551-x. doi: 10.1016/0735-1097(95)00551-X. [DOI] [PubMed] [Google Scholar]
- 17.Glagov S, Zarins C, Giddens DP, Ku DN. Hemodynamics and atherosclerosis. Insights and perspectives gained from studies of human arteries. Arch Pathol Lab Med. 1988;112:1018–31. [PubMed] [Google Scholar]
- 18.Svindland A. The localization of sudanophilic and fibrous plaques in the main left coronary bifurcation. Atherosclerosis. 1983;48:139–45. doi: 10.1016/0021-9150(83)90100-4. doi: 10.1016/0021-9150(83)90100-4. [DOI] [PubMed] [Google Scholar]
- 19.Watanabe H, Yoshikawa J, Yoshida K, Akasaka T, Hozumi T, Takagi T, et al. Intravascular ultrasound scanning assessment of plaque distribution in the left anterior descending coronary artery just distal to the bifurcation. J Cardiol. 1995;26:325–9. [PubMed] [Google Scholar]
- 20.Perktold K, Nerem RM, Peter RO. A numerical calculation of flow in a curved tube model of the left main coronary artery. J Biomech. 1991;24:175–89. doi: 10.1016/0021-9290(91)90176-n. doi: 10.1016/0021-9290(91)90176-N. [DOI] [PubMed] [Google Scholar]
- 21.He X, Ku DN. Pulsatile flow in the human left coronary artery bifurcation: Average conditions. J Biomech Eng. 1996;118:74–82. doi: 10.1115/1.2795948. doi: 10.1115/1.2795948. [DOI] [PubMed] [Google Scholar]
- 22.Giannoglou GD, Soulis JV, Farmakis TM, Giannakoulas GA, Parcharidis GE, Louridas GE. Wall pressure gradient in normal left coronary artery tree. Med Eng Phys. 2005;27:455–64. doi: 10.1016/j.medengphy.2004.12.015. doi: 10.1016/j.medengphy.2004.12.015. [DOI] [PubMed] [Google Scholar]
- 23.Badak O, Schoenhagen P, Tsunoda T, Magyar WA, Coughlin J, Kapadia S, et al. Characteristics of atherosclerotic plaque distribution in coronary artery bifurcations:An intravascular ultrasound analysis. Coron Artery Dis. 2003;14:309–16. doi: 10.1097/01.mca.0000076511.29238.f1. doi: 10.1097/01.mca.0000076511.29238.f1. [DOI] [PubMed] [Google Scholar]
- 24.Koskinas KC, Chatzizisis YS, Baker AB, Edelman ER, Stone PH, Feldman CL. The role of low endothelial shear stress in the conversion of atherosclerotic lesions from stable to unstable plaque. Curr Opin Cardiol. 2009;24:580–90. doi: 10.1097/HCO.0b013e328331630b. doi: 10.1097/HCO.0b013e328331630b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Papafaklis MI, Koskinas KC, Chatzizisis YS, Stone PH, Feldman CL. In-vivo assessment of the natural history of coronary atherosclerosis: Vascular remodeling and endothelial shear stress determine the complexity of atherosclerotic disease progression. Curr Opin Cardiol. 2010;25:627–38. doi: 10.1097/HCO.0b013e32833f0236. doi: 10.1097/HCO.0b013e32833f0236. [DOI] [PubMed] [Google Scholar]
- 26.Suo J, Ferrara DE, Sorescu D, Guldberg RE, Taylor WR, Giddens DP. Hemodynamic shear stresses in mouse aortas: Implications for atherogenesis. Arterioscler Thromb Vasc Biol. 2007;27:346–51. doi: 10.1161/01.ATV.0000253492.45717.46. doi: 10.1161/01.ATV.0000253492.45717.46. [DOI] [PubMed] [Google Scholar]
- 27.Chen SL, Louvard Y, Runlin G. Perspective on bifurcation PCI. J Interv Cardiol. 2009;22:99–109. doi: 10.1111/j.1540-8183.2009.00441.x. doi: 10.1111/j.1540-8183.2009.00441.x. [DOI] [PubMed] [Google Scholar]