Dystonia is a neurologic disorder characterized by sustained involuntary movements or abnormal posture. Oromandibular dystonia is a form of focal dystonia that is characterized by forceful contractions of face, jaw, or tongue, which can cause difficulty in opening or closing the mouth, often affecting chewing and speech.[1] The course of the disease is chronic and no cure is available. Currently, reports concerning globus pallidus internus (GPi)-deep brain stimulation (DBS) for medically refractory generalized or segmental dystonia[2] are increasing. In addition, subthalamic nucleus (STN) DBS has shown similar therapeutic potential for dystonia.[3]
A 74-year-old woman presented with oromandibular dystonia 2 years ago. Symptoms were induced by a shock caused by an accident of her son falling from the roof. From then on, her mouth began to move involuntarily as she stuck out her tongue repeatedly for about 10 s every few minutes. Drugs (including haloperidol, tiapride, and botulinum toxin) could not relieve her discomfort. The disease has been progressing gradually for the past 2 months. Eating and speaking have become difficult.
Before surgery, the Burke-Fahn-Marsden dystonia rating scale (BFMDRS, movement and disability score) and SF-36 were completed to examine the patient's condition. Montreal cognitive assessment and Hamilton depression scale were used to exclude serious cognition and depression issues. In light of the limited response to GPi-DBS in patients with secondary dystonia reported in the literature,[4] it was decided to test multitarget subthalamic and pallidal stimulation. The patient was informed of this approach and agreed to carry out this surgery.
The surgical procedure has been described previously.[5] Quadripolar electrodes (L301 for STN; L302 for GPi, PINS, Beijing, China) were implanted into the targets. Tentative test stimulations were used to exclude possible side effects. Postoperative computed tomography and magnetic resonance imaging (MRI) confirmed that the leads were put in the appropriate target points. Figure 1 shows the location of the implanted electrodes in a postoperative MRI. An external neurostimulator (ENS) was connected to the DBS leads with a screening cable specific for the ENS and DBS. A trial stimulation was applied for 5 days to determine the preliminary therapeutic effects.
Figure 1.
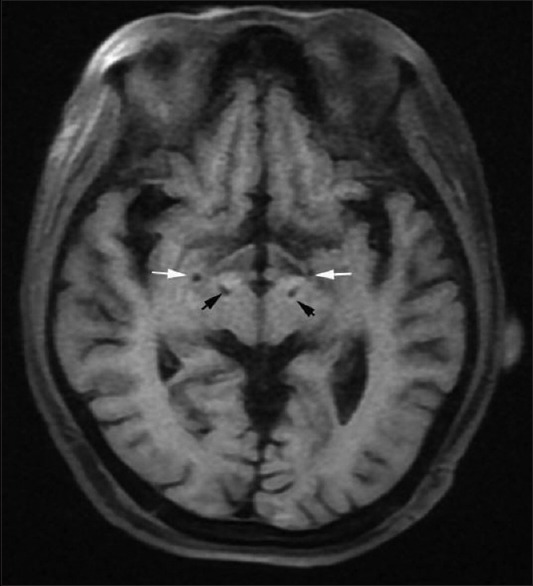
Postoperative magnetic resonance imaging showing the electrode location. The white arrows indicate the GPi and the black arrows indicate the STN. GPi: Globus pallidus internus, STN: Subthalamic nucleus.
We first tested the bilateral STN-DBS stimulation effects starting at a general parameter (60 μs, 130 Hz, 1.5 V), and the lead configurations were set to bipolar: 1−, 4+, 2 off, and 3 off (distal to proximal). One hour later, we observed that the patient's typical symptom got worse. Instead of increasing the amplitude, we turned off the stimulator and allowed a wash out period of 30 min. After restarting the stimulator using the same lead configurations, symptoms aggravated although the stimulation parameter was low (60 μs, 130 Hz, 1.0 V). We then changed to bilateral GPi-DBS (60 μs, 130 Hz, 1.0 V) with unchanged lead configurations. The patient felt better than before. Thus, we increased the pulse width to 70 μs to obtain a stronger effect. During stimulation for 2 h, the patient described a reduced frequency and duration of her typical symptom. To test the effect of combined stimulation, we stimulated both targets at the same time. When both stimulators were turned on, symptoms immediately worsened. Finally, we chose the GPi as the permanent stimulating target. An implantable pulse generator (PINS, Beijing, China) was implanted subcutaneously in the left subclavicular region, thereafter under general anesthesia and connected to the leads in the GPi while the leads in the STN were removed.
Programming began 3 weeks later. The lead configurations were set to monopolar on both sides, stimulating the most dorsal contacts. Stimulation parameters were 130 Hz, 70 μs, and 2.1 V. No obvious side effects were observed.
To the best of our knowledge, there are few reports on choosing the best targets as described above. At first, the patient was implanted with two leads in the STN and two leads in the GPi. Following careful tentative test stimulations, GPi proved to be more effective to alleviate symptoms. The leads were removed in the STN but retained in the GPi and connected to the implanted pulse generator. Previous studies may use STN alone, GPi alone, or both for long-term stimulation. We tested both targets and chose the one that was more effective and economical for outpatient.
In this case, STN-DBS seemed to induce dyskinesia, similar to its effect in treating Parkinson's disease, which made the patient felt uncomfortable although stimulation was slight. On the contrary, GPi-DBS stimulation relieved her discomfort. Since her typical symptom is repeated tongue protrusion, the BFMDRS may not adequately reflect her situation. We mainly acted according to her feelings and the frequency and duration of each “seizure” to quantify her symptom. However, the tentative test stimulations are rough and long-term effects still need to be confirmed. We will continue to follow-up on this patient in the future and will perform more tests.
Financial support and sponsorship
This study was supported by grants from the National Natural Science Foundation of China (No. 81471315), the Beijing Municipal Administration of Hospitals Clinical Medicine Development of Special Funding (No. ZYLX201305), and the Scientific Research Common Program of Beijing Municipal Commission of Education (No. KZ201510025029).
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Qiang Shi
REFERENCES
- 1.Marsden CD. Blepharospasm-oromandibular dystonia syndrome (Brueghel's syndrome). A variant of adult-onset torsion dystonia? J Neurol Neurosurg Psychiatry. 1976;39:1204–9. doi: 10.1136/jnnp.39.12.1204. doi: 10.1136/jnnp.39.12.1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Volkmann J, Wolters A, Kupsch A, Müller J, Kühn AA, Schneider GH, et al. Pallidal deep brain stimulation in patients with primary generalised or segmental dystonia:5-year follow-up of a randomised trial. Lancet Neurol. 2012;11:1029–38. doi: 10.1016/S1474-4422(12)70257-0. doi: 10.1016/s1474-4422(12)70257-0. [DOI] [PubMed] [Google Scholar]
- 3.Fonoff ET, Campos WK, Mandel M, Alho EJ, Teixeira MJ. Bilateral subthalamic nucleus stimulation for generalized dystonia after bilateral pallidotomy. Mov Disord. 2012;27:1559–63. doi: 10.1002/mds.25127. doi: 10.1002/mds.25127. [DOI] [PubMed] [Google Scholar]
- 4.Eltahawy HA, Saint-Cyr J, Giladi N, Lang AE, Lozano AM. Primary dystonia is more responsive than secondary dystonia to pallidal interventions:Outcome after pallidotomy or pallidal deep brain stimulation. Neurosurgery. 2004;54:613–19. doi: 10.1227/01.neu.0000108643.94730.21. doi: 10.1227/01.neu.0000108643.94730.21. [DOI] [PubMed] [Google Scholar]
- 5.Wang X, Zhang C, Wang Y, Liu C, Zhao B, Zhang JG, et al. Deep brain stimulation for craniocervical dystonia (Meige Syndrome):A report of four patients and a literature-based analysis of its treatment effects. Neuromodulation. 2015 doi: 10.1111/ner.12345. [Epub ahead of print] doi:10.1111/ner.12345. [DOI] [PubMed] [Google Scholar]


