Abstract
Clinical evidence strongly suggests that certain live vaccines, in particular Bacille Calmette–Guérin (BCG) and measles vaccines, can reduce all-cause mortality, likely via protection against non-targeted pathogens in addition to the targeted pathogen. The underlying mechanisms are currently unknown. We discuss how heterologous lymphocyte activation and innate immune memory could promote protection beyond the intended target pathogen and consider how vaccinologists could leverage heterologous immunity to improve outcomes in vulnerable populations, in particular the very young and the elderly.
The goal of vaccination is to induce an immune response against one or more antigens that results in persistent antibody production (to maintain circulating and mucosal antibody levels) and/or more efficient (faster and/or increased) mobilization of adaptive immune cells (specifically, T cells and B cells) when an individual subsequently encounters a pathogen containing those antigens months or years later. Antigens can be delivered as purified molecules (for example, in subunit vaccines) or in a more complex format (for example, as components of live attenuated or inactivated microbes). Purified antigens are generally poorly immunogenic, and therefore adjuvants are used to boost their immunogenicity. Whole microbes often have intrinsic adjuvant activity due in part to their immunostimulatory molecules, such as cell wall components and nucleotides that engage pattern recognition receptors (PRRs). However, even vaccines that deliver intact microbes may be enhanced by an additional adjuvant, especially in the case of inactivated vaccines. Aluminum salts (alum) are the most commonly used adjuvants in human vaccines, but microbe-derived components and their synthetic congeners, and/or oil-in-water emulsions that engage PRR pathways are increasingly attractive alternative adjuvants in the development of new vaccines 1, 2.
Vaccines have classically been thought to generate specificity and memory via activation of antigen-induced adaptive immune responses mediated by T cells and B cells. Adjuvants may promote these responses by stimulating antigen acquisition and immunogenic presentation by antigen presenting cells (APCs) of the innate immune system (principally dendritic cells, DCs). However, as discussed below, accumulating evidence from clinical and laboratory studies indicates that heterologous activation of lymphocytes and innate immune memory mechanisms also shape the host response to vaccination.
Moreover, several lines of evidence suggest that certain vaccines influence immune responses against either other vaccines, or pathogens not targeted by the vaccine. These effects have variously been called ‘heterologous’, ‘non-specific’ or ‘off-target’ effects. As defined in Box 1, we herein use the term ‘heterologous’ to describe a vaccine that is designed to target a specific pathogen, but also impacts the host’s response to unrelated pathogens (or potentially to the host itself), with unanticipated effects on morbidity and mortality that are not attributable to prevention of the disease(s) targeted by the vaccine. We also use the term ‘heterologous’ to describe the activation of lymphocyte responses (antigen-specific or non-specific) that are directed against non-target antigens.
Box 1. Definitions: heterologous effects and mechanisms.
A vaccine that confers protection against unrelated pathogens, in addition to the target pathogen, is described as having heterologous effects (see Box 2 and Table 1 for examples). Heterologous effects of vaccination may persist for long periods (see Box 3). Deleterious (negative) heterologous effects are also possible if vaccination impairs the ability of the host to combat infection with non-targeted pathogens. Vaccines may also have heterologous effects (positive or negative) that are directed against host tissues, such as induction of an anti-tumor response or autoimmune disease.
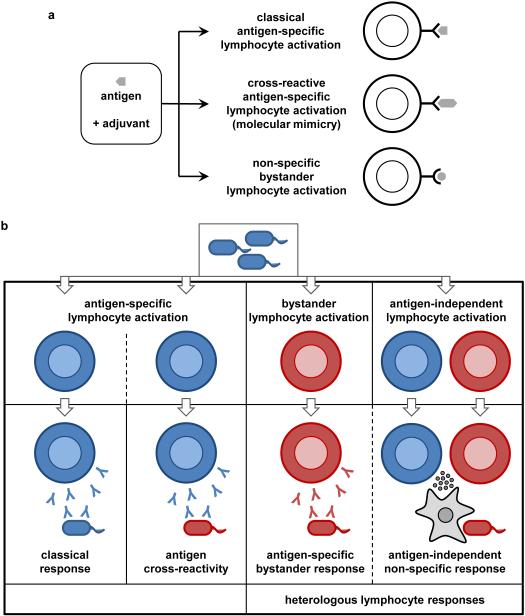
In some cases, heterologous effects can be attributed to antigen cross-reactivity, whereby lymphocytes specific for the vaccine antigen also recognize other antigens due to molecular mimicry. However, most heterologous effects of vaccination cannot be explained by molecular mimicry.
Heterologous effects of vaccination may alternatively be mediated by heterologous immune responses that are not specifically directed against the vaccine antigen (see Figures 1 and 2). Heterologous lymphocyte responses include the broad effects of cytokines produced by activated T cells (for example, macrophage activation by IFNγ) and activation of ‘bystander’ lymphocytes that are specific for non-targeted antigens. Heterologous immune responses can also involve lymphocyte-independent activation of innate immune cells. These effects may persist as a result of ‘innate memory’ mechanisms involving macrophages or NK cells. For example, epigenetic reprogramming due to sustained changes in gene expression and cell physiology, without permanent genetic changes (mutations or recombination), underlies ‘trained immunity’.
In this Opinion article, we discuss how complex effects of antigens and adjuvants underlie immune responses to vaccines, with parallels to the development of immunity following natural infection. Moreover, we consider how these effects could account for heterologous clinical effects of vaccination, and reflect on the implications of vaccine-induced heterologous immunity for the optimization of immunization programmes.
Heterologous effects of vaccination
A major goal of modern vaccinology is to influence the magnitude, quality and durability of the T and B cell response using adjuvants, viral vectors, virus-like particles and other formulations and delivery vehicles to enhance immune responses 2. The aim is to generate protective immunity in immunologically naive or less immunocompetent populations (especially the very young, who are the target of most vaccines, and the elderly) by boosting responses against ‘weak’ antigens and inducing responses that are more broadly protective against a range of microbial strains (for example, against multiple strains of influenza virus).
In addition to providing immune protection against their intended targets, vaccines may have other beneficial effects, such as broadening the diversity of cross-clade protection within an individual species. For example, the MF59-adjuvanted influenza vaccine provided expanded coverage to include related but antigenically distinct influenza virus strains in addition to the target strain 3. Vaccines may also provide broader benefit by preventing opportunistic secondary infections. Indeed, a recent population-level analysis describing the post-disease immunosuppressive effects of measles infections argued that measles vaccination not only provides protection against the measles virus itself, but also prevents the prolonged immunosuppression that occurs as a consequence of this disease; as such, the vaccine also reduces non-measles mortality 4.
Moreover, there is increasing evidence that some vaccines can broadly enhance immune responses to a range of distinct pathogens or other vaccines (see Boxes 2, 3), which indicates that immune protection may be influenced by prior exposure to unrelated microbes or microbial components. The strongest evidence of such effects in humans relates to the apparent ability of the Bacille Calmette-Guerin (BCG) vaccine (live attenuated Mycobacterium bovis) to reduce all-cause infant mortality in high mortality settings 5-7 (see Table 1). Similar benefits have been reported for other live attenuated vaccines, specifically measles vaccine and oral polio vaccine 8, 9. The observed reductions in all-cause mortality appear to extend beyond the direct protection induced by these vaccines against their target pathogens, and likely reflect the induction of resistance to unrelated pathogens via enhancement of heterologous adaptive immune responses and/or antigen non-specific innate immune mechanisms. These positive heterologous effects of certain live attenuated vaccines may not be limited to resource-poor settings. Indeed, certain live attenuated vaccines, such as measles, smallpox and polio vaccines, may also reduce infection-related hospitalization in developed countries by providing protection against unrelated pathogens 10-13.
Box 2. Heterologous effects of vaccination.
The strongest evidence for heterologous effects of vaccination relates to the apparent ability of certain live attenuated vaccines to enhance immunity in an antigen non-specific manner, resulting in reduced all-cause mortality that likely reflects the induction of resistance to unrelated pathogens 5, 89. Specifically, the World Health Organization (WHO) Strategic Advisory Group of Experts (SAGE) on Immunization recently concluded that available evidence suggests a possible beneficial effect of immunization with Bacille Calmette-Guerin (BCG; a live attenuated Mycobacterium bovis strain) and live attenuated measles vaccine on all-cause mortality in high risk populations during the first weeks of life 5, 7 (see Table 1). Oral polio vaccine has also been reported to reduce all-cause mortality in high mortality settings 9.
Such benefits of live attenuated vaccines may not be limited to resource poor settings. A retrospective analysis of epidemiological data from Spain suggested that BCG given at birth may decrease hospitalization due to sepsis and respiratory infection 10. Also, cohort studies from Denmark have suggested that measles, smallpox and oral polio vaccines reduce the rate of infection-related hospitalization 11-13.
Research is ongoing to determine whether other types of vaccines, including inactivated vaccines, also exhibit heterologous effects and to evaluate the potential for negative as well as positive effects 5.
Vaccines can also have heterologous effects (positive or negative) on host tissues. For example, the anti-tumour effects of BCG have been leveraged to treat non-invasive bladder cancer 14. Other vaccines have been implicated in the development of autoimmunity, such as the increased incidence of narcolepsy among children who received the AS03-adjuvanted H1N1 influenza vaccine 18, 21.
Box 3. Durability of heterologous effects of vaccination.
Data describing the durability of heterologous protection following vaccination are limited. In the randomized controlled trials that provide some of the strongest evidence of a beneficial effect, BCG given at birth in a high-risk population in Guinea-Bissau was associated with a reduction in all-cause mortality of 58%, 48% and 21% over the first few days, first month and first year of life respectively 90. This might suggest that protection waned rapidly. However, it is possible that the apparent reduction in all-cause mortality over time was related to a gain in protection among individuals in the control group who subsequently received the vaccine (from 6 weeks of age onwards).
Similarly, a study of all-cause mortality following measles vaccination in infants also suggested that protection is greatest in the first weeks to months after vaccination 8, but the apparent diminution in heterologous protection may also be due in part to confounding factors.
At least one study suggests that heterologous effects can persist for more than a decade. An epidemiological study of 10-14 year old children in Spain reported 69.6% fewer incidents of hospitalization due to respiratory infections not attributable to tuberculosis among those who had received BCG at birth, compared with those who had not received the BCG vaccine 10.
Mechanistic studies are consistent with the possibility that at least some heterologous effects may be durable. Heterologous lymphocyte responses may persist for decades in memory cells, and innate immune memory may be maintained by epigenetic reprogramming of long-lived differentiated cells (including tissue-resident macrophages) or hematopoietic progenitors. Indeed, some heterologous responses to non-mycobacterial stimuli have been reported to remain strongly elevated 1 year after BCG vaccination of healthy adults 91.
Table 1.
Impact of BCG and measles vaccines on all-cause mortality.*
| Vaccine | Country (year) |
Sample size |
Observed % reduction in all-cause mortality (95% CI) |
References |
|---|---|---|---|---|
| BCG | Canada (1933-1945) |
609 | 6% (−32, 33) | 101 |
| BCG | Guinea Bissau (2002-2008) |
2343 | 45% (11, 66) | 90, 102 |
| BCG | Guinea Bissau (2002-2008) |
105 | 72% (−37, 94) | 90 |
| BCG | USA (1935) | 3008 | 9% (−99, 59) | 103 |
| BCG | USA (1941) | 451 | 58% (−35, 87) | 104 |
| Measles | Guinea Bissau (2002-2008) |
6417 | 33% (−19, 62) | 8 |
| Measles | Guinea Bissau (1989-1999) |
300 | 0% (−392, 80) | 105 |
| Measles | Guinea Bissau (1989-1999) |
8511 | 6% (−67, 47) | 106 |
| Measles | Nigeria (1961) | 1962 | 59% (15, 86) | 107 |
CI – confidence interval
Randomised trials of the impact of BCG and Measles vaccine on all-cause mortality identified in a systematic review conducted for the World Health Organisation 7. Data were derived from the 2014 Strategic Advisory Group of Experts (SAGE) on immunization report: www.who.int/immunization/sage/meetings/2014/april/3_NSE_Epidemiology_review_Report_to_SAGE_14_Mar_FINAL.pdf
Heterologous effects do not just influence immune responses to pathogens, but have also been harnessed in the treatment of non-invasive bladder cancer, where local instillation of BCG is used to induce anti-tumour responses 14. Negative heterologous effects have also been reported, including an association between the AS03-adjuvanted pandemic influenza vaccine that was widely used in Europe during the 2009 H1N1 influenza pandemic 15-17 and the development of narcolepsy in some children and infants 18, 19. It has also been suggested that inactivated vaccines ― such as DTP (diphtheria, tetanus, pertussis) ― may either have negative heterologous effects or oppose the positive heterologous effects of live vaccines 5, although evidence for this is currently limited 7.
The above examples of heterologous effects indicate that the immunological consequences of infectious diseases, and the vaccines that prevent them, may influence the host’s immediate and future interaction with antigens from unrelated organisms or the host. These observations provide both opportunities and challenges in the design of vaccines and vaccination programmes, and call for a deeper, evidence-based understanding of the mechanisms underlying immune responses to both infection and vaccination. While it is apparent that the nature of the antigen and adjuvant can have profound effects on the immune response to that antigen, it is less clear how they can also influence subsequent immune responses to other antigens, resulting in heterologous effects.
Heterologous lymphocyte responses
Antigen cross-reactivity resulting from molecular mimicry may be responsible for some heterologous effects (see Figure 1). For example, adult humans (but not neonates) have been shown to possess memory T cells reactive to viral antigens to which they had not been exposed (including HIV, herpes simplex virus (HSV) and cytomegalovirus (CMV)) and cross-reactive to antigenic peptides from non-targeted microbes 20. The development of narcolepsy in some patients who received the AS03-adjuvanted influenza (H1N1) pandemic vaccine may also be due to cross-reactivity, in this case to host antigens 21. Specifically, molecular mimicry between a fragment of one of the influenza antigens (nucleoprotein) and a portion of the human brain receptor that promotes wakefulness (hypocretin receptor 2) provides a plausible explanation for this heterologous effect, although other mechanisms have been suggested 22. Interestingly, during the same H1N1 influenza pandemic, a spike in childhood narcolepsy cases was also observed in China, where vaccination rates were very low 23. It is possible that the same antigen cross-reactivity mechanism (that is, between the nucleoprotein of the infectious virus and the host hypocretin receptor 2) was responsible for the increased narcolepsy in this population, but this has not yet been demonstrated. Antigen cross-reactivity cannot, however, explain all the diverse heterologous effects observed with other vaccines.
Figure 1. Heterologous lymphocyte responses.
a | Some heterologous effects may be attributed to antigen-specific mechanisms that generate lymphocytes whose antigen receptors are cross-reactive for distinct microbial or host antigens due to molecular mimicry. Alternatively, a vaccine may induce bystander activation of memory lymphocytes to sustain levels of antibodies directed against other targets. b | Heterologous lymphocyte responses can induce protection against unrelated pathogens via antigen-specific bystander lymphocytes or induction of antigen non-specific innate mechanisms such as cytokine production to activate macrophages.
Heterologous lymphocyte activation may account for some of the heterologous effects of these vaccines. ‘Bystander’ activation of unrelated B cells and/or T cells following infectious or vaccine-induced immune stimulation may also help to sustain pre-existing antigen-specific immunity (see Figure 1). Microbial components or cytokines may stimulate polyclonal activation of T cells or antibody production by memory B cells. Indeed, a recent report demonstrated that memory CD8+ T cells can be re-activated by inflammatory monocyte-derived interleukin-15 (IL-15) and IL-18 24. Similarly, it has been reported that immunizing adults with tetanus toxoid not only increases concentrations of tetanus-specific antibody, but also stimulates increased levels of antibodies that recognize measles and Toxoplasma gondii, either through enriched T cell help or other polyclonal stimuli 25, 26. However, others failed to see a substantial impact of heterologous immunization on pre-existing antibody titres in humans 27 or on memory B cell responses in mouse models 28. Thus, while polyclonal stimulation of B cell responses could contribute to sustained antibody responses throughout life, the evidence for this is inconsistent. When given at or around the same time as other vaccines, BCG may enhance the antibody response to those vaccines, acting as or like an adjuvant, although the specific effects/outcomes are a matter of debate 29-31. In any case, this is not likely to be the main mechanism for the beneficial heterologous effects of BCG or measles vaccine in infants because they often lack pre-existing immunity to most pathogens and durability of antibody responses is more limited than at older ages, perhaps attributable in part to suboptimal support in the bone marrow 32, 33.
Cytokine production has also been suggested to underlie the heterologous effects of BCG (see Figure 1b). Experiments conducted half a century ago demonstrated infection-induced ‘cross-protection’ between unrelated bacterial pathogens, and these pioneering observations played a crucial role in defining the concept of cell-mediated immunity 34. This classical form of cross-protection is mediated by lymphocytes that produce interferon-γ (IFN-γ) after stimulation by the first pathogen encountered (for example, BCG), thereby activating macrophages. This generates a state of heightened innate immunity against a secondary infection, which wanes rapidly once the initial pathogen is eliminated 35.
Some, but not all, studies in humans have reported that peripheral blood mononuclear cells harvested at various time points after BCG immunization exhibit increased production of IFN-γ and other pro-inflammatory cytokines upon in vitro stimulation with mitogens or unrelated antigens 36. Enhanced in vitro IFN-γ production following measles vaccination has also been reported in some studies 36. In contrast, several studies have consistently shown reduced mitogen-induced proliferative responses following vaccination 36. These apparently divergent results may reflect variation in randomization, controls, antigenic stimuli and the in vitro assays used. It is therefore difficult to draw robust conclusions regarding the impact of live attenuated vaccines on cytokine induction from the available data.
An enhanced pro-inflammatory cytokine milieu after measles and BCG vaccination might contribute to the increased protective immunity and reduced all-cause mortality observed with these vaccines. However, a clear pattern has not been determined for the antigenic requirements, timing, nature or persistence of these effects, nor their influence on subsequent antibody or T cell responses. Moreover, there is currently no direct evidence in humans that such immunological phenomena are the basis for the epidemiological observations of reduced mortality following the administration of live vaccines in high mortality settings. Indeed the immunological mechanisms underlying both the mycobacteria-targeting and the heterologous effects of BCG remain incompletely defined despite over 50 years of laboratory and animal studies highlighting its immunomodulatory properties 37-39.
Innate immune memory
In addition to the impact of lymphocyte-derived cytokines such as IFNγ on innate immune cells, recent evidence suggests that innate immune cells, especially monocytes/macrophages and natural killer (NK) cells, possess intrinsic memory characteristics and may make a greater contribution to the heterologous protective effects of vaccines than T and B cell-based adaptive immune mechanisms.
Of note, immunization with certain live microbes or microbial components that activate innate immune cells can protect mice against lethal infection with distinct pathogens. For example, heterologous immunity is induced by fungal β-glucans against infection with Staphylococcus aureus bacteria 40, 41, by the peptidoglycan component muramyl dipeptide against Toxoplasma parasites 42, by CpG oligodeoxynucleotide against Escherichia coli meningitis 43, and by flagellin against S. pneumoniae and rotavirus 44, 45. The broad characteristics of this protection implicate innate immune mechanisms rather than classical antigen-specific adaptive immune memory.
Bacteria, fungi, parasites and viruses can exert long-term immunomodulatory effects that have been demonstrated, using T and B cell-deficient or lymphocyte-depleted animals, to be independent of adaptive immunity. BCG vaccination of animals induces T and B cell-independent protection against secondary infections with Candida albicans or Schistosoma mansoni 46, 47, demonstrating that innate immune mechanisms mediate at least some of the heterologous protective effects of vaccination in these models. Moreover, protection against disseminated candidiasis (CA-6 strain of C. albicans) conferred by an attenuated (PCA-2) strain of C. albicans is dependent on macrophages 48 and pro-inflammatory cytokines 49, and is induced in athymic mice and Rag1-deficient animals, demonstrating a T and B cell-independent mechanism 50, 51. Latent infection of mice with gamma-herpesvirus also increases T cell-independent resistance to unrelated bacterial pathogens such as Listeria monocytogenes and Yersinia pestis 52, due to sustained IFNγ production and systemic macrophage activation. Similarly, the helminth parasite Nippostrongylus brasiliensis induces a long-term macrophage phenotype that damages the parasite and induces protection against re-infection independently of T and B cells 53.
‘Trained immunity’, whereby the responses of innate immune cells such as monocytes and macrophages are primed by prior exposure to the same or an unrelated stimulus 54 (see Figure 2), may underlie some of the heterologous effects of vaccination. For example, exposure of monocytes or macrophages to C. albicans or β-glucan enhances their subsequent response to stimulation with unrelated pathogens or pathogen-associated molecular patterns 51. Innate immune memory can induce various functional programs, some characterized by a combination of suppressive and stimulatory effects. This is best exemplified by lipopolysaccharide (LPS)-induced ‘tolerance’. LPS confers long-lasting innate immune effects on monocytes and macrophages, resulting in reduced inflammatory cytokine production upon either re-stimulation with LPS or subsequent exposure to unrelated microbial components. However, in contrast to its effects on inflammatory cytokine production, anti-microbial responses are primed by prior LPS exposure 55. While the functional programme may be different from that induced by β–glucan or other vaccine adjuvants, conceptually the long-term functional reprogramming of innate immune cells by LPS can therefore be considered similar to trained immunity. Thus innate memory is shaped by a combination of positive and negative effects at the molecular level (often simultaneously within the same cell – Figure 2b) that ultimately influence subsequent responses to related or unrelated pathogens.
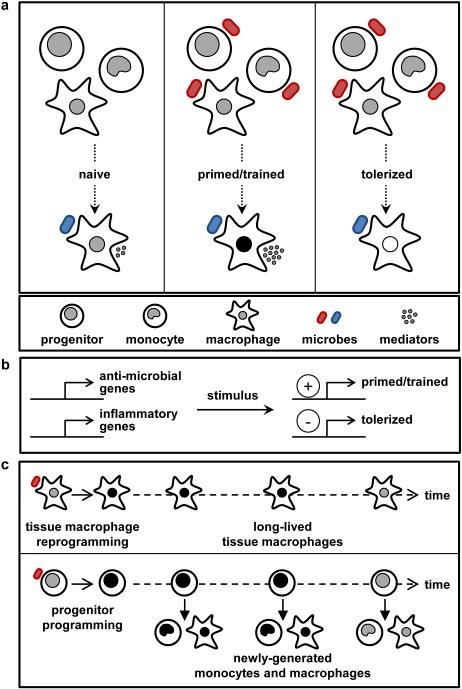
Figure 2. Mechanisms of innate memory in monocytes and macrophages.
Initial exposure of monocytes/macrophages or their progenitors to certain microbial stimuli induces epigenetic marks and metabolic changes that persist for extended periods and influence the subsequent responses of these cells to the same or distinct stimuli. a | Priming/training increases the subsequent response, whereas tolerance reduces responsiveness. b | Priming/training and tolerance can occur simultaneously within the same cell, with expression of some genes being promoted and others suppressed. c | Innate memory effects may be long-lived due to the persistence of self-renewing macrophages in tissues, or exposed progenitors that continue to yield monocytes and macrophages with altered function for extended periods. Innate memory may be maintained indefinitely or wane over time.
Epigenetic reprogramming likely underlies these effects. LPS stimulation of macrophages induces gene-specific chromatin modifications that simultaneously silence genes coding for inflammatory molecules (tolerance) while promoting the expression of genes coding for anti-microbial molecules (priming) 55. Monocyte training by C. albicans and β-glucan is also accompanied by distinct reprogramming of chromatin marks, and blockade of histone methylation has been shown to impair the induction of trained immunity 51, 56. Metabolic shifts in the trained monocytes from oxidative phosphorylation to glycolysis (known as the ‘Warburg effect’) are also important for the induction of trained immunity 57. Several metabolites may function as co-factors for epigenetic enzymes 58, and investigation of the impact of changes in cellular metabolism on the epigenetic programme of innate immune cells is an important focus of ongoing research.
Autophagy has also been implicated in the development of trained immunity. A recent study showed that epigenetic reprogramming of monocytes by BCG in vitro is dependent on autophagy 59. Blocking autophagy abrogated the in vitro training effect of BCG, and monocyte training in vitro and in vivo was also influenced by genetic variation in the autophagy gene ATG2B. Moreover, the same polymorphism correlates with responsiveness to intravesical BCG instillation in patients with non-invasive bladder cancer 59.
NK cells can also respond more vigorously after previous encounters with pathogens or other activation events. Indeed, the heterologous protective effects of BCG vaccination may be mediated in part by activation of NK cells. NK cells from BCG-vaccinated individuals exhibit enhanced pro-inflammatory cytokine production in response to mycobacteria and other unrelated pathogens, and in mice, NK cells help mediate the heterologous protective effects of BCG against C. albicans 60. Moreover, several recent studies have reported that NK cells develop adaptive immune characteristics after infection with murine cytomegalovirus (MCMV) 53, 61-63. NK cells bearing the Ly49H receptor persist for months in lymphoid and non-lymphoid organs after MCMV infection, and upon re-infection, these ‘memory’ NK cells undergo a secondary clonal expansion, rapidly degranulating and releasing cytokines, thereby inducing a protective immune response 53. NK cell memory may also involve the IL-12/IFNγ axis 63 and the activation of the co-stimulatory molecule DNAM1 (DNAX accessory molecule-1, also known as CD226) 64.
Evidence in support of NK cell memory is almost entirely derived from rodent studies 65, but a recent report showed that simian immunodeficiency virus infection and viral vectored-vaccines induce NK cell memory in macaques that can persist for up to 5 years 66. However, this immunity, like NK cell memory induced by MCMV or hapten sensitization in mice 67-69, was specific to the priming agent. Thus unlike the heterologous effects seen in trained monocytes and macrophages, NK cell memory may provide pathogen specificity. MCMV-induced NK memory, for example, appears to be specific for MCMV and to impair NK cell responses to other pathogens such as influenza and L. monocytogenes 68. Depending on the conditions, NK cells are therefore able to employ both trained immunity, which may be mediated by epigenetic changes 62, 70, and antigen-specific memory.
An important aspect to be considered regarding trained immunity is the lifespan of innate immune cells, particularly monocytes and the macrophages derived from them. In humans, trained monocytes can be observed in the circulation for at least three months after BCG vaccination 60. This observation suggests that reprogramming also takes place at the level of progenitor cells to account for the persistence of modified populations of these relatively short-lived cells (Figure 2c). Indeed, innate immune memory effects of microbial exposure can be transferred from haematopoietic stem and progenitor cells to their progeny. For instance, haematopoietic stem and progenitor cells exposed to Toll-like receptor 2 (TLR2) agonists generate macrophages that produce lower amounts of inflammatory cytokines and reactive oxygen species 71. Moreover, NK cells prime monocytes for regulatory function in the bone marrow in response to T. gondii infection, an effect that is mediated at least in part by IFNγ programming of monocyte progenitors 72. Epigenetic imprinting of haematopoietic progenitors may enable chronic persistence of training/tolerance effects. Indeed, UV radiation of the skin induces prostaglandin E2 (PGE2)-mediated systemic immunosuppression due to modulation of DC function, and this effect can be transferred to naive mice upon serial bone marrow transplantation due to long-lasting epigenetic reprogramming of DC progenitors (presumably haematopoietic stem cells) 73-75.
Impact of age on heterologous immunity
The heterologous effects of infection and vaccination are likely impacted by age. Indeed, the beneficial heterologous effects of BCG were most evident when given early in life 7. Moreover, narcolepsy following the monovalent AS03-adjuvanted H1N1 pandemic influenza vaccine was primarily observed in children and adolescents, and decreased as the subjects approached adulthood 76-78. In contrast, in elderly subjects a related vaccine enhanced protection against some influenza subtypes compared to the non-adjuvanted trivalent seasonal influenza vaccine and decreased all-cause morbidity and mortality, despite not meeting the main criteria for efficacy in this clinical study 79, 80. A recent study reported that the early immune response of healthy adults to the monovalent AS03-adjuvanted H1N1 vaccine changes significantly after the age of ~35 years 81.
The immune system undergoes development throughout childhood. Th1-type cytokines (such as tumour necrosis factor (TNF), IL-12 and IFNγ), NK cell cytotoxicity, antigen-specific Th1 cell responses and high affinity antibody production are all low during the neonatal period and gradually increase over time (see Box 4). Immune function is shaped in early life by colonization with commensal microbes and exposure to pathogens and vaccines. Given this immune ontogeny and the fact that, to date, most beneficial heterologous effects have been noted with paediatric vaccines given in early life, innate immune memory mechanisms may play a particularly prominent role in early life, likely exceeding that of heterologous lymphocyte activation 82. Indeed, blood cells isolated 4 weeks after neonatal BCG immunization exhibit increased TLR-induced cytokine production in vitro, suggesting that BCG may accelerate the development of innate cytokine responses 83.
Box 4. The immune system in early life.
Humans (unlike rodents) have high numbers of T and B cells by mid-gestation, although immune development continues for many years after birth 92. A ‘layered’ immune system, comprising a mix of fetal liver-derived and adult-like bone marrow-derived leukocytes, exists in early life 1.
Regulatory T cells dominate in utero 93, and at birth almost all T and B cells are phenotypically and functionally naive. Regulatory and Th2-type immune responses are favoured in neonates, and a progressive shift to Th17-type and then type I interferon responses follows over the first year. Th1-type responses gradually develop later. Newborns exhibit impaired production of Th1-promoting cytokines 1, consistent with high susceptibility to intracellular pathogens, which declines throughout infancy 84.
Infants also have a lower capacity to mount robust and durable antibody responses. T cell-dependent antibody responses reach near adult competence by 1 year of age, and T cell-independent responses by 2-4 years 94. Infant NK cells are approximately half as effective as adult NK cells at killing most targets, although their cytotoxic capacity can be augmented by Th1 cell-promoting cytokines and approaches that of adult cells by 1 year of age 92. Limited neutrophil mobilization and mononuclear phagocyte function (including their responsiveness to IFNγ) may also contribute to susceptibility to infection in neonates.
Postnatal immune development is profoundly impacted by the commensal microbiota, which is initially acquired from the mother during vaginal delivery and matures thereafter in an age- and environment-dependent manner 95-98, as well as pathogens and live vaccines. Human newborn and adult monocytes exhibit distinct responses to adjuvants and licensed adjuvanted vaccines in vitro that demonstrate age- and adjuvant-specific correlations to adjuvanted vaccine-induced responses in vivo 99. Moreover, TLR agonist administration to newborn mice enhances their subsequent cytokine and phagocytic responses to polymicrobial sepsis 100, and early inflammatory/innate immune-activating events in humans are associated with reduced risk of late-onset sepsis 82, 83.
Among the elderly, decreased innate and adaptive immune function underlies increased susceptibility to infection and reduced vaccine efficacy 84, 85. Thymic involution, restricted lymphocyte clonality and declining anti-microbial innate immune function contribute to sub-optimal responses to many vaccine antigens and adjuvants. Little is known about the heterologous effects of vaccination in elderly populations or the impact of immunosenescence on heterologous lymphocyte responses and trained immunity. Dysregulated cytokine responses associated with immunosenescence following a lifetime of microbial exposure may lead to diminished trained immunity, but this remains to be investigated.
Perspectives for vaccinology
The studies and mechanisms described above present vaccinologists with exciting opportunities, as well as a number of challenges, in relation to the design of new vaccines and the development of vaccination programmes. Positive heterologous effects mediated by innate and/or adaptive immune mechanisms could be leveraged to confer broader protection against a range of pathogens. If a ‘super-protective’ state could be induced by vaccination, even if only for a brief window of exquisite infectious susceptibility, such as the neonatal period, important improvements in health could be realized. On the other hand, the potential for negative heterologous effects must also be considered in order to improve vaccine efficacy and safety.
High quality randomized controlled trials are needed to establish heterologous effects (both pathogen-specific and non-specific) of vaccines in diverse populations in order to define, for example, the impact of age, genetics, geographical location, and sociological factors. These studies must be accompanied by thorough characterization of immunologic correlates of clinically observed heterologous effects, including phenotypic and molecular changes in monocytes, NK cells, lymphocytes and other leukocytes. Model organisms should also be employed to establish the role of proposed immune mechanisms in the observed effects. Bioinformatics tools will enable the refinement of vaccine antigens to exclude those that are potentially cross-reactive, thus limiting the negative effects. In addition, positive and negative heterologous effects could be predicted by taking advantage of new technologies such as human in vitro platforms that model age-specific immunity 1 and powerful systems biology approaches 81, 86, 87 that can now be employed using small sample volumes 88.
Defining mechanisms of heterologous lymphocyte activation and innate immune memory in early life and the elderly is of particular importance because infection-induced mortality is highest in the first year of life and aging-associated susceptibility to infection is placing increasing pressure on healthcare resources in the developed world. Given the burden of infection in those at the extremes of age, an effective plan is needed that engages funding agencies and other stakeholders to mobilize the resources required to leverage these newly discovered immunologic pathways to reduce infection-induced morbidity and mortality.
Key Points.
A vaccine that confers protection against unrelated pathogens in addition to the target pathogen is described as having heterologous effects. For example, some live vaccines (in particular BCG and measles vaccine) have been shown to reduce all-cause mortality in high risk populations.
Heterologous effects may persist for long periods (months or even years).
Most heterologous effects cannot be attributed to antigen cross-reactivity.
Heterologous effects may be mediated by heterologous lymphocyte activation or by innate immune memory mechanisms such as ‘trained immunity’.
The age of the vaccinated individual impacts the immune response and therefore the heterologous effects. It is particularly important to study heterologous effects and mechanisms in infants and the elderly.
Leveraging heterologous effects could reduce infection-induced morbidity and mortality in vulnerable populations.
Acknowledgements
This work was supported by funding from the National Institutes of Health (NIH) Infant Immunity Program (RO1 5R01AI100135-03 to OL), the European Research Council (Consolidator Grant #310372 to MGN), the Michael Smith Foundation for Health Research (Career Investigator Award to TRK), the Canadian Institute of Health Research (300819 to TRK), the BC Children’s Hospital Foundation (to TRK), and the NIHR Oxford Biomedical Research Centre (to AJP).
Biographies
Helen S. Goodridge received her Ph.D. from the University of Glasgow, U.K. After postdoctoral training there and at Cedars-Sinai Medical Center, Los Angeles, USA, she joined the Cedars-Sinai faculty and is currently an Associate Professor. Her research focuses on the production and function of myeloid cells in infectious and inflammatory diseases, cancer and aging.
S. Sohail Ahmed received his M.D. degree from the University of Texas Medical School in Houston where he completed his general medicine residency training and clinical immunology/rheumatology subspecialty training. In 2006, he joined Novartis Pharmaceuticals (Translational Medicine) and in 2011, he was appointed Global Head of Clinical Sciences at Novartis Vaccines & Diagnostics Srl. He is currently affiliated with VisMederi Srl in Siena, Italy.
Nigel Curtis completed medical training in Cambridge and London, received his PhD from the University of London, and did further clinical and laboratory training in London and Vancouver before moving to Melbourne, Australia. He is Head of Infectious Diseases at the Royal Children’s Hospital Melbourne, Professor at the University of Melbourne, and Leader of the Infectious Diseases & Microbiology Research Group at Murdoch Children’s Research Institute. His current research focuses on the heterologous effects of BCG vaccination.
Tobias R. Kollmann completed his MD and PhD at the Albert of Einstein College of Medicine and his residency and fellowship at the University of Washington, Seattle. He is now consulting physician in pediatric infectious disease at BC Children’s Hospital, Canada. His research focuses on development of the immune system, with special emphasis on vaccines for early life. His team has developed high-throughput platforms to dissect molecular events in small samples from newborns and small infants.
Ofer Levy received his MD and PhD (Microbiology) from New York University. He completed his residency in Pediatrics and fellowship in Pediatric Infectious Diseases at Boston Children’s Hospital, along with postdoctoral research training at the Brigham & Women’s Hospital. He is currently staff physician and Director of the Precision Vaccines Program at Boston Children’s Hospital and Associate Professor of Pediatrics at Harvard Medical School. His laboratory focuses on employing human in vitro systems and adjuvant discovery to develop vaccine formulations tailored to those at the extremes of life - the very young and the elderly.
Mihai G. Netea studied medicine in Cluj-Napoca, Romania and completed his PhD at the Radboud University Nijmegen, The Netherlands. After postdoctoral training at the University of Colorado, he returned to Nijmegen to finish his clinical training as an infectious diseases specialist. He currently heads the division of Experimental Medicine, Department of Internal Medicine, Radboud University Medical Center. His main research interests are sepsis and immunoparalysis, pattern recognition of fungal pathogens, primary immunodeficiencies in the innate immune system, and the study of the memory traits of innate immunity.
Andrew J. Pollard, FRCPCH PhD, is Professor of Paediatric Infection and Immunity at the University of Oxford, UK. He trained in paediatric infectious diseases in the UK and Canada. His research includes the design, development and clinical evaluation of vaccines in the UK and Nepal. He has supervised 23 PhD students and has published over 300 manuscripts.
Reinout van Crevel is an internist-infectious diseases specialist. His primary research focus is the interaction of mycobacteria with innate host defense, combining patient cohorts, mainly in Indonesia, with in vitro studies. He leads a consortium on the interaction between diabetes and tuberculosis, with field work and laboratory studies in eight different countries.
Chris B. Wilson, M.D., is Director of the Global Health Discovery & Translational Sciences program at the Bill & Melinda Gates Foundation. He is a member of the Advisory Committee to the Director, NIH and the Advisory Council of the National Institute of Allergy & Infections Diseases, and an elected fellow of the American Association for the Advancement of Science.
Footnotes
Further information
Systematic review of the non-specific effects of BCG, DTP and measles containing vaccines: www.who.int/immunization/sage/meetings/2014/april/3_NSE_Epidemiology_review_Report_to_SAGE_14_Mar_FINAL.pdf
Systematic review of the non-specific immunological effects of selected routine childhood immunizations:
References
- 1.Dowling DJ, Levy O. Ontogeny of early life immunity. Trends Immunol. 2014;35:299–310. doi: 10.1016/j.it.2014.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coffman RL, Sher A, Seder RA. Vaccine adjuvants: putting innate immunity to work. Immunity. 2010;33:492–503. doi: 10.1016/j.immuni.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Khurana S, et al. MF59 adjuvant enhances diversity and affinity of antibody-mediated immune response to pandemic influenza vaccines. Sci Transl Med. 2011;3:85ra48. doi: 10.1126/scitranslmed.3002336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mina MJ, Metcalf CJ, de Swart RL, Osterhaus AD, Grenfell BT. Long-term measles-induced immunomodulation increases overall childhood infectious disease mortality. Science. 2015;348:694–9. doi: 10.1126/science.aaa3662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aaby P, Kollmann TR, Benn CS. Nonspecific effects of neonatal and infant vaccination: public-health, immunological and conceptual challenges. Nat Immunol. 2014;15:895–9. doi: 10.1038/ni.2961. [DOI] [PubMed] [Google Scholar]
- 6.Flanagan KL, et al. Heterologous ("nonspecific") and sex-differential effects of vaccines: epidemiology, clinical trials, and emerging immunologic mechanisms. Clin Infect Dis. 2013;57:283–9. doi: 10.1093/cid/cit209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Organization, W.H. Meeting of the Strategic Advisory Group of Experts on immunization, April 2014 - conclusions and recommendations. Weekly epidemiological record. 2014;89:221–236. [PubMed] [Google Scholar]
- 8.Aaby P, et al. Non-specific effects of standard measles vaccine at 4.5 and 9 months of age on childhood mortality: randomised controlled trial. BMJ. 2010;341:c6495. doi: 10.1136/bmj.c6495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lund N, et al. The Effect of Oral Polio Vaccine at Birth on Infant Mortality: A Randomized Trial. Clin Infect Dis. 2015;61:1504–11. doi: 10.1093/cid/civ617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Castro MJ, Pardo-Seco J, Martinon-Torres F. Nonspecific (Heterologous) Protection of Neonatal BCG Vaccination Against Hospitalization Due to Respiratory Infection and Sepsis. Clin Infect Dis. 2015;60:1611–9. doi: 10.1093/cid/civ144. [DOI] [PubMed] [Google Scholar]
- 11.Sorup S, et al. Live vaccine against measles, mumps, and rubella and the risk of hospital admissions for nontargeted infections. JAMA. 2014;311:826–35. doi: 10.1001/jama.2014.470. [DOI] [PubMed] [Google Scholar]
- 12.Sorup S, et al. Smallpox vaccination and all-cause infectious disease hospitalization: a Danish register-based cohort study. Int J Epidemiol. 2011;40:955–63. doi: 10.1093/ije/dyr063. [DOI] [PubMed] [Google Scholar]
- 13.Sorup S, et al. Oral Polio Vaccination and Hospital Admissions With Non-Polio Infections in Denmark: Nationwide Retrospective Cohort Study. Open Forum Infect Dis. 2016;3:ofv204. doi: 10.1093/ofid/ofv204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kamat AM, Porten S. Myths and mysteries surrounding bacillus Calmette-Guerin therapy for bladder cancer. Eur Urol. 2014;65:267–9. doi: 10.1016/j.eururo.2013.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clark TW, et al. Trial of 2009 influenza A (H1N1) monovalent MF59-adjuvanted vaccine. N Engl J Med. 2009;361:2424–35. doi: 10.1056/NEJMoa0907650. [DOI] [PubMed] [Google Scholar]
- 16.Mbow ML, De Gregorio E, Valiante NM, Rappuoli R. New adjuvants for human vaccines. Curr Opin Immunol. 2010;22:411–6. doi: 10.1016/j.coi.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 17.Roman F, Vaman T, Kafeja F, Hanon E, Van Damme P. AS03(A)-Adjuvanted influenza A (H1N1) 2009 vaccine for adults up to 85 years of age. Clin Infect Dis. 2010;51:668–77. doi: 10.1086/655830. [DOI] [PubMed] [Google Scholar]
- 18.Ahmed SS, Schur PH, MacDonald NE, Steinman L. Narcolepsy, 2009 A(H1N1) pandemic influenza, and pandemic influenza vaccinations: what is known and unknown about the neurological disorder, the role for autoimmunity, and vaccine adjuvants. J Autoimmun. 2014;50:1–11. doi: 10.1016/j.jaut.2014.01.033. [DOI] [PubMed] [Google Scholar]
- 19.Pasquale AD, Preiss S, Silva FT, Garcon N. Vaccine Adjuvants: from 1920 to 2015 and Beyond. Vaccines (Basel) 2015;3:320–43. doi: 10.3390/vaccines3020320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Su LF, Kidd BA, Han A, Kotzin JJ, Davis MM. Virus-specific CD4(+) memory-phenotype T cells are abundant in unexposed adults. Immunity. 2013;38:373–83. doi: 10.1016/j.immuni.2012.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ahmed SS, et al. Antibodies to influenza nucleoprotein cross-react with human hypocretin receptor 2. Sci Transl Med. 2015;7:294ra105. doi: 10.1126/scitranslmed.aab2354. [DOI] [PubMed] [Google Scholar]
- 22.Tesoriero C, et al. H1N1 influenza virus induces narcolepsy-like sleep disruption and targets sleep-wake regulatory neurons in mice. Proc Natl Acad Sci U S A. 2016;113:E368–77. doi: 10.1073/pnas.1521463112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Han F, et al. Narcolepsy onset is seasonal and increased following the 2009 H1N1 pandemic in China. Ann Neurol. 2011;70:410–7. doi: 10.1002/ana.22587. [DOI] [PubMed] [Google Scholar]
- 24.Soudja SM, Ruiz AL, Marie JC, Lauvau G. Inflammatory monocytes activate memory CD8(+) T and innate NK lymphocytes independent of cognate antigen during microbial pathogen invasion. Immunity. 2012;37:549–62. doi: 10.1016/j.immuni.2012.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Traggiai E, Puzone R, Lanzavecchia A. Antigen dependent and independent mechanisms that sustain serum antibody levels. Vaccine. 2003;21(Suppl 2):S35–7. doi: 10.1016/s0264-410x(03)00198-1. [DOI] [PubMed] [Google Scholar]
- 26.Bernasconi NL, Traggiai E, Lanzavecchia A. Maintenance of serological memory by polyclonal activation of human memory B cells. Science. 2002;298:2199–202. doi: 10.1126/science.1076071. [DOI] [PubMed] [Google Scholar]
- 27.Di Genova G, Roddick J, McNicholl F, Stevenson FK. Vaccination of human subjects expands both specific and bystander memory T cells but antibody production remains vaccine specific. Blood. 2006;107:2806–13. doi: 10.1182/blood-2005-08-3255. [DOI] [PubMed] [Google Scholar]
- 28.Benson MJ, et al. Distinction of the memory B cell response to cognate antigen versus bystander inflammatory signals. J Exp Med. 2009;206:2013–25. doi: 10.1084/jem.20090667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ota MO, et al. Influence of Mycobacterium bovis bacillus Calmette-Guerin on antibody and cytokine responses to human neonatal vaccination. J Immunol. 2002;168:919–25. doi: 10.4049/jimmunol.168.2.919. [DOI] [PubMed] [Google Scholar]
- 30.Ritz N, Mui M, Balloch A, Curtis N. Non-specific effect of Bacille Calmette-Guerin vaccine on the immune response to routine immunisations. Vaccine. 2013;31:3098–103. doi: 10.1016/j.vaccine.2013.03.059. [DOI] [PubMed] [Google Scholar]
- 31.Leentjens J, et al. BCG Vaccination Enhances the Immunogenicity of Subsequent Influenza Vaccination in Healthy Volunteers: A Randomized, Placebo-Controlled Pilot Study. J Infect Dis. 2015;212:1930–8. doi: 10.1093/infdis/jiv332. [DOI] [PubMed] [Google Scholar]
- 32.Belnoue E, et al. APRIL is critical for plasmablast survival in the bone marrow and poorly expressed by early-life bone marrow stromal cells. Blood. 2008;111:2755–64. doi: 10.1182/blood-2007-09-110858. [DOI] [PubMed] [Google Scholar]
- 33.PrabhuDas M, et al. Challenges in infant immunity: implications for responses to infection and vaccines. Nat Immunol. 2011;12:189–94. doi: 10.1038/ni0311-189. [DOI] [PubMed] [Google Scholar]
- 34.Mackaness GB. The Immunological Basis of Acquired Cellular Resistance. J Exp Med. 1964;120:105–20. doi: 10.1084/jem.120.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mackaness GB. The influence of immunologically committed lymphoid cells on macrophage activity in vivo. J Exp Med. 1969;129:973–92. doi: 10.1084/jem.129.5.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Organization, W.H. Systematic review of the non-specific immunological effects of selected routine childhood immunisations. 2014. [DOI] [PMC free article] [PubMed]
- 37.Freyne B, Marchant A, Curtis N. BCG-associated heterologous immunity, a historical perspective: experimental models and immunological mechanisms. Trans R Soc Trop Med Hyg. 2015;109:46–51. doi: 10.1093/trstmh/tru196. [DOI] [PubMed] [Google Scholar]
- 38.Jasenosky LD, Scriba TJ, Hanekom WA, Goldfeld AE. T cells and adaptive immunity to Mycobacterium tuberculosis in humans. Immunol Rev. 2015;264:74–87. doi: 10.1111/imr.12274. [DOI] [PubMed] [Google Scholar]
- 39.Freyne B, Marchant A, Curtis N. BCG-associated heterologous immunity, a historical perspective: intervention studies in animal models of infectious diseases. Trans R Soc Trop Med Hyg. 2015;109:52–61. doi: 10.1093/trstmh/tru197. [DOI] [PubMed] [Google Scholar]
- 40.Di Luzio NR, Williams DL. Protective effect of glucan against systemic Staphylococcus aureus septicemia in normal and leukemic mice. Infect Immun. 1978;20:804–10. doi: 10.1128/iai.20.3.804-810.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Marakalala MJ, et al. Dectin-1 plays a redundant role in the immunomodulatory activities of beta-glucan-rich ligands in vivo. Microbes Infect. 2013;15:511–5. doi: 10.1016/j.micinf.2013.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Krahenbuhl JL, Sharma SD, Ferraresi RW, Remington JS. Effects of muramyl dipeptide treatment on resistance to infection with Toxoplasma gondii in mice. Infect Immun. 1981;31:716–22. doi: 10.1128/iai.31.2.716-722.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ribes S, et al. Intraperitoneal prophylaxis with CpG oligodeoxynucleotides protects neutropenic mice against intracerebral Escherichia coli K1 infection. J Neuroinflammation. 2014;11:14. doi: 10.1186/1742-2094-11-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Munoz N, et al. Mucosal administration of flagellin protects mice from Streptococcus pneumoniae lung infection. Infect Immun. 2010;78:4226–33. doi: 10.1128/IAI.00224-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang B, et al. Viral infection. Prevention and cure of rotavirus infection via TLR5/NLRC4-mediated production of IL-22 and IL-18. Science. 2014;346:861–5. doi: 10.1126/science.1256999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kleinnijenhuis J, et al. Bacille Calmette-Guerin induces NOD2-dependent nonspecific protection from reinfection via epigenetic reprogramming of monocytes. Proc Natl Acad Sci U S A. 2012;109:17537–42. doi: 10.1073/pnas.1202870109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tribouley J, Tribouley-Duret J, Appriou M. [Effect of Bacillus Callmette Guerin (BCG) on the receptivity of nude mice to Schistosoma mansoni] C R Seances Soc Biol Fil. 1978;172:902–4. [PubMed] [Google Scholar]
- 48.Bistoni F, et al. Evidence for macrophage-mediated protection against lethal Candida albicans infection. Infect Immun. 1986;51:668–74. doi: 10.1128/iai.51.2.668-674.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vecchiarelli A, et al. Protective immunity induced by low-virulence Candida albicans: cytokine production in the development of the anti-infectious state. Cell Immunol. 1989;124:334–44. doi: 10.1016/0008-8749(89)90135-4. [DOI] [PubMed] [Google Scholar]
- 50.Bistoni F, et al. Immunomodulation by a low-virulence, agerminative variant of Candida albicans. Further evidence for macrophage activation as one of the effector mechanisms of nonspecific anti-infectious protection. J Med Vet Mycol. 1988;26:285–99. doi: 10.1080/02681218880000401. [DOI] [PubMed] [Google Scholar]
- 51.Quintin J, et al. Candida albicans infection affords protection against reinfection via functional reprogramming of monocytes. Cell Host Microbe. 2012;12:223–32. doi: 10.1016/j.chom.2012.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Barton ES, et al. Herpesvirus latency confers symbiotic protection from bacterial infection. Nature. 2007;447:326–9. doi: 10.1038/nature05762. [DOI] [PubMed] [Google Scholar]
- 53.Sun JC, Beilke JN, Lanier LL. Adaptive immune features of natural killer cells. Nature. 2009;457:557–61. doi: 10.1038/nature07665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Netea MG, Quintin J, van der Meer JW. Trained immunity: a memory for innate host defense. Cell Host Microbe. 2011;9:355–61. doi: 10.1016/j.chom.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 55.Foster SL, Hargreaves DC, Medzhitov R. Gene-specific control of inflammation by TLR-induced chromatin modifications. Nature. 2007;447:972–8. doi: 10.1038/nature05836. [DOI] [PubMed] [Google Scholar]
- 56.Saeed S, et al. Epigenetic programming of monocyte-to-macrophage differentiation and trained innate immunity. Science. 2014;345:1251086. doi: 10.1126/science.1251086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cheng SC, et al. mTOR- and HIF-1alpha-mediated aerobic glycolysis as metabolic basis for trained immunity. Science. 2014;345:1250684. doi: 10.1126/science.1250684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Donohoe DR, Bultman SJ. Metaboloepigenetics: interrelationships between energy metabolism and epigenetic control of gene expression. J Cell Physiol. 2012;227:3169–77. doi: 10.1002/jcp.24054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Buffen K, et al. Autophagy controls BCG-induced trained immunity and the response to intravesical BCG therapy for bladder cancer. PLoS Pathog. 2014;10:e1004485. doi: 10.1371/journal.ppat.1004485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kleinnijenhuis J, et al. BCG-induced trained immunity in NK cells: Role for non-specific protection to infection. Clin Immunol. 2014;155:213–9. doi: 10.1016/j.clim.2014.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nabekura T, Girard JP, Lanier LL. IL-33 receptor ST2 amplifies the expansion of NK cells and enhances host defense during mouse cytomegalovirus infection. J Immunol. 2015;194:5948–52. doi: 10.4049/jimmunol.1500424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schlums H, et al. Cytomegalovirus infection drives adaptive epigenetic diversification of NK cells with altered signaling and effector function. Immunity. 2015;42:443–56. doi: 10.1016/j.immuni.2015.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sun JC, et al. Proinflammatory cytokine signaling required for the generation of natural killer cell memory. J Exp Med. 2012;209:947–54. doi: 10.1084/jem.20111760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nabekura T, et al. Costimulatory molecule DNAM-1 is essential for optimal differentiation of memory natural killer cells during mouse cytomegalovirus infection. Immunity. 2014;40:225–34. doi: 10.1016/j.immuni.2013.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.O'Sullivan TE, Sun JC, Lanier LL. Natural Killer Cell Memory. Immunity. 2015;43:634–45. doi: 10.1016/j.immuni.2015.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Reeves RK, et al. Antigen-specific NK cell memory in rhesus macaques. Nat Immunol. 2015;16:927–32. doi: 10.1038/ni.3227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hendricks DW, et al. Cutting edge: NKG2C(hi)CD57+ NK cells respond specifically to acute infection with cytomegalovirus and not Epstein-Barr virus. J Immunol. 2014;192:4492–6. doi: 10.4049/jimmunol.1303211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Min-Oo G, Lanier LL. Cytomegalovirus generates long-lived antigen-specific NK cells with diminished bystander activation to heterologous infection. J Exp Med. 2014;211:2669–80. doi: 10.1084/jem.20141172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Paust S, et al. Critical role for the chemokine receptor CXCR6 in NK cell-mediated antigen-specific memory of haptens and viruses. Nat Immunol. 2010;11:1127–35. doi: 10.1038/ni.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lee J, et al. Epigenetic modification and antibody-dependent expansion of memory-like NK cells in human cytomegalovirus-infected individuals. Immunity. 2015;42:431–42. doi: 10.1016/j.immuni.2015.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yanez A, et al. Detection of a TLR2 agonist by hematopoietic stem and progenitor cells impacts the function of the macrophages they produce. Eur J Immunol. 2013;43:2114–25. doi: 10.1002/eji.201343403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Askenase MH, et al. Bone-Marrow-Resident NK Cells Prime Monocytes for Regulatory Function during Infection. Immunity. 2015;42:1130–42. doi: 10.1016/j.immuni.2015.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ng RL, et al. Altered immunity and dendritic cell activity in the periphery of mice after long-term engraftment with bone marrow from ultraviolet-irradiated mice. J Immunol. 2013;190:5471–84. doi: 10.4049/jimmunol.1202786. [DOI] [PubMed] [Google Scholar]
- 74.Ng RL, Bisley JL, Gorman S, Norval M, Hart PH. Ultraviolet irradiation of mice reduces the competency of bone marrow-derived CD11c+ cells via an indomethacin-inhibitable pathway. J Immunol. 2010;185:7207–15. doi: 10.4049/jimmunol.1001693. [DOI] [PubMed] [Google Scholar]
- 75.Scott NM, et al. Prostaglandin E2 imprints a long-lasting effect on dendritic cell progenitors in the bone marrow. J Leukoc Biol. 2014;95:225–32. doi: 10.1189/jlb.0513294. [DOI] [PubMed] [Google Scholar]
- 76.Partinen M, et al. Increased incidence and clinical picture of childhood narcolepsy following the 2009 H1N1 pandemic vaccination campaign in Finland. PLoS One. 2012;7:e33723. doi: 10.1371/journal.pone.0033723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Stowe J, et al. Risk of Narcolepsy after AS03 Adjuvanted Pandemic A/H1N1 2009 Influenza Vaccine in Adults: A Case-Coverage Study in England. Sleep. 2016 doi: 10.5665/sleep.5752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jokinen J, Nohynek H. Increased risk of narcolepsy observed also among adults vaccinated with Pandemrix in Finland. National Institute for Health and Welfare; Finland: 2013. [Google Scholar]
- 79.Ledgerwood JE. AS03-adjuvanted influenza vaccine in elderly people. Lancet Infect Dis. 2013;13:466–7. doi: 10.1016/S1473-3099(13)70038-0. [DOI] [PubMed] [Google Scholar]
- 80.McElhaney JE, et al. AS03-adjuvanted versus non-adjuvanted inactivated trivalent influenza vaccine against seasonal influenza in elderly people: a phase 3 randomised trial. Lancet Infect Dis. 2013;13:485–96. doi: 10.1016/S1473-3099(13)70046-X. [DOI] [PubMed] [Google Scholar]
- 81.Sobolev O, et al. Adjuvanted influenza-H1N1 vaccination reveals lymphoid signatures of age-dependent early responses and of clinical adverse events. Nat Immunol. 2016;17:204–13. doi: 10.1038/ni.3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Levy O, Netea MG. Innate immune memory: implications for development of pediatric immunomodulatory agents and adjuvanted vaccines. Pediatr Res. 2014;75:184–8. doi: 10.1038/pr.2013.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jensen KJ, et al. Heterologous immunological effects of early BCG vaccination in low-birth-weight infants in Guinea-Bissau: a randomized-controlled trial. J Infect Dis. 2015;211:956–67. doi: 10.1093/infdis/jiu508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kollmann TR, Levy O, Montgomery RR, Goriely S. Innate immune function by Toll-like receptors: distinct responses in newborns and the elderly. Immunity. 2012;37:771–83. doi: 10.1016/j.immuni.2012.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Targonski PV, Jacobson RM, Poland GA. Immunosenescence: role and measurement in influenza vaccine response among the elderly. Vaccine. 2007;25:3066–9. doi: 10.1016/j.vaccine.2007.01.025. [DOI] [PubMed] [Google Scholar]
- 86.Nakaya HI, Pulendran B. Vaccinology in the era of high-throughput biology. Philos Trans R Soc Lond B Biol Sci. 2015;370 doi: 10.1098/rstb.2014.0146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Nakaya HI, et al. Systems biology of immunity to MF59-adjuvanted versus nonadjuvanted trivalent seasonal influenza vaccines in early childhood. Proc Natl Acad Sci U S A. 2016;113:1853–8. doi: 10.1073/pnas.1519690113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Amenyogbe N, Levy O, Kollmann TR. Systems vaccinology: a promise for the young and the poor. Philos Trans R Soc Lond B Biol Sci. 2015;370 doi: 10.1098/rstb.2014.0340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Blok BA, Arts RJ, van Crevel R, Benn CS, Netea MG. Trained innate immunity as underlying mechanism for the long-term, nonspecific effects of vaccines. J Leukoc Biol. 2015;98:347–56. doi: 10.1189/jlb.5RI0315-096R. [DOI] [PubMed] [Google Scholar]
- 90.Biering-Sorensen S, et al. Small randomized trial among low-birth-weight children receiving bacillus Calmette-Guerin vaccination at first health center contact. Pediatr Infect Dis J. 2012;31:306–8. doi: 10.1097/INF.0b013e3182458289. [DOI] [PubMed] [Google Scholar]
- 91.Kleinnijenhuis J, et al. Long-lasting effects of BCG vaccination on both heterologous Th1/Th17 responses and innate trained immunity. J Innate Immun. 2014;6:152–8. doi: 10.1159/000355628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hong DK, Lewis DB. In: Remington and Klein’s Infectious diseases of the fetus and newborn infant. Wilson CB, Nizet V, Maldonado YA, editors. Elsevier Saunders; Philadelphia: 2015. pp. 81–188. [Google Scholar]
- 93.Mold JE, et al. Fetal and adult hematopoietic stem cells give rise to distinct T cell lineages in humans. Science. 2010;330:1695–9. doi: 10.1126/science.1196509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Siegrist CA, Aspinall R. B-cell responses to vaccination at the extremes of age. Nat Rev Immunol. 2009;9:185–94. doi: 10.1038/nri2508. [DOI] [PubMed] [Google Scholar]
- 95.Arrieta MC, Stiemsma LT, Amenyogbe N, Brown EM, Finlay B. The intestinal microbiome in early life: health and disease. Front Immunol. 2014;5:427. doi: 10.3389/fimmu.2014.00427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Arrieta MC, et al. Early infancy microbial and metabolic alterations affect risk of childhood asthma. Sci Transl Med. 2015;7:307ra152. doi: 10.1126/scitranslmed.aab2271. [DOI] [PubMed] [Google Scholar]
- 97.Lim ES, et al. Early life dynamics of the human gut virome and bacterial microbiome in infants. Nat Med. 2015;21:1228–34. doi: 10.1038/nm.3950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Subramanian S, et al. Cultivating healthy growth and nutrition through the gut microbiota. Cell. 2015;161:36–48. doi: 10.1016/j.cell.2015.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Oh DY, et al. Adjuvant-induced human monocyte secretome profiles reveal adjuvant- and age-specific protein signatures. Mol Cell Proteomics. 2016 doi: 10.1074/mcp.M115.055541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wynn JL, et al. Defective innate immunity predisposes murine neonates to poor sepsis outcome but is reversed by TLR agonists. Blood. 2008;112:1750–8. doi: 10.1182/blood-2008-01-130500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ferguson RG, Simes AB. BCG vaccination of Indian infants in Saskatchewan. Tubercle. 1949;30:5–11. doi: 10.1016/s0041-3879(49)80055-9. [DOI] [PubMed] [Google Scholar]
- 102.Aaby P, et al. Randomized trial of BCG vaccination at birth to low-birth-weight children: beneficial nonspecific effects in the neonatal period? J Infect Dis. 2011;204:245–52. doi: 10.1093/infdis/jir240. [DOI] [PubMed] [Google Scholar]
- 103.Aronson JD. Protective vaccination against tuberculosis with special reference to BCG vaccination. Am Rev Tuberc. 1948;58:255–81. doi: 10.1164/art.1948.58.3.255. [DOI] [PubMed] [Google Scholar]
- 104.Rosenthal SR, et al. BCG vaccination in tuberculous households. Am Rev Respir Dis. 1961;84:690–704. doi: 10.1164/arrd.1961.84.5P1.690. [DOI] [PubMed] [Google Scholar]
- 105.Benn CS, Bale C, Sommerfelt H, Friis H, Aaby P. Hypothesis: Vitamin A supplementation and childhood mortality: amplification of the non-specific effects of vaccines? Int J Epidemiol. 2003;32:822–8. doi: 10.1093/ije/dyg208. [DOI] [PubMed] [Google Scholar]
- 106.Aaby P, et al. Increased female-male mortality ratio associated with inactivated polio and diphtheria-tetanus-pertussis vaccines: Observations from vaccination trials in Guinea-Bissau. Pediatr Infect Dis J. 2007;26:247–52. doi: 10.1097/01.inf.0000256735.05098.01. [DOI] [PubMed] [Google Scholar]
- 107.Hartfield J, Morley D. Efficacy of measles vaccine. J Hyg (Lond) 1963;61:143–7. doi: 10.1017/s0022172400020817. [DOI] [PMC free article] [PubMed] [Google Scholar]