Abstract
Objectives:
Several studies have revealed that systemic hypertension is strongly associated with cataractogenesis. However, the pathophysiology and treatment is often unclear. In this study, we evaluated the anti-cataractogenic effect of cinnamaldehyde (CA), a natural organic compound, in rats with fructose-induced hypertension.
Methods:
The rats were divided into six groups. For six weeks, the normal group received a suspension of 0.5% carboxy methyl cellulose (10 mL/kg/day, p.o.) while five other groups received a 10% (w/v) fructose solution in their drinking water to induce hypertension. By the end of the third week hypertension had been induced in all the animals receiving fructose. From the beginning of the fourth week to the end of the sixth week, one of those five groups (control) continued to receive only 10% (w/v) fructose solution, one group (standard) received ramipril (1 mg/kg/day, p.o.) plus 10% (w/v) fructose solution, and three groups (experimental) received CA at doses of 20, 30, and 40 mg/kg/day p.o., plus 10% (w/v) fructose solution. Blood pressure was measured weekly using a non-invasive blood pressure apparatus. After six weeks, the animals were sacrificed, and the anti-cataractogenic effects on the eye lenses were evaluated.
Results:
Administration of fructose elevated both the systolic and the diastolic blood pressures, which were significantly reduced by CA at all dose levels. In the control group, a significant increase in the malonaldehyde (MDA) level and decreases in the total protein, Ca2+adenosine triphosphate (ATP)ase activity, glutathione peroxidase, catalase, superoxide dismutase and glutathione levels, as compared to the normal group, were observed. Administration of CA at all doses significantly restored the enzymatic, non-enzymatic, antioxidants, total protein, and Ca2+ATPase levels, but decreased the MDA level, as compared to the control group.
Conclusion:
The present study revealed that CA modulated the antioxidant parameters of the serum and lens homogenates in hypertension-induced cataractogenic animals.
Keywords: cataract, cinnamaldehyde, fructose, hypertension, oxidative stress
1. Introduction
High blood pressure is one of the most common diseases in the world, which had affected nearly 1 billion of the adult population in 2000, and it is predicted that this proportion will increase to 29% (1.56 billion) by 2025 [1, 2]. Hypertension carries a high-risk factor for arteriosclerosis, myocardial infarction, end-stage renal disease and various ocular disorders [3]. In recent years, various epidemiological studies have indicated that hypertension plays an important role in the development of cataracts, but the mode of cataractogenesis is unclear [4-7]. Hypertension has profound effects, such as hypertensive retinopathy, hypertensive choroidopathy and hypertensive optic neuropathy, on the structure and function of the eye [8]. Apart from this, hypertension is thought to cause elevations of inflammatory cytokines, such as tumor necrosis factor-alpha, interleukin-6 and C-reactive protein, which are closely related to cataract formation through intense systemic inflammation [9, 10]. Lee et al reported that hypertension could induce conformational structure alteration of proteins in lens capsules, thereby exacerbating cataract formation [11]. Although several plausible mechanisms have been proposed based on laboratory results, the conclusions from epidemiologic studies remain inconsistent.
Although extensive studies have been performed to investigate the effects of fructose-induced hypertension on various organs [12, 13], data on lens integrity and its composition in a rat model are lacking. Several previous studies postulated that administration of cinnamaldehyde (CA) would improve oxidative stress, lipid abnormalities and inflammatory markers in the liver and the muscles of a fructose-fed rat [14-16]. Evidence from epidemiological in vitro and animal studies supports the idea that CA may reduce the cataractogenic effect in a hypertensive state.
CA is a naturally-occurring, organic compound that has a wide range of biological activities, such as anti-bacterial [17], anti-inflammatory [18], immunomodulatory [19], anti-diabetic [20], and aldose reductase inhibition activities [21]. Aldose reductase is well known to be a key enzyme in the polyol pathway which may be accelerated in fructose-induced hypertension leading to alterations in the morphology of the lens and its function. Hence, the objective of the present study was to explore the effects of different doses of CA on hypertension and on the levels of various biochemical parameters of the lens and serum in albino rats fed with a high-fructose diet.
2. Materials and Methods
CA and fructose were purchased from Himedia Laboratories Ltd., Mumbai (India). Ramipril was obtained as a gift sample from Cipla Ltd. (Mumbai, India). All chemicals and reagents that were used were of analytical grade. Sprague-Dawley albino male rats (150 − 180 g) were used for the present study. They were housed in standard polypropylene cages (three rats per cage) and kept in a lightdark cycle of equal durations (12:12 hours) under constant environmental conditions (22 ± 2°C with 55% ± 5% humidity) according to the guidelines of the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), Government of India. The rats were fed commercially-available normal pellet diet and water ad libitum under hygienic conditions. The experimental protocol was approved by the Institutional Animals Ethics Committee (IAEC, 994/GO/ERe/S/06/CPCSEA) of the SLT Institute of Pharmaceutical Sciences, Guru Ghasidas Vishwavidyalaya (A Central University), Bilaspur, India.
Animals were randomly selected and divided into six groups, with six animals per group. Group 1 (normal) received a suspension of 0.5% carboxy methyl cellulose (10 mL/kg/day, p.o.) for six weeks. Groups 2 to 6 received a 10% (w/v) fructose solution in their drinking water (equivalent to a diet containing 48% − 57% fructose) for six weeks to induce hypertension [22]. By the end of the third week, hypertension had been induced in all animals in Groups 2 − 6. After induction of hypertension, from the beginning of week four until the end of week six, Group 2 (control) continued to receive 10% (w/v) fructose only, Group 3 (standard) received ramipril (1 mg/kg/day, p.o.), as well as 10% (w/v) fructose, and Groups 4 to 6 received CA at three different dose levels (20, 30 and 40 mg/kg/day, p.o.) respectively, as well as 10% (w/v) fructose. Blood pressure (systolic and diastolic) was monitored weekly by using a non-invasive blood pressure apparatus NIBP (CODA-08 Channel, Kent scientific, USA). Various pathophysiological parameters of the cataractogenic effects were evaluated after completion of the experimental protocol (six weeks). Blood samples from animals were collected by cardiac puncture, and serum was separated and stored at 2 − 8°C for further analysis of biochemical parameters.
After the completion of the experimental protocol, animals were sacrificed and their eyeballs were removed. Both lenses were separated from the eye balls and kept on graph paper. Their opacities were measured by using the photographic method. The graph lines would appear clearly in the transparent lens and cloudy or not visible in the cataractous lens. Then, the lenses were rapidly desiccated, washed with saline and carefully dried over fine filter paper, weighed and placed in clean, sterilized vials, and stored at -20°C until analysis. A lens homogenate was prepared from both lenses of each animal in 10 volumes of 0.1-M phosphate buffer, pH 7. The homogenate was centrifuged at 10,000 rpm for 1 hour, and the supernatant was separated and used for biochemical assays [23].
The supernatant from the lens and serum were used for the biochemical analysis. Enzymatic antioxidants were measured: The glutathione peroxidase (GPx) activity was measured by using the procedure of Tappel [24], and the enzyme activity of catalase (CAT) was measured spectrophotometrically at 240 nm by following the decomposition of H2O2 [25]. Superoxide dismutase (SOD) activity was determined by the addition of NADH (nicotinamide adenine dinucleotide) after incubation at 30°C for 1 minute, after which the reaction was stopped by the addition of 1.0 mL of glacial acetic acid into lens homogenate and the absorbance was measured at 560 nm [26]. The estimate of the non-enzymatic anti-oxidant reduced glutathione (GSH) was based on the reaction of Ellman’s reagent [27]. Malondialdehyde (MDA) in the lens homogenate and serum was estimated by using the colorimetric methods of Ohkawa et al [28]. Total protein (TP) in the lens homogenate was assayed by using an ultraviolet (UV)-visible spectrophotometer at 610 nm and the method of Lowry et al. The protein content was calculated from a standard curve prepared with bovine serum albumin and expressed as μg/mg lens tissue [29]. The activity of Ca2+adenosine triphosphate (ATP)ase in the lens sample was measured by using the method of Rorive and Kleinzeller [30].
The data obtained were expressed as means ± standard errors of the mean (SEMs). The significance of the differences in mean values between multiple groups was analyzed by using the one-way analysis of variance (ANOVA) followed by the Newman-Keuls post test and the two-way ANOVA followed by Bonferroni’s test. The level of statistical significance was set at P < 0.05. Statistical analyses were performed using Graph Pad Prism 5.0 software.
3. Results
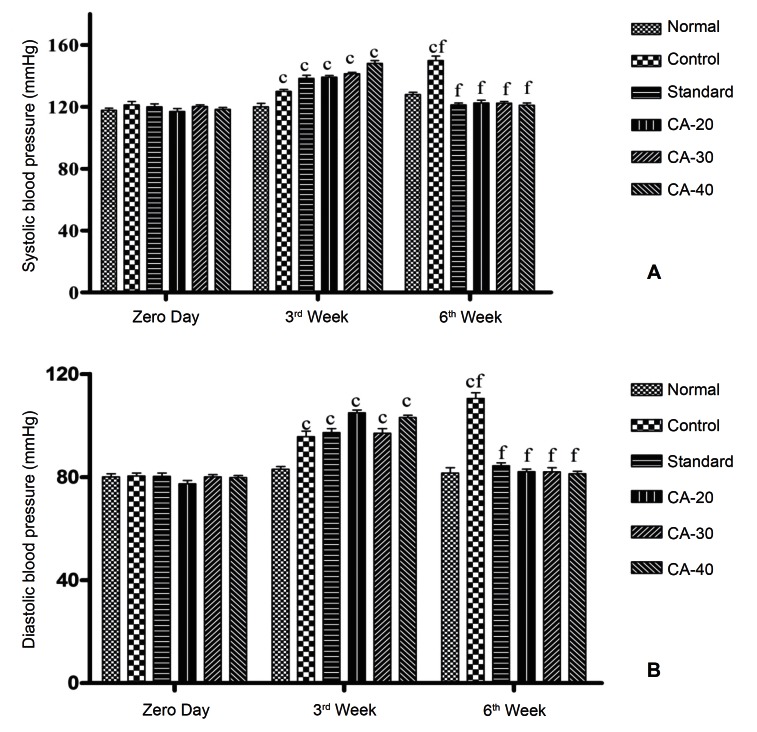
Fructose-fed rats (Groups 2 – 6) showed significant (P < 0.001) development of hypertension in terms of systolic blood pressure (SBP) and diastolic blood pressure (DBP) within three weeks compared to day zero. The administration of ramipril in Group 3 (standard) at 1 mg/kg/day p.o. for 3 weeks and the administration of CA in Groups 4, 5, 6 at 20, 30, and 40 mg/kg/day p.o., respectively, for 3 weeks significantly (P < 0.001) reduced the values of the blood pressure compared to those at the end of the third week (Fig. 1).
Fig. 1. Effect of cinnamaldehyde on (a) the systolic blood pressure and (b) the diastolic blood pressure. Results are expressed as means ± SEMs, with n = 6. Data were analyzed by using the two-way ANOVA, followed by Bonferroni post tests. aP < 0.05, bP < 0.01, and cP < 0.001 as compared to day 0; dP < 0.05, eP < 0.01, fP < 0.001 as compared to week 3. CA-20 = cinnamaldehyde at 20 mg/kg/day; CA-30 = cinnamaldehyde at 30 mg/kg/day; CA-40 = cinnamaldehyde at 40 mg/kg/day.
SEMs, standard errors of the mean; ANOVA, analysis of variance.
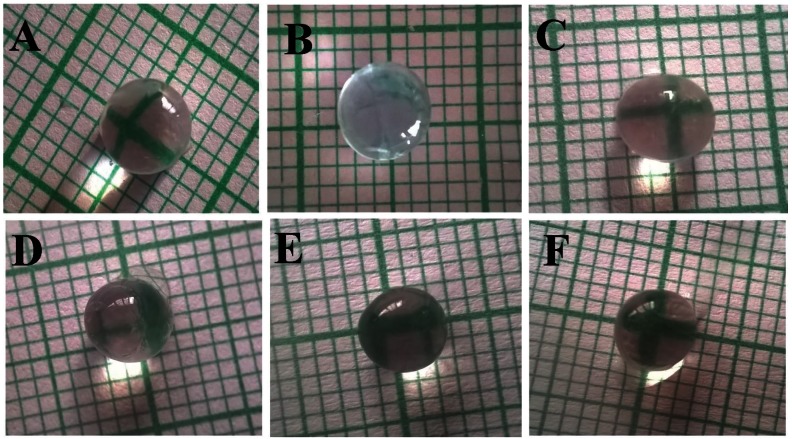
After the completion of the experimental protocol, the lenses of the eyes of the rats in all groups were removed and examined. The lenses of the eyes of the rats that had received the 10% (w/v) fructose solution (Groups 2 – 6) were opaque (Fig. 2)B - 2F) as compared to the normal groups (Fig. 2A). Treatments of rats with ramipril at 1 mg/ kg/day, p.o., as well as with CA at 20, 30, and 40 mg/kg/day, p.o., seemed to retard the progression of lens opacification (Fig. 2C - 2F) as compared to the control group (Fig. 2B). At doses of 30 and 40 mg/kg/day, CA had a very significant effect as lenses were more transparent than they were at a 20 mg/kg/day dose of CA.
Fig. 2. Photographs of lenses in the normal and the experimental groups: (A) normal, (B) control, (C) standard, and cinnamaldehyde at (D) 20 mg/kg/day (CA-20), (E) 30 mg/kg/day (CA-30), and (F) 40 mg/kg/day (CA-40).
Fructose administration significantly (P < 0.001) decreased the level of enzymatic anti-oxidants (GPx, CAT, SOD) and non-enzymatic anti-oxidant GSH and increased the level of MDA in the serum of control group when compared to the normal group. Treatment with CA at 20, 30 and 40 mg/kg/day, p.o. and with ramipril at 1 mg/kg/day, p.o. simultaneously with fructose caused a significant (P < 0.001) increase in the enzymatic antioxidants. The level of the non-enzymatic antioxidant GSH increased at in the rats treated with ramipril at 1 mg/kg/day (P < 0.05) and with CA at 20, 30 and 40 mg/kg/day (P < 0.05, P < 0.01 and P < 0.001, respectively). Ramipril and CA at all doses significantly (P < 0.001) protected the test groups from lipid peroxidation, as indicated by a reduction in the level of MDA as compared to the control group (Table 1).
Table. 1. Effect of cinnamaldehyde on GPx, CAT, SOD, GSH and MDA levels in serum.
| Groups | GPx (μM/min/mg protein) | CAT (μM of H2O2 consumed/min/ mg protein) | SOD (μM/mg protein) | GSH (μM/mg protein) | MDA (μM/mg protein) |
|---|---|---|---|---|---|
| Normal | 15.37 ± 0.39 | 0.63 ± 0.02 | 3.05 ± 0.20 | 2.88 ± 0.23 | 2.62 ± 0.12 |
| Control | 5.11 ± 0.26c | 0.33 ± 0.02c | 1.08 ± 0.06c | 1.33 ± 0.28c | 3.5 ± 0.15c |
| Standard | 13.08 ± 0.35cf | 0.53 ± 0.02bf | 2.37 ± 0.08af | 2.10 ± 0.18ad | 2.55 ± 0.19f |
| CA-20 | 11.27 ± 0.35cfi | 0.44 ± 0.02cfg | 2.34 ± 0.10af | 1.94 ± 0.08bd | 2.78 ± 0.10f |
| CA-30 | 13.36 ± 0.17cf | 0.48 ± 0.02cf | 2.69 ± 0.10f | 2.38 ± 0.06e | 2.11 ± 0.12afg |
| CA-40 | 15.33 ± 0.21fi | 0.64 ± 0.02fh | 2.78 ± 0.29f | 2.63 ± 0.18f | 1.87 ± 0.11bfh |
Values are expressed as means ± SEMs, with n = 6. Data were analyzed by using the one-way ANOVA, followed by the Newman-Keuls post test. aP < 0.05, bP < 0.01, and cP < 0.001 compared with the normal group, dP < 0.05, eP < 0.01, and fP < 0.001 compared with the control group, and gP < 0.05, hP < 0.01, iP < 0.001 compared with the standard group. CA-20 = cinnamaldehyde at 20 mg/kg/day; CA-30 = cinnamaldehyde at 30 mg/kg/day; CA-40 = cinnamaldehyde at 40 mg/kg/day. GPx, glutathione peroxidase; CAT, catalase; SOD, superoxide dismutase; GSH, glutathione; MDA, malonaldehyde; SEMs, standard errors of the mean; ANOVA, analysis of variance.
Treatment with fructose in control group led to significant (P < 0.001) decreases in the levels of enzymatic antioxidants (GPx, CAT, SOD) and in the level of the non-enzymatic antioxidant GSH (P < 0.01), but to an increase in the level of MDA (P < 0.001) in the lens, as compared to the levels in the normal group. The significant restoration of such antioxidants, GPx (P < 0.001, P < 0.001), CAT (P < 0.001, P < 0.001), SOD (P < 0.001, P < 0.001), and GSH (P < 0.05, P < 0.01) and MDA (P < 0.001, P < 0.001) level was observed in CA-treated group at 30 and 40 mg/kg/day respectively. Whereas, ramipril and CA (20 mg/kg/day) treated group showed significant restoration of GPx (P < 0.001, P < 0.001), CAT (P < 0.01, P < 0.01), SOD (P < 0.001, P < 0.05) and MDA (P < 0.001, P < 0.001) level respectively, and failed to significant restoration of GSH level as compared to the control group (Table 2).
Table. 2. Effects of the cinnamaldehyde GPx, CAT, SOD, GSH and MDA levels in the lens.
| Group | GPx (μM/min/mg lens protein) | CAT (μM of H2O2 consumed/min/mg protein) | SOD (μM/mg lens protein) |
GSH (μM/mg protein) | MDA (μM/mg lens protein) |
|---|---|---|---|---|---|
| Normal | 8.24 ± 0.46 | 0.46 ± 0.02 | 2.74± 0.05 | 2.51 ± 0.33 | 0.19 ± 0.02 |
| Control | 2.16 ± 0.13c | 0.26 ± 0.01c | 0.82 ± 0.02c | 1.54 ± 0.09b | 0.61 ± 0.02c |
| Standard | 4.97 ± 0.15cf | 0.35 ± 0.02e | 2.08 ± 0.22f | 1.90 ± 0.01a | 0.13 ± 0.02f |
| CA-20 | 5.49 ± 0.43cf | 0.40 ± 0.02e | 1.52 ± 0.08bd | 1.73 ± 0.06b | 0.12 ± 0.01f |
| CA-30 | 5.99 ± 0.08cfg | 0.44 ± 0.03f | 2.28 ± 0.36f | 2.02 ± 0.03d | 0.09 ± 0.02af |
| CA-40 | 7.65 ± 0.02fi | 0.52 ± 0.05cfi | 2.45 ± 0.20f | 2.46 ± 0.01eg | 0.06 ± 0.01bf |
Values are expressed as means ± SEMs, with n = 6. Data were analyzed by using the one-way ANOVA, followed by the Newman-Keuls post test. aP < 0.05, bP < 0.01, and cP < 0.001 compared with the normal group, dP < 0.05, eP < 0.01, and fP < 0.001 compared with the control group, and gP < 0.05, hP < 0.01, and iP < 0.001 compared with the standard group. CA-20 = cinnamaldehyde at 20 mg/kg/day; CA-30 = cinnamaldehyde at 30 mg/kg/day; CA-40 = cinnamaldehyde at 40 mg/kg/day. GPx, glutathione peroxidase; CAT, catalase; SOD, superoxide dismutase; GSH, glutathione; MDA, malonaldehyde; SEMs, standard errors of the mean; ANOVA, analysis of variance.
Significant (P < 0.001) decreases in the levels of total protein and Ca2+ATPase were observed in the eye lenses of the animals in the control group, as compared to the normal group. Treatment with CA at the doses 30 and 40 mg/kg, along with fructose, caused significant increases in the total protein (P < 0.05, P < 0.001) and Ca2+ATPase activity (P < 0.001, P < 0.001) in the lens, while CA at 20 mg/kg, showed non-significant effects on total protein and Ca2+ATPase activity, as compared to the control group. Moreover, standard group significantly (P < 0.001) increased the total protein only (Table 3).
Table. 3. Effect of cinnamaldehyde on the total protein and Ca2+ATPase levels in the lens.
| Groups | TP (mg/mg lens tissue) | Ca2+ATPase (μM/min/mg inorganic phosphate) |
|---|---|---|
| Normal | 0.70 ± 0.01 | 16.78 ± 0.39 |
| Control | 0.27 ± 0.01c | 11.28 ± 0.24c |
| Standard | 0.68 ± 0.03f | 11.00 ± 0.20c |
| CA-20 | 0.36 ± 0.04ci | 11.96 ± 0.20c |
| CA-30 | 0.40 ± 0.04cdi | 12.90 ± 0.03cfi |
| CA-40 | 0.68 ± 0.01f | 15.19 ± 0.40cfi |
Values are expressed as means ± SEMs, with n = 6. Data were analyzed by using the one-way ANOVA, followed by the Newman- Keuls post test. aP < 0.05, bP < 0.01, and cP < 0.001 compared with the normal group, dP < 0.05, eP < 0.01, and fP < 0.001 compared with the control group, and gP < 0.05, hP < 0.01, and iP < 0.001 compared with the standard group. CA-20 = cinnamaldehyde at 20 mg/kg/day; CA-30 = cinnamaldehyde at 30 mg/kg/day; CA-40 = cinnamaldehyde at 40 mg/kg/day. ATP, adenosine triphosphate; TP, total protein; SEMs, standard errors of the mean; ANOVA, analysis of variance.
4. Discussion
Some experimental and clinical evidence has identified enhanced oxidative stress as an important responsible factor for hypertension [31] while other epidemiological studies have speculated that oxidative stress is increased in hypertensive subjects [32]. However, whether enhanced oxidative stress is primary or secondary to the pathogenicity in hypertension has never been clearly established. In hypertension, the mechanisms responsible for oxidative stress are still not well understood, even though a decrease in the disposal of anti-oxidant mechanisms has been proposed.
Recent studies indicate that a high fructose diet is associated with high blood pressure [33, 34]. Various studies have shown that the excessive provision of fructose enhances the rate of production of reactive oxygen species in mitochondria, which may be responsible for oxidative damage to cellular constituents that results in several dysfunctions, namely, diminished anti-oxidant defense mechanism [35], decreased enzymatic and non-enzymatic anti-oxidants [36], metabolic syndrome with hyperlipidemia, insulin resistance [37], production of high non-enzymatic glycosylation [38] and formation of advanced glycation end products (AGEs) formation [39], which further leads to hypertension. This is reflected in our study. We found that 10% (w/v) fructose in drinking water for six weeks significantly raised the SBP and the DBP from the third week to the sixth week and augmented the systemic oxidative stress via depletion of serum antioxidants, including GPx, CAT, SOD, and GSH, and enhancement of the serum MDA level in the control group. On the other hand, similar to treatment with ramipril (1 mg/kg, p.o.), treatment with CA at three doses levels (20, 30 and 40 mg/kg, p.o.) significantly decreased the SBP and the DBP and restored the levels of serum antioxidants and MDA, suggesting that fructose-induced hypertension is strongly associated with oxidative stress.
Further, various epidemiological studies indicate that hypertension plays an important role in the development of cataracts [5, 7]. According to various experimental and clinical studies, impaired hypertension induces a conformational structural alteration of proteins in lens capsules [11], oxidative stress in the eye [40], activation of the polyol pathway of the glucose metabolism and formation of AGEs in the lens [41].
Enhanced oxidative stress in the lenses is a key mechanism of cataract formation. In the present study, fructose- fed animals (control group) showed marked cataractogenic effects through depletion of antioxidants, such as GPx, CAT, SOD, and GSH, and increased MDA level in the lens as compared to the animals in the normal group. Treatments with CA and with the standard antihypertensive drug ramipril (ACE inhibitor) concurrent with fructose solution showed significant protection against cataractogenesis via reduction of oxidative stress in the lenses of the eye. Both CA and ramipril significantly increased the levels of GPx, CAT, SOD, and GSH in the lens and decreased the level of MDA. That antioxidants play an important role in protection against cellular damage is well know: GPx decreases the levels of H2O2 in cells [42], and SOD is a chain-breaking anti-oxidant that converts superoxide to H2O2 and scavenges the superoxide anion to form hydrogen peroxide. CAT helps to keep the level of free radicals below toxic levels [43]. GSH is synthesized in the lens and is responsible for protecting the thiol groups on the proteins in the lens, thereby preventing the cross-linkage of lens crystalline. A reduced level of GSH is expected to cause the formation of disulphide bonds through sulfhydryl oxidation of lens crystalline which leads to cataractogenesis [44], while MDA is a product of membrane lipid peroxidation and decreases the content of water-soluble proteins. MDA protects against aggregation and insolubilization of proteins in the lens, and an increased level of MDA may be linked mainly or secondarily to a reduction in the content of antioxidants in the lens. MDA is known to play a role in lens opacification and can form cross-links between membrane lipids and proteins [45].
The transparency of the lens depends highly on the total content, order and structural integrity of the proteins in the lens. Relatively small changes, such as decrease the level of total protein and the Ca2+ATPase activity, may lead to the development of lenticular opacification and later cataracts. However, Ca2+ATPase is a major factor involved in maintaining homeostasis of lenticular Ca2+ levels, which counteracts the inward passive diffusion of Ca2+. In the present study, CA at 30- and 40-mg/kg dose levels significantly eliminated the cataractogenic effect via elevations of the total protein content and the Ca2+ATPase activity in the lens. The reduction in the total protein content in the lens might be due to the loss of Ca2+ATPase activity, which results in enhanced Ca2+ levels and leads to the formation of insoluble protein aggregates, the development of opacification, and ultimately cataractogenesis [46, 47].
Aldose reductase is the key enzyme in the polyol pathway that is accelerated in cataracts and elicits accumulation of polyol in the lens’ cells. Its poor penetration through cellular membranes leads to osmotic swelling, changes in membrane permeability, leakage of GSH, and perhaps even the production of free radicals and hydrogen peroxide, causing generation of osmotic and oxidative insult [48-50]. CA is reported to have aldose reductase inhibitory activity [51]. Further, El-Bassossy et al (2011) reported that CA reduced hypertension associated with diabetes due to its having a direct protective effect on vascular function [16]. Thus, the anti-cataract activity shown by CA might be due, in addition to its antioxidant property, to its aldose reductase inhibitory action and the resulting protective effect on vascular function.
The fructose-fed rats developed hypertension and cataracts due to oxidative stress, as indicated by reductions in the levels of various antioxidant enzymes. Administration of CA reduced the SBP and the DBP due to its having protective effects on the vascular function, as well as antioxidant properties. CA may also inhibit the aldose reductase activity, the key enzyme in the polyol pathway responsible for cataractogenesis. Thus, we can conclude that CA attenuates cataractogenesis in fructose-fed hypertensive rats due to its having antihypertensive, anti-oxidant and aldose reductase inhibition activities.
References
- 1.Lira RP, Nascimento MA, Arieta CEL, Duarte LE, Hirata FE, Nadruz W. Incidence of preoperative high blood pressure in cataract surgery among hypertensive and normotensive patients. Indian J Opthalmol. 2010;58(6):493–495. doi: 10.4103/0301-4738.71679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kearney PM, Whelton M, Reynolds K, Muntner P, Whelton PK, He J. Global burden of hypertension: analysis of worldwide data. Lancet. 2005;365(9455):217–223. doi: 10.1016/S0140-6736(05)70151-3. [DOI] [PubMed] [Google Scholar]
- 3.Lin L, Lv S, Li B. Angiotensin-I-converting enzyme (ACE)-inhibitory and antihypertensive properties of squid skin gelatin hydrolysates. Food Chem. 2012;131(1):225–230. doi: 10.1016/j.foodchem.2011.08.064. [DOI] [Google Scholar]
- 4.Mukesh BN, Le A, Dimitrov PN, Ahmed S, Taylor HR, McCarty CA. Development of cataract and associated risk factors. Arch Ophthalmol. 2006;124(1):79–85. doi: 10.1001/archopht.124.1.79. [DOI] [PubMed] [Google Scholar]
- 5.Rim TH, Kim MH, Kim WC, Kim TI, Kim EK. Cataract subtype risk factors identified from the Korea national health and nutrition examination survey 2008-2010. BMC Ophthalmol. 2014;14 doi: 10.1186/1471-2415-14-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tsai SY, Hsu WM, Cheng CY, Liu JH, Chou P. Epidemiologic study of age-related cataracts among an elderly Chinese population in Shih-Pai, Taiwan. Ophthalmology. 2003;110(6):1089–1095. doi: 10.1016/S0161-6420(03)00243-4. [DOI] [PubMed] [Google Scholar]
- 7.Yu X, Lyu D, Dong X, He J, Yao K. Hypertension and risk of cataract: a meta-analysis. PloS one. 2014;9(12) doi: 10.1371/journal.pone.0114012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wong TY, Mitchell P. The eye in hypertension. Lancet. 2007;369(9559):425–435. doi: 10.1016/S0140-6736(07)60198-6. [DOI] [PubMed] [Google Scholar]
- 9.Bautista LE, Vera LM, Arenas IA, Gamrara G. Independent association between inflammatory markers (C-reactive protein, interleukin-6, and TNF-alpha) and essential hypertension. J Hum Hypertension. 2005;19(2):149–154. doi: 10.1038/sj.jhh.1001785. [DOI] [PubMed] [Google Scholar]
- 10.Schaumberg DA, Ridker PM, Glynn RJ, Christen WG, Dana MR, Hennekens CH. High levels of plasma C-reactive protein and future risk of age-related cataract. Ann Epidemiol. 1999;9(3):166–171. doi: 10.1016/S1047-2797(98)00049-0. [DOI] [PubMed] [Google Scholar]
- 11.Lee SM, Lin SY, Li MJ, Liang RC. Possible mechanism of exacerbating cataract formation in cataractous human lens capsules induced by systemic hypertension or glaucoma. Ophthalmic Res. 1997;29(2):83–90. doi: 10.1159/000268001. [DOI] [PubMed] [Google Scholar]
- 12.Klein AV, Kiat H. The mechanisms underlying fructose- induced hypertension: a review. J Hypertens. 2015;33(5):912–920. doi: 10.1097/HJH.0000000000000551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Balasaraswathi K, Rajasekar P, Anuradha CV. Changes in redox ratio and protein glycation in precataractous lens from fructose-fed rats: effects of exogenous L-carnitine. Clin Exp Pharmacol Physiol. 2008;35(2):168–173. doi: 10.1111/j.1440-1681.2007.04815.x. [DOI] [PubMed] [Google Scholar]
- 14.Mang B, Wolters M, Schmitt B, Kelb K, Lichtinghagen R, Stichtenoth DO et al. Effects of a cinnamon extract on plasma glucose, HbA1c and serum lipids in diabetes mellitus type 2. Eur J Clin Invest. 2006;36(5):340–344. doi: 10.1111/j.1365-2362.2006.01629.x. [DOI] [PubMed] [Google Scholar]
- 15.Mahfouz MH, Ghanem HM, Mohamed MA. Therapeutic effect of L-carnitine on sialic acid, soluble Fas (sFas) and other biochemical variables in hyperinsulinemic rats. Life Sci J. 2009;6(2):76–82. [Google Scholar]
- 16.El-Bassossy HM, Fahmy A, Badawy D. Cinnamaldehyde protects from the hypertension associated with diabetes. Food Chem Toxicol. 2011;49(11):3007–3012. doi: 10.1016/j.fct.2011.07.060. [DOI] [PubMed] [Google Scholar]
- 17.Chang ST, Chen PF, Chang SC. Antibacterial activity of leaf essential oils and their constituents from Cinnamomum osmophloeum. J Ethnopharmacol. 2001;77(1):123–127. doi: 10.1016/S0378-8741(01)00273-2. [DOI] [PubMed] [Google Scholar]
- 18.Chao LK, Hua KF, Hsu HY, Cheng SS, Lin IF, Chen CJ et al. Cinnamaldehyde inhibits pro-inflammatory cytokines secretion frommonocytes/macrophages through suppression of intracellular signaling. Food Chem Toxicol. 2008;46(1):220–231. doi: 10.1016/j.fct.2007.07.016. [DOI] [PubMed] [Google Scholar]
- 19.Youn HS, Lee JK, Choi YJ, Saitoh SI, Miyake K, Hwang DH et al. Cinnamaldehyde suppresses toll-like receptor 4 activation mediated through the inhibition of receptor oligomerization. Biochem Pharmacol. 2008;75(2):494–502. doi: 10.1016/j.bcp.2007.08.033. [DOI] [PubMed] [Google Scholar]
- 20.Zhang W, Xu YC, Guo FJ, MengY, Li ML. Anti-diabetic effects of cinnamaldehyde and berberine and their impacts on retinol-binding protein 4expression in rats with type 2 diabetes mellitus. Chin Med J (Engl) 2008;121(21):2124–2128. [PubMed] [Google Scholar]
- 21.Lee HS. Inhibitory activity of Cinnamomum cassia bark-derived component against rat lens aldose reductase. J Pharm Pharm Sci. 2002;5(3):226–230. [PubMed] [Google Scholar]
- 22.Dai S, McNeil JH. Fructose-induced hypertension in rats is concentration and duration dependent. J Pharmacol Toxicol Methods. 1995;33(2):101–107. doi: 10.1016/1056-8719(94)00063-A. [DOI] [PubMed] [Google Scholar]
- 23.Son HY, Kim H, H Kwon., Y Taurine prevents oxidative damage of high glucose – induced cataractogenesis in isolated rat lenses. J Nutr Sci Vitaminol. 2007;53(4):324–330. doi: 10.3177/jnsv.53.324. [DOI] [PubMed] [Google Scholar]
- 24.Tappel AL. Glutathione peroxidase and hydroperoxides. Methods Enzymol. 1978;52(53):506–513. doi: 10.1016/S0076-6879(78)52055-7. [DOI] [PubMed] [Google Scholar]
- 25.Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121–126. doi: 10.1016/s0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- 26.Kakkar P, Das B, Viswanathan PN. A modified spectrophotometric assay of superoxide dismutase. Indian J Biochem Biophys. 1984;21(2):130–132. [PubMed] [Google Scholar]
- 27.Ellman GL. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959;82(1):70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 28.Ohkawa H, Ohishi N, Yagi K. Assay of lipid peroxide in animal tissue by thiobarbituric acid reaction. Anal Biochem. 1979;95(2):351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 29.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with Folin phenol reagent. J Biol Chem. 1951;193(1):265–275. [PubMed] [Google Scholar]
- 30.Rorive G, Kleinzeller A. Ca2+-Activated ATPase from renal tubular cells. Methods Enzymol. 1974;32(28):303–306. doi: 10.1016/0076-6879(74)32031-9. [DOI] [PubMed] [Google Scholar]
- 31.de Champlain, J, Wu R, Girouard H, Karas M, EL Midaoui, A, Laplante MA et al. Oxidative stress in hypertension. Clin Exp Hypertens. 2004;26(7-8):593–601. doi: 10.1081/ceh-200031904. [DOI] [PubMed] [Google Scholar]
- 32.Redon J, Oliva MR, Tormos C, Giner V, Chaves J, Iradi A et al. Antioxidant activities and oxidative stress byproducts in human hypertension. Hypertension. 2003;41(5):1096–1101. doi: 10.1161/01.HYP.0000068370.21009.38. [DOI] [PubMed] [Google Scholar]
- 33.Dimo T, Rakotonirina SV, Tan PV, Azay J, Dongo E, Cros G. Leaf methanol extract of Bidens pilosa prevents and attenuates the hypertension induced by high-fructose diet in Wistar rats. J Ethnopharmacol. 2002;83(3):183–191. doi: 10.1016/S0378-8741(02)00162-9. [DOI] [PubMed] [Google Scholar]
- 34.Khitan Z, Kim DH. Fructose: a key factor in the development of metabolic syndrome and hypertension. Nutr Metab. 2013;2013 doi: 10.1155/2013/682673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brownlee M. The pathobiology of diabetic complications: a unifying mechanism. Diabetes. 2005;54(6):1615–1625. doi: 10.2337/diabetes.54.6.1615. [DOI] [PubMed] [Google Scholar]
- 36.Rebolledo OR, Marra CA, Raschia A, Rodriguez S, Gagliardino JJ. Abdominal adipose tissue: early metabolic dysfunction associated to insulin resistance and oxidative stress induced by an unbalanced diet. Horm Metab Res. 2008;40(11):794–800. doi: 10.1055/s-2008-1081502. [DOI] [PubMed] [Google Scholar]
- 37.Gersch MS, Mu W, Cirillo P, Reungjui S, Zhang L, Roncal C et al. Fructose, but not dextrose, accelerates the progression of chronic kidney disease. Am J Physiol Renal physiol. 2007;293(4):1256–1261. doi: 10.1152/ajprenal.00181.2007. [DOI] [PubMed] [Google Scholar]
- 38.Rajasekar P, Anuradha CV. Effect of L-carnitine on skeletal muscle lipids and oxidative stress in rats fed high-fructose diet. 72741Exp Diabetes Res. 2007;2007 doi: 10.1155/2007/72741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guglielmotto M, Aragno M, Tamagno E, Vercellinatto I, Visentin S, Medana C et al. AGEs/RAGE complex upregulates BACE1 via NF-kappa B pathway activation. Neurobiol Aging. 2012;33(1):13–27. doi: 10.1016/j.neurobiolaging.2010.05.026. [DOI] [PubMed] [Google Scholar]
- 40.Lyle BJ, Mares-Periman JA, Klein BE, Klein R, Greger JL. Antioxidant intake and risk of incident age-related nuclear cataracts in the beaver dam eye study. Am J Epidemiol. 1999;149(9):801–809. doi: 10.1093/oxfordjournals.aje.a009895. [DOI] [PubMed] [Google Scholar]
- 41.Takagi Y, Kashiwagi A, Tanaka Y, Ashahin T, Kikkawa R, Shigeta Y. Significance of fructose-induced protein oxidation and formation of advanced glycation end product. J Diabetes Complications. 1995;9(2):87–91. doi: 10.1016/1056-8727(94)00022-G. [DOI] [PubMed] [Google Scholar]
- 42.Paglia DE, Valentine WN. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med. 1967;70(1):158–169. [PubMed] [Google Scholar]
- 43.Shearer TR, Azuma M, David LL, Murachi T. Amelioration of cataracts and proteolysis in cultured lenses by cysteine protease inhibitor E64. Invest Ophthalmol Vis Sci. 1991;32(3):533–540. [PubMed] [Google Scholar]
- 44.Awasthi S, Srivatava SK, Piper JT, Singhal SS, Chaubey M, Awasthi YC. Curcumin protects against 4-hydroxy- 2-trans-nonenal-induced cataract formation in rat lenses. AM J Clin Nutr. 1996;64(5):761–766. doi: 10.1093/ajcn/64.5.761. [DOI] [PubMed] [Google Scholar]
- 45.Grattagliano I, Vendemiale G, Boscia F, Micelli-Ferrari T, Cardia L, Altomare E. Oxidative retinal products and ocular damages in diabetic patients. Free Radic Biol Med. 1998;25(3):369–372. doi: 10.1016/S0891-5849(98)00059-8. [DOI] [PubMed] [Google Scholar]
- 46.Gupta PD, Johar K, Vasavada A. Causative and preventive action of calcium in cataracto-genesis. Acta Pharmacol Sin. 2004;25(10):1250–1256. [PubMed] [Google Scholar]
- 47.Liu L, Paterson CA, Borchman D. Regulation of sarco/ endoplasmic Ca2+-ATPase expression by calcium in human lens cells. Exp Eye Res. 2002;75(5):583–590. doi: 10.1006/exer.2002.2049. [DOI] [PubMed] [Google Scholar]
- 48.Bodakhe SH, Ram A, Verma S, Pandey DP. Anticataract activity of rhamnocitrin isolated from Bauhinia variegata stem bark. Orient Pharm Exp Med. 2012;12(3):227–232. doi: 10.1007/s13596-012-0059-1. [DOI] [Google Scholar]
- 49.Jung HA, Islam MD, Kwon YS, Jin SE, Son YK, Park JJ et al. Extraction and identification of three major aldose reductase inhibitors from Artemisia montana. Food Chem Toxicol. 2011;49(2):376–384. doi: 10.1016/j.fct.2010.11.012. [DOI] [PubMed] [Google Scholar]
- 50.Lee AY, Chung SK, Chung SS. Demonstration that polyol accumulation is responsible for diabetic cataract by the use of transgenic mice expressing the aldose reductase gene in the lens. Proc Natl Acad Sci USA. 1995;92(7):2780–2784. doi: 10.1073/pnas.92.7.2780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lee HS. Inhibitory activity of Cinnamomum cassia bark-derived component against rat lens aldose reductase. J Pharm Pharm Sci. 2002;5(3):226–230. [PubMed] [Google Scholar]