Abstract
Objective: Childhood-onset schizophrenia (COS) is a rare but severe form of the disorder, which is often treatment refractory. Short-term studies have indicated a greater differential efficacy, evident through effect sizes, favoring clozapine over other agents in alleviating negative symptoms in COS patients compared with adult-onset patients (AOS). There have been no data for COS patients on long-term compliance with clozapine treatment. Therefore, we wanted to know, over a span of up to 24 years, how many of our COS cohort had remained on clozapine for at least 2 years. We review short-term treatment data and present updated long-term data on compliance and functioning for our patients.
Methods: We present the results for long-term medication maintenance over a 24 year observation period for our cohort of 131 patients. Of this cohort, 91.6% (120) were available for follow-up information from either in-person or telephone contact with the patient and/or family members. We defined clozapine compliance as ≥2 years receiving this medication and doing well.
Results: We were able to contact 120 of the 131 patients. In spite of the additional cost and inconvenience of regular blood monitoring, 87 patients (72.5%, 87/120) adhered to long-term clozapine maintenance therapy with dosages ranging from 50 to 900 mg, and a median dosage of 500 mg. This rate exceeds the long-term clozapine maintenance rates reported for AOS patients.
Conclusions: Short-term data on differential efficacy and long-term maintenance data suggest a possibly greater efficacy of clozapine, relative to other antipsychotics, in COS than in AOS. Our overall findings indicate that very early-onset schizophrenic patients may be more responsive to clozapine. This extends other support for clozapine as an option in the treatment of early-onset schizophrenia.
Introduction
Childhood-onset schizophrenia (COS), defined as onset before age 13, is a rare and severe form of the illness (McKenna et al. 1994). Since 1990, the National Institutes of Mental Health (NIMH) has been conducting a study of the diagnosis, prepsychotic developmental history, biological markers, and treatment of COS.
The Child Psychiatry Branch of the NIMH has assembled a unique cohort of COS patients (n = 131, to date) which had a pattern of chronic, treatment-resistant illness, with insidious onset, resembling that of poor outcome adult-onset schizophrenia (AOS) (Nicolson et al. 2003; Rapoport et al. 2012). Converging evidence shows clinical and biological continuity of COS with AOS (Gordon et al. 1994; McKenna et al. 1994; Frazier et al. 1996; Jacobsen et al. 1996a,b; Zahn et al. 1997; Kumra et al. 2000; Asarnow et al. 2001; Levitt et al. 2001; Nicolson et al. 2003; Sporn et al. 2005; Kranzler et al. 2006; Gogtay 2008; Addington and Rapoport 2009; Rapoport et al. 2012; Ahn et al. 2014). The focus of the present report is to summarize our longer-term experience of treating COS patients with clozapine.
As will be reviewed, short-term studies suggest that COS patients may show a stronger response to clozapine than to other antipsychotics, and when compared with AOS patients treated with clozapine. First, we summarize our double-blind trials in relation to similar trials in AOS patients. The significant findings of our short-term double-blind studies of 6 and 8 weeks with small sample sizes suggest larger effect sizes compared with that seen in comparable adult trials. Because of this, we became further interested in our cohort's long-term clozapine adherence during our unusually long follow-up observation period of (up to 24 years).
Short-term studies
We compare the relative short-term superiority of clozapine, evident through effect sizes, for our two short-term (6–8 weeks) random, double-blind trials in small samples of treatment-refractory COS (Table 1) to large samples of AOS patients (Table 2). An independent study was also included that compared clozapine in relation to “high dose” olanzapine in early-onset schizophrenic (EOS) patients with a mean age of onset of 11.8 ± 2.9 years. This EOS study was included, because the unusually young age for adolescent onset included many COS patients (Kumra et al. 1996; Shaw et al. 2006; Kumra et al. 2008).
Table 1.
Clozapine Versus Active Treatment Studies for Adult-and Early-Onset Schizophrenia, Early-Onset Schizophrenia Studies
| Study | Design | Length (wks) | Age at enrollment (yrs) | Comparison drug dosage (mg/d) | Clozapine dosage (mg/d) | n | Cohen's d | p |
|---|---|---|---|---|---|---|---|---|
| Kumra et al. (1996) | Parallel | 6 | Mean: 14.0 ± 2.3 | Typical: Haldoperidol | Clozapine | 21 | SANS: 1.156 | 0.002 |
| randomized DB | Range: 6–18 | 16 ± 8 | 176 ± 149 | SAPS: 0.675 | 0.01 | |||
| BPRS: 0.258 | 0.04 | |||||||
| CGAS: 1.370 | 0.01 | |||||||
| Shaw et al. (2006) | Parallel | 8 | Mean: 12.3 | Atypical: Olanzapine | Clozapine | 25 | SANS: 0.7 | 0.08 |
| randomized DB | Range: 7–16 | 18.1 ± 4.3 | 327 ± 113 | SAPS: 0.4 | 0.14 | |||
| BPRS: 1 | 0.12 | |||||||
| CGI-S: 0.6 | 0.39 | |||||||
| Kumra et al. (2008) | Parallel | 12 | Mean: 15.6 ± 2.1a | Atypical: Olanzapine | Clozapine | 39 | SANS: 0.92 | 0.02 |
| randomized DB | Range: 10–18 | 26.2 ± 6.5 | 403.1 ± 201.8 | BPRS: 0.29 | 0.78 | |||
| CGI-S: 0.4 | 0.8 | |||||||
| CGI-Improvement: 0.59 | 0.38 |
The rationale for including this early-onset schizophrenia study was the unusually young mean age of onset: 11.8 ± 2.9 years.
DB, double-blind; SANS, Scale for the Children's Global Assessment Scale; Assessment of Negative Symptoms; SAPS, Scale for the Assessment of Positive Symptoms; BPRS, Brief Psychiatric Rating Scale; CGAS, CGI, Clinical Global Impressions Scale; CGI-S, Clinical Global Impressions Severity of Symptom Scale.
Table 2.
Clozapine Versus Active Treatment Studies for Adult- and Early-Onset Schizophrenia, Adult-Onset Schizophrenia Studies
| Study | Design | Length (wks) | Age at enrollment (yrs) | Comparison drug dosage (mg/d) | Clozapine dosage (mg/d) | n | Cohen's d | p |
|---|---|---|---|---|---|---|---|---|
| Pickar et al. (1992) | Crossover comparison | Range: 20–43 | Typical: Fluphenazine | Clozapine | 21 | SANS: 0.222 | ||
| Varied | Mean: 29.4 ± 6.1 | 28.9 ± 21.2 | 542.9 ± 207.4 | BPRS Negative: 0.566 | <0.01 | |||
| BPRS Positive: 0.685 | <0.05 | |||||||
| BPRS: 0.986 | <0.001 | |||||||
| Azorin et al. (2001) | Parallel | 12 | Range: 18–65 | Atypical: Risperidone | Clozapine | 273 | PANSS Total: 0.327 | 0.02 |
| randomized DB | Mean: 38.7 | Median: 9 | Median: 600 | PANSS Negative: 0.243 | 0.06 | |||
| BPRS: 0.410 | 0.006 | |||||||
| CGI: 0.332 | 0.008 | |||||||
| Bitter et al. (2004) | Parallel | 18 | Range: 18–65 | Atypical: Olanzapine | Clozapine | 147 | PANSS Total: 0.009 | 0.562 |
| randomized DB | Mean: 37.6 | 17.2 ± 4.8 | 216.2 ± 107.9 | PANSS Negative: 0.017 | 0.838 | |||
| PANSS Positive: 0.013 | 0.574 | |||||||
| CGI-S: 0.087 | 0.829 | |||||||
| Meltzer et al. (2008) | Parallel | 6 | Range: 18–58 | Atypical: Olanzapine | Clozapine | 40 | PANSS Total: 0.5 | |
| randomized DB | Mean: 36.8 | 32.7 ± 5.94 | 400 ± 158 | PANSS Negative: 0.021 | ||||
| PANSS Positive: 0.145 GAF: 0.28 CGI: 0.325 CGI-S: 0.5 |
DB, double-blind; SANS, Scale for the Assessment of Negative Symptoms; BPRS, Brief Psychiatric Rating Scale; PANSS, Positive and Negative Syndrome Scale; CGI, Clinical Global Impressions Scale; CGI-S, Clinical Global Impressions Severity of Symptom Scale; GAF, Global Assessment of Functioning.
The superior efficacy of clozapine in controlled, comparative trials with treatment-refractory AOS has been well documented (Kane et al. 1989; Pickar et al. 1992; Bondolfi et al. 1998; Azorin et al. 2001; Tollefson et al. 2001; Volavka et al. 2002; Conley et al. 2003; Bitter et al. 2004; Meltzer et al. 2008). As seen in Table 2, clozapine was more efficacious in alleviating negative symptoms than comparison antipsychotics. This efficacy was more pronounced when clozapine was compared with a first generation antipsychotic (d = 0.22, Scale for the Assessment of Negative Symptoms [SANS]; d = 0.566, p < 0.01, Brief Psychiatric Rating Scale for Children [BPRS] Negative) than when it was compared with a few second generation antipsychotics (d = 0.243, p = 0.06; d = 0.017, p = 0.84; d = 0.021) (Pickar et al. 1992; Azorin et al. 2001; Bitter et al. 2004; Meltzer et al. 2008). Regardless, these differential effect sizes are considered small.
As summarized in Table 1, Kumra et al. (1996) conducted the first randomized, double-blind study comparing clozapine to the typical neuroleptic haloperidol in our sample of treatment-refractory COS patients. At 6 weeks, clozapine reduced both positive and negative symptoms and was superior to haloperidol on all end-point measures of psychosis, including the BPRS for Children (BPRS-C), Children's Global Assessment Scale (CGAS), SANS, and Scale for the Assessment of Positive Symptoms (SAPS) (p = 0.04, p = 0.01, p = 0.002, and p = 0.01, respectively) (Andreasan 1983; Shaffer et al. 1983; Andreasan 1984; Overall and Pfefferbaum 1984; Kumra et al. 1996).
A later study comparing clozapine to olanzapine in our COS patients produced similar findings (Shaw et al. 2006). At the conclusion of this 8 week, randomized, double-blind comparative study, clozapine significantly improved all outcome measures, including the Clinical Global Impressions Scale and Severity of Symptoms Scale (CGI and CGI-S), SANS, SAPS, BPRS B1-7B, 24-item version, and the Bunney-Hamburg psychosis scales (p = 0.005, p = 0.005, p = 0.03, p = 0.006, and p = 0.003, respectively), from admission measures (Overall and Gorham 1962; Bunney and Hamburg 1963; Guy 1976; Shaw et al. 2006). Meanwhile, olanzapine showed clinical improvement only from medication-free baseline measures, and not admission measures. Responder status for clozapine versus olanzapine did not significantly differ.
In an independent study, Kumra et al. evaluated the effectiveness of clozapine and “high dose” olanzapine in treatment-refractory children and adolescents with EOS (2008). At the conclusion of the 12 week, randomized, double-blind comparison trial, clozapine was significantly superior to olanzapine in alleviating negative symptoms from medication-free baseline measures (p = 0.04) and was trending toward significance from admission baseline measures (p = 0.08). Additionally, significantly more clozapine-treated patients (12 of 18, 66%) than olanzapine-treated patients (7 of 21, 33%) qualified as responders (p = 0.038), making a differential categorical response rate of 33% (Kumra et al. 2008).
Despite the limitations in comparing the statistic of Cohen's d scores across studies of differing populations, designs, active comparison agents, and measures, clozapine generally appeared to be more efficacious in pediatric than in adult populations (McGough and Faraone 2009). This is particularly true with respect to alleviating negative symptoms. Tables 1 and 2 show that clozapine generally had small differential effect sizes in AOS, as evident through the SANS and PANSS Negative scales, compared with the large differential effect sizes seen in EOS studies, using the SANS scale (Kumra: 1.15; Shaw: 0.7; Kumra: 0.92) (Kumra et al. 1996; Shaw et al. 2006; Kumra et al. 2008).
These data suggest that COS patients generally benefit more from clozapine than AOS patients do. Ideally, these two patient populations should be compared in a single, large, randomized, controlled, double-blind study.
In this present report, we follow our COS cohort's medication maintenance over 24 years. Our survey indicates a high rate of long-term clozapine use, monitored by outside referring psychiatrists, in our COS cohort, despite the cost and inconvenience of regular blood monitoring. We were interested to see if long-term maintenance rates mirrored short-term response data, supporting a relatively greater long-term preference for clozapine treatment in our COS cohort.
Methods
Because COS is rare, this study required national recruitment. Inclusion criteria for the COS study were based on unmodified criteria for schizophrenia (Diagnostic and Statistical Manual of Mental Disorders, 3rd, ed., revised, and 4th ed. [DSM-IIIR/DSM-IV]) with onset of positive symptoms, such as delusions and/or hallucinations, documented prior to the subject's 13th birthday (American Psychiatric Association 1987, 1994). Diagnosis was determined on the basis of a review of medical and school records as well as an interview and administration of the Kiddie-Schedule for Affective Disorders and Schizophrenia for School-Aged Children—Present and Lifetime Version (K-SADS-PL) with the child and parents (Kaufman et al. 1997). Most of our patients had negative symptoms, and all had had clear deterioration of at least 6 months' duration. Premorbid intelligence quotient (IQ) <70 and/or comorbid neurological disorder was exclusionary. Since 1990, the Child Psychiatry Branch of the National Institutes of Health (NIH) has assembled a unique cohort of COS cases (n = 131, to date).
Screening and inpatient baseline evaluation
Participation in the COS study involved a 2 day outpatient screening. For those considered to have probable COS, the screening was followed by a 3 month inpatient observation period, including a 3 week, medication-free period for diagnostic and treatment purposes. Most COS patients received either a double-blind or an open trial of clozapine before discharge. With initial patient recruits, we conducted two short-term, double-blind, controlled trials, (Kumra et al. 1996; Shaw et al. 2006). Patient entry into either of our two psychopharmacological trials required previous failure to respond to two different neuroleptics, typical or atypical, at adequate dosages (>100 mg chlorpromazine equivalents) and at adequate durations (≥ 4 weeks unless terminated for adverse effects). Failure was operationalized as insufficient response of symptoms such that the persistence of symptoms significantly impaired the child's functioning (evidence of impairment was obtained from the child, parent, and medical and school records) or intolerable adverse side effects (Kumra et al. 1996; Shaw et al. 2006).
Patients not in either of our double-blind studies typically received a structured, 6–8 week open trial of clozapine, with weekly ratings for symptoms and side effects.
For all COS patients, initial weekly ratings included scores on the CGI, CGAS, SANS, SAPS, BPRS, and the Bunney-Hamburg psychosis, depression, mania, and anxiety rating scales.
To evaluate the efficacy of antipsychotic treatment, outcome ratings from the end-point of the double-blind study or open-label trial were compared with initial ratings on the aforementioned measures of the CGI, CGI-S, CGAS, SANS, SAPS, BPRS, BPRS-C, and the Bunney-Hamburg psychosis, depression, mania, and anxiety rating scales.
In both of our double-blind studies, as well as open treatment with clozapine, adverse effects were evaluated using the Subjective Treatment Emergent Symptoms Scale, which was modified to include adverse events known to be associated with clozapine (Campbell and Palig 1985). Extrapyramidal symptoms and involuntary movements were rated on the Abnormal Involuntary Movement Scale (Rapoport et al. 1985) and the Simpson-Angus Neurological Rating Scale (Simpson and Angus 1970).
Long-term maintenance
The initial focus of the COS study required long-term follow-up at 2 year intervals as part of an imaging study of brain development. Therefore, the initial 70 COS subjects enrolled in our cohort were systematically followed. Many of these initial COS patients, in addition to participating in a double-blind trial, returned in person to the NIH for as many as six follow-up visits (Fig 1.).
FIG. 1.
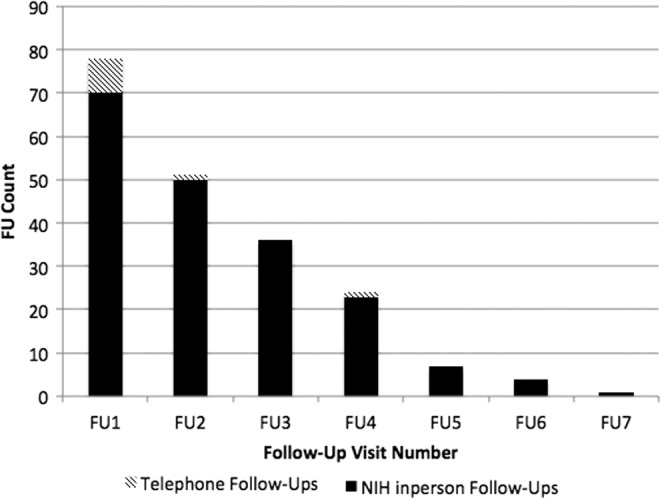
Frequency of long-term in-person and phone follow-up visits.
The remainder of our COS cohort were not followed quite as stringently. Later in our study, patients typically returned for the acquisition of biological measures or new treatment trials. After discharge and between follow-up visits to the NIH, all patients were managed independently by their individual private physicians. During follow-up visits, we collected information regarding interim and current medication information.
Medication updates were obtained through in-person follow-up returns, parents, or phone interviews. For those patients returning to the NIH, we received exact medication dosages. When feasible, we contacted patients, specifically those not returning to the NIH, by phone at regular intervals to obtain incomplete information to assess their interim medication history. Through these telephone interviews, we discovered that some of our patients who were not discharged from the NIH on clozapine were started and maintained on clozapine by their personal physician.
Since 1990, we have had the opportunity to collect patients' medication histories prospectively for as many as 24 years. For the purpose of this report, we identified reachable COS patients doing well on clozapine for at least 2 years. Therefore, long-term clozapine maintenance was operationalized as ≥2 years of adherence to clozapine treatment with descriptive information identifying that the patient had optimal results on clozapine compared with the same measures with other antipsychotics. Patients included in this study were either discharged from the NIH on clozapine, or, in a small number of cases, the patients were placed on clozapine by an outside provider for at least 2 years and were considered to be functioning relatively better than when on other agents.
Results
Since 1990, a total of 131 COS patients have been accrued. This study included 127 patients who were discharged from the NIH as of January 2014, in order to have at least a 2 year treatment history. Over time, we lost contact with six patients discharged on clozapine and one patient discharged on an alternate antipsychotic from the NIH. Therefore, there were a total of 120 (91.6%, 120/131) reachable patients with the potential to have at least a 2 year follow-up. These 120 patients were the population used to determine medication maintenance. Our updated information regarding adherence to drug regimen is summarized in Figure 2.
FIG. 2.
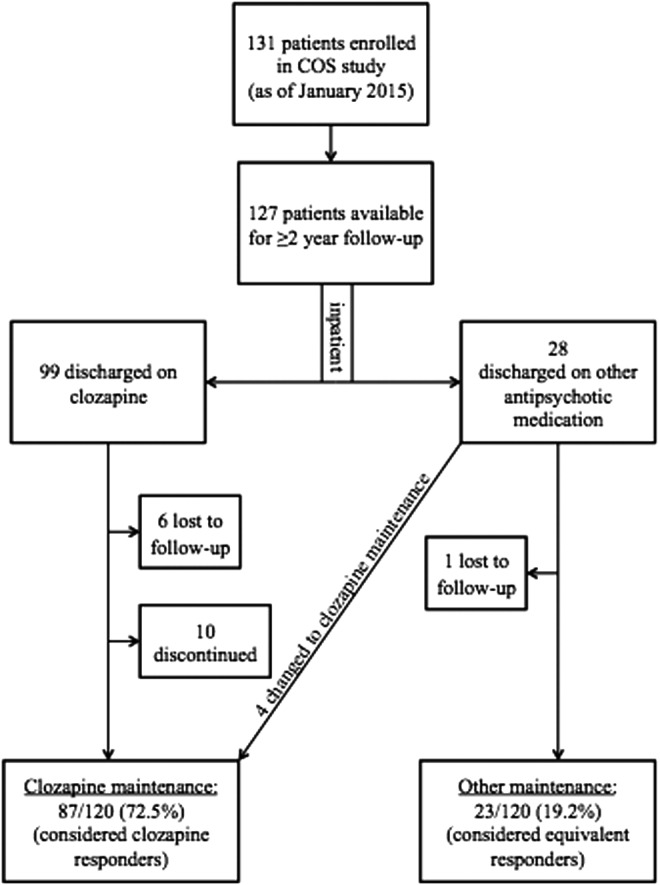
Long-term clozapine maintenance rate in childhood-onset schizophrenia.
Twenty-eight patients were discharged from the NIH on an alternative medication regime because either they were not receiving maximal benefit and/or they were unable to tolerate the antipsychotic. Of the 28 patients discharged on an alternative medication regime, four were later put on clozapine by their outside providers and returned to the NIH for follow-up visits on clozapine after at least 2 years on clozapine. They did better on clozapine than on other antipsychotics, meeting our criteria for clozapine maintenance. The remaining 23 of these patients maintained their alternative medication regime for their follow-up visits, as they were judged to have “equivalent” maintenance to an alternate agent.
Complimentary to this, 99 patients who tolerated clozapine well in the NIH study were discharged on clozapine. Of these 99 patients discharged from the NIH on clozapine, 10 discontinued clozapine because of various side effects prior to their 2 year follow-up visit. Two of these patients had clozapine-induced seizures, and two had neutropenia. Four additional patients of our cohort discontinued clozapine because of other side effects including weight gain, palpitations, violence and aggression, and poor impulse control. Two patients discontinued for unknown reasons.
The remaining 83 COS patients tolerated clozapine in the NIH study, were discharged on clozapine, and were maintained by their outside physician for at least 2 years, assuming maximum benefit. In addition, we included the four patients, discharged on other antipsychotics and later changed to clozapine maintenance, resulting in a total of 87 patients. Therefore, of the 120 reachable COS patients, 72.5% (87/120) were maintained on clozapine compared with the 19.2% (23/120) who maintained an alternative medication regime. The remaining 8.3% (10/120) of the cohort did not meet criteria for either clozapine or alternative maintenance.
For patients meeting the criteria for clozapine maintenance, their dosage of clozapine at their most recent follow-up visit to the NIH ranged from 50 to 900 mg, with a median dosage of 500 mg. The lower quartile dosage was 275 mg, and the upper quartile dosage was 500 mg (n = 84; we could not confirm exact clozapine dosage for three patients).
The literature on long-term, open clozapine maintenance reports in AOS patients was reviewed for comparison purposes with these data (Table 3). The high maintenance rate of 72.5% of COS patients adhering to clozapine treatment exceeds the long-term clozapine maintenance data in AOS that range from 32% to 57% (Juul Povlsen et al. 1985; Lindström 1988; Mattes 1989; Gaszner and Makkos 2004). Although we cannot statistically compare across studies, compliance with clozapine treatment is greater in COS than in AOS patients.
Table 3.
Long-Term Clozapine Maintenance Rates for AOS and the NIH COS Population
| Study | Design | FU length (yrs) | n | Population description of subjects | Mean CLZ duration | Range of CLZ duration | Discontinued | Maintained |
|---|---|---|---|---|---|---|---|---|
| Adult-onset | ||||||||
| Juul Povlsen et al. (1985) | Retrospective open, chart | 12 | 216 | Primary diagnosis of SCH: 182 | 2.75 yrs | 0.08–12 yrs | 57.4% (124) | 42.6% (92) |
| Lindstrom (1988) | Retrospective | 13 | 96 | Chronic, treatment-resistant SCH: 89 Schizoaffective: 7 |
36.4% (35) | 57.3% (55)a | ||
| Mattes (1989) | Open study | 2 | 14 | Chronic, treatment-resistant | Two years after initiating study, 4.3 ± 3.6 mos | 57.1% (8) | 42.9% (6) | |
| Childhood-onset | ||||||||
| Rapoport et al. (2015) | Open study | 24 | 120 | Chronic, treatment-resistant | 6.9 ± 4.98 yrs | 1.024–23.5 yrs | 8.3% (10/120) | 72.5% (87/120) |
As calculated from manuscript data.
AOS, adult-onset schizophrenia; NIH, National Institutes of Health; COS, childhood-onset schizophrenia; FU, follow-up; SCH, schizophrenia.
The high maintenance rate of COS exceeds the limited evidence from randomized, double-blind controlled studies of treatment-refractory adult-onset schizophrenia. Moreover, despite additional cost and inconvenience, these patients continue with a clear preference to remain on clozapine ranging from 2 to ≥20 years, with an average of 6.9 ± 4.98 years of maintenance.
Discussion
Our short-term double-blind symptoms measures, differential efficacy, and long-term medication adherence suggest a possible greater efficacy of clozapine in COS than in AOS. With small sample sizes of inpatient and treatment-refractory COS patients, it was striking that significant results were seen in both of our double-blind studies. Because of the rarity of COS, small sample sizes of 21 (Kumra et al. 1996), 25 (Shaw et al. 2006), and 39 (Kumra et al. 2008) were available for comparison with the relatively large sample sizes employed in the AOS studies that ranged from 40 to nearly 300 patients (Table 2).
Our findings are consistent with the recent Scandinavian population study that found that 88.8% of EOS patients (mean age of onset is 16.2 years) prescribed clozapine appeared to have a favorable outcome as indicated by continued prescription redemption for 6 consecutive months (Schneider et al. 2015).
Prescription redemption for a minimum of 6 consecutive months following initiation may serve as a good operationalized definition of treatment response, although our operational definition of at least 2 years was more conservative. Periods shorter than this may reflect discontinuation caused by adverse drug reactions during titration, whereas discontinuation after 6 months is likely to be influenced by nonspecific factors affecting long-term adherence, including refusal of ongoing hematological monitoring (Schneider et al. 2015). Additionally, symptomatic improvement may continue from 6 weeks to ≥6 months. Approximately 30% of treatment-resistant AOS patients require up to 6 months of treatment with clozapine to show improvement in psychopathology (Meltzer et al. 1989). Consequentially, 88.8% may be considered responders in Schneider et al.'s study (2015), and this parallels our finding that 72.5% of our cohort consisted of long-term responders. Uniquely, our long-term findings suggest that COS may be more responsive to clozapine, as many children in our study continued to improve after discharge from initial NIH contact.
One interpretation seen in in our short-term studies is that greater efficacy manifests in the long-term compliance for patients with COS, evidenced by their greater long-term follow-up (72.5%) compared with AOS rates that range from 32% to 57% (Table 3). If the superiority of clozapine in the long-term is true, then this may encourage treatment retention and, therefore, prevent relapse (Meltzer et al. 2010).
This finding stands despite the rate of clozapine-related adverse effects in children exceeding those reported for adults (Frazier et al. 2003; Sporn et al. 2007). There is a greater toxicity and risk of agranulocytosis in younger subjects, as only 1–2% of adults experience neutropenia, whereas 6% of children do (Alvir et al. 1993). Children also have akathisia at a higher rate, 15%, in comparison with the 3% seen in adults (Physicians' Desk Reference 2004). Undesirable side effects, such as weight gain and metabolic changes, may also warrant discontinuation of clozapine usage.
In addition, clozapine maintenance is more expensive and inconvenient because of the need for blood tests and monthly renewal of medication. Because long-term controlled studies have not been feasible, our follow-up findings are open to other possible explanations for the high percentage of children on clozapine maintenance.
Limitations
We cannot rule out an alternative explanation of a “halo” effect, which is based on the deep need for families of very ill children to believe in a treatment independent of strong evidence. We are aware that, for example, parents of children with autism spectrum disorders (ASDs) may have faith in unsubstantiated treatments. Children with ASDs have disturbances in their metabolism of copper (Cu) and zinc (Zn) that manifest as a Zn deficiency and a toxicity of Cu (Bjorklund 2013). Because of these nutritional disturbances, there is great loyalty to supplemental nutrients (Curtis and Patel 2008) despite little support in the literature to suggest efficacy.
Similar to parents of ASD children, parents of COS patients are coping with chronically ill children, and these families may cling to the hope of the presumed effectiveness of clozapine, widely viewed as the “gold standard” medication for treatment-refractory schizophrenia (Findling et al. 2007). This may influence parental enforcement of compliance in our families that may result in greater long-term management. Parents of COS children are likely to be more reliably compliant with the regulations of clozapine treatment than are AOS patients, who must comply on their own. The most common reason for adult schizophrenic patients to discontinue clozapine treatment is lack of compliance with mandatory blood draws monitoring white blood cells (Mustafa et al. 2015). Therefore, our higher rate of compliance may be the result of parental support, which is less consistently available for AOS patients with respect to clozapine renewal.
Nevertheless, other prestigious institutions have also conducted clozapine comparison treatment trials, and their percentage of those maintained on clozapine who are short-term differential responders appears lower than our findings. However, it should be noted that at this point in our ongoing study, the average age is 27.2 (SD 7.7) years within our COS cohort, and we have not seen reduced compliance as our subjects become older.
A second limitation is that we do not have accurate dosage information for all participants meeting the criteria for clozapine maintenance. We were able to consistently obtain exact clozapine dosages only for participants personally returning to the NIH for a follow-up visit. Our efforts to gather dosages through follow-up phone calls to either patients or parents cannot be considered as accurate as our inpatient data collection. Despite being unable to follow up and/or receive exact clozapine dosages for some of our patients, the high proportion of reachable patients maintaining clozapine is remarkable.
Finally, our small sample size, necessitated by the rarity of the disease and the difficulty of conducting a long-term trial with a nationally recruited population, precludes easy replication.
A multicentered, European study (Optimise) will address some questions with regard to clozapine response and maintenance in AOS. At six various sites, clozapine will be compared with olanzapine in a long-term, double-blind study in AOS patients. Arango et al. found that 20% of AOS patients end up on clozapine, and approximately half of those patients maintain clozapine treatment (http://www.optimisetrial.eu/). This will provide insightful long-term information for adults, and will eventually be extended to adolescents.
Conclusions
Our short-term data suggest greater efficacy, and our long-term maintenance data suggest greater compliance, and these data taken together indicate a greater efficacy of clozapine in COS than in AOS.
Clinical Significance
There is good support for clozapine as an option in the treatment of EOS. Both the short-term and the long-term maintenance data indicate this to be the case.
Disclosures
No competing financial interests exist.
References
- Addington AM, Rapoport JL: The genetics of childhood-onset schizophrenia: When madness strikes the prepubescent. Curr Psychiatry Rep 2:156–161, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn K, Gotay N, Andersen TM, Anvari AA, Gochman P, Lee Y, Sanders S, Guha S, Darvasi A, Glessner JT, Hakonarson H, Lencz T, State MW, Shugart YY, Rapoport JL: High rate of disease-related copy number variations in childhood onset schizophrenia. Mol Psychiatry 19:568–572, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvir JM, Lieberman JA, Safferman AZ, Schwimmer JL, Schaaf JA: Clozapine-induced agranulocytosis. Incidence and risk factors in the United States. N Engl J Med 329:162–167, 1993 [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association: Diagnostic and Statistical Manual of Mental Disorders, 3rd ed., revised. Washington, DC: American Psychiatric Association; 1987 [Google Scholar]
- American Psychiatric Association: Diagnostic and Statistical Manual of Mental Disorders, 4th ed., Text Revision. Washington, DC: American Psychiatric Association; 1994 [Google Scholar]
- Andreasan NC: Scale for the Assessment of Negative Symptoms (SANS). Iowa City, University of Iowa, 1983 [Google Scholar]
- Andreasan NC: Scale for the Assessment of Positive Symptoms (SAPS). Iowa City, University of Iowa, 1984 [Google Scholar]
- Asarnow RF, Nuechterlein KH, Fogelson D, Subotnik KL, Payne DA, Russell AT, Asamen J, Kuppinger H, Kendler KS: Schizophrenia and schizophrenia-spectrum personality disorders in the first-degree relatives of children with schizophrenia: The UCLA family study. Arch Gen Psychiatry 58:581–588, 2001 [DOI] [PubMed] [Google Scholar]
- Azorin JM, Spiegel R, Remington G, Vanelle JM, Péré JJ, Giguere M, Bourdeix I: A double-blind comparative study of clozapine and risperidone in the management of severe chronic schizophrenia. Am J Psychiatry 158:1305–1313, 2001 [DOI] [PubMed] [Google Scholar]
- Bitter I, Dossenbach MR, Brook S, Feldman PD, Metcalfe S, Gagiano CA, Füredi J, Bartko G, Janka Z, Banki CM, Kovacs G, Breier A, Olanzapine HGCK Study Group: Olanzapine versus clozapine in treatment-resistant or treatment-intolerant schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry 28:173–180, 2004 [DOI] [PubMed] [Google Scholar]
- Bjorklund G: The role of zinc and copper in autism spectrum disorders. Acta Neurobiol Exp (Wars) 73:225–236, 2013 [DOI] [PubMed] [Google Scholar]
- Bondolfi G, Dufour H, Patris M, May JP, Billeter U, Eap CB, Baumann P: Risperidone versus clozapine in treatment-resistant chronic schizophrenia: A randomized double-blind study. The Risperidone Study Group. Am J Psychiatry 155:499–504, 1998 [DOI] [PubMed] [Google Scholar]
- Bunney WE, Hamburg DA: Methods for reliable longitudinal observation of behavior: Development of a method for systematic observation of emotional behavior on psychiatric wards. Arch Gen Psychiatry 3:280–294, 1963 [DOI] [PubMed] [Google Scholar]
- Campbell M, Palig M: Subjective Treatment Emergent Symptoms Scale (STESS). Psychopharmacol Bull 21:1063–1082, 1985. 4089101 [Google Scholar]
- Conley RR, Kelly DL, Richardson CM, Tamminga CA, Carpenter WT, Jr: The efficacy of high-dose olanzapine versus clozapine in treatment-resistant schizophrenia: A double-blind crossover study. J Clin Psychopharmacol 23:668–671, 2003 [DOI] [PubMed] [Google Scholar]
- Curtis LT, Patel K: Nutritional and environmental approaches to preventing and treating autism and attention deficit hyperactivity disorder (ADHD): A review. J Altern Complement Med 14:79–85, 2008 [DOI] [PubMed] [Google Scholar]
- Findling RL, Frazier JA, Gerbino–Rosen G, Kranzler HN, Kumra S, Kratochvil CJ: Is there a role for clozapine in the treatment of children and adolescents? J Am Acad Child Adolesc Psychiatry 46:423–428, 2007 [DOI] [PubMed] [Google Scholar]
- Frazier JA, Cohen LG, Jacobsen L, Grothe D, Flood J, Baldessarini RJ, Piscitelli S, Kim GS, Rapoport JL: Clozapine pharmacokinetics in children and adolescents with childhood-onset schizophrenia. J Clin Psychopharmacol 23:87–91, 2003 [DOI] [PubMed] [Google Scholar]
- Frazier JA, Giedd JN, Hamburger SD, Albus KE, Kaysen D, Vaituzis AC, Rajapakse JC, Lenane MC, McKenna K, Jacobsen LK, Gordon CT, Breier A, Rapoport JL: Brain anatomic magnetic resonance imaging in childhood-onset schizophrenia. Arch Gen Psychiatry 53:617–624, 1996 [DOI] [PubMed] [Google Scholar]
- Gaszner P, Makkos Z: Clozapine maintenance therapy in schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry 28:465–469, 2004 [DOI] [PubMed] [Google Scholar]
- Gogtay N: Cortical brain development in schizophrenia: Insights from neuroimaging studies in childhood-onset schizophrenia. Schizophr Bull 34:30–36, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon CT, Frazier JA, McKenna K, Giedd J, Zametkin A, Zahn T, Hommer D, Hong W, Kaysen D, Albus KE, et al. . Childhood-onset schizophrenia: An NIMH study in progress. Schizophr Bull 20:697–712, 1994 [DOI] [PubMed] [Google Scholar]
- Guy W: Abnormal involuntary movement scale (AIMS) ECDEU Assessment Manual for Psychopharmacology, Revised. Rockville, MD: National Institute of Mental Health; 338:534–537, 1976 [Google Scholar]
- Jacobsen LK, Giedd JN, Vaituzis AC, Hamburger SD, Rajapakse JC, Frazier JA, Kaysen D, Lenane MC, McKenna K, Gordon CT, Rapoport JL: Temporal lobe morphology in childhood-onset schizophrenia. Am J Psychiatry 153:355–361, 1996a [DOI] [PubMed] [Google Scholar]
- Jacobsen LK, Hong WL, Hommer DW, Hamburger SD, Castellanos FX, Frazier JA, Giedd JN, Gordon CT, Karp BI, McKenna K, Rapoport JL: Smooth pursuit eye movements in childhood-onset schizophrenia: Comparison with attention-deficit hyperactivity disorder and normal controls. Biol Psychiatry 40:1144–1154, 1996b [DOI] [PubMed] [Google Scholar]
- Juul Povlsen U, Noring U, Fog R, Gerlach J. Tolerability and therapeutic effect of clozapine. A retrospective investigation of 216 patients treated with clozapine for up to 12 years. Acta Psychiatr Scand 71:176–185, 1985 [DOI] [PubMed] [Google Scholar]
- Kane J, Honigfeld G, Singer J, Meltzer H: Clozapine for the treatment-resistant schizophrenic. A double-blind comparison with chlorpromazine. Arch Gen Psychiatry 45:789–796, 1988 [DOI] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Williamson D, Ryan N: Schedule for Affective Disorders and Schizophrenia for School-Age Children–Present and Lifetime Version (K-SADS-PL): Initial reliability and validity data. J Am Acad Child Adolesc Psychiatry 36:980–988, 1997 [DOI] [PubMed] [Google Scholar]
- Kranzler HN, Kester HM, Gerbino–Rosen G, Henderson IN, Youngerman J, Beauzile G, Ditkowsky K, Kumra S: Treatment-refractory schizophrenia in children and adolescents: An update on clozapine and other pharmacologic interventions. Child Adolesc Psychiatr Clin N Am 15:135–159, 2006 [DOI] [PubMed] [Google Scholar]
- Kumra S, Frazier JA, Jacobsen LK, McKenna K, Gordon CT, Lenane MC, Hamburger SD, Smith AK, Albus KE, Alaghband-Rad J, Rapoport JL: Childhood-onset schizophrenia. A double-blind clozapine-haloperidol comparison. Arch Gen Psychiatry 53:1090–1097, 1996 [DOI] [PubMed] [Google Scholar]
- Kumra S, Kranzler H, Gerbino–Rosen G, Kester HM, De Thomas C, Kafantaris V, Correll CU, Kane JM: Clozapine and “high-dose” olanzapine in refractory early-onset schizophrenia: A 12-week randomized and double-blind comparison. Biol Psychiatry 63:524–529, 2008 [DOI] [PubMed] [Google Scholar]
- Kumra S, Wiggs E, Bedwell J, Smith AK, Arling E, Albus K, Hamburger SD, McKenna K, Jacobsen LK, Rapoport JL, Asarnow RF: Neuropsychological deficits in pediatric patients with childhood-onset schizophrenia and psychotic disorder not otherwise specified. Schizophr Res 42:135–144, 2000 [DOI] [PubMed] [Google Scholar]
- Levitt JG, Blanton RE, Caplan R, Asarnow R, Guthrie D, Toga AW, Capetillo–Cunliffe L, McCracken JT: Medial temporal lobe in childhood-onset schizophrenia. Psychiatry Res 108:17–27, 2001 [DOI] [PubMed] [Google Scholar]
- Lindström LH: The effect of long-term treatment with clozapine in schizophrenia: A retrospective study in 96 patients treated with clozapine for up to 13 years. Acta Psychiatr Scand 77:524–529, 1988 [DOI] [PubMed] [Google Scholar]
- Mattes JA: Clozapine for refractory schizophrenia: An open study of 14 patients treated up to 2 years. J Clin Psychiatry 50:389–391, 1989 [PubMed] [Google Scholar]
- McGough JJ, Faraone SV: Estimating the size of treatment effects: moving beyond p values. Psychiatry (Edgmont) 6:21–9, 2009 [PMC free article] [PubMed] [Google Scholar]
- McKenna K, Gordon CT, Lenane M, Kaysen D, Fahey K, Rapoport JL: Looking for childhood–onset schizophrenia: The first 71 cases screened. J Am Acad Child Adolesc Psychiatry 33:636–644, 1994 [DOI] [PubMed] [Google Scholar]
- Meltzer HY, Bastani B, Kwon KY, Ramirez LF, Burnett S, Sharpe J: A prospective study of clozapine in treatment-resistant schizophrenic patients. I. Preliminary report. Psychopharmacology (Berl) 99 Suppl:S68–72, 1989 [DOI] [PubMed] [Google Scholar]
- Meltzer HY, Bobo WV, Lee MA, Cola P, Jayathilake K: A randomized trial comparing clozapine and typical neuroleptic drugs in non-treatment-resistant schizophrenia. Psychiatry Res 177:286–293, 2010 [DOI] [PubMed] [Google Scholar]
- Meltzer HY, Bobo WV, Roy A, Jayathilake K, Chen Y, Ertugrul A, Anil Yağcioğlu AE, Small JG: A randomized, double-blind comparison of clozapine and high-dose olanzapine in treatment-resistant patients with schizophrenia. J Clin Psychiatry 69:274–285, 2008 [DOI] [PubMed] [Google Scholar]
- Mustafa FA, Burke JG, Abukmeil SS, Scanlon JJ, Cox M: “Schizophrenia past clozapine”: Reasons for clozapine discontinuation, mortality, and alternative antipsychotic prescribing. Pharmacopsychiatry 48:11–4, 2015 [DOI] [PubMed] [Google Scholar]
- Nicolson R, Brookner FB, Lenane M, Gochman P, Ingraham LJ, Egan MF, Kendler KS, Pickar D, Weinberger DR, Rapoport JL: Parental schizophrenia spectrum disorders in childhood-onset and adult-onset schizophrenia. Am J Psychiatry 160:490–495, 2003 [DOI] [PubMed] [Google Scholar]
- Overall JE, Gorham DR: The brief psychiatric rating scale (BPRS). Psychological Reports 10: 799–812, 1962 [Google Scholar]
- Overall JE, Pfefferbaum B: A brief scale for rating psychopathology in children. Innovations 3:257–266, 1984 [Google Scholar]
- Physicians' Desk Reference 2004. Medical Economics Company, 2003
- Pickar D, Owen RR, Litman RE, Konicki E, Gutierrez R, Rapaport MH: Clinical and biologic response to clozapine in patients with schizophrenia. Crossover comparison with fluphenazine. Arch Gen Psychiatry 49:345–353, 1992 [DOI] [PubMed] [Google Scholar]
- Rapoport J, Connors C, Reatig N: Rating scales and assessment instruments for use in pediatric psychopharmacology research. Psychopharmacol Bull 21:714–1124, 1985 [PubMed] [Google Scholar]
- Rapoport JL, Giedd JN, Gogtay N: Neurodevelopmental model of schizophrenia: Update 2012. Mol Psychiatry 17:1228–1238, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider C, Papachristou E, Wimberley T, Gasse C, Dima D, MacCabe JH, Mortensen PB, Frangou S: Clozapine use in childhood and adolescent schizophrenia: A nationwide population-based study. Eur Neuropsychopharmacol 25:857–863, 2015 [DOI] [PubMed] [Google Scholar]
- Shaffer D, Gould MS, Brasic J, Ambrosini P, Fisher P, Bird H, Aluwahlia S: A children's global assessment scale (CGAS). Arch Gen Psychiatry 11:1228–1231, 1983 [DOI] [PubMed] [Google Scholar]
- Shaw P, Sporn A, Gogtay N, Overman GP, Greenstein D, Gochman P, Tossell JW, Lenane M, Rapoport JL: Childhood-onset schizophrenia: A double-blind, randomized clozapine-olanzapine comparison. Arch Gen Psychiatry 63:721–730, 2006 [DOI] [PubMed] [Google Scholar]
- Simpson GM, Angus JW: A rating scale for extrapyramidal side effects. Acta Psychiatr Scand Suppl. 212:11–19, 1970 [DOI] [PubMed] [Google Scholar]
- Sporn A, Greenstein D, Gogtay N, Sailer F, Hommer DW, Rawlings R, Nicolson R, Egan MF, Lenane M, Gochman P, Weinberger DR, Rapoport JL: Childhood-onset schizophrenia: Smooth pursuit eye-tracking dysfunction in family members. Schizophr Res 73:243–252, 2005 [DOI] [PubMed] [Google Scholar]
- Sporn AL, Vermani A, Greenstein DK, Bobb AJ, Spencer EP, Clasen LS, Tossell JW, Stayer CC, Gochman PA, Lenane MC, Rapoport JL, Gogtay N: Clozapine treatment of childhood-onset schizophrenia: Evaluation of effectiveness, adverse effects, and long-term outcome. J Am Acad Child Adolesc Psychiatry 46:1349–1356, 2007 [DOI] [PubMed] [Google Scholar]
- Tollefson GD, Birkett MA, Kiesler GM, Wood AJ; Lilly Resistant Schizophrenia Study Group: Double-blind comparison of olanzapine versus clozapine in schizophrenic patients clinically eligible for treatment with clozapine. Biol Psychiatry 49:52–63, 2001 [DOI] [PubMed] [Google Scholar]
- Volavka J, Czobor P, Sheitman B, Lindenmayer JP, Citrome L, McEvoy JP, Cooper TB, Chakos M, Lieberman JA: Clozapine, olanzapine, risperidone, and haloperidol in the treatment of patients with chronic schizophrenia and schizoaffective disorder. Am J Psychiatry 159: 255–262, 2002 [DOI] [PubMed] [Google Scholar]
- Zahn TP, Jacobsen LK, Gordon CT, McKenna K, Frazier JA, Rapoport JL: Autonomic nervous system markers of psychopathology in childhood-onset schizophrenia. Arch Gen Psychiatry 54:904–912, 1997 [DOI] [PubMed] [Google Scholar]


