Abstract
Although based on very limited M and L segment sequences, Artybash virus (ARTV) was proposed previously as a unique hantavirus harbored by the Laxmann's shrew (Sorex caecutiens). To verify this conjecture, lung tissues from 68 Laxmann's shrews, captured during 2006 to 2014 in eastern Siberia, Russia, and Hokkaido, Japan, were analyzed for ARTV RNA using reverse transcription polymerase chain reaction (RT-PCR). ARTV RNA was detected in six Laxmann's shrews. Pairwise alignment and comparison of partial- and full-length S, M, and L segment sequences from these Laxmann's shrews, as well as phylogenetic analyses, using maximum likelihood and Bayesian methods indicated that ARTV was distinct from other soricine shrew-borne hantaviruses and representative hantaviruses harbored by rodents, moles, and bats. Taxonomic identity of the ARTV-infected Laxmann's shrews was confirmed by full-length cytochrome b mitochondrial DNA sequence analysis. Our data indicate that the hantavirus previously known as Amga virus (MGAV) represents genetic variants of ARTV. Thus, the previously proposed designation of ARTV/MGAV should be replaced by ARTV.
Key Words: : Hantavirus, Japan, RT-PCR, Russia, Shrew
Introduction
Recent discovery of genetically distinct hantaviruses (family Bunyaviridae and genus Hantavirus) in multiple species of soricine and crocidurine shrews (order Eulipotyphla, family Soricidae, and subfamilies Soricinae and Crocidurinae), as well as in moles (family Talpidae and subfamilies Talpinae and Scalopinae) and insectivorous bats (order Chiroptera), challenges the conventional view that rodents (order Rodentia and families Muridae and Cricetidae) are the principal reservoir hosts. Broad taxonomic and geographic distribution of hosts indicates that small mammals other than rodents played a significant role in the evolutionary history of hantaviruses and points to the urgent need to understand the diversity, distribution, and phylogeography of hantaviruses worldwide (Bennett et al. 2014, Yanagihara et al. 2014).
Seewis virus (SWSV), originally detected in the Eurasian common shrew (Sorex araneus) in Switzerland (Song et al. 2007), has now been found across the vast distribution of its soricine reservoir host in the Czech Republic (Schlegel et al. 2012), Finland (Kang et al. 2009a, Ling et al. 2014), Germany (Schlegel et al. 2012), Hungary (Kang et al. 2009a), Poland (Gu et al. 2014), Russia (Yashina et al. 2010), Slovakia (Schlegel et al. 2012), and Slovenia (Korva et al. 2013; Resman et al. 2013).
Genetically distinct hantaviruses have also been detected in other Sorex species, including Ash River virus in the North American masked shrew (Sorex cinereus) and Jemez Springs virus in the dusky shrew (Sorex monticolus) (Arai et al. 2008a), Kenkeme virus in the Asian flat-skulled shrew (Sorex roboratus) (Kang et al. 2010), Asikkala virus in the Eurasian pygmy shrew (Sorex minutus) (Radosa et al. 2013), Yakeshi virus in the taiga shrew (Sorex isodon) (Guo et al. 2013), Qian Hu Shan virus in the stripe-back shrew (Sorex cylindricauda) (Zuo et al. 2014), and Sarufutsu virus in the long-clawed shrew (Sorex unguiculatus) (Arai et al. unpublished data).
In addition, based on very limited M and L segment sequences detected in a Laxmann's shrew (Sorex caecutiens) captured near Teletskoye Lake in the Altai Republic of western Siberia, a new hantavirus, named Artybash virus (ARTV), has been proposed. In an attempt to validate its reservoir host species and explore the genetic diversity of ARTV, we analyzed lung tissues collected from Laxmann's shrews trapped in eastern Siberia, Russia, and Hokkaido, Japan. Genetic and phylogenetic analyses, based on partial- and full-length genomes of a hantavirus formerly called Amga virus (MGAV), show that it represents genetic variants of ARTV. Thus, instead of referring to this hantavirus as ARTV/MGAV, as previously proposed (Bennett et al. 2014), ARTV should be the preferred designation.
Materials and Methods
Trapping and sample collection
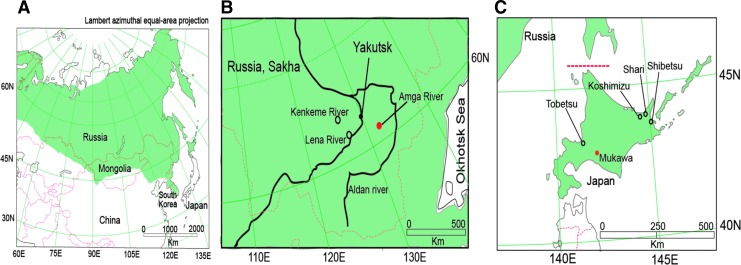
Laxmann's shrews, which are widely distributed from northern Fennoscandia through Siberia and across northern Japan (Fig. 1A), were captured at three sites in the Sakha Republic, Russia, during July and August 2006 (Fig. 1B and Table 1) and five sites in Hokkaido, Japan, between 2008 and 2014 (Fig. 1C and Table 1). Protocols for trapping, euthanasia, and tissue processing were performed according to well-established guidelines (Animal Care and Use Committee 1998).
FIG. 1.
(A) Map showing the geographic distribution of the Laxmann's shrew (Sorex caecutiens) across Eurasia, where it inhabits broad-leaved forests on lowland and mountainous areas. (B) Map showing trap sites of Laxmann's shrews along the Lena River (N61°45′14.399 E129°31′30.0), Amga River (N61°35′34.810 E132°56′24.053), and Kenkeme River (N62°04′11.996 E128°56′16.821) in the Sakha Republic of Russia. (C) Map showing trap sites of Laxmann's shrews in Koshimizu (N43°56′10.615 E144°26′31.750), Shibetsu (N43°37′43.589 E145°11′34.594), Mukawa (N42°50′30.073 E142°10′01.675), Shari (N43°55′23.998, E144°50′39.800), and Tobetsu (N43°12′11.084 E141°26′58.999) in Hokkaido, Japan. Red circles indicate trap sites of ARTV-positive shrews.
Table 1.
Capture Sites of Laxmann's Shrews Tested for Hantavirus RNA
| Total | Positive | ||||||
|---|---|---|---|---|---|---|---|
| Country | Republic/prefecture | Location | Year | Male | Female | Male | Female |
| Russia | Sakha | Lena River | 2006 | 4 | 1 | 0 | 0 |
| Amga River | 2006 | 7 | 7 | 4 | 1 | ||
| Kenkeme River | 2006 | 15 | 5 | 0 | 0 | ||
| Japan | Hokkaido | Koshimizu | 2008 | 1 | 2 | 0 | 0 |
| Shibetsu | 2008 | 0 | 1 | 0 | 0 | ||
| Mukawa | 2010 | 3 | 1 | 1 | 0 | ||
| Shari | 2011 | 0 | 1 | 0 | 0 | ||
| Tobetsu | 2013 | 2 | 2 | 0 | 0 | ||
| 2014 | 9 | 7 | 0 | 0 | |||
| Total | 41 | 27 | 5 | 1 | |||
RNA extraction and reverse transcription polymerase chain reaction
Total RNA was extracted from lung tissues using the PureLink Micro-to-Midi Total RNA Purification Kit (Invitrogen, San Diego, CA) or MagDEA® RNA 100 Kit (Precision System Science; PSS, Matstudo, Japan). cDNA was synthesized using SuperScript III First-Strand Synthesis System (Invitrogen) or PrimeScript™ II 1st strand cDNA Synthesis Kit (Takara Bio, Otsu, Japan) and an oligonucleotide primer (OSM55: 5′-TAGTAGTAGACTCC-3′) designed from the genus-specific conserved 3′-end of the S, M, and L segments of all hantaviruses. For initial screening by RT-PCR, primers were based on highly conserved regions of shrew-borne hantavirus genomes: S (outer: OSM55F, HTN-S6: 5′-AGCTCNGGATCCATNTCATC-3′; inner: Cro2F: 5′-AGYCCNGTNATGRGWGTNRTYGG-3′ and Cro2R: 5′-ANGAYTGRTARAANGANGAYTTYTT-3′) and L (outer: Han-L1880F: 5′-ATGAARNTNTGTGCNATNTTTGA-3′ and Han-L3000R: 5′-GCNGARTTRTCNCCNGGNGACCA-3′; inner: Han-L2520F: 5′-ATNWGHYTDAARGGNATGTCNGG-3′ and Han-L2970R: 5′-CCNGGNGACCAYTTNGTDGCATC-3′). Oligonucleotide primers designed for amplification and sequencing of the full genome of ARTV are shown in Table 2. Nested PCR cycling conditions and methods for DNA sequencing have been previously described (Arai et al. 2008a, 2008b, Kang et al. 2009b).
Table 2.
Oligonucleotide Primers for the Amplification of the S, M, and L Genomic Segments of Artybash Virus
| Segment | Primer | Sequence (5′→3′) | Length | Polarity |
|---|---|---|---|---|
| S | OSM55 | TAG TAG TAG ACT CC | 14 | + |
| CAS-381F | CAN GTG GNC ARA CWG CWG AYT GG | 23 | + | |
| Han-S604F | GCH GAD GAR HTN ACA CCN GG | 20 | + | |
| Cro2F | AGY CCN GTN ATG RGW GTN RTY GG | 24 | + | |
| Han-S974R | TCN GGN GCH CHN GCA AAN AHC CA | 23 | − | |
| Cro2R | ANG AYT GRT ARA ANG ANG AYT TYT T | 25 | − | |
| HTN-S6 | AGC TCN GGA TCC ATN TCA TC | 20 | − | |
| MKWS-1612R | AGA GTG TTT GAG GTA GTG GAG TG | 23 | − | |
| Han-S3R1 | TAG TAG TAN NCT CCN | 15 | − | |
| PHS-3endR | TAG TAG TAT ACT CCT TGA AAA GC | 23 | − | |
| M | OSM55 | TAG TAG TAG ACT CC | 14 | + |
| JAM-241F | GTT CAT GYA GYA TGG ATG TRC A | 22 | + | |
| MKWM-433R | TNC GCA TCT TGTA RGC CTG YTC | 22 | − | |
| HTM-1490F | TGT GTN CCW GGN TTY CAT GGN T | 22 | + | |
| CAM-1685F | ACN AAG GGY TCW ATG GTN TGT GA | 23 | + | |
| SHM-1706R | CAT ACA TCA CAN ACC ATW GAA CC | 23 | − | |
| SHM-2371F | TGY AAC CCN GTN GAT TGY CCW GG | 23 | + | |
| MKWM-2458R | ATA GGC AGT CCC GAC AGC CTT | 21 | − | |
| MKWM-2631R | CAT GAT RTC NCC AGG RTC NCC | 21 | − | |
| TM-2957R | GAA CCC CAD GCC CCNTCY AT | 20 | − | |
| AM-3280F | CCA TAT CTA TGA TGA TGG TGC | 21 | + | |
| PHM-3endR | TAG TAG TAG ACT CCG CAA GAA | 21 | − | |
| L | OSM55 | TAG TAG TAG ACT CC | 14 | + |
| PHL-173F | GAT WAA GCA TGA YTG GTC TGA | 21 | + | |
| CAL-539F | TAT NTC MAC ACA RTG GCC WAG T | 22 | + | |
| HTL-577R | CMC CNK CAT THC KYC TAC TNG GC | 23 | − | |
| SL-761F | CCT ATT TNA GTA YTG TAA GGW NTG G | 25 | + | |
| Han-L1880F | CAR AAR ATG AAR NTN TGT GC | 20 | + | |
| CS-L-2319R | CTT CYT CAT TYA CAT TMC CAT G | 22 | − | |
| Han-L2520F | ATN WGH YTD AAR GGN ATG TCN GG | 23 | + | |
| Tal-L2855F | GAA AGG GCA TTN MGA TGG GCN TCA GG | 26 | + | |
| Tal-L2928F | GNA AAY TNA TGT ATG TNA GTG C | 22 | + | |
| HAN-L-F1 | ATG TAY GTB AGT GCW GAT GC | 20 | + | |
| HAN-L-F2 | TGC WGA TGC HAC NAA RTG GTC | 21 | + | |
| Han-L3000R | GCN GAR TTR TCN CCN GGN GAC CA | 23 | − | |
| HAN-L-R2 | GCR TCR TCW GAR TGR TGD GCA A | 22 | − | |
| HAN-L-R1 | AAC CAD TCW GTY CCR TCA TC | 20 | − | |
| MKWL-4020F | YAA YCA TGA RAG GTT GGG NGA G | 22 | + | |
| MKWL-4132F | GAG ACT TGG AGT GAN CAA CAY CC | 23 | + | |
| SL-4321R | AAYGTTACCCAYTCACCAYCCA | 22 | − | |
| PHL-5167R | CAT AYT GYT THC CTG AAT AWG C | 22 | − | |
| CAL-6375F | GKR TWG TKA ARG GYT GGG GWG A | 22 | − | |
| HNL-6388R | CTC WGT YAA RTC ATA WGG ATC | 21 | − | |
| HNL-3R | TAG TAG TAK GCT CCG | 15 | − |
Mixed bases: B = G, T, C; D = G, A, T; H = A, T, C; K = G, T; M = A, C; N = A, T, G, C; R = A, G; W = A, T; and Y = C, T.
Genetic and phylogenetic analysis
Pairwise alignment and comparison of partial- and full-length S, M, and L segment sequences of hantaviruses from Laxmann's shrews, as well as other representative rodent-, shrew-, mole-, and bat-borne hantaviruses, were performed using the ClustalW method (Thompson et al. 1994). Phylogenetic analyses were conducted using maximum likelihood and Bayesian methods, implemented in MrBayes 3.1 (Ronquist and Huelsenbeck 2003) and RAxML (Stamatakis et al. 2008), under the best-fit GTR + I + Γ model of evolution using MrModeltest 2.3 (Posada 2008). Two replicate Bayesian Metropolis–Hastings Markov chain Monte Carlo runs, each consisting of six chains of 10 million generations sampled every 100 generations with a burn-in of 25,000 (25%), resulted in 150,000 trees overall. Topologies were evaluated by bootstrap analysis of 100 iterations, and posterior node probabilities were based on 10 million generations and estimated sample sizes more than 100 (implemented in MrBayes).
Host species identification
Because shrews are inherently difficult to identify by morphological features alone, host verification of ARTV-infected shrews was confirmed by analyzing the entire 1140-base pair cytochrome b gene of mitochondrial DNA (mtDNA), amplified by PCR, using modified universal primers (Cy-14726F: 5′-GACYARTRRCATGAAAAAYCAYCGTTGT-3′ and Cy-15909R: 5′-CYYCWTYIYTGGTTTACAAGACYAG-3′) (Arai et al. 2008b). A Bayesian approach, as described above, with midpoint rooting was used to determine the phylogenetic relationship between Laxmann's shrews and other shrew and mole species known to harbor distinct hantaviruses.
Results and Discussion
ARTV RNA was detected by RT-PCR in five of 39 (12.8%) and one of 29 (3.4%) Laxmann's shrews captured in Russia and Japan, respectively (Table 1). All but one of the six ARTV-infected shrews were males.
Analysis of the full-length nucleotide and amino acid sequences of the S, M, and L segments (GenBank accession numbers: KF974360, KF974359, and KF974361, respectively) of ARTV strain Mukawa AH301 showed considerable divergence from representative hantaviruses harbored by shrews in the Soricinae subfamily in Eurasia (Table 3): S, 22.9–27.3% and 13.3–16.0%; M, 20.9–23.8% and 8.4–12.7%; and L, 20.2–22.1% and 5.7–8.7%, respectively. In contrast, alignment and comparison between ARTV strain Mukawa AH301 and the very limited S, M, and L segments of ARTV strains ART502, Galkino2712, and Parnaya1205 showed higher sequence similarity at the amino acid level (Table 3).
Table 3.
Sequence Identities of the Full-Length S, M, and L Segments of ARTV Strain Mukawa AH301 from Sorex caecutiens in Japan to Other Representative Hantaviruses
| S | M | L | ||||
|---|---|---|---|---|---|---|
| Virus | 1290 nt | 429 aa | 3420 nt | 1139 aa | 6456 nt | 2151 aa |
| ARTV Amga MSB148558 | 1290 nta | 429 aa | 1088 nt | 362 aa | 4598 nt | 1532 aa |
| 79.8% | 93.5% | 78.1% | 95.0% | 79.7% | 96.0% | |
| ARTV Amga MSB148559 | — | — | — | — | 1401 nt | 467 aa |
| 79.8% | 97.4% | |||||
| ARTV Amga MSB148436 | — | — | 727 nt | 242 aa | 2454 nt | 818 aa |
| 78.3% | 97.1% | 80.2% | 97.3% | |||
| ARTV Amga MSB148457 | — | — | 683 nt | 227 aa | 2397 nt | 799 aa |
| 78.2% | 96.9% | 80.0% | 97.4% | |||
| ARTV Amga MSB148347 | 582 nt | 194 aa | 729 nt | 243 aa | 1302 nt | 434 aa |
| 76.5% | 88.7% | 78.2% | 97.5% | 80.2% | 97.2% | |
| ARTV ART502 | 837 nt | 279 aa | 247 nt | 82 aa | 347 nt | 115 aa |
| 76.9% | 92.8% | 79.8% | 96.3% | 80.1% | 96.5% | |
| ARTV Galkino2712 | 559 nt | 186 aa | — | — | — | — |
| 75.9% | 89.2% | |||||
| ARTV Parnaya1205 | 767 nt | 255 aa | — | — | 347 nt | 115 aa |
| 76.8% | 92.6% | 80.4% | 95.7% | |||
| SWSV mp70 | 1290 nta | 429 aa | 250 nt | 83 aa | 3288 nt | 1096 aa |
| 77.1% | 86.7% | 78.0% | 91.6% | 77.9% | 94.3% | |
| KKMV MSB148794 | 1290 nta | 429 aa | 1002 nt | 334 aa | 4295 nt | 1432 aa |
| 74.6% | 83.3% | 79.1% | 91.6% | 77.9% | 91.5% | |
| QHSV YN05-284 | 1290 nta | 429 aa | 1430 nt | 476 aa | 450 nt | 149 aa |
| 72.7% | 83.5% | 76.2% | 89.9% | 79.8% | 93.3% | |
| YKSV Si-210 | 1290 nta | 429 aa | 3420 nta | 1139 aa | 339 nt | 113 aa |
| 74.3% | 84.0% | 76.8% | 88.2% | 79.4% | 91.3% | |
| ASIV Drahany | 1290 nta | 429 aa | 3420 nta | 1139 aa | 1729 nt | 576 aa |
| 73.2% | 84.7% | 76.8% | 87.3% | 79.3% | 93.8% | |
| ARRV MSB73418 | 1059 nt | 353 aa | — | — | 347 nt | 115 aa |
| 62.5% | 64.0% | 75.8% | 86.1% | |||
| JMSV MSB144475 | 1290 nta | 429 aa | 1412 nt | 470 aa | 4928 nt | 1642 aa |
| 65.8% | 69.1% | 72.6% | 79.8% | 74.8% | 85.7% | |
| CBNV CBN-3 | 1287 nta | 428 aa | 3420 nta | 1139 aa | 412 nt | 137 aa |
| 64.7% | 69.0% | 68.9% | 73.9% | 75.0% | 84.9% | |
| TGNV Tan826 | 442 nt | 147 aa | — | — | 412 nt | 137 aa |
| 62.4% | 61.2% | 71.8% | 77.4% | |||
| BOWV VN1512 | 1296 nta | 431 aa | 3438 nta | 1145 aa | 6477 nta | 2158 aa |
| 63.6% | 67.8% | 67.0% | 66.8% | 70.9% | 78.2% | |
| AZGV KBM15 | 540 nt | 180 aa | 687 nt | 229 aa | 4556 nt | 1518 aa |
| 60.7% | 60.6% | 71.0% | 73.8% | 71.4% | 77.7% | |
| JJUV SH42 | 1287 nta | 428 aa | 3408 nt | 1135 aa | 6474 nta | 2157 aa |
| 60.8% | 61.8% | 66.2% | 65.8% | 71.3% | 77.6% | |
| MJNV Cl 05-11 | 1311 nta | 436 aa | 3363 nta | 1120 aa | 6450 nta | 2149 aa |
| 53.8% | 45.3% | 51.7% | 42.5% | 62.3% | 61.7% | |
| TPMV VRC66412 | 1308 nta | 435 aa | 3366 nta | 1121 aa | 6450 nta | 2149 aa |
| 50.9% | 44.2% | 52.3% | 43.1% | 61.6% | 61.7% | |
| ULUV FMNH158302 | 1187 nt | 395 aa | 1389 nt | 463 aa | 6459 nta | 2152 aa |
| 52.8% | 45.7% | 52.8% | 42.8% | 61.9% | 61.9% | |
| KMJV FMNH174124 | 1269 nta | 423 aa | 1240 nt | 413 aa | 6447 nta | 2148 aa |
| 53.7% | 47.6% | 53.8% | 45.1% | 63.0% | 62.7% | |
| RKPV MSB57412 | 1287 nta | 428 aa | 3411 nt | 1136 aa | 6462 nta | 2153 aa |
| 60.1% | 60.3% | 57.7% | 51.8% | 65.3% | 67.9% | |
| OXBV Ng1453 | 1287 nta | 428 aa | 3426 nt | 1141 aa | 4345 nt | 1448 aa |
| 64.4% | 68.3% | 66.9% | 67.4% | 72.4% | 80.3% | |
| ASAV N10 | 1302 nta | 433 aa | 3423 nta | 1140 aa | 6456 nta | 2151 aa |
| 65.1% | 70.0% | 71.6% | 77.6% | 75.4% | 85.0% | |
| NVAV Te34 | 1287 nta | 428 aa | 3384 nta | 1127 aa | 6474 nta | 2157 aa |
| 56.1% | 49.7% | 53.5% | 44.8% | 63.6% | 62.1% | |
For virus names and hosts, refer to legend of Figure 2.
Complete coding region.
—, sequences unavailable; aa, amino acids; ARTV, Artybash virus; nt, nucleotides; SWSV, Seewis virus.
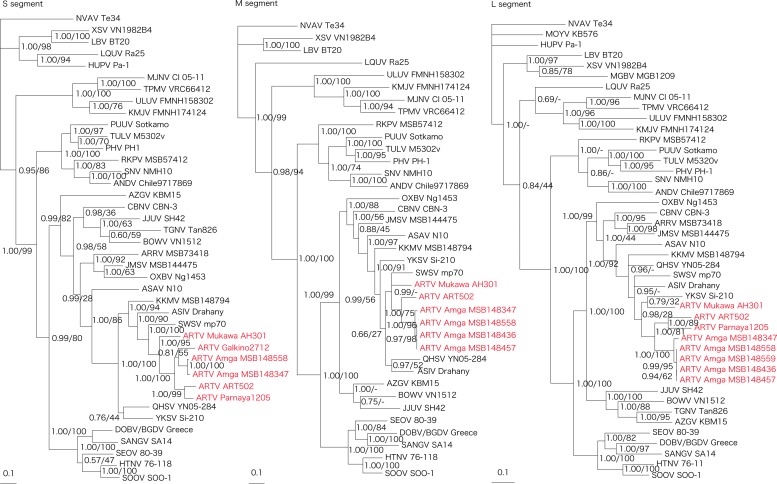
Phylogenetic analyses of ARTV strain Mukawa AH301, based on the full-length coding regions (comprising 1290-nucleotide S, 3420-nucleotide M, and 6456-nucleotide L segment sequences), showed similar topologies, with ARTV strains from the Sakha Republic and ARTV strains detected independently in Laxmann's shrews captured in the Altai Republic and Khabarovsk Krai of Russia forming a separate geographic-specific cluster (Fig. 2). Moreover, ARTV strains were distinct from recently detected hantaviruses harbored by other Sorex shrew species in Eurasia. In contrast, differences in the phylogenetic positions of some soricine shrew-borne hantaviruses may be attributed to the limited sequences, especially for the M and L segments.
FIG. 2.
Phylogenetic trees generated by Bayesian and maximum likelihood methods under the best-fit GTR + I + Γ model of evolution as estimated based on the entire coding regions of the full-length 1290-nucleotide S, 3420-nucleotide M, and 6456-nucleotide L genomic segments of Artybash virus (ARTV) strain Mukawa AH301. Phylogenetic trees show the positions of ARTV Mukawa AH301 (GenBank accession numbers: S: KF974360; M: KF974359; and L: KF974361), ARTV Amga MSB148558 (S: KM201411; M: KM201412; and L: KM201413), ARTV Amga MSB148559 (S: KM201414), ARTV Amga MSB148436 (M: KM201415 and L: KM201416), ARTV Amga MSB148457 (M: KM201417 and L: KM201418), ARTV Amga MSB148347 (S: KM201419; M: KM201420; and L: KM201421), ARTV ART502 (S: KM288698; M: EU424340; and L: EU424339), ARTV Galkino2712 (S: KM288699), and ARTV Parnaya1205 (S: KU253274 and L: KU253275) from Sorex caecutiens. Also shown are Ash River virus (ARRV MSB73418, S: EF650086 and L: EF619961) from Sorex cinereus, Jemez Springs virus (JMSV MSB144475, S: FJ593499; M: FJ593500; and L: FJ593501) from Sorex monticolus, Seewis virus (SWSV mp70, S: EF636024; M: EF636025; and L: EF636026) from Sorex araneus, Asikkala virus (ASIV Drahany, S: KC880342; M: KC880345; and L: KC880348) from Sorex minutus, Kenkeme virus (KKMV MSB148794, S: GQ306148; M: GQ306149; and L: GQ306150) from Sorex roboratus, Qian Hu Shan virus (QHSV YN05-284, S: GU566023; M: GU566022; and L: GU566021) from Sorex cylindricauda, Yakeshi virus (YKSV Si-210, S: JX465423; M: JX465403; and L: JX465389) from Sorex isodon, and Cao Bang virus (CBNV CBN-3, S: EF543524; M: EF543526; and L: EF543525) from Anourosorex squamipes, as well as Thottapalayam virus (TPMV VRC66412, S: AY526097 and L: EU001330) from Suncus murinus, Imjin virus (MJNV Cl 05-11, S: EF641804; M: EF641798; and L: EF641806) from Crocidura lasiura, Azagny virus (AZGV KBM15, S: JF276226; M: JF276227; and L: JF276228) from Crocidura obscurior, Tanganya virus (TGNV Tan826, S: EF050455 and L: EF050454) from Crocidura theresea, Bowé virus (BOWV VN1512, S: KC631782; M: KC631783; and L: KC631784) from Crocidura douceti, Jeju virus (JJUV SH42, S: HQ663933; M: HQ663934; and L: HQ663935) from Crocidura shantungensis, Uluguru virus (ULUV FMNH158302, S: JX193695; M: JX193696; and L: JX193697) from Myosorex geata, and Kilimanjaro virus (KMJV FMNH174124, S: JX193698; M: JX193699; and L: JX193700) from Myosorex zinki. Mole-borne hantaviruses include Asama virus (ASAV N10, S: EU929072; M: EU929075; and L: EU929078) from Urotrichus talpoides, Nova virus (NVAV Te34, S: KR072621; M: KR072622; and L: KR072623) from Talpa europaea, Oxbow virus (OXBV Ng1453, S: FJ5339166; M: FJ539167; and L: FJ593497) from Neurotrichus gibbsii, and Rockport virus (RKPV MSB57412, S: HM015223; M: HM015222; and L: HM015221) from Scalopus aquaticus. Bat-borne hantaviruses include Magboi virus (MGBV MGB1209, L: JN037851) from Nycteris hispida, Mouyassué virus (MOYV KB576, L: JQ287716) from Neoromicia nanus, Huangpi virus (HUPV Pa-1, S: JX473273 and L: JX465369) from Pipistrellus abramus, Longquan virus (LQUV Ra-25, S: JX465415; M: JX465397; and L: JX465381) from Rhinolophus sinicus, Laibin virus (LBV BT20, S: KM102247; M: KM102248; and L: KM102249) from Taphozous melanopogon, and Xuan Son virus (XSV VN1982B4, S: KC688335; M: KU976427; and L: JX912953) from Hipposideros pomona. Other taxa include Sin Nombre virus (SNV NMH10, S: NC_005216; M: NC_005215; and L: NC_005217), Andes virus (ANDV Chile9717869, S: AF291702; M: AF291703; and L: AF291704), Prospect Hill virus (PHV PH-1, S: Z49098; M: X55129; and L: EF646763), Tula virus (TULV M5302v, S: NC_005227; M: NC_005228; and L: NC_005226), Puumala virus (PUUV Sotkamo, S: NC_005224; M: NC_005223; and L: NC_005225), Dobrava virus/Belgrade virus (DOBV/BGDV Greece, S: NC_005233; M: NC_005234; and L: NC_005235), Hantaan virus (HTNV 76-118, S: NC_005218; M: NC_005219; and L: NC_005222), Soochong virus (SOOV SOO-1, S: AY675349; M: AY675353; and L: DQ056292), Sangassou virus (SANGV SA14, S: JQ082300; M: JQ082301; and L: JQ082302) and Seoul virus (SEOV 80-39, S: NC_005236; M: NC_005237; and L: NC_005238). The numbers at each node are posterior node probabilities (left) based on 150,000 trees: two replicate Markov chain Monte Carlo runs, consisting of six chains of 10 million generations each sampled every 100 generations with a burn-in of 25,000 (25%) and bootstrap values (right), based on 100 replicates executed on the RAxML BlackBox Web server.
The identities of the six ARTV-infected shrews were confirmed as Laxmann's shrews (GenBank no. KF974362 for strain AH301; GU562415, GU562416, GU562417, GU562418, and GU562422 for strains MSB148558, MSB148559, MSB148436, MSB148457, and MSB148347, respectively). The cytochrome b mtDNA sequences of the ARTV-infected Laxmann's shrews from Japan and Russia differed by 4.0–6.4%. The ARTV-negative shrews from Japan were also identified as Laxmann's shrews by analysis of the 1140-nucleotide cytochrome b mtDNA gene (GenBank nos. KU760858 to KU760884). However, the full-length cytochrome b mtDNA sequence variation was 0–1.1% and 0.5–1.1% among Laxmann's shrews in Japan and Russia, respectively.
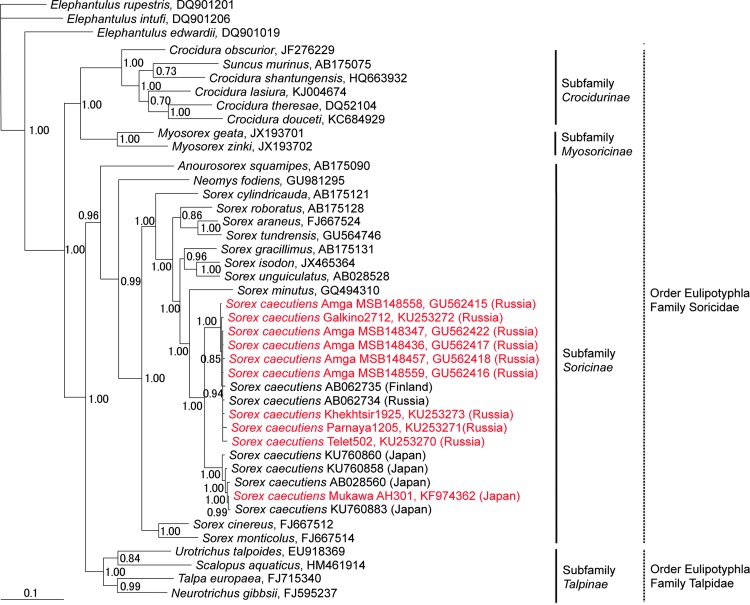
Host phylogenies based on mtDNA cytochrome b sequences of shrew and mole species, which harbor genetically distinct hantaviruses, showed two separate lineages of Laxmann's shrews (Fig. 3). This was consistent with previous studies suggesting at least two genetic races of Laxmann's shrews distributed in Eurasia and Japan (Ohdachi et al. 2003). That is, based on analysis of the full-length cytochrome b gene, Laxmann's shrews were separated into Hokkaido and Eurasian Continent–Sakhalin–Cheju clusters. Individuals in the latter cluster do not always reflect the geographical proximity of their capture locations, which is consistent with an ancestral isolation of the Hokkaido population, occurring ∼13,000 years before present (Tanabe et al. 2015), and recent rapid range expansion of the modern Eurasian Continent–Sakhalin–Cheju population (Ohdachi et al. 2003).
FIG. 3.
Bayesian phylogenetic tree based on 1140 nucleotides of the cytochrome b mtDNA of shrews and moles (order Eulipotyphla and families Talpidae and Soricidae). The tree was rooted using Elephantulus (order Macroscelidea, GenBank nos. DQ901019, DQ901206, and DQ901201) as the outgroup. Numbers at nodes indicate posterior probability values based on 150,000 trees: two replicate Markov chain Monte Carlo runs, consisting of six chains of 10 million generations each sampled every 100 generations with a burn-in of 25,000 (25%). GenBank numbers for all taxa are provided in the tree.
The phylogeographic variation in ARTV reflects the distinct evolutionary histories of these variants. Similar patterns of geographic variation have also been reported for rodent-borne hantaviruses. For example, geographic-specific genetic variants have been reported for Puumala virus in the bank vole (Myodes glareolus) (Garanina et al. 2009), Tula virus in the European common vole (Microtus arvalis) (Song et al. 2004), and Andes virus in the colilargo (Oligoryzomys longicaudus) (Torres-Perez et al. 2011). Both Muju virus in the royal vole (Myodes regulus) (Lee et al. 2014) and Hokkaido virus in the grey red-backed vole (Myodes rufocanus) and northern red-backed vole (Myodes rutilus) (Yashina et al. 2015) may represent genotypes of Puumala virus. These examples point to the urgency of studying the phylogeographic variation of viruses and their hosts to provide an essential foundation for understanding their evolutionary histories and as a prelude to forecasting their future emergence under changing environmental conditions (Hope et al. 2013, Campbell et al. 2015).
Although ARTV was detected in a single Laxmann's shrew captured in the Mukawa area in Japan, the evidence is compelling that this Sorex shrew species harbors ARTV across its broad geographic range. Our findings suggest that Laxmann's shrews, resident on Hokkaido Island before the geologic separation from the Eurasian continent, might have already been infected with ARTV. Renewed attempts to isolate ARTV from Laxmann's shrews captured in Eurasian Continent–Sakhalin–Cheju and Japan are warranted to better understand its evolutionary origins and phylogeography, as well as its pathogenic potential in humans.
Acknowledgments
We thank Masashi Hamada, Yu Ikeyama, and Keita Aoki for their technical assistance and Kimiyuki Tsuchiya, Hidenori Nishizawa, and Kyle R. Taylor for supporting field investigations. We also thank Nikolai Dokuchaev, Andrew G. Hope, Anson Koehler, Stephen O. MacDonald, Robert A. Nofchissey, and Albina Tsvetkova for assistance with field collections made in Russia through the National Science Foundation support (DEB0415668). In addition, this research was supported in part by a grant-in-aid for Research on Emerging and Re-emerging Infectious Diseases, Health Labour Sciences Research Grant in Japan (H22-Shinko-Ippan-006 and H25-Shinko-Ippan-008), a grant-in aid for Research Program on Emerging and Re-emerging Infectious Diseases, Japan Agency for Medical Research and Development (AMED), and a grant-in-aid from the Japan Society for the Promotion of Science (S13205 Invitation Fellowship for Research in Japan), as well as by the U.S. Public Health Service grants R01AI075057 from the National Institute of Allergy and Infectious Diseases and P20GM103516 and P30GM114737 (Centers of Biomedical Research Excellence) from the National Institute of General Medical Sciences, National Institutes of Health.
Author Disclosure Statement
No competing financial interests exist.
References
- Animal Care and Use Committee. Guidelines for the capture, handling, and care of mammals as approved by the American Society of Mammalogists. J Mammal 1998; 79:1416–1431 [Google Scholar]
- Arai S, Bennett SN, Sumibcay L, Cook JA, et al. Phylogenetically distinct hantaviruses in the masked shrew (Sorex cinereus) and dusky shrew (Sorex monticolus) in the United States. Am J Trop Med Hyg 2008a; 78:348–351 [PMC free article] [PubMed] [Google Scholar]
- Arai S, Ohdachi SD, Asakawa M, Kang HJ, et al. Molecular phylogeny of a newfound hantavirus in the Japanese shrew mole (Urotrichus talpoides). Proc Natl Acad Sci USA 2008b; 105:16296–16301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett SN, Gu SH, Kang HJ, Arai S, et al. Reconstructing the evolutionary origins and phylogeography of hantaviruses. Trends Microbiol 2014; 22:473–482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell LP, Luther C, Moo-Llanes D, Ramsey JM, et al. Climate change influences on global distributions of dengue and chikungunya virus vectors. Philos Trans R Soc Lond B Biol Sci 2015; 370:ii, 20140135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garanina SB, Platonov AE, Zhuravlev VI, Murashkina AN, et al. Genetic diversity and geographic distribution of hantaviruses in Russia. Zoonoses Public Health 2009; 56:297–309 [DOI] [PubMed] [Google Scholar]
- Gu SH, Hejduk J, Markowski J, Kang HJ, et al. Co-circulation of soricid- and talpid-borne hantaviruses in Poland. Infect Genet Evol 2014; 28:296–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo WP, Lin XD, Wang W, Tian JH, et al. Phylogeny and origins of hantaviruses harbored by bats, insectivores and rodents. PLoS Pathog 2013; 9:e1003159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hope AG, Waltari EC, Payer DC, Cook JA, et al. Future distribution of tundra refugia in Alaska. Nature Climate Change 2013; 3:931–938 [Google Scholar]
- Kang HJ, Arai S, Hope AG, Song JW, et al. Genetic diversity and phylogeography of Seewis virus in the Eurasian common shrew in Finland and Hungary. Virol J 2009a; 6:208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang HJ, Bennett SN, Sumibcay L, Arai S, et al. Evolutionary insights from a genetically divergent hantavirus harbored by the European common mole (Talpa europaea). PLoS One 2009b; 4:e6149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korva M, Knap N, Rus KR, Fajs L, et al. Phylogeographic diversity of pathogenic and non-pathogenic hantaviruses in Slovenia. Viruses 2013; 5:3071–3087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JG, Gu SH, Baek LJ, Shin OS, et al. Muju virus, harbored by Myodes regulus in Korea, might represent a genetic variant of Puumala virus, the prototype arvicolid rodent-borne hantavirus. Viruses 2014; 6:1701–1714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling J, Sironen T, Voutilainen L, Hepojoki S, et al. Hantaviruses in Finnish soricomorphs: Evidence for two distinct hantaviruses carried by Sorex araneus suggesting ancient host-switch. Infect Genet Evol 2014; 96:1664–1675 [DOI] [PubMed] [Google Scholar]
- Ohdachi S, Abe H, Han SH. Phylogenetical positions of Sorex sp. (Insectivore, Mammlia) from Cheju island and S. caecutiens from Korean peninsula, inferred from mitochondrial cytochrome b gene sequences. Zool Sci 2003; 20:91–95 [DOI] [PubMed] [Google Scholar]
- Posada D. jModelTest: phylogenetic model averaging. Mol Biol Evol 2008; 25:1253–1256 [DOI] [PubMed] [Google Scholar]
- Radosa L, Schlegel M, Gebauer P, Ansorge H, et al. Detection of shrew-borne hantavirus in Eurasian pygmy shrew (Sorex minutus) in Central Europe. Infect Genet Evol 2013; 19:403–410 [DOI] [PubMed] [Google Scholar]
- Resman K, Korva M, Fajs L, Zidarič T, et al. Molecular evidence and high genetic diversity of shrew-borne Seewis virus in Slovenia. Virus Res 2013; 177:113–117 [DOI] [PubMed] [Google Scholar]
- Ronquist F, Huelsenbeck JP. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 2003; 19:1572–1574 [DOI] [PubMed] [Google Scholar]
- Schlegel M, Radosa L, Rosenfeld UM, Schmidt S, et al. Broad geographical distribution and high genetic diversity of shrew-borne Seewis hantavirus in Central Europe. Virus Genes 2012; 45:48–55 [DOI] [PubMed] [Google Scholar]
- Song JW, Baek LJ, Song KJ, Skrok A, et al. Characterization of Tula virus from common voles (Microtus arvalis) in Poland: evidence for geographic-specific phylogenetic clustering. Virus Genes 2004; 29:239–247 [DOI] [PubMed] [Google Scholar]
- Song JW, Gu SH, Bennett SN, Arai S, et al. Seewis virus, a genetically distinct hantavirus in the Eurasian common shrew (Sorex araneus). Virol J 2007; 4:114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamatakis A, Hoover P, Rougemont J. A rapid bootstrap algorithm for the RAxML Web servers. Syst Biol 2008; 57:758–771 [DOI] [PubMed] [Google Scholar]
- Tanabe S, Nakanishi T, Ishihara Y, Nakashima R. Millennial-scale stratigraphy of a tide-dominated incised valley during the last 14 kyr: spatial and quantitative reconstruction in the Tokyo Lowland, central Japan. Sedimentology 2015 [Google Scholar]
- Thompson JD, Higgins DG, Gibson TJ. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res 1994; 22:4673–4680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres-Perez F, Hjelle B, Holmes EC, Cook JA. Spatial but not temporal co-divergence of a virus and its mammalian host. Mol Ecol 2011; 20:4109–4122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagihara R, Gu SH, Arai S, Kang HJ, et al. Hantaviruses: rediscovery and new beginnings. Virus Res 2014; 187:6–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yashina LN, Abramov SA, Dupal TA, Danchinova GA, et al. Hokkaido genotype of Puumala virus in the grey red-backed vole (Myodes rufocanus) and northern red-backed vole (Myodes rutilus) in Siberia. Infect Genet Evol 2015; 33:304–313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yashina LN, Abramov SA, Gutorov VV, Dupal TA, et al. Seewis virus: phylogeography of a shrew-borne hantavirus in Siberia, Russia. Vector Borne Zoonotic Dis 2010; 10:585–591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo SQ, Gong ZD, Fang LQ, Jiang JF, et al. A new hantavirus from the stripe-backed shrew (Sorex cylindricauda) in the People's Republic of China. Virus Res 2014; 184:82–86 [DOI] [PubMed] [Google Scholar]