Abstract
We described recently a subacute serum autoantibody response toward glial fibrillary acidic protein (GFAP) and its breakdown products 5–10 days after severe traumatic brain injury (TBI). Here, we expanded our anti-GFAP autoantibody (AutoAb[GFAP]) investigation to the multicenter observational study Transforming Research and Clinical Knowledge in TBI Pilot (TRACK-TBI Pilot) to cover the full spectrum of TBI (Glasgow Coma Scale 3–15) by using acute (<24 h) plasma samples from 196 patients with acute TBI admitted to three Level I trauma centers, and a second cohort of 21 participants with chronic TBI admitted to inpatient TBI rehabilitation. We find that acute patients self-reporting previous TBI with loss of consciousness (LOC) (n = 43) had higher day 1 AutoAb[GFAP] (mean ± standard error: 9.11 ± 1.42; n = 43) than healthy controls (2.90 ± 0.92; n = 16; p = 0.032) and acute patients reporting no previous TBI (2.97 ± 0.37; n = 106; p < 0.001), but not acute patients reporting previous TBI without LOC (8.01 ± 1.80; n = 47; p = 0.906). These data suggest that while exposure to TBI may trigger the AutoAb[GFAP] response, circulating antibodies are elevated specifically in acute TBI patients with a history of TBI. AutoAb[GFAP] levels for participants with chronic TBI (average post-TBI time 176 days or 6.21 months) were also significantly higher (15.08 ± 2.82; n = 21) than healthy controls (p < 0.001). These data suggest a persistent upregulation of the autoimmune response to specific brain antigen(s) in the subacute to chronic phase after TBI, as well as after repeated TBI insults. Hence, AutoAb[GFAP] may be a sensitive assay to study the dynamic interactions between post-injury brain and patient-specific autoimmune responses across acute and chronic settings after TBI.
Key words: : autoantibody, autoimmunity, biomarkers, glia, traumatic brain injury
Introduction
Traumatic brain injury (TBI) causes transient opening of the brain–blood barrier, which is often followed by neural cell damage or death. During the acute phase of TBI, a number of brain-specific proteins are released into the cerebrospinal fluid and/or blood (serum/plasma). A partial list includes neuronal proteins (ubiquitin-C-terminal hydrolase-L1 ([UCH-L1]), microtubule associated protein tau (MAPT/Tau), neuron specific enolase (NSE), axonal proteins (neurofilament-H, αII-spectrin breakdown products [SBDPs]), dendritic protein (MAP2), glial proteins (glial fibrillary acidic protein [GFAP], S100β) oligodendrocyte proteins (myelin basic protein [MBP]), and endothelial cell derived proteins (e.g. von Willebrand factor [VWF]).1–4
Because the brain is a site of immune-privilege, most of these proteins are not generally accessible to the immune system. TBI represents a situation where high concentrations of brain proteins are transiently released into the circulation and become accessible to the immune system.
Previous reports have documented brain-directed autoimmunity in neurological and neurodegenerative diseases such as Alzheimer disease, stroke, epilepsy, spinal cord injury, and paraneoplastic syndromes.5–11 In human TBI, however, autoimmunity has only been examined in a limited way and focused on autoantibodies against preselected antigens such as MBP, S100β, and glutamate receptors.12–18 Among investigators in the areas of autoimmunity and biomarkers,19–23 Tanriverdi and associates20 showed the presence of antipituitary antibodies in patients serum 3 years after head trauma. In other investigations,24,25 Marchi and colleagues25 demonstrated that antiglial protein S100β autoantibody levels are elevated in football players with repeated concussions. In parallel, we recently reported a rather unexpected immunodominant autoantibody response to GFAP and its breakdown products (BDPs) in a subset of patients with severe TBI.26
Based on our previous anti-GFAP-autoantibody study,26 we observed that GFAP appeared to be a dominant brain-derived autoantigen after severe TBI. We hypothesized that TBI causes protease-mediated GFAP-BDP formation in injured glial cells. This is followed by the subsequent release of GFAP-BDPs in substantive quantity through a compromised brain–blood barrier into the circulation.24,27–29 This combination allows GFAP and GFAP-BDPs to become accessible to and recognized by the immune cells as nonself-proteins, triggering autoantibody response in those individuals.
While GFAP is an intracellular antigen and the central nervous system (CNS) is normally considered immune-privileged, it is still conceivable that autoantibodies can gain assess to the CNS tissue where such an antigen is localized. For example MBP, myelin oligodendrocyte glycoprotein (MOG), and other intracellular myelin proteins in the spinal cord appear to be attacked by the immune system in multiple sclerosis and other demyelination diseases.30 Hence, it is possible that an autoantibody specifically targeting a major brain protein such as GFAP might trigger a persistent autoimmune activation, which could negatively impact on long-term recovery from TBI.
Thus, we sought to expand our anti-GFAP autoantibody (AutoAb[GFAP]) investigation to the Transforming Research and Clinical Knowledge in TBI Pilot (TRACK-TBI pilot) study,31 a multicenter observational study that covers the full spectrum of TBI (Glasgow Coma Scale [GCS] 3–15) with acute (<24 h) plasma samples available from 196 patients with acute TBI admitted to Level I trauma centers, as well as a second cohort of 21 participants with chronic TBI admitted to an inpatient rehabilitation center.
Methods
TBI patients
Patients with acute TBI were identified and recruited on arrival at one of three Level I trauma centers and one inpatient TBI rehabilitation center as part of the multicenter prospective TRACK-TBI pilot study.31 Study protocols were approved by the Institutional Review Boards of participating centers—acute sites: San Francisco General Hospital (SFGH); University of Pittsburgh Medical Center (UPMC), University Medical Center Brackenridge (UMCB); rehabilitation site: Mount Sinai Rehabilitation Center (MSRC). All participants or their legal authorized representatives provided written informed consent. At follow-up outcome time points, consent from participants from whom previous consent was obtained from a legally authorized representative was obtained for continuation in the study if the patient was neurologically improved to be capable of self-consent.
To be eligible for the TRACK-TBI pilot study, patients with acute TBI presented within 24 h of injury to the emergency department and had a history of trauma to the head sufficient to triage to noncontrast head computed tomography (CT) scan using the American College of Emergency Physicians/Centers for Disease Control evidence-based joint practice guideline, while patients with chronic TBI had sufficient neurologic impairment to triage to inpatient TBI rehabilitation.
Details of loss of consciousness (LOC), amnesia, and source of trauma were recorded on screening, and informed consent was obtained. GCS score was assessed by a neurosurgeon at admission and was reconfirmed by study personnel at the time of biomarker collection. For those with chronic TBI, plasma samples were collected on presentation to rehabilitation at MSMC with an average post-injury time of 188 days (6.2 months). We further identified patients with acute TBI with self-reported previous TBI with or without LOC (Table 1).
Table 1.
Demographics and Injury Characteristics of Patients with Traumatic Brain Injury
| Acute TBI | Chronic TBI* | |
|---|---|---|
| Age | N = 196 | N = 21 |
| Mean, SD | 42.4, 17.8 | 44.4, 20.5 |
| Range | 16–86 | 19–81 |
| Sex | N = 196 | N = 21 |
| Male | 151 (73%) | 16 (76%) |
| Female | 55 (27%) | 5 (24%) |
| GCS | N = 196 | N = 21 |
| 3–8 | 12 (6%) | — |
| 9–12 | 6 (3%) | — |
| 13–15 | 160 (82%) | — |
| Unknown | 18 (9%) | — |
| Previous TBI | N = 196 | N = 21 |
| None | 106 (54%) | 4 (19%) |
| Yes, without LOC | 47 (24%) | 4 (19%) |
| Yes, with LOC | 43 (22%) | 13 (62%) |
| Admission Head CT | N = 196 | N = 21 |
| Negative | 108 (55%) | — |
| Extra-axial only | 22 (11%) | 5 (24%) |
| Intra-axial only | 24 (12%) | 3 (14%) |
| Extra + Intra-axial | 42 (21%) | 3 (14%) |
| Unknown | — | 10 (48%) |
| Outcome (6-month) | N = 137 | N = 17 |
| GOS-E = 1 | 7 (5%) | 0 (0%) |
| GOS-E = 2 | 1 (1%) | 0 (0%) |
| GOS-E = 3 | 10 (7%) | 1 (5%) |
| GOS-E = 4 | 4 (2%) | 5 (24%) |
| GOS-E = 5 | 5 (10%) | 5 (24%) |
| GOS-E = 6 | 21 (15%) | 1 (5%) |
| GOS-E = 7 | 39 (28%) | 1 (5%) |
| GOS-E = 8 | 44 (32%) | 4 (19%) |
TBI, traumatic brain injury; SD, standard deviation; GCS, Glasgow Coma Scale; LOC, loss of consciousness; CT, computed tomography; GOS-E, Glasgow Outcome Scale-Extended,
GCS data was unavailable for chronic TBI patients. CT pathology was positive for all chronic TBI patients with CT data.
Biosample collection
Blood samples were collected from patients with acute TBI who consented to genetic and proteomic analysis within 24 h of injury (n = 196). Blood samples from those with chronic TBI were collected at the indicated time point. Plasma was extracted as supernatant after centrifugation of whole blood in ethylenediaminetetracetic acid (EDTA) blood tubes for 5–7 min at 4000 rpm according to the National Institutes of Health/National Institute of Neurological Disorders and Stroke TBI Common Data Elements Biospecimens and Biomarkers Working Group recommendations.32 In addition, 16 commercial control plasma samples collected with EDTA blood tubes (Bioreclamation Inc., mean ± standard deviation [SD] 39.1 ± 17.2 years old) were age-matched with the acute (n = 196; 42.1 ± 18.1 years old) and chronic TBI (n = 21; age 44.4 ± 20.5 years old) samples and assayed for AutoAb[GFAP].
Measurement of AutoAb[GFAP]
To detect and quantify AutoAb[GFAP] levels in biosamples, we used our previously published manifold autoantibody immunoblotting assay format26 (see Supplementary Fig. 1 for assay set-up; see online supplementary material at ftp.liebertpub.com). Briefly, human brain GFAP protein or human brain fraction enriched in GFAP protein (20 μg) were subjected to sodium dodecyl sulfate polyacrylamide gel electrophoresis on 4–20% Tris-glycine 1-well gel and electrotransferred to polyvinylidene fluoride (PVDF) membrane. PVDF membranes were then clamped into the Mini-Protean II Multiscreen apparatus (Bio-Rad), and individual lanes were blocked and probed with human sera diluted at 1:100, unless otherwise noted.
This manifold autoantibody immunoblot assay26 requires only a 1/100 dilution (e.g., 1 μL in 100 μL). We serially diluted the plasma to verify that the signal is plasma concentration-dependent provided that it is within the optical density (OD) readings for the spectrometer. Secondary antibodies used were either alkaline phosphatase (AP)-conjugated goat antihuman immunoglobulin G (IgG) or AP-conjugated donkey antihuman IgG diluted 1:10,000 (Jackson ImmunoResearch).
Blots were developed at room temperature with substrate 5-bromo-4-chloro-3'-indolyphosphate p-toluidine salt and nitro-blue tetrazolium chloride solution for 10 min. We also routinely performed in-solution pre-absorption with GFAP protein (2 μg/100 μL) as a control study. The bands of interest on the blotting membrane disappear after pre-absorption (data not shown). Quantification of autoantibody reactivity on immunoblots was performed via computer-assisted densitometric scanning (Epson 8836XL high-resolution scanner and NIH Image J densitometry software). Autoantibody levels were expressed in arbitrary densitometry units.
Values are reported as mean and standard error (SE) unless stated otherwise. Analysis of variance (ANOVA) was used for multigroup analysis; Tukey post hoc test used to assess mean differences between subgroups as well as distinguish homogeneous subsets. Statistical significance was assessed at p < 0.05. Statistics were performed using GraphPad Prism 5.0 (GraphPad Software, La Jolla, CA) and Statistical Analysis System (SAS) version 9.2, (SAS Institute, Inc., Cary, NC) unless stated otherwise.
Results
Anti-GFAP autoantibody levels in acute plasma samples from TRACK-TBI pilot study
Because we previously identified a dominant autoantibody response to glial intermediate filament protein GFAP among patients with severe TBI,26,33–35 here we sought to expand these findings by using the TRACK-TBI pilot study cohorts and plasma samples.1,36,37 Of 586 subjects with acute TBI from the TRACK-TBI pilot, we identified 196 with available acute plasma samples (collected within 24 h of injury) for this autoantibody study. Study patients covered the range of initial GCS of 3–15, which are reported with age, sex, and admission head CT distributions in Table 1. The TRACK-TBI pilot study also recorded self-reported previous TBI history (apart from the index TBI of enrollment), with the following categories: no previous TBI (n = 106), previous TBI without LOC (n = 47), and previous TBI with LOC (n = 43) (Table 1).
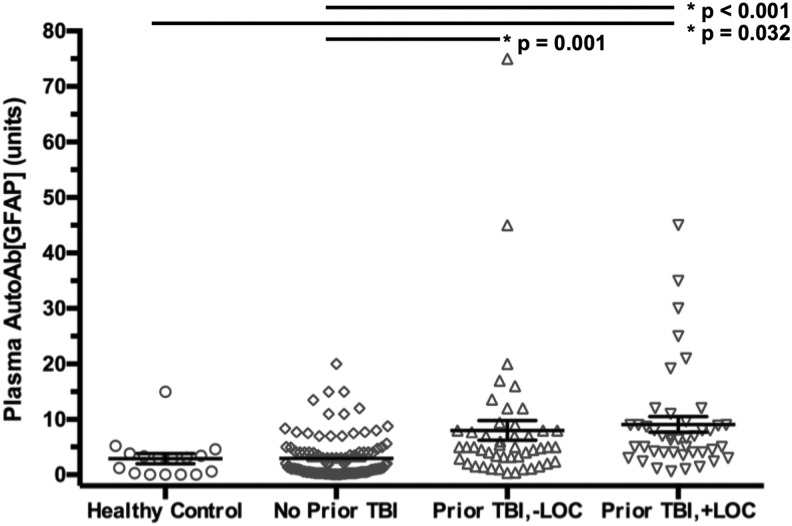
Autoantibodies reacting with intact GFAP (50 kDa) and its various BDPs (48–38 kDa) were assayed using quantitative manifold immunblotting developed previously.3,26 The distribution for AutoAb[GFAP] (mean ± SE) was 2.90 ± 0.92 units for healthy controls, 2.97 ± 0.37 units for patients with acute TBI reporting no previous TBI, 8.01 ± 1.80 units for patients with acute TBI reporting previous TBI without LOC, and 9.11 ± 1.42 units for patients with acute TBI reporting previous TBI with LOC.
ANOVA showed a significant difference across groups (p < 0.001); Tukey post hoc test demonstrated that healthy controls and patients with acute TBI reporting no previous TBI constituted a statistically different subgroup in AutoAb[GFAP] levels than patients with acute TBI reporting previous TBI either with or without LOC (Fig. 1). Specifically, patients with acute TBI reporting previous TBI with LOC showed significantly elevated AutoAb[GFAP] levels than healthy controls (mean increase 6.21 ± 2.26, p = 0.032) and patients reporting no previous TBI (mean increase 6.14 ± p < 0.001), but not with patients with acute TBI reporting previous TBI without LOC (mean increase 1.10 ± 1.62, p = 0.906); patients with acute TBI reporting previous TBI without LOC showed significantly elevated AutoAb[GFAP] levels when compared with patients with acute TBI reporting no previous TBI (mean increase 5.04 ± 1.35, p = 0.001), but not with healthy controls (mean increase 5.11 ± 2.23, p = 0.103). No difference was observed in AutoAb[GFAP] between healthy controls and patients with acute TBI reporting no previous TBI (mean increase 0.07 ± 2.07, p = 0.999).
FIG. 1.
Mean and standard error of the mean are shown for each respective patient subgroup (healthy control, acute traumatic brain injury [TBI] reporting no prior TBI, acute TBI reporting prior TBI without loss of consciousness [LOC], acute TBI reporting prior TBI with LOC). The plasma AutoAb[GFAP] (glial fibrillary acidic protein autoantibody) is shown in units as described in the Methods section of the article. Statistically significant differences across subgroups are denoted with (*) and the respective p value.
In previous reports, we have assayed the same patient plasma samples for GFAP (and its BDP) levels.36,37 Thus, we examined whether there is a correlation between GFAP antigen levels and AutoAb[GFAP] levels in these samples. As expected, we did not find a correlation between the two (data not shown).
In addition, we sought to examine acute AutoAb[GFAP] distributions across different initial GCS scores. Because of the relatively small number of samples for those with lower GCS, by convention we grouped acute patients to three GCS categories for autoantibody comparison purposes: GCS 3–8 (n = 12; mean ± SE: 3.35 ± 0.87), GCS 9–12 (n = 6, 4.37 ± 1.59), GCS 13–15 (n = 169, 6.16 ± 0.72). Results on ANOVA showed no statistically significant differences in acute AutoAb[GFAP] levels across the three GCS categories (p = 0.197).
We also examined acute AutoAb[GFAP] distributions by presence of intracranial pathology on admission head CT, across categories of “no intracranial pathology” (n = 108), “extra-axial pathology only” (n = 22), “intra-axial pathology only” (n = 24), and “both extra-axial and intra-axial pathology” (n = 42). Results on ANOVA showed no statistically significant differences in acute AutoAb[GFAP] levels across the four categories (mean ± SE: 6.48 ± 0.87; 5.02 ± 2.01; 5.72 ± 2.04; 3.22 ± 0.49, respectively; p = 0.197).
To further explore the relationship between pathological injury severity and AutoAb[GFAP], we analyzed the distribution of AutoAb[GFAP] across Marshall CT categories. Because of the small numbers of individual Marshall CT scores of 3 (n = 9), 4 (n = 2), 5 (n = 12), and 6 (n = 1), we combined Marshall score 3–6 into a single category “3+”. AutoAb[GFAP] distributions were as follows: Marshall 1 (n = 96, mean ± SE: 6.20 ± 0.91), Marshall 2 (n = 78, 5.57 ± 0.96), Marshall 3+ (n = 22, 2.47 ± 0.64) and showed no statistically significant differences across Marshall CT categories (p = 0.148).
Anti-GFAP autoantibody levels in chronic plasma samples from TRACK-TBI pilot study
We previously demonstrated that post-TBI serum AutoAb[GFAP] shows a delayed increase, beginning about 5–6 days after severe TBI and sustained to at least 10 days.26,38 Here, we examined AutoAb[GFAP] levels in chronic TBI plasma samples collected from 21 subjects during rehabilitation. The demographics of these subjects are tabulated in Table 1. Initial GCS and CT Marshall scores or Glasgow Outcome Score-Extended data were unavailable for patients with chronic TBI.
All patients triaged to the rehabilitation facility were assessed with an index injury severe enough to warrant inpatient rehabilitation, with Rancho Los Amigos-Revised (RLA) score distributions of the following on admission to the rehabilitation facility: RLA 1 (No Response, Total Assistance, n = 1); RLA 2 (Generalized Response, Total Assistance, n = 2), RLA 3 (Localized Response, Total Assistance, n = 2), RLA 4 (Confused/Agitated, Maximal Assistance, n = 1), RLA 5 (Confused, Inappropriate/Nonagitated, Maximal Assistance, n = 6), RLA 6 (Confused, Appropriate, Moderate Assistance, n = 4), RLA 7 (Automatic, Appropriate, Minimal Assistance for Activities of Daily Living (ADL), n = 1), RLA 8 (Purposeful, Appropriate, Stand-By Assistance, n = 0), RLA 9 (Purposeful, Appropriate, With Standby Assist on Request, n = 0), RLA 10 (Purposeful, Appropriate, Modified Independent, n = 0), RLA Unknown, (n = 5).
Thus, all patients with chronic TBI with known RLA had a score of 7 or less, with 16 (76%) of the 21 total patients needing moderate assistance for ADL because of their brain injury (5 [24%] needing total assistance, 7 [33%] needing maximal assistance, and 4 [19%] needing moderate assistance). Hence, we observe that the chronic TBI population in this study is one of overall moderate to total impairment in ADL. CT data were available for 11 of 21 patients with chronic TBI (5 extra-axial hemorrhage only, 3 intra-axial hemorrhage only, 3 both extra- and intra-axial hemorrhage).
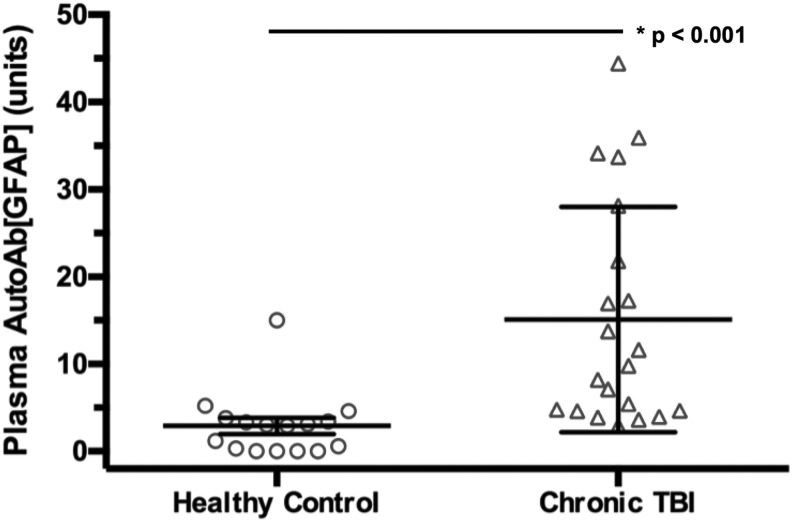
The post-injury time ranged 16–250 days after injury, with an average of 176.4 days (or 6.4 months) post-injury (Table 2). Using ANOVA, we show that the AutoAb[GFAP] levels were significantly elevated in patients with chronic TBI (mean 15.08 ± 2.82 units, p < 0.001) compared with healthy controls as previously reported (mean 2.90 ± 0.93 units) (Fig. 2).
Table 2.
Plasma Glial Fibrillary Acidic Protein Autoantibody Levels in Patients with Traumatic Brain Injury*
| Acute TBI | Chronic TBI | |||||
|---|---|---|---|---|---|---|
| Post-injury time | N = 196 | N = 21 | ||||
| Mean ± SD | 10.6 ± 6.3 (h) | 176.4 ± 44.5 (days) | ||||
| Range | 0.5 to 23.9 (h) | 16.0 to 250.0 (days) | ||||
| GCS | N | Mean (SE) | Sig. (p) | N | Mean (SE) | Sig. (p) |
| 3–8 | 12 | 3.35 (0.87) | 0.136 | — | — | — |
| 9–12 | 6 | 4.37 (1.59) | — | — | ||
| 13–15 | 120 | 6.16 (0.72) | — | — | ||
| Unknown | 18 | 1.73 (2.35) | — | — | ||
| Previous TBI | N | Mean (SE) | Sig. (p) | N | Mean (SE) | Sig. (p) |
| None | 106 | 2.97 (0.37) [a] | <0.001 | 4 | 13.25 (2.43) | 0.956 |
| Yes, without LOC | 47 | 8.01 (1.80) [b] | 4 | 15.41 (6.56) | ||
| Yes, with LOC | 43 | 9.11 (1.42) [b] | 13 | 15.54 (4.18) | ||
| Admission head CT | N | Mean (SE) | Sig. (p) | N | Mean (SE) | Sig. (p) |
| Negative | 108 | 6.48 (0.87) | 0.197 | — | — | 0.168 |
| Extra-axial only | 22 | 5.02 (2.01) | 5 | 14.32 (6.01) | ||
| Intra-axial only | 24 | 5.72 (2.04) | 3 | 8.27 (2.88) | ||
| Extra- + intra-axial | 42 | 3.22 (0.49) | 3 | 13.82 (7.98) | ||
| Unknown | — | — | 10 | 13.07 (3.86) |
TBI, traumatic brain injury; SD, standard deviation; GCS, Glasgow Coma Scale; SE, standard error of the mean; LOC, loss of consciousness; CT, computed tomography;.
Blood draw for GFAP-AutoAb post-injury time calculated from time of injury. GCS data were unavailable for patients with chronic TBI. CT pathology was positive for all patients with chronic TBI with CT data. [a] and [b] denote statistically significant subgroups on the Tukey post hoc test.
FIG. 2.
Mean and standard error of the mean are shown for healthy control versus patients with chronic traumatic brain injury (TBI). The plasma AutoAb[GFAP] (glial fibrillary acidic protein autoantibody) is shown in units as described in the Methods section of the article. Statistically significant differences across subgroups are denoted with (*) and the respective p value.
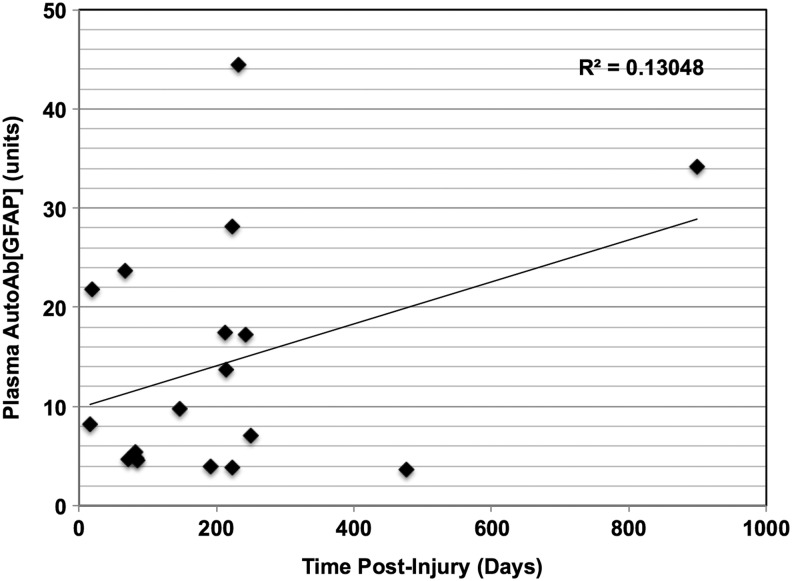
We also plotted a graph of the plasma AutoAb[GFAP] against the time post-injury based on this set of 21 patients. Each patient with chronic TBI only had one timed plasma sample drawn as part of the TRACK-TBI pilot study (Fig. 3); while the sample size is limited, no significant correlation was found between post-injury time and AutoAb[GFAP] levels (Spearman rank correlation test, data not shown).
FIG. 3.
Scatterplot for plasma AutoAb[GFAP] (glial fibrillary acidic protein auto-antibody) plotted against time post-injury for 21 patients with chronic traumatic brain injury (TBI). The plasma AutoAb[GFAP] is shown in units as described in the Methods section of the article. The correlation coefficient (R2) is shown.
We also examined the relationship between CT intracranial lesion and AutoAb[GFAP] levels in these patients with chronic TBI. Results on ANOVA showed no statistically significant differences across the four categories (mean ± SE): extra-axial only, 14.32 ± 6.01; intra-axial only, 8.27 ± 2.88; both extra- and intra-axial, 13.82 ± 7.98, unknown CT pathology, 13.07 ± 3.86; p = 0.168.
Discussion
In this study, we expand on our previous finding that there is a dominant anti-GFAP autoantibody response within 5–10 days among a subset of patients with severe TBI.26,39 While the number of plasma samples is still relatively small within the cohort, the TRACK-TBI pilot dataset was selected for this study because it is well-characterized with 13 published articles regarding various components of these TBI patients across the full range of TBI severity—including proteomic and genetic biomarkers, neuroimaging, and outcome data.31,36,37,40–43
Based on the 217 subjects with available biosamples from this cohort, we identified that anti-GFAP autoantibody levels were elevated in acute plasma samples from brain injury subjects who had a self-reported history of previous TBI with or without LOC when compared with patients with acute TBI without self-reported previous TBI (Fig. 1). There is no correlation between GFAP antigen levels and GFAP autoantibody levels in these acute samples and to initial GCS.
We also found no statistically significant differences between AutoAb[GFAP] and acute CT pathology—widely used as the current clinical standard for TBI diagnosis and a surrogate marker of brain injury after acute TBI.1,44–46 Because newly acquired anti-GFAP antibody response usually takes about 5 days to manifest,26,47,48 it is unlikely that the acute post-TBI autoantibody levels we report here were from a de novo response to current TBI, but rather to a sustained increase because of previous head injuries. At present, however, we cannot rule out whether the acute TBI event might serve to be an antigen-boosting event for those with pre-existing anti-GFAP antibody titers. It is also interesting to consider that repeated mild TBI/concussion can potentially serve as an autoantigen-boosting event.
Our study is the first to report AutoAb[GFAP] values across the spectrum of acute TBI. The reason for anti-GFAP reactivity in a subset of healthy controls is not completely known. We have reported similar results in our first study on AutoAb[GFAP].26 We also noted that autoantibodies to other human autoantigens have been reported in normal populations.49,50 We suspect that the baseline anti-GFAP autoantibody levels we observed in certain healthy controls likely reflect the TBI health history of those subjects—e.g., they may have experienced previous unreported concussions or other subclinical neurological events.25
It is also presently unclear as to why AutoAb[GFAP] was statistically significantly elevated in those with an acute TBI and history of previous TBI when compared with those with acute TBI without a previous history of TBI, but not healthy controls. The samples captured from the auto rehabilitation cohort with confirmed previous TBI, however, did demonstrate statistically higher GFAP autoantibody levels. Whether this contradiction is reflective of the small sample size, a high prevalence of unreported TBI in the control group, and/or a combination thereof remains to be determined.
It is also possible that the GFAP autoantibody level represents not only initial injury severity/mortality, but also individual variability in the immune response and/or clearance of autoantibodies. Hence, our study should be considered preliminary and future studies with serial collection of GFAP autoantibodies are therefore needed to better quantitate the time course in individuals to better characterize the hypothesized variability.
All samples were collected within 24 h after the current TBI event and thus the plasma AutoAb[GFAP] we measured in these patients with acute TBI likely reflects previous brain injury or perturbation incidents. Patients reporting previous TBI without LOC had a slightly lower AutoAb[GFAP] level on average than those reporting previous TBI with LOC. This preliminarily suggests that the severity of previous exposure exerts some effect on the magnitude of the AutoAb[GFAP] response measureable in plasma. Future studies with a larger population of post-TBI patients in which initial injury characteristics are available is needed to further validate this finding, however.
While preliminary, this is the first report of significant plasma AutoAb[GFAP] elevation in patients with TBI at the chronic time point (mean >6 month) compared with age-matched controls (Fig. 2). Because the autoantibody response is a marker of sustained immunological memory, it may be the case that neuronal or glial autoantibody biomarkers can be useful to confirm a diagnosis of chronic TBI in cases where history is vague or incomplete.
Some of the limitations of the current study are as follows. Currently, we focused on IgG responses; in future studies, we plan to examine in parallel IgM-based autoantibody responses to investigate acute changes. To increase the throughput of the anti-GFAP autoantibody assays, it will be desirable to use microplate-based enzyme-linked immunosorbent assays; we are working toward this direction. In addition, because of institutional-specific differences in medical record documentation, all previous injury information was patient-reported and additional injury characteristics (e.g., acute GCS in the rehabilitation setting) could not be independently confirmed and/or clarified.
Another limitation is the lack of longitudinal blood samples within the same patient, and thus we were unable to follow the temporal profile of AutoAb[GFAP] response. To this end, we will be expanding our AutoAb[GFAP] studies to the ongoing, NIH-funded prospective multicenter TRACK-TBI study51 with acute (day 1, 3, 5), subacute (2 weeks), and chronic (6 month) blood samples from up to 2700 patients with TBI across injury severities, as well as 300 non-TBI controls, as part of the U.S. Department of Defense TBI Endpoints Development Initiative.52 Data from these future studies will allow us to examine whether elevations of post-injury AutoAb[GFAP] associate with patient outcome.
Conclusion
AutoAb[GFAP] assays may be useful to study the dynamic interactions among brain autoimmune mechanisms post-TBI across acute and chronic injury settings. There are two important new findings reported in this study: (1) We find that in the setting of acute TBI, plasma AutoAb[GFAP] levels associate with a history of past exposure to TBI; (2) Further, this is the first study to report elevated AutoAb[GFAP] levels at a chronic time point (average of 6 months post-injury) among patients with moderate to severe TBI. With emerging attention on reexamining TBI as a chronic condition with various comorbidities,3,38,53–56 we can now add brain protein-targeting autoantibodies to a growing list of potential useful biomarkers for studying at-risk acute and chronic TBI populations.
Supplementary Material
Contributor Information
Collaborators: and the TRACK-TBI Investigators (including
Acknowledgments
This study is supported in part by NIH RC2 NS069409 (G.T.M.), NIH 1U01 NS086090-01 (G.T.M.), U.S. DOD Grant W81XWH-14-2-0176 (G.T.M.), U.S. DOD Grant W81XWH-13-1-04 (G.T.M.), NIH R21NS085455-01 (K.K.W.), and UF Psychiatry Development Fund (K.K.W.).
Author Disclosure Statement
K.K.W. holds stocks of Banyan Biomarkers, Inc., a company interested in commercialization of diagnostic tests for TBI. For the remaining authors, no competing financial interests exist.
References
- 1.Hergenroeder G.W., Redell J.B., Moore A.N., and Dash P.K. (2008). Biomarkers in the clinical diagnosis and management of traumatic brain injury. Mol. Diagn. Ther. 12, 345–358 [DOI] [PubMed] [Google Scholar]
- 2.Zhang Z., Mondello S., Kobeissy F., Rubenstein R., Streeter J., Hayes R.L., and Wang K.K. (2011). Protein biomarkers for traumatic and ischemic brain injury: from bench to bedside. Transl. Stroke Res. 2, 455–462 [DOI] [PubMed] [Google Scholar]
- 3.Yang Z., and Wang K.K. (2015) Glial fibrillary acidic protein: from intermediate filament assembly and gliosis to neurobiomarkers. Trends Neurosci. 38, 364–374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Agoston D.V., and Elsayed M.M. (2012). Serum-based protein biomarkers in blast-induced traumatic brain injury spectrum disorder. Front. Neurol. 3, 107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu M.C., Akinyi L., Scharf D., Mo J., Larner S.F., Muller U., Oli M.W., Zheng W., Kobeissy F., Papa L., Lu X.C., Dave J.R., Tortella F.C., Hayes R.L., and Wang K.K.W. (2010). Ubiquitin C-terminal hydrolase-L1 as a biomarker for ischemic and traumatic brain injury in rats. Eur. J. Neurosci. 31, 722–732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dambinova S.A., Khounteev G.A., Izykenova G.A., Zavolokov I.G., Ilyukhina A.Y., and Skoromets A.A. (2003). Blood test detecting autoantibodies to N-methyl-D-aspartate neuroreceptors for evaluation of patients with transient ischemic attack and stroke. Clin. Chem. 49, 1752–1762 [DOI] [PubMed] [Google Scholar]
- 7.Colasanti T., Barbati C., Rosano G., Malorni W., and Ortona E. (2010). Autoantibodies in patients with Alzheimer's disease: pathogenetic role and potential use as biomarkers of disease progression. Autoimmun. Rev. 9, 807–811 [DOI] [PubMed] [Google Scholar]
- 8.D'Andrea M.R. (2005). Add Alzheimer's disease to the list of autoimmune diseases. Med. Hypotheses 64, 458–463 [DOI] [PubMed] [Google Scholar]
- 9.Ankeny D.P., Lucin K.M., Sanders V.M., McGaughy V.M., and Popovich P.G. (2006). Spinal cord injury triggers systemic autoimmunity: evidence for chronic B lymphocyte activation and lupus-like autoantibody synthesis. J. Neurochem. 99, 1073–1087 [DOI] [PubMed] [Google Scholar]
- 10.Popovich P.G., Stokes B.T., and Whitacre C.C. (1996). Concept of autoimmunity following spinal cord injury: possible roles for T lymphocytes in the traumatized central nervous system. J. Neurosci. Res. 45, 349–363 [DOI] [PubMed] [Google Scholar]
- 11.Lancaster E., and Dalmau J. (2012). Neuronal autoantigens—pathogenesis, associated disorders and antibody testing. Nat. Rev. Neurol. 8, 380–390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Svetlov S.I., Prima V., Kirk D.R., Gutierrez H., Curley K.C., Hayes R.L., and Wang K.K. (2010). Morphologic and biochemical characterization of brain injury in a model of controlled blast overpressure exposure. J. Trauma 69, 795–804 [DOI] [PubMed] [Google Scholar]
- 13.Ponomarenko N.A., Durova O.M., Vorobiev I.I., Belogurov A.A., Telegin G.B., Suchkov S.V., Misikov V.K., Morse H.C., III, and Gabibov A.G. (2006). Catalytic activity of autoantibodies toward myelin basic protein correlates with the scores on the multiple sclerosis expanded disability status scale. Immunol. Lett. 103, 45–50 [DOI] [PubMed] [Google Scholar]
- 14.Svetlov S.I., Prima V., Glushakova O., Svetlov A., Kirk D.R., Gutierrez H., Serebruany V.L., Curley K.C., Wang K.K., and Hayes R.L. (2012). Neuro-glial and systemic mechanisms of pathological responses in rat models of primary blast overpressure compared to “composite” blast. Front. Neurol. 3, 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hedegaard C.J., Chen N., Sellebjerg F., Sørensen P.S., Leslie R.G., Bendtzen K., and Nielsen C.H. (2009). Autoantibodies to myelin basic protein (MBP) in healthy individuals and in patients with multiple sclerosis: a role in regulating cytokine responses to MBP. Immunology 128, Suppl 1, e451–e461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cox A.L., Coles A.J., Nortje J., Bradley P.G., Chatfield D.A., Thompson S.J., and Menon D.K. (2006). An investigation of auto-reactivity after head injury. J. Neuroimmunol. 174, 180–186 [DOI] [PubMed] [Google Scholar]
- 17.Sorokina E.G., Semenova Z.B., Granstrem O.K., Karaseva O.V., Meshcheriakov S.V., Reutov V.P., Sushkevich G.N., Pinelis V.G., and Roshal L.M. (2010). [S100B protein and autoantibodies to S100B protein in diagnostics of brain damage in craniocerebral trauma in children]. (Rus) Zh. Nevrol. Psikhiatr. Im. S. S. Korsakova 110, 30–35 [PubMed] [Google Scholar]
- 18.Goryunova A.V., Bazarnaya N.A., Sorokina E.G., Semenova N.Y., Globa O.V., Semenova Z.B., Pinelis V.G., Roshal L.M., and Maslova O.I. (2007). Glutamate receptor autoantibody concentrations in children with chronic post-traumatic headache. Neurosci. Behav. Physiol. 37, 761–764 [DOI] [PubMed] [Google Scholar]
- 19.Siman R., Toraskar N., Dang A., McNeil E., McGarvey M., Plaum J., Maloney E., and Grady M.S. (2009). A panel of neuron-enriched proteins as markers for traumatic brain injury in humans. J. Neurotrauma 26, 1867–1877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tanriverdi F., De Bellis A., Bizzarro A., Sinisi A.A., Bellastella G., Pane E., Bellastella A., Unluhizarci K., Selcuklu A., Casanueva F.F., and Kelestimur F. (2008). Antipituitary antibodies after traumatic brain injury: is head trauma-induced pituitary dysfunction associated with autoimmunity? Eur. J. Endocrinol. 159, 7–13 [DOI] [PubMed] [Google Scholar]
- 21.Papa L., Akinyi L, Liu MC, Pineda JA, Tepas JJ, 3rd, Oli MW, Zheng W, Robinson G, Robicsek SA, Gabrielli A, Heaton SC, Hannay HJ, Demery JA, Brophy GM, Layon J, Robertson CS, Hayes RL, and Wang KK. (2010). Ubiquitin C-terminal hydrolase is a novel biomarker in humans for severe traumatic brain injury. Crit. Care Med. 38, 138–144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tanriverdi F., De Bellis A., Battaglia M., Bellastella G., Bizzarro A., Sinisi A.A., Bellastella A., Unluhizarci K., Selcuklu A., Casanueva F.F., and Kelestimur F. (2010). Investigation of antihypothalamus and antipituitary antibodies in amateur boxers: is chronic repetitive head trauma-induced pituitary dysfunction associated with autoimmunity? Eur. J. Endocrinol. 162, 861–867 [DOI] [PubMed] [Google Scholar]
- 23.Brophy G.M., Mondello S., Papa L., Robicsek S.A., Gabrielli A., Tepas J., Buki A., III, Robertson C., Tortella F.C., Hayes R.L., and Wang K.K. (2011). Biokinetic analysis of ubiquitin C-terminal hydrolase-L1 (UCH-L1) in severe traumatic brain injury patient biofluids. J. Neurotrauma 28, 861–870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zoltewicz J.S., Mondello S., Yang B., Newsom K.J., Kobeissy F., Yao C., Lu X.C., Dave J.R., Shear D.A., Schmid K., Rivera V., Cram T., Seaney J., Zhang Z., Wang K.K., Hayes R.L., and Tortella F.C. (2013). Biomarkers track damage after graded injury severity in a rat model of penetrating brain injury. J. Neurotrauma 30, 1161–1169 [DOI] [PubMed] [Google Scholar]
- 25.Marchi N., Bazarian J.J., Puvenna V., Janigro M., Ghosh C., Zhong J., Zhu T., Blackman E., Stewart D., Ellis J., Butler R., and Janigro D. (2013). Consequences of repeated blood-brain barrier disruption in football players. PLoS ONE 8, e56805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang Z., Zoltewicz J.S., Mondello S., Newsom K.J., Yang Z., Yang B., Kobeissy F., Guingab J., Glushakova O., Robicsek S., Heaton S., Buki A., Hannay J., Gold M.S., Rubenstein R., Lu X.C., Dave J.R., Schmid K., Tortella F., Robertson C.S., and Wang K.K. (2014). Human traumatic brain injury induces autoantibody response against glial fibrillary acidic protein and its breakdown products. PLoS ONE 9, e92698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Giacoppo S., Bramanti P., Barresi M., Celi D., Foti Cuzzola V., Palella E., and Marino S. (2012). Predictive biomarkers of recovery in traumatic brain injury. Neurocrit. Care 16, 470–477 [DOI] [PubMed] [Google Scholar]
- 28.Yang S.H., Gustafson J., Gangidine M., Stepien D., Schuster R., Pritts T.A., Goodman M.D., Remick D.G., and Lentsch A.B. (2013). A murine model of mild traumatic brain injury exhibiting cognitive and motor deficits. J. Surg. Res. 184, 981–988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yokobori S., Hosein K., Burks S., Sharma I., Gajavelli S., and Bullock R. (2013). Biomarkers for the clinical differential diagnosis in traumatic brain injury—a systematic review. CNS Neurosci. Ther. 19, 556–565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Derfuss T., and Meinl E. (2012). Identifying autoantigens in demyelinating diseases: valuable clues to diagnosis and treatment? Curr. Opin. Neurol. 25, 231–238 [DOI] [PubMed] [Google Scholar]
- 31.Yue J.K., Vassar M.J., Lingsma H.F., Cooper S.R., Okonkwo D.O., Valadka A.B., Gordon W.A., Maas A.I., Mukherjee P., Yuh E.L., Puccio A.M., Schnyer D.M., and Manley G.T.; TRACK-TBI Investigators. (2013). Transforming research and clinical knowledge in traumatic brain injury pilot: multicenter implementation of the common data elements for traumatic brain injury. J. Neurotrauma. 30, 1831–1844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Manley G.T., Diaz-Arrastia R., Brophy M., Engel D., Goodman C., Gwinn K., Veenstra T.D., Ling G., Ottens A.K., Tortella F., and Hayes R.L. (2010). Common Data Elements for Traumatic Brain Injury: Recommendations from the Biospecimens and Biomarkers Working Group. Arch. Phys. Med. Rehabil. 91, 1667–1672 [DOI] [PubMed] [Google Scholar]
- 33.Mondello S., Jeromin A., Buki A., Bullock R., Czeiter E., Kovacs N., Barzo P., Schmid K., Tortella F., Wang K.K., and Hayes R.L. (2012). Glial neuronal ratio: a novel index for differentiating injury type in patients with severe traumatic brain injury. J. Neurotrauma 29, 1096–1104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Papa L., Lewis L.M., Falk J.L., Zhang Z., Silvestri S., Giordano P., Brophy G.M., Demery J.A., Dixit N.K., Ferguson I., Liu M.C., Mo J., Akinyi L., Schmid K., Mondello S., Robertson C.S., Tortella F.C., Hayes R.L., and Wang K.K. (2012). Elevated levels of serum glial fibrillary acidic protein breakdown products in mild and moderate traumatic brain injury are associated with intracranial lesions and neurosurgical intervention. Ann. Emerg. Med. 59, 471–483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Papa L., Lewis L.M., Silvestri S., Falk J.L., Giordano P., Brophy G.M., Demery J.A., Liu M.C., Mo J., Akinyi L., Mondello S., Schmid K., Robertson C.S., Tortella F.C., Hayes R.L., and Wang K.K. (2012). Serum levels of ubiquitin C-terminal hydrolase distinguish mild traumatic brain injury from trauma controls and are elevated in mild and moderate traumatic brain injury patients with intracranial lesions and neurosurgical intervention. J. Trauma Acute Care Surg. 72, 1335–1344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Okonkwo D.O., Yue J.K., Puccio A.M., Panczykowski D.M., Inoue T., McMahon P.J., Sorani M.D., Yuh E.L., Lingsma H.F., Maas A.I.R., Valadka A.B., and Manley G.T.; TRACK-TBI Investigators. (2013). GFAP-BDP as an acute diagnostic marker in traumatic brain injury: results from the prospective transforming research and clinical knowledge in traumatic brain injury study. J. Neurotrauma 30, 1490–1497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Diaz-Arrastia R., Wang K.K.Papa L., Sorani M.D., Yue J.K., Puccio A.M., McMahon P.J., Inoue T., Yuh E.L., Lingsma H.F., Maas A.I., Valadka A.B., Okonkwo D.O., and Manley G.T.; TRACK-TBI Investigators. (2014). Acute biomarkers of traumatic brain injury: relationship between plasma levels of ubiquitin C-terminal hydrolase-L1 and glial fibrillary acidic protein. J. Neurotrauma 31, 19–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kobeissy F.H., Guingab-Cagmat J.D., Razafsha M., O'Steen L., Zhang Z., Hayes R.L., Chiu W.T., and Wang K.K. (2011). Leveraging biomarker platforms and systems biology for rehabilomics and biologics effectiveness research. PM R 3, Suppl, S139–S147 [DOI] [PubMed] [Google Scholar]
- 39.Yang Z., Lin F., Robertson C.S., and Wang K.K. (2014). Dual vulnerability of TDP-43 to calpain and caspase-3 proteolysis after neurotoxic conditions and traumatic brain injury. J. Cereb. Blood Flow Metab. 34, 1444–1452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yuh E.L., Cooper S.R., Mukherjee P., Yue J.K., Lingsma H.F., Gordon W.A., Valadka A.B., Okonkwo D.O., Schnyer D.M., Vassar M.J., Maas A.I., and Manley G.T. (2014). Diffusion tensor imaging for outcome prediction in mild traumatic brain injury: a TRACK-TBI study. J. Neurotrauma. 31, 1457–1477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yue J.K., Pronger A.M., Ferguson A.R., Temkin N.R., Sharma S., Rosand J., Sorani M.D., McAllister T.W., Barber J., Winkler E.A., Burchard E.G., Hu D., Lingsma H.F., Cooper S.R., Puccio A.M., Okonkwo D.O., Diaz-Arrastia R., and Manley G.T.; COBRIT Investigators; TRACK-TBI Investigators. (2015). Association of a common genetic variant within ANKK1 with six-month cognitive performance after traumatic brain injury. Neurogenetics 16, 169–180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ratcliff J.J., Adeoye O., Lindsell C.J., Hart K.W., Pancioli A., McMullan J.T., Yue J.K., Nishijima D.K., Gordon W.A., Valadka A.B., Okonkwo D.O., Lingsma H.F., Maas A.I., and Manley G.T.; TRACK-TBI Investigators. (2014). ED disposition of the Glasgow Coma Scale 13 to 15 traumatic brain injury patient: analysis of the Transforming Research and Clinical Knowledge in TBI study. Am. J. Emerg. Med. 32, 844–850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dams-O'Connor K., Spielman L., Singh A., Gordon W.A., Lingsma H.F., Maas A.I., Manley G.T., Mukherjee P., Okonkwo D.O., Puccio A.M., Schnyer D.M., Valadka A.B., Yue J.K., and Yuh E.L. (2013). The impact of previous traumatic brain injury on health and functioning: a TRACK-TBI study. 30, 2014–2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Herrmann M., Jost S., Kutz S., Ebert A.D., Kratz T., Wunderlich M.T., and Synowitz H. (2000). Temporal profile of release of neurobiochemical markers of brain damage after traumatic brain injury is associated with intracranial pathology as demonstrated in cranial computerized tomography. J. Neurotrauma. 17, 113–122 [DOI] [PubMed] [Google Scholar]
- 45.Raabe A., Grolms C., Keller M., Döhnert J., Sorge O., and Seifert V. (1998). Correlation of computed tomography findings and serum brain damage markers following severe head injury. Acta Neurochir. (Wien) 140, 787–792 [DOI] [PubMed] [Google Scholar]
- 46.Ruan S., Noyes K., and Bazarian J.J. (2009). The economic impact of S-100B as a pre-head CT screening test on emergency department management of adult patients with mild traumatic brain injury. J. Neurotrauma. 26,1655–1664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rubenstein R., Chang B., Gray P., Piltch M., Bulgin M.S., Sorensen-Melson S., and Miller M.W. (2010). A novel method for preclinical detection of PrPSc in blood. J. Gen. Virol. 91, 1883–1892 [DOI] [PubMed] [Google Scholar]
- 48.Chang B., Gray P., Piltch M., Bulgin M.S., Sorensen-Melson S., Miller M.W., Davies P., Brown D.R., Coughlin D.R., and Rubenstein R. (2009). Surround optical fiber immunoassay (SOFIA): an ultra-sensitive assay for prion protein detection. J. Virol. Methods 159, 15–22 [DOI] [PubMed] [Google Scholar]
- 49.Watanabe M., Uchida K., Nakagaki K., Trapnell B.C., and Nakata K. (2010). High avidity cytokine autoantibodies in health and disease: pathogenesis and mechanisms. Cytokine Growth Factor Rev. 21, 263–273 [DOI] [PubMed] [Google Scholar]
- 50.Iseme R.A., McEvoy M., Kelly B., Agnew L., Attia J., and Walker F.R. (2014) Autoantibodies and depression: evidence for a causal link? Neurosci. Biobehav. Rev. 40, 62–79 [DOI] [PubMed] [Google Scholar]
- 51.Transforming Research and Clinical Knowledge in Traumatic Brain Injury (TRACK-TBI). Available at: http://tracktbi.ucsf.edu Accessed: September8, 2015
- 52.TBI Endpoints Development (TED) Initiative. Available at: http://tbiendpoints.ucsf.edu Accessed: September8, 2015
- 53.de Olmos J.S., Beltramino C.A., and de Olmos de Lorenzo S. (1994). Use of an amino-cupric-silver technique for the detection of early and semiacute neuronal degeneration caused by neurotoxicants, hypoxia, and physical trauma. Neurotoxicol. Teratol. 16, 545–561 [DOI] [PubMed] [Google Scholar]
- 54.Hall E.D., Bryant Y.D., Cho W., and Sullivan P.G. (2008). Evolution of post-traumatic neurodegeneration after controlled cortical impact traumatic brain injury in mice and rats as assessed by the de Olmos silver and fluorojade staining methods. J. Neurotrauma. 25, 235–247 [DOI] [PubMed] [Google Scholar]
- 55.Saltzman J.W., Battaglino R.A., Stott H.L., and Morse L.R. (2013). Rehabilitation considerations for traumatic brain injury in the geriatric population: epidemiology, neurobiology, prognosis, and management. Curr. Tran. Geriatr. Gerontol. Rep. 1, 149–158 [Google Scholar]
- 56.Smith D.H., Johnson V.E., and Stewart W. (2013). Chronic neuropathologies of single and repetitive TBI: substrates of dementia? Nat. Rev. Neurol. 9, 211–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.