Abstract
Sport-related concussion (SRC) is a major health problem, affecting millions of athletes each year. While the clinical effects of SRC (e.g., symptoms and functional impairments) typically resolve within several days, increasing evidence suggests persistent neurophysiological abnormalities beyond the point of clinical recovery after injury. This study aimed to evaluate cerebral blood flow (CBF) changes in acute SRC, as measured using advanced arterial spin labeling (ASL) magnetic resonance imaging (MRI). We compared CBF maps assessed in 18 concussed football players (age, 17.8 ± 1.5 years) obtained within 24 h and at 8 days after injury with a control group of 19 matched non-concussed football players. While the control group did not show any changes in CBF between the two time-points, concussed athletes demonstrated a significant decrease in CBF at 8 days relative to within 24 h. Scores on the clinical symptom (Sport Concussion Assessment Tool 3, SCAT3) and cognitive measures (Standardized Assessment of Concussion [SAC]) demonstrated significant impairment (vs. pre-season baseline levels) at 24 h (SCAT, p < 0.0001; SAC, p < 0.01) but returned to baseline levels at 8 days. Two additional computerized neurocognitive tests, the Automated Neuropsychological Assessment Metrics and Immediate Post-Concussion and Cognitive Testing, showed a similar pattern of changes. These data support the hypothesis that physiological changes persist beyond the point of clinical recovery after SRC. Our results also indicate that advanced ASL MRI methods might be useful for detecting and tracking the longitudinal course of underlying neurophysiological recovery from concussion.
Key words: : arterial spin labeling, cerebral blood flow, concussion, MRI, recovery
Introduction
Sport-related concussion (SRC) has become recognized as a major health problem, affecting millions of people each year during athletic participation.1 Concussion is induced by traumatic biomechanical forces, resulting in a complex pathophysiological process that affects the brain structure and function.2 A concussion typically occurs following transmission of direct or indirect impulsive forces to the head, which result in a rapid onset of short-lived neurological impairments and presents clinically with cognitive, physical, and behavioral signs and symptoms.2,3 Although most individuals with an SRC experience symptom resolution relatively rapidly (within 7–10 days),2,4–8 important clinical issues, such as the time course of neurophysiological recovery and the relationship between underlying neural recovery, return to play, and risk for developing long-term problems, have yet to be fully addressed.2,5–10
Previous research has provided great insight into the neurometabolic and biomechanical changes following mild traumatic brain injury (mTBI).3,6,8,9,11–15 However, studies of the biological substrates of SRC have been limited in scope, and knowledge regarding the functional physiological deficits following mTBI remains relatively unknown.11,13,16–22 Although the pathogenesis of concussion has yet to be fully elucidated, it is becoming increasingly clear that cerebrovascular alterations play a significant role in the evolution of injury sequelae, as well as in the process of post-traumatic brain repair.4,11,22–24 Reduced cerebral blood flow (CBF) signifies one of the most lasting markers of concussion in animal models of traumatic brain injury (TBI),14,25,26 while CBF has been shown to be decreased immediately after injury in an animal model27,28 and decreased regional CBF persisted after injury.29
Reduced CBF during the acute and subacute phases of mTBI has been detected using imaging methods such as single-photon emission computed tomography30,31 and perfusion computed tomography,32 which have several disadvantages, including financial cost, ionizing radiation, and limited repetition of acquisitions. Arterial spin labeling (ASL) is an advanced magnetic resonance imaging (MRI) technique capable of measuring CBF non-invasively by using magnetically labeled arterial blood water as an endogenous contrast tracer, without radiation exposure, and is hence very suitable for longitudinal studies of CBF in healthy and diseased individuals, or as a surrogate marker of brain function and metabolism.33–35 The three dimensional (3D) pseudo-continuous ASL (pCASL) technique with a higher signal-to-noise ratio (SNR) and efficiency has been developed.36–38 Until recently, only very limited research has applied this advanced MRI technique in concussion research.39 To evaluate the recovery trajectory during the acute stage after SRC, we examined clinical signs and CBF changes as measured using 3D pCASL in football athletes who sustained acute SRC, compared to matched uninjured teammates.
Methods
Participants
Participants were recruited from an ongoing prospective study of clinical recovery following concussion, Project Head to Head. Project Head to Head enrolled contact and collision sport athletes from nine high schools and four colleges in southeastern Wisconsin from August 2012 through November 2014. For the purposes of this paper, a sample of male football players who sustained concussions (n = 22) in the Fall 2014 sport season were recruited for neuroimaging assessment as part of a concurrent General Electric-National Football League (GE-NFL) Head Health Challenge study, in addition to completing the larger project's standard clinical post-injury protocol. Four concussed patients were excluded due to severe head motion during the imaging procedure; therefore, only 18 patients were included in this report. Also, the non-injured controls (n = 19) were selected to match injured athletes based on age, gender, sport, estimated premorbid level of academic achievement (see Wechsler Test of Adult Reading [WTAR] measure listed below), and grade point average. Table 1 summarizes the sample characteristics and degree of matching between the groups used in these analyses. All subjects except one reported engaging in a graded program of exertion. The one subject who did not was injured in the last game of the season and therefore did not need to return to play after injury.
Table 1.
Sample Characteristics
| Concussed (n = 18) M (SD) or N (%) | Control (n = 19) M (SD) or N (%) | Between groups p | |
|---|---|---|---|
| Demographics | |||
| College (vs. high school) | 14 (77.8%) | 15 (78.9%) | 0.931 |
| Age (years.) | 17.72 (1.53) | 18.00 (1.76) | 0.613 |
| Race | 0.571 | ||
| White | 14 (77.8%) | 13 (68.4%) | |
| Black | 4 (22.2%) | 5 (26.3%) | |
| Other/unknown | 0 (0.0%) | 1 (5.3%) | |
| Height (inches) | 75.11 (17.54) | 71.37 (2.61) | 0.364 |
| Weight (pounds) | 217.34 (55.45) | 198.32 (36.40) | 0.230 |
| ADHD | 2 (11.1%) | 2 (10.5%) | 0.954 |
| Learning disability | 0 (0.0%) | 2 (10.5%) | 0.157 |
| Number of prior concussions | 1.00 (1.03) | .42 (.77) | 0.060 |
| WTAR standard score | 98.67 (14.87) | 97.42 (15.19) | 0.803 |
| Acute injury characteristics | |||
| LOC | 1 (5.6%) | - | |
| PTA | 1 (5.6%) | - | |
| RGA | 4 (22.2%) | - | |
Sample included male football players only. One concussed athlete did not complete the pre-season baseline measures.
M, mean ; SD, standard deviation; ADHD, attention-deficit hyperactivity disorder; WTAR, Wechsler Test of Adult Reading (estimate of premorbid verbal intellectual ability); LOC, loss of consciousness; PTA, posttraumatic amnesia; RGA, retrograde amnesia.
In this study, as a requirement of the funding mechanism, we used the U.S. Department of Defense's definition for mild traumatic brain injury: “an injury to the brain resulting from an external force and/or acceleration/deceleration mechanism from an event such as a blast, fall, direct impact, or motor vehicle accident which causes an alteration in mental status typically resulting in the temporally related onset of symptoms such as headache, nausea, vomiting, dizziness/balance problems, fatigue, insomnia/sleep disturbances, drowsiness, sensitivity to light/noise, blurred vision, difficulty remembering, and/or difficulty concentrating.”40
Adult athletes and parents of minor athletes completed informed consent, and minor participants completed assent prior to their first evaluation. Participants were compensated $30 for completing pre-season baseline assessments, $50 for each follow-up clinical assessment, and $100 for each MRI session. All testing procedures were approved by the Institutional Review Board at the Medical College of Wisconsin.
Baseline and post-injury clinical assessments
Participants completed a 90-min clinical assessment protocol at pre-season baseline testing and at each follow-up (i.e., post-injury or control) evaluation. Post-injury testing occurred within 24 h of injury (mean [M] = 20.33 h; range, 14–24 h) and at 8 days post-injury (M = 8.39 days; range, 7–11 days). Follow-up assessments for controls were performed as soon as possible after identification and 7 days after the first follow-up (M = 7.21; range, 6–10 days). Baseline clinical assessments were individually proctored by paid research assistants and included a demographics and health history interview, WTAR, Sport Concussion Assessment Tool 3 (SCAT3) symptom checklist,2 Standardized Assessment of Concussion (SAC),41 Balance Error Scoring System (BESS),42 the Automated Neuropsychological Assessment Metrics (ANAM)43,44 and Immediate Post-Concussion and Cognitive Testing (ImPACT) computerized neurocognitive test (CNT) batteries,45 and other self-report and computerized measures. The version of ANAM used in this study was comprised of eight subtests: Simple Reaction Time (SRT), Code Substitution-Learning (CDS), Procedural Reaction Time (PRO), Mathematical Processing (MTH), Matching to Sample (M2S), Code Substitution-Delayed (CDD), Simple Reaction Time 2 (SR2), and Go/No-Go (GNG). The test produces nine indices—one per test and a composite score. ImPACT produces four neurocognitive composite scores: Verbal Memory (VERM), Visual Memory (VISM), Visual Motor Speed (VMS), and Reaction Time (RT). Follow-up assessments were similar to baseline assessments but obtained injury/recovery (rather than health history) information and did not repeat the WTAR.
MRI acquisition
Participants were scanned on a GE Discovery MR750 whole–body 3-Tesla MRI scanner with a receive-only 32-channel coil array head coil () at the Center for Imaging Research, Medical College of Wisconsin. CBF measurements were performed using the 3D pCASL sequence with Fast Spin Echo spiral multi-slice readout using the following parameters: repetition time (TR) = 4632 msec; echo time (TE) = 10.54 msec; field of view (FOV) = 240 mm; matrix = 128 × 128; spiral readout of eight arms and 512 samples; number of excitations = 3; post label delay = 1.52 sec; label duration = 1.45 sec; in-plane resolution = 1.875 × 1.875 mm; slice thickness = 4 mm, total 36 slices; and sampling bandwidth = 62.5 Hz. To minimize blurring, the spiral acquisition was short (4 msec), and the required resolution was achieved with eight spiral interleaves. Three pairs of tagged–untagged images were collected. Background suppression pulse was applied to maximize the sensitivity to blood perfusion,46 scan time = 4:29 min. In addition, T1-weighted 3D fast spoiled gradient echo (FSPGR) sequence with sensitivity encoding was acquired for anatomical reference with the following parameters: FOV = 256 mm; 180 axial slices; slice thickness = 1 mm; voxel size 1 × 1 × 1 mm3; image matrix = 256 × 256; TR/TE = 8.124/3.164 msec; acceleration factor R = 2 in the phase encoding direction; flip angle = 12°; sampling bandwidth = 31.25 kHz; scan time = 3:41 min.
Imaging processing
Individual T1 FSPGR and ASL image quality was checked for potential distortion and artifact. Three-dimensional pCASL data analysis was conducted with MATLAB software programs developed as described previously.47 Images were corrected for motion, pair-wise subtracted between label and control images followed by averaging to generate the mean difference image. CBF quantification (in mL/100 g/min) was performed for the 3D pCASL based on a single compartment model using the equation:
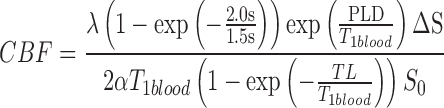 |
where λ is the brain-to-blood partition coefficient (0.9 mL/g), PLD is the post label delay, T1blood is the T1 of arterial blood at 3T (1.6 sec),48 α is the labeling efficiency (0.85),49 TL is the labeling duration (1.5 sec), ΔS is the ASL difference signal, and S0 is the proton-density signal intensity. The term (1-exp[-2.0sec/1.5sec]) in the numerator reflects the presence of a saturation pulse that is applied in the proton-density images and allows conversion between measured MR signal (S0) and the unperturbed longitudinal gray matter magnetization. The quantified CBF maps were co-registered with anatomical FSPGR, which was segmented into gray matter (GM), white matter (WM), and cerebrospinal fluid (CSF) compartments using SPM8 (www.fil.ion.ucl.ac.uk/spm). Co-registered MRI maps were further normalized using the canonical Montréal Neurological Institute (MNI) template, resampled to 2 mm3 voxels, and smoothed with a full width at half maximum (FWHM) kernel of 6 mm.
Statistical analysis
For voxel-wise group comparison on individual CBF maps, the Analysis of Functional NeuroImages (AFNI; www.afni.nimh.nih.gov) mixed-effects multilevel analysis tool was applied to perform a two-way analysis of variance (ANOVA) with repeated measures that incorporates both the variability across subjects and the precision estimate of each effect of interest from individual subject analyses.50 Since age has an established effect on CBF,35,51 we included age as covariate to control for potential confounds, although no group difference was found in our sample. Since there was a trend for the concussed group to report more prior concussions, compared with the control group (p = 0.06; Table 1), we conducted group analyses twice, with and without number of prior concussions as an additional covariate. For all the CBF analyses described above, the height threshold was set at p < 0.05 and the spatial extent threshold was set at cluster volume >1072 μL, which corresponds to p < 0.05 corrected for family-wise error of multiple comparisons according to Monte Carlo simulations (10,000 iterations) implemented in AFNI's 3dClustSim.52,53 In additional analyses, results also were evaluated using a more conservative threshold of corrected p < 0.01, as determined using 3dCluStim by setting the height threshold at p < 0.01 with the minimal cluster size of 448 μL.
Statistical Package for Social Sciences (SPSS, version 21.0; ) was used to assess differences in demographic and clinical measures between groups. For continuous variables, ANOVAs were employed with correction for multiple comparisons, while χ2 tests were used to examine group differences in categorical variables.
Results
Participant characteristics
All participants in this study were male. Concussed and control groups did not differ significantly with respect to age, years of education, height, or weight (Table 1). In addition, no significant difference was found in pre-existing conditions, including diagnosis of attention-deficit hyperactivity disorder, learning disability, or WTAR standard score. However, in light of the trend showing a difference in number of prior concussions between groups (p = 0.06), this variable was tested as a potential confounding covariate in group analyses as stated above. In the concussed group, one participant (5.6%) had any loss of consciousness associated with injury, one (5.6%) reported post-traumatic amnesia, and four (22.2%) reported retrograde amnesia.
Clinical measures
Between-group comparisons
Concussed subjects were equivalent in ratings on the SCAT3 symptom checklist and performance on the SAC and BESS at baseline (Table 2). Within 24 h of injury, concussed subjects produced higher symptom ratings (p < 0.0001) and performed more poorly than controls on the SAC (p = 0.029), with these impairments resolving by day 8 (p = 0.398 for group comparison at Day 8). The majority of the sample also completed the ANAM and ImPACT CNTs (concussed/control group, ANAM n = 16/18; ImPACT n = 11/18; Table 3). Across the CNTs' 13 indices, two demonstrated significantly different performance between groups at baseline (ANAM PRO throughput, p = 0.023; ImPACT VERM, p = 0.002). Consequently, ANOVA models evaluating group differences for these subtests at later time-points included baseline performance as a covariate. At 24 h, six of 13 indices demonstrated significant impairment for concussed versus control subjects (ANAM Composite p = 0.013, CDS p = 0.037, M2S p = 0.025, CDD p = 0.003; ImPACT VISM p = 0.011, VMS p = 0.020; all other p values >0.06). At the Day 8 assessment, concussed athletes were impaired (vs. controls) on only one of the 13 indices (ANAM CDS p = 0.043; Table 3).
Table 2.
Group Comparisons in Core Clinical Measures
| Concussed (n = 18) M (SD) | Control (n = 19) M (SD) | Between groups p | |
|---|---|---|---|
| SCAT3 symptom severity | |||
| Baseline | 4.82 (4.59) | 4.53 (5.38) | 0.860 |
| 24 h | 28.22 (18.98)a | 3.16 (3.11) | < 0.001 |
| 8 days | 6.06 (8.91)b | 2.53 (3.85) | 0.203 |
| SAC total score | |||
| Baseline | 26.82 (2.19) | 27.16 (1.50) | 0.593 |
| 24 h | 24.61 (2.64)a | 26.26 (1.70) | 0.029 |
| 8 days | 27.00 (2.70)b | 27.63 (1.71) | 0.398 |
| BESS total score | |||
| Baseline | 14.53 (4.64) | 12.84 (4.59) | 0.281 |
| 24 h | 13.31 (3.81) | 12.05 (4.16) | 0.360 |
| 8 days | 12.22 (4.28) | 10.47 (4.74) | 0.248 |
significantly worse at 24 h than the baseline.
significantly improved at 8 days from 24 h. No measure showed a difference between baseline and 8 days in either group.
M, mean; SD, standard deviation; SCAT3, Sport Concussion Assessment Tool 3; SAC, Standardized Assessment of Concussion; BESS, Balance Error Scoring System.
Table 3.
Group Comparisons on Computerized Neurocognitive Tests (CNTs)
| Concussed M (SD) | Control M (SD) | Between groups p | |
|---|---|---|---|
| ANAM | |||
| Composite BL | −0.16 (1.07) | 0.56 (0.96) | 0.059 |
| Composite 24 h | −0.76 (1.56) | 0.38 (0.64) | 0.013 |
| Composite 8 days | 0.27 (1.29)b | 0.47 (0.74) | 0.592 |
| SRT BL | 231 (25.72) | 240.78 (24.93) | 0.296 |
| SRT 24 h | 211.19 (53.59) | 223.11 (25.29) | 0.404 |
| SRT 8 days | 231.69 (32.43) | 227.44 (27.36) | 0.682 |
| CDS BL | 58.08 (11.09) | 59.72 (13.15) | 0.717 |
| CDS 24 h | 53.63 (10.29) | 61.06 (9.56) | 0.037 |
| CDS 8 days | 58.31 (11.32) | 65.11 (7.31)b | 0.043 |
| PRO BL | 94.69 (15.35) | 106.44 (8.40) | 0.023 |
| PRO 24 h | 92.25 (23.26) | 108.33 (8.95) | 0.061* |
| PRO 8 days | 99.25 (18.57) | 104.17 (10.31) | 0.340* |
| MTH BL | 19.08 (4.42) | 22.56 (5.75) | 0.079 |
| MTH 24 h | 20.88 (6.13) | 23.67 (6.67) | 0.215 |
| MTH 8 days | 25.13 (7.79)b | 26.11 (7.60) | 0.711 |
| M2S BL | 38.08 (10.80) | 44.61 (14.71) | 0.185 |
| M2S 24 h | 31.25 (13.76) | 42.22 (13.42) | 0.025 |
| M2S 8 days | 39.88 (12.53)b | 42.17 (11.60) | 0.584 |
| CDD BL | 56.23 (10.33) | 56.78 (12.45) | 0.898 |
| CDD 24 h | 48.19 (13.92)a | 62.61 (12.70) | 0.003 |
| CDD 8 days | 52.19 (12.59) | 57.39 (11.93) | 0.225 |
| SR2 BL | 217.69 (50.64) | 238.11 (29.03) | 0.166 |
| SR2 24 h | 203.25 (46.26) | 219.67 (31.70) | 0.232 |
| SR2 8 days | 238.06 (35.33)b | 222.33 (26.24) | 0.147 |
| AN GNG BL | 3.54 (1.51) | 3.44 (1.42) | 0.861 |
| AN GNG 24 h | 3.69 (1.58) | 3.78 (1.26) | 0.854 |
| AN GNG 8 days | 4.19 (1.72) | 4.89 (1.41)b | 0.201 |
| ImPACT | |||
| VERM BL | 77.4 (9.06) | 89.78 (8.80) | 0.002 |
| VERM 24 h | 75 (20.70) | 91.78 (9.26) | 0.233* |
| VERM 8 days | 85.2 (11.48) | 90.72 (7.96) | 0.145* |
| VISM BL | 74.5 (10.99) | 82.39 (10.64) | 0.074 |
| VISM 24 h | 64.18 (17.57)a | 80.89 (7.00) | 0.011 |
| VISM 8 days | 72.5 (14.39) | 77.61 (9.90) | 0.276 |
| VMS BL | 37.49 (8.01) | 40.18 (5.69) | 0.309 |
| VMS 24 h | 35.16 (7.05) | 41.28 (6.09) | 0.020 |
| VMS 8 days | 38.18 (8.65) | 41.27 (5.04) | 0.319 |
| RT BL | 0.58 (.07) | 0.59 (0.06) | 0.885 |
| RT 24 h | 0.64 (.17) | 0.56 (0.05) | 0.149 |
| RT 8 days | 0.55 (.07) | 0.55 (0.06) | 0.968 |
models adjusted for baseline performances between groups on this scale. Concussed/control ANAM n = 16/18; ImPACT n = 11/18.
significantly worse at 24 h than the baseline.
significantly improved at 8 days from 24 h. Higher scores reflect better performance for all ANAM and ImPACT tests with the exception of ImPACT RT, for which lower reaction time reflects better performance.
M, mean; SD, standard deviation; ANAM, Automated Neuropsychological Assessment Metrics; BL, baseline; SRT, Simple Reaction Time; CDS, Code Substitution-Learning; PRO, Procedural Reaction Time; MTH, Mathematical Processing; M2S, Matching to Sample; CDD, Code Substitution Delayed; SR2, Simple Reaction Time 2; GNG, Go/No-Go (all ANAM analyses used throughput scores with the exception of Go/No-Go, for which d-prime scores were used); ImPACT, Immediate Post-Concussion and Cognitive Testing; VERM, Verbal Memory; VISM, Visual Memory; VMS, Visual Motor Speed; RT, Reaction Time.
Within-group longitudinal change
For the SCAT3 symptom checklist, SAC, and BESS, there were no significant changes between any two time-points in the control group. For the concussion group, scores on the clinical symptom (SCAT3 symptom severity) and cognitive (SAC total score) measures demonstrated significant impairment (versus pre-season baseline levels) at 24 h (SCAT3 p < 0.0001; SAC p < 0.01) but returned to baseline levels at 8 days (Table 2). Among the 17 concussed subjects with baseline symptom data, 10 (59%) provided SCAT3 symptom severity scores equal to or lower than their baseline levels at Day 8, while the remaining seven reported higher (4–33 points) than baseline symptom scores at this time-point. The median length of graded exertion protocol did not differ between the groups whose symptoms were at baseline levels versus not at baseline levels at Day 8 (Mann-Whitney U test, p = 0.345).
At 24 h, concussed athletes demonstrated worse performance (vs. baseline levels) on two of 13 CNT indices (ANAM CDD p = 0.032 and ImPACT VISM p = 0.020; other p values >0.08). In comparison, the control group demonstrated significant improvement from baseline levels on two of 13 CNT scores at 24 h (with no significant declines from baseline). On Day 8, both the concussed and control groups performed at or better than baseline levels on all CNT indices, with both groups demonstrating significant improvements from baseline on four of 13 indices (Table 3).
CBF changes
Between-group comparisons
At 24 h, the concussion group showed significantly lower CBF, predominantly in the right supplementary motor area (SMA) and pre-SMA regions, relative to the control group (p < 0.05 corrected; Fig. 1). At 8 days, concussed subjects demonstrated significantly lower CBF diffusely across cortical gray matter, mainly in bilateral prefrontal regions, temporal lobes, some parietal regions, as well as the thalamus, compared with the control group (p < 0.05 corrected; Fig. 2). No region showed significantly more CBF in the patient group related to the control group at any time-point.
FIG. 1.
Regions (in blue color) show significantly less cerebral blood flow (CBF) in concussion group at 24 h after injury, compared with the control group. No region shows significantly more CBF in the concussion group compared to the control group. Images reflect family-wise error correction at p < 0.05. Color bar indicates the t score. Color image is available online at www.liebertpub.com/neu
FIG. 2.
Diffuse cortical and subcortical regions (in blue color) show significantly less cerebral blood flow (CBF) in concussion group at 8 days after injury, compared with the control group. No region shows significant more CBF in the concussion group compared to the control group. Images reflect family-wise error correction at p < 0.05. Color bar indicates the t scores. Color image is available online at www.liebertpub.com/neu
Within-group longitudinal change
Spread cortical area in frontal and temporal lobes demonstrated significantly decreased CBF at 8 days, compared with 24 h after concussion in the patient group (p < 0.05 corrected; Fig. 3), while the control group did not show any changes in CBF between the two time-points. Significant group-by-time interaction of CBF changes was found mainly in bilateral frontal and temporal area (p < 0.05 corrected; Fig. 4).
FIG. 3.
Spread cortical and subcortical regions (in blue color) show significantly decreased cerebral blood flow (CBF) in concussion group at 8 days compared to 24 h after injury. No region shows significantly increased CBF in the concussion group at 8 days. Images reflect family-wise error correction at p < 0.05. Color bar indicates the t scores. Color image is available online at www.liebertpub.com/neu
FIG. 4.
Significant group-by-time interaction of cerebral blood flow changes is found mainly in bilateral frontal and temporal regions (in hot color). Images reflect family-wise error correction at p < 0.05. Color bar indicates the t scores. Color image is available online at www.liebertpub.com/neu
In additional analysis, we repeated the above voxel-wise CBF group comparisons with and without the number of prior concussions as the covariate but found no significant effect of number of prior concussion on the final CBF results in this sample. Further, we also evaluated the results by applying a more conservative threshold of corrected p < 0.01. Although the spatial size of significant clusters was attenuated, decreased CBF at 8 days within the concussion group and less CBF in the concussion group relative to controls at the same time-point in the original analysis remained significant, illustrating decreased CBF in spread regions of frontal and temporal lobes in concussed patients at 8 days after injury (Table 4).
Table 4.
Clusters Showing Decreased CBF in Concussed Subjects at 8 days, Compared with 24 H after Injury (p < 0.01, Corrected)
| Center | Peak | |||||
|---|---|---|---|---|---|---|
| Location | X | Y | Z | X | Y | Z |
| Left SFG | −23.8 | 42.8 | 23.3 | −22 | 40 | 31 |
| Left IFG | −46.0 | 40.2 | −11.2 | −51 | 36 | −13 |
| Left orbital frontal gyrus | −13.4 | 17.5 | −22.3 | −12 | 15 | −23 |
| Left IFG | −44.9 | 2.1 | 30.3 | −47 | 3 | 35 |
| Left STG (extended to MTG/ITG) | −54.3 | −26.7 | 2.3 | −63 | −36 | 9 |
| Left MTG | −42.9 | −77.1 | 24.8 | −49 | −78 | 13 |
| Right SFG | 19.4 | 59.4 | −16.3 | 20 | 65 | −13 |
| Right MFG (extended to SFG) | 27.8 | 38.2 | 22.1 | 27 | 46 | 15 |
| Right MFG | 20.6 | 7.9 | 51.7 | 22 | 6 | 53 |
| Right insula | 45.1 | −2.4 | −6.5 | 41 | −18 | −5 |
| Right MTG | 63.9 | −35.3 | 12.3 | 68 | −33 | 3 |
| Right lingual gyrus | 12.4 | −64.0 | 0.0 | 10 | −63 | −1 |
| Cingulate cortex | −3.6 | 12.3 | 27.6 | −2 | −22 | 31 |
| Thalamus | 0.7 | −19.1 | −1.8 | 5 | −17 | 3 |
| Left cerebellum | −20.0 | −71.7 | −40.4 | −16 | −78 | −43 |
| Right cerebellum | 11.3 | −56.5 | 30.7 | 16 | −57 | −37 |
SFG, superior frontal gyrus; IFG, inferior frontal gyrus; STG, superior temporal gyrus; MTG, middle temporal gyrus; MFG, middle frontal gyrus.
Discussion
In this study, concussed football players demonstrated abnormally reduced CBF 24 h after sustaining SRC, and further decreased CBF at 8 days post-injury. Findings were evident in both within (compared with preseason baseline) and between (compared with matched controls) subjects analyses. Notably, significant clinical symptoms and impaired neurocognitive performance were observed at 24 h post-injury relative to the baseline, while at a group level both clinical measures returned to baseline levels at 8 days. Further, neurocognitive recovery at 8 days was observed for both a paper-and-pencil screening measure (SAC) and two computerized measures (ANAM and ImPACT).
These findings are considered to have important clinical implications in the context of understanding the natural time course of clinical and physiological recovery after SRC and mTBI. Current guidelines for management of SRC call for a step-wise, symptom-limited program of exertion that is initiated after resolution of clinical symptoms.2,10 However, as symptoms are self-reported, underlying physiological effects of SRC still may be present when these subjective markers are reported to have resolved.22,54 Even after symptom resolution, neurons under a state of physiologic stress function abnormally and may remain susceptible to second injury.55,56 Supporting this notion, our recent studies indicate that abnormalities on advanced electrophysiological testing and working memory functional MRI are detectable in athletes who otherwise report a complete symptom recovery and perform normally on clinical measures (i.e., cognitive and balance testing) after SRC.7,57,58
These findings fuel the critical concern that a window of cerebral vulnerability (WoCV) extends beyond the point of clinical recovery after SRC, when the brain remains physiologically compromised and at heightened risk of repetitive injury. However, no measurable biological indicators of this WoCV are available for clinical use, and existing data are insufficient to determine the likely time course or the end point of physiological abnormalities after SRC. Our results are in line with the notion that underlying neurophysiological changes persist even after clinical recovery from SRC, which supports the hypothesis of the WoCV. In particular, CBF alterations might present a possible mechanism underlying the WoCV during recovery after SRC.
Recent research has suggested that SRC may produce a pathophysiologic process resulting in altered CBF values with a variable and possibly protracted time frame for resolution.39,55,59 CBF perturbations have been identified in experimental models as the cause of a “metabolic mismatch” between supply and demand for oxygen and glucose.60 Neurons under such a state of physiologic stress function abnormally and become more susceptible to secondary injury.55 If a concussed athlete returns too quickly to strenuous physical activity or experiences a second SRC, symptoms and neuropsychological testing deficits frequently worsen.2,6,55,61 Therefore, our data enrich the theory that a protracted state of physiologic abnormality exists for some concussed athletes, even when clinical assessments appear normal. The current findings have significant implications for the management of concussion. Return-to-play decisions are currently based on clinical judgment that is informed by neurocognitive batteries and self-report symptom metrics rather than on evidence-based biomarkers.10,22,39,62 Our study suggests a potential objective marker of physiological recovery indicating that the WoCV has elapsed and the individual is safe to resume activity.10
Yet to be fully elucidated, there are several possible pathophysiologic mechanisms of decreased CBF after mTBI.11,22 Following TBI, primary injury at the moment of impact damages brain tissue by disrupting blood vessels; this event facilitates secondary injury cascades affecting the neurovascular unit (NVU) physiology.11 The NVU is a physiological entity that is structurally defined by interactions occurring between endothelial cells, pericytes, smooth muscle cells, astrocytes, and neurons.11,63 The post-traumatic changes in the NVU are mostly observed during the first week after injury, although the evolution of these changes in the NVU over a long period of time is still unknown.11 Moreover, cerebral autoregulation, the intrinsic ability of the brain to maintain a constant CBF in response to variations in systemic blood pressure, has been found to be lost or impaired following mTBI for up to 14 days.22,64–66 In addition, research has illustrated that the autonomic and cardiovascular systems become uncoupled following acute brain injury67; it has also been suggested that deficits in neuroautonomic control after brain injury are correlated with abnormal cerebrovascular responses.68 CBF changes after TBI also may be related to changes in basic properties of the cerebral vasculature, and decreased CBF early after injury is a common signature of TBI.11
Our findings of scattered perfusion deficit (i.e., decreased CBF) in acute SRC are in accord with our previous study showing significantly lower CBF in bilateral dorsal prefrontal and temporal lobe regions in asymptomatic pediatric SRC subjects at 7 months post injury.59 Our data also are consistent with another magnetic resonance perfusion study of mTBI.69 Further, it has been shown the most reliable patterns of parenchymal changes after TBI were observed in the frontal, temporal, and cingulate regions, although effects were observed to varying degrees in nearly every brain region.70 Most recently, Meier and colleagues39 found longitudinal (1 day and 1 week vs. 1 month post-injury) CBF recovery only in the right insular and superior temporal cortex in a sample of collegiate athletes. The reasons for discrepancy of results might be due to different sample characteristics and/or the use of different methodology in assessments. Another recent study reported increased CBF in acute mTBI relative to controls, but with a very limited sample size (seven patients) and no longitudinal within-subject comparisons.71 Nevertheless, animal research has demonstrated that diffuse and mild injuries could result in very low reductions or even increases in blood flow, at least at initial time-points following TBI,11,72–74 which might provide an explanation of our observing relatively small difference in CBF between SRC at 24 h post-injury and controls. It should be noted that the concussed sample of this study represents the mildest end of the continuum of TBI. This might also give context to the fact that we observed CBF deficit within 24 h, despite the mild injuries, and provides a call for additional study of more severe mTBI to look for a CBF dose response based on injury severity.
Relevant limitations of this study require acknowledgment. A modest sample size and confined follow-up period are the primary limitations of this preliminary investigation. It would be important to follow up with existing subjects to detect a complete recovery course of the underlying perfusion deficit after SRC. In this study, we only recruited male athletes and were therefore unable to examine gender differences. As age may play a role in the moderation of CBF after mTBI,22 further research is warranted to evaluate the effect of age on duration of the WoCV after SRC. Further, although the sample demonstrated clinical recovery at 8 days post-injury at a group level, there were seven of 18 patients who were not completely symptom-free at the time-point. Limited sample size did not allow for the separate analyses of CBF based on clinical recovery status at an individual level. While group comparisons showed a similar course of changes in clinical symptoms and neurocognitive performance, we do not have enough evidence to draw conclusions about which clinical measure might be more or less sensitive to detect changes at 8 days post-injury. Future work accumulating larger samples and athletes of more varied in demographic characteristics will allow for more complete elucidation of the interplay between clinical symptoms and physiological recovery for a wide range of athletes. A multi-modal approach to combine 3D pCASL with other imaging methods, such as functional and diffusion MRI, would be useful to characterize the physiological effects of concussion. Future studies may demonstrate that such advanced imaging modalities could potentially direct rehabilitation after concussion and possibly aid in determining when it is safe for an athlete to return to play, although there is no evidence at the present time to support such conclusions.15
Acknowledgments
This work was supported by the GE-NFL Head Health Challenge I and the U.S. Army Medical Research and Materiel Command under award number W81XWH-12-1-0004. Opinions, interpretations, conclusions, and recommendations are those of the authors and are not necessarily endorsed by the NFL, GE, or the U.S. Army. This publication was also supported by the Clinical and Translational Science Institute grant 1UL1-RR031973 (-01) and by the National Center for Advancing Translational Sciences, National Institutes of Health grant 8UL1TR000055. The contents of this publication are the sole responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.(2007). Nonfatal traumatic brain injuries from sports and recreation activities–United States, 2001–2005. MMWR Morbid. Mortal. Wkly. Rep. 56, 733–737 [PubMed] [Google Scholar]
- 2.McCrory P., Meeuwisse W.H., Aubry M., Cantu B., Dvořák J., Echemendia R.J., Engebretsen L., Johnston K., Kutcher J.S., Raftery M., Sills A., Benson B.W., Davis G.A., Ellenbogen R.G., Guskiewicz K., Herring S.A., Iverson G.L., Jordan B.D., Kissick J., McCrea M., McIntosh A.S., Maddocks D., Makdissi M., Purcell L., Putukian M., Schneider K., Tator C.H., and Turner M. (2013). Consensus statement on concussion in sport: the 4th International Conference on Concussion in Sport held in Zurich, November 2012. Br. J. Sports Med. 47, 250–258 [DOI] [PubMed] [Google Scholar]
- 3.Jordan B.D. (2013). The clinical spectrum of sport-related traumatic brain injury. Nat. Rev. Neurol. 9, 222–230 [DOI] [PubMed] [Google Scholar]
- 4.Tan C.O., Meehan W.P., Iverson G.L., and Taylor J.A. (2014). Cerebrovascular regulation, exercise, and mild traumatic brain injury. Neurology 83, 1665–1672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McCrea M., Guskiewicz K., Randolph C., Barr W.B., Hammeke T.A., Marshall S.W., Powell M.R., Woo Ahn K., Wang Y., and Kelly J.P. (2013). Incidence, clinical course, and predictors of prolonged recovery time following sport-related concussion in high school and college athletes. J. Int. Neuropsychol. 19, 22–33 [DOI] [PubMed] [Google Scholar]
- 6.McCrea M., Guskiewicz K.M., Marshall S.W., Barr W., Randolph C., Cantu R.C., Onate J.A., Yang J., and Kelly J.P. (2003). Acute effects and recovery time following concussion in collegiate football players: the NCAA Concussion Study. JAMA 290, 2556–2563 [DOI] [PubMed] [Google Scholar]
- 7.McCrea M., Prichep L., Powell M.R., Chabot R., and Barr W.B. (2010). Acute effects and recovery after sport-related concussion: a neurocognitive and quantitative brain electrical activity study. J. Head Trauma Rehabil. 25, 283–292 [DOI] [PubMed] [Google Scholar]
- 8.Slobounov S., Gay M., Johnson B., and Zhang K. (2012). Concussion in athletics: ongoing clinical and brain imaging research controversies. Brain Imaging Behav. 6, 224–243 [DOI] [PubMed] [Google Scholar]
- 9.Manley G.T. and Maas A.R. (2013). Traumatic brain injury: an international knowledge-based approach. JAMA 310, 473–474 [DOI] [PubMed] [Google Scholar]
- 10.McCrea M. and Guskiewicz K. (2014). Evidence-based management of sport-related concussion. Prog. Neurology. Surg. 28, 112–127 [DOI] [PubMed] [Google Scholar]
- 11.Pop V. and Badaut J. (2011). A neurovascular perspective for long-term changes after brain trauma. Transl. Stroke Res. 2, 533–545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.King D., Brughelli M., Hume P., and Gissane C. (2014). Assessment, management and knowledge of sport-related concussion: systematic review. Sports Med. 44, 449–471 [DOI] [PubMed] [Google Scholar]
- 13.Guskiewicz K.M. and Valovich McLeod T.C. (2011). Pediatric sports-related concussion. PM R 3, 353–364 [DOI] [PubMed] [Google Scholar]
- 14.Giza C.C. and Hovda D.A. (2014). The new neurometabolic cascade of concussion. Neurosurgery 75 Suppl 4, S24–S33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dashnaw M.L., Petraglia A.L., and Bailes J.E. (2012). An overview of the basic science of concussion and subconcussion: where we are and where we are going. Neurosurg. Focus 33, 1–9 [DOI] [PubMed] [Google Scholar]
- 16.Choe M.C., Babikian T., DiFiori J., Hovda D.A., and Giza C.C. (2012). A pediatric perspective on concussion pathophysiology. Curr. Opin. Pediatr. 24, 689–695 [DOI] [PubMed] [Google Scholar]
- 17.Schrader H., Mickeviciene D., Gleizniene R., Jakstiene S., Surkiene D., Stovner L.J., and Obelieniene D. (2009). Magnetic resonance imaging after most common form of concussion. BMC Med. Imaging 9, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Prabhu S.P. (2011). The role of neuroimaging in sport-related concussion. Clin. Sports Med. 30, 103–114 [DOI] [PubMed] [Google Scholar]
- 19.Cubon V.A., Putukian M., Boyer C., and Dettwiler A. (2011). A diffusion tensor imaging study on the white matter skeleton in individuals with sports-related concussion. J. Neurotrauma 28, 189–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lange R.T., Iverson G.L., Brubacher J.R., Madler B., and Heran M.K. (2012). Diffusion tensor imaging findings are not strongly associated with postconcussional disorder 2 months following mild traumatic brain injury. J. Head Trauma Rehabil. 27, 188–198 [DOI] [PubMed] [Google Scholar]
- 21.Niogi S.N., Mukherjee P., Ghajar J., Johnson C., Kolster R.A., Sarkar R., Lee H., Meeker M., Zimmerman R.D., Manley G.T., and McCandliss B.D. (2008). Extent of microstructural white matter injury in postconcussive syndrome correlates with impaired cognitive reaction time: a 3T diffusion tensor imaging study of mild traumatic brain injury. A.J.N.R. Am. J. Neuroradiol. 29, 967–973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Len T.K. and Neary J.P. (2011). Cerebrovascular pathophysiology following mild traumatic brain injury. Clin. Physiol. Funct. Imaging 31, 85–93 [DOI] [PubMed] [Google Scholar]
- 23.Dijkhuizen R.M. (2011). Advances in MRI-Based detection of cerebrovascular changes after experimental traumatic brain injury. Transl. Stroke Res. 2, 524–532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gardner A.J., Tan C.O., Ainslie P.N., van Donkelaar P., Stanwell P., Levi C.R., and Iverson G.L. (2014). Cerebrovascular reactivity assessed by transcranial Doppler ultrasound in sport-related concussion: a systematic review. Br. J. Sports Med. 49, 1050–1055 [DOI] [PubMed] [Google Scholar]
- 25.Pasco A., Lemaire L., Franconi F., Lefur Y., Noury F., Saint-Andre J.P., Benoit J.P., Cozzone P.J., and Le Jeune J.J. (2007). Perfusional deficit and the dynamics of cerebral edemas in experimental traumatic brain injury using perfusion and diffusion-weighted magnetic resonance imaging. J. Neurotrauma 24, 1321–1330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McGoron A.J., Capille M., Georgiou M.F., Sanchez P., Solano J., Gonzalez-Brito M., and Kuluz J.W. (2008). Post traumatic brain perfusion SPECT analysis using reconstructed ROI maps of radioactive microsphere derived cerebral blood flow and statistical parametric mapping. BMC Med. Imaging 8, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ginsberg M.D., Zhao W., Alonso O.F., Loor-Estades J.Y., Dietrich W.D., and Busto R. (1997). Uncoupling of local cerebral glucose metabolism and blood flow after acute fluid-percussion injury in rats. Am J Physiol. 272(6 Pt 2), H2859–H2868 [DOI] [PubMed] [Google Scholar]
- 28.Muir J.K., Boerschel M., and Ellis E.F. (1992). Continuous monitoring of posttraumatic cerebral blood flow using laser-Doppler flowmetry. J. Neurotrauma 9, 355–362 [DOI] [PubMed] [Google Scholar]
- 29.Yamakami I. and McIntosh T.K. (1989). Effects of traumatic brain injury on regional cerebral blood flow in rats as measured with radiolabeled microspheres. J. Cereb. Blood Flow Metab. 9, 117–124 [DOI] [PubMed] [Google Scholar]
- 30.Audenaert K., Jansen H.M., Otte A., Peremans K., Vervaet M., Crombez R., de Ridder L., van Heeringen C., Thirot J., Dierckx R., and Korf J. (2003). Imaging of mild traumatic brain injury using 57Co and 99mTc HMPAO SPECT as compared to other diagnostic procedures. Med. Sci. Monit. 9, MT112–117 [PubMed] [Google Scholar]
- 31.Gowda N.K., Agrawal D., Bal C., Chandrashekar N., Tripati M., Bandopadhyaya G.P., Malhotra A., and Mahapatra A.K. (2006). Technetium Tc-99m ethyl cysteinate dimer brain single-photon emission CT in mild traumatic brain injury: a prospective study. A.J.N.R. Am. J. Neuroradiol. 27, 447–451 [PMC free article] [PubMed] [Google Scholar]
- 32.Metting Z., Spikman J.M., Rödiger L.A., and van der Naalt J. (2014). Cerebral perfusion and neuropsychological follow up in mild traumatic brain injury: acute versus chronic disturbances? Brain Cogn. 86, 24–31 [DOI] [PubMed] [Google Scholar]
- 33.Detre J.A., Wang J., Wang Z., and Rao H. (2009). Arterial spin-labeled perfusion MRI in basic and clinical neuroscience. Curr. Opin. Neurol. 22, 348–355 [DOI] [PubMed] [Google Scholar]
- 34.Wang J., Aguirre G.K., Kimberg D.Y., Roc A.C., Li L., and Detre J.A. (2003). Arterial spin labeling perfusion fMRI with very low task frequency. Magn. Reson. Med. 49, 796–802 [DOI] [PubMed] [Google Scholar]
- 35.Wang Y., Saykin A.J., Pfeuffer J., Lin C., Mosier K.M., Shen L., Kim S., and Hutchins G.D. (2011). Regional reproducibility of pulsed arterial spin labeling perfusion imaging at 3T. NeuroImage 54, 1188–1195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen Y., Wang D.J., and Detre J.A. (2011). Test-retest reliability of arterial spin labeling with common labeling strategies. J. Magn. Reson. Imaging 33, 940–949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vidorreta M., Wang Z., Rodriguez I., Pastor M.A., Detre J.A., and Fernandez-Seara M.A. (2012). Comparison of 2D and 3D single-shot ASL perfusion fMRI sequences. NeuroImage 66C, 662–671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu W.C., Fernandez-Seara M., Detre J.A., Wehrli F.W., and Wang J. (2007). A theoretical and experimental investigation of the tagging efficiency of pseudocontinuous arterial spin labeling. Magn. Reson. Med. 58, 1020–1027 [DOI] [PubMed] [Google Scholar]
- 39.Meier T.B., Bellgowan P.S., Singh R., Kuplicki R., Polanski D.W., and Mayer A.R. (2015). Recovery of cerebral blood flow following sports-related concussion. JAMA Neurol. 72, 530–538 [DOI] [PubMed] [Google Scholar]
- 40.Helmick K., Guskiewicz K., Barth J., Cantu R., Kelly J.P., McDonald E., Flaherty S., Bazarian J., Bleigberg J., Carter T., Cooper J., and Warden D. (2006). Defense and Veterans Brain Injury Center Working Group on the Acute Management of Mild Traumatic Brain Injury in Military Operational Settings. Defense and Veterans Brain Injury Center: Washington, DC [Google Scholar]
- 41.McCrea M., Kelly J.P., Kluge J., Ackley B., and Randolph C. (1997). Standardized assessment of concussion in football players. Neurology 48, 586–588 [DOI] [PubMed] [Google Scholar]
- 42.Riemann B.L. and Guskiewicz K.M. (2000). Effects of mild head injury on postural stability as measured through clinical balance testing. J. Athl. Train. 35, 19–25 [PMC free article] [PubMed] [Google Scholar]
- 43.Bleiberg J., Kane R.L., Reeves D.L., Garmoe W.S., and Halpern E. (2000). Factor analysis of computerized and traditional tests used in mild brain injury research. Clin. Neuropsychol. 14, 287–294 [DOI] [PubMed] [Google Scholar]
- 44.Nelson L.D., Pfaller A.Y., Rein L.E., and McCrea M.A. (2015). Rates and predictors of invalid baseline test performance in high school and collegiate athletes for 3 computerized neurocognitive tests: ANAM, Axon Sports, and ImPACT. Am. J. Sports Med. 43, 2018–2026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Iverson G.L., Brooks B.L., Collins M.W., and Lovell M.R. (2006). Tracking neuropsychological recovery following concussion in sport. Brain Inj. 20, 245–252 [DOI] [PubMed] [Google Scholar]
- 46.Alsop D.C., Detre J.A., Golay X., Günther M., Hendrikse J., Hernandez-Garcia L., Lu H., MacIntosh B.J., Parkes L.M., Smits M., van Osch M.J.P., Wang D.J.J., Wong E.C., and Zaharchuk G. (2015). Recommended implementation of arterial spin-labeled perfusion MRI for clinical applications: a consensus of the ISMRM perfusion study group and the European consortium for ASL in dementia. Magn. Reson. Med. 73, 102–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang D.J., Alger J.R., Qiao J.X., Hao Q., Hou S., Fiaz R., Gunther M., Pope W.B., Saver J.L., Salamon N., and Liebeskind D.S.; UCLA Stroke Investigators. (2012). The value of arterial spin-labeled perfusion imaging in acute ischemic stroke: comparison with dynamic susceptibility contrast-enhanced MRI. Stroke 43, 1018–1024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wu W.C., Jain V., Li C., Giannetta M., Hurt H., Wehrli F.W., and Wang D.J. (2010). In vivo venous blood T1 measurement using inversion recovery true-FISP in children and adults. Magn. Reson. Med. 64, 1140–1147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Aslan S., Xu F., Wang P.L., Uh J., Yezhuvath U.S., van Osch M., and Lu H. (2010). Estimation of labeling efficiency in pseudocontinuous arterial spin labeling. Magn. Reson. Med. 63, 765–771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen G., Saad Z.S., Nath A.R., Beauchamp M.S., and Cox R.W. (2012). FMRI group analysis combining effect estimates and their variances. NeuroImage 60, 747–765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Amin-Hanjani S., Du X., Pandey D.K., Thulborn K.R., and Charbel F.T. (2015). Effect of age and vascular anatomy on blood flow in major cerebral vessels. J. Cereb. Blood Flow Metab. 35, 312–318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chow H.M., Horovitz S.G., Carr W.S., Picchioni D., Coddington N., Fukunaga M., Xu Y., Balkin T.J., Duyn J.H., and Braun A.R. (2013). Rhythmic alternating patterns of brain activity distinguish rapid eye movement sleep from other states of consciousness. Proc. Natl. Acad. Sci. U. S. A. 110, 10300–10305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Okonkwo O.C., Xu G., Oh J.M., Dowling N.M., Carlsson C.M., Gallagher C.L., Birdsill A.C., Palotti M., Wharton W., Hermann B.P., LaRue A., Bendlin B.B., Rowley H.A., Asthana S., Sager M.A., and Johnson S.C. (2014). Cerebral blood flow is diminished in asymptomatic middle-aged adults with maternal history of Alzheimer's disease. Cerebr. Cortex 24, 978–988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gall B., Parkhouse W.S., and Goodman D. (2004). Exercise following a sport induced concussion. Br. J. Sports Med. 38, 773–777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Maugans T.A., Farley C., Altaye M., Leach J., and Cecil K.M. (2012). Pediatric sports-related concussion produces cerebral blood flow alterations. Pediatrics 129, 28–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Len T.K., Neary J.P., Asmundson G.J., Goodman D.G., Bjornson B., and Bhambhani Y.N. (2011). Cerebrovascular reactivity impairment after sport-induced concussion. Med. Sci. Sports Exerc. 43, 2241–2248 [DOI] [PubMed] [Google Scholar]
- 57.Barr W.B., Prichep L.S., Chabot R., Powell M.R., and McCrea M. (2012). Measuring brain electrical activity to track recovery from sport-related concussion. Brain Inj. 26, 58–66 [DOI] [PubMed] [Google Scholar]
- 58.Hammeke T.A., McCrea M., Coats S.M., Verber M.D., Durgerian S., Flora K., Olsen G.S., Leo P.D., Gennarelli T.A., and Rao S.M. (2013). Acute and subacute changes in neural activation during the recovery from sport-related concussion. J. Int. Neuropsychol. Soc. 19, 863–872 [DOI] [PubMed] [Google Scholar]
- 59.Wang Y., West J.D., Bailey J.N., Westfall D.R., Xiao H., Arnold T.W., Kersey P.A., Saykin A.J., and McDonald B.C. (2015). Decreased cerebral blood flow in chronic pediatric mild TBI: an MRI perfusion study. Dev. Neuropsychol. 40, 40–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Giza C.C. and Hovda D.A. (2001). The neurometabolic cascade of concussion. J. Athl. Training 36, 228–235 [PMC free article] [PubMed] [Google Scholar]
- 61.McCrea M., Guskiewicz K., Randolph C., Barr W.B., Hammeke T.A., Marshall S.W., and Kelly J.P. (2009). Effects of a symptom-free waiting period on clinical outcome and risk of reinjury after sport-related concussion. Neurosurgery 65, 876–882 [DOI] [PubMed] [Google Scholar]
- 62.Kumar N.S., Chin M., O'Neill C., Jakoi A.M., Tabb L., and Wolf M. (2014). On-field performance of national football league players after return from concussion. Am. J. Sports Med. 42, 2050–2055 [DOI] [PubMed] [Google Scholar]
- 63.Iadecola C. and Nedergaard M. (2007). Glial regulation of the cerebral microvasculature. Nat. Neurosci. 10, 1369–1376 [DOI] [PubMed] [Google Scholar]
- 64.Junger E.C., Newell D.W., Grant G.A., Avellino A.M., Ghatan S., Douville C.M., Lam A.M., Aaslid R., and Winn H.R. (1997). Cerebral autoregulation following minor head injury. J. Neurosurg. 86, 425–432 [DOI] [PubMed] [Google Scholar]
- 65.Strebel S., Lam A.M., Matta B.F., and Newell D.W. (1997). Impaired cerebral autoregulation after mild brain injury. Surg. Neurol. 47, 128–131 [DOI] [PubMed] [Google Scholar]
- 66.Rangel-Castilla L., Gasco J., Nauta H.J., Okonkwo D.O., and Robertson C.S. (2008). Cerebral pressure autoregulation in traumatic brain injury. Neurosurg. Focus 25, E7. [DOI] [PubMed] [Google Scholar]
- 67.Goldstein B., Toweill D., Lai S., Sonnenthal K., and Kimberly B. (1998). Uncoupling of the autonomic and cardiovascular systems in acute brain injury. Am. J. Physiol. 275, R1287–1292 [DOI] [PubMed] [Google Scholar]
- 68.Zhang R., Zuckerman J.H., Iwasaki K., Wilson T.E., Crandall C.G., and Levine B.D. (2002). Autonomic neural control of dynamic cerebral autoregulation in humans. Circulation 106, 1814–1820 [DOI] [PubMed] [Google Scholar]
- 69.Liu W., Wang B., Wolfowitz R., Yeh P.H., Nathan D.E., Graner J., Tang H., Pan H., Harper J., Pham D., Oakes T.R., French L.M., and Riedy G. (2013). Perfusion deficits in patients with mild traumatic brain injury characterized by dynamic susceptibility contrast MRI. NMR Biomed. 26, 651–663 [DOI] [PubMed] [Google Scholar]
- 70.Levine B., Kovacevic N., Nica E.I., Cheung G., Gao F., Schwartz M.L., and Black S.E. (2008). The Toronto traumatic brain injury study: injury severity and quantified MRI. Neurology 70, 771–778 [DOI] [PubMed] [Google Scholar]
- 71.Doshi H., Wiseman N., Liu J., Wang W., Welch R.D., O'Neil B.J., Zuk C., Wang X., Mika V., Szaflarski J.P., Haacke E.M., and Kou Z. (2015). Cerebral hemodynamic changes of mild traumatic brain injury at the acute stage. PloS One 10, e0118061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zweckberger K., Eros C., Zimmermann R., Kim S.W., Engel D., and Plesnila N. (2006). Effect of early and delayed decompressive craniectomy on secondary brain damage after controlled cortical impact in mice. J. Neurotrauma 23, 1083–1093 [DOI] [PubMed] [Google Scholar]
- 73.Bryan R.M., Jr., Cherian L., and Robertson C. (1995). Regional cerebral blood flow after controlled cortical impact injury in rats. Anesth. Analg. 80, 687–695 [DOI] [PubMed] [Google Scholar]
- 74.Engel D.C., Mies G., Terpolilli N.A., Trabold R., Loch A., De Zeeuw C.I., Weber J.T., Maas A.I., and Plesnila N. (2008). Changes of cerebral blood flow during the secondary expansion of a cortical contusion assessed by 14C-iodoantipyrine autoradiography in mice using a non-invasive protocol. J. Neurotrauma 25, 739–753 [DOI] [PubMed] [Google Scholar]






