Abstract
We investigated and describe change in the Multidimensional Prognostic Index (MPI) score between admission and discharge in 960 older patients admitted to 20 geriatric units for an acute disease or a relapse of a chronic disease. The MPI was calculated at admission and at discharge. Subjects were divided into three groups of MPI score, low risk (MPI-1 value ≤0.33), moderate risk (MPI-2 value 0.34–0.66), and severe risk of mortality (MPI-3 value ≥0.67), on the basis of previously established cutoffs. Variation of MPI values over length of hospital stay (LOS) was analyzed with a multivariable longitudinal linear model for repeated measurements. At admission, 23.5% subjects had an MPI-1 score, 33.3% had an MPI-2 score, and 43.0% had an MPI-3 score. Overall, for almost 60% of the patients, MPI score at hospital discharge was different compared with the score at admission, although the difference was not statistically significant (−0.003; p = 0.708). Patients with high and intermediate MPI scores at admission had a decrease of MPI score at discharge (delta-MPI −0.026, p < 0.001, and delta-MPI −0.066, p = 0.569, respectively), whereas patients in the MPI-low group, experienced a significant increase in MPI score (delta-MPI 0.041, p < 0.001). The evolution of MPI score as a function of LOS had a curvilinear shape because it significantly decreased for patients with short hospitalization (1–6 days) and tended to increase for those with longer LOS. The MPI, a well-established prognostic tool, is sensitive to change of patient's health status and might be used to objectively track and monitor the clinical evolution of acutely ill geriatric patients admitted to the hospital.
Introduction
In older frail patients, hospitalization is characterized by a heterogeneous and frequently unpredictable trajectory of the clinical picture, often independently from the outcome of the specific disease that caused the admission to the hospital and of appropriate medical treatment of the acute underlying disease.1 Indeed, besides the negative effect of the acute event, hospitalization itself might represent an additional stressor2 in terms of environmental hazard, reduced caloric intake, low physical activity or prolonged bed rest, depressed mood, and social isolation; furthermore, many older inpatients experience new geriatric syndromes and worsening of existing syndromes during hospitalization.3
Decline in health status during hospitalization has important consequences in terms of quality of life and healthcare utilization as it has been associated with the risk of longer hospital stay, institutionalization, and mortality.4,5
Availability of objective and standardized tools to assess and track health status changes during hospitalization is of great importance for appropriate management and to properly monitor the effect of medical therapy.6 Nevertheless, the ability of medical diagnoses and traditional clinical assessment to discriminate heterogeneous patients is limited.7 Several studies have been published on instruments assessing functional change during hospitalization, whereas little is known about multidimensional and more comprehensive tools' ability to track variation in the overall patient's health condition.8,9
The Multidimensional Prognostic Index (MPI) is a validated index based on six commonly used geriatric assessment scales exploring cognitive, functional, nutritional, and clinical status, as well as on information about drugs taken and patient's social support.10 Its long-term predictive value has been established in the overall hospitalized population11 as well as in older subjects hospitalized for specific clinical conditions.12–16 Recently, we have demonstrated the validity of MPI in predicting in-hospital mortality and length of hospital stay (LOS) among patients with multiple types of diseases and causes of hospitalization,17 but no data are available on change in MPI score over hospital stay and on its sensitivity to patient's clinical modification over time.
The aim of this multicenter study was to describe change in the MPI score between admission and discharge in older patients admitted to geriatric units for an acute disease or a relapse of a chronic disease. We also investigated the relationship between variation of the MPI as a function of LOS and baseline MPI value.
Materials and Methods
Study population
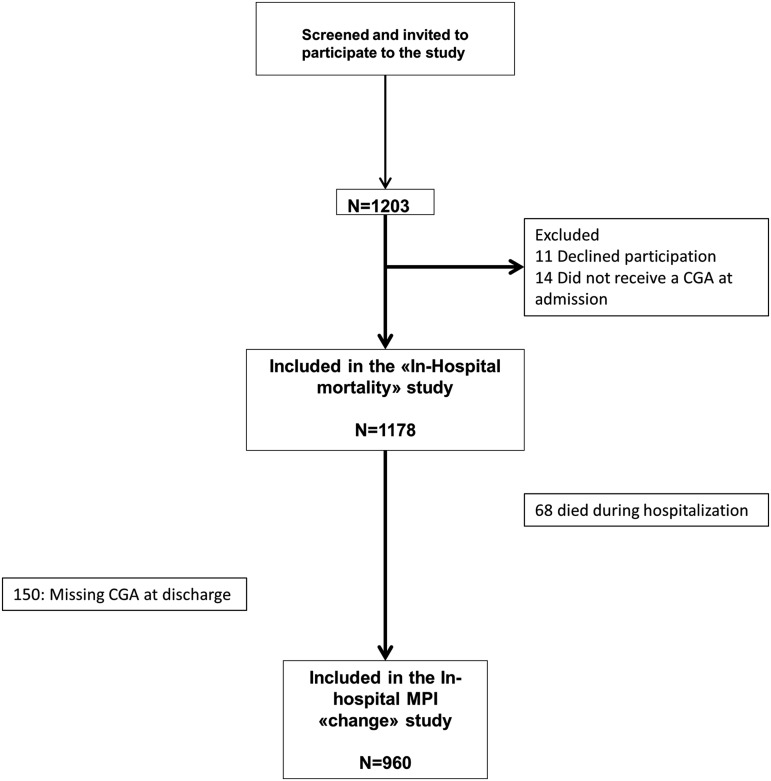
All patients with age greater or equal to 65 years consecutively admitted for acute illness or relapse of chronic disease to 20 Acute Geriatric Wards located in the northeastern area of Italy from January 1, 2012, to March 31, 2012, were screened for inclusion (Fig. 1). The study was conducted according to the principles of the Declaration of Helsinki. Information on demographics, including age and gender, housing status (i.e., living with family or caregivers, institutionalized, or living alone), medical history, and medications taken, was collected using interview and/or medical records.
FIG. 1.
Participant flowchart. CGA, comprehensive geriatric assessment.
A comprehensive geriatric assessment (CGA) was performed within 48 hours from admission to collect information on basic activities of daily living (ADL) and instrumental activities of daily living (IADL) according to the Katz18 and the Lawton–Brody19 scales, respectively. Cognitive status was evaluated using the Short Portable Mental Status Questionnaire (SPMSQ).20 Comorbidity burden was summarized using the comorbidity subscale of the cumulative illness rating scale (CIRS),21 and nutritional status was assessed through the mininutritional assessment (MNA).22 The Exton–Smith scale (ESS) was used to evaluate the risk of developing pressure ulcers.23 The number of medications taken at home was recorded.
At discharge, the number of hospitalization days and the first five diagnoses reported in the discharge form and coded according to the International Classification of Diseases, Tenth Revision (ICD-10), were collected. For patients who were discharged from the hospital, the same CGA was performed within 24 hours before hospital discharge.
The MPI
At admission, the MPI was calculated using the CGA-based validated algorithm.10 Scores of each of the aforementioned multidimensional assessment scales (ADL, IADL, SPMSQ, CIRS, MNA, ESS), information of housing status, and number of medications prescribed were recategorized based on a tripartite hierarchy and a new score was assigned (0 = no problems/low burden; 0.5 = minor problems/intermediate burden; and 1 = major problems/major burden). The specific thresholds used to define the three hierarchic categories were reported elsewhere13,16 and were based on either validated cutoffs (SPMSQ, MNA, EES, ADL, and IADL) or frequency of distribution in the previous validation study (for CIRS and number of medications10).
The newly adjudicated scores were summed and the result obtained was divided by eight (the total number of domains) to obtain an average value, namely the MPI score, ranging from 0 ( = low mortality risk) to 1 ( = high mortality risk). For clinical purposes, three grades of MPI were identified according to previously validated cutoff: MPI-1 (low risk of mortality, MPI values from 0 to 0.33), MPI-2 (moderate risk of mortality, MPI values from 0.34 to 0.66), and MPI-3 (severe risk of mortality, MPI values from 0.67 to 1.0). To calculate the MPI, software for Windows may be downloaded (available for free) at the following address: www.ulss16.padova.it/all/MPISetup.exe (English version). The full GCA testing and MPI computation took, on average, between 30 and 45 minutes to complete, depending on patient's collaboration.
Statistical methods
Patients baseline characteristics are reported as mean ± standard deviation, median and interquartile range (first and third quartiles: Q1–Q3), or frequencies and percentage for continuous and categorical variables, respectively. Baseline differences according to MPI at admission grades were assessed with ANOVA F test for trend on ranks or Mantel–Haenszel chi-square test for continuous and categorical variables, respectively. Differences of MPI at discharge and MPI at admission (delta-MPI) were calculated for each patient. Crude delta-MPI means between MPI groups at admission were calculated. Patients with delta-MPI <0 were defined as improved, those with delta-MPI = 0 were defined as unchanged, and those with delta-MPI >0 were defined as worsened.
The evolution of MPI values over LOS was analyzed with a multivariable longitudinal linear model for repeated measurements. A spatial power covariance structure was used to account for unequally spaced time occasions during the follow-up.24,25 This method borrows strength from correlated measures within each subject across time occasions. Furthermore, the presence of nonlinear trend over time was investigated with the inclusion of LOS as a categorical ordinal time variable, with categories defined according to their tertiles. The categorization is useful to explore the functional shape of the MPI means over time.
Model included the LOS variable and the following baseline covariates: MPI at admission grades, age, sex, the presence of heart failure, arrhythmia, pneumonia, chronic obstructive pulmonary disease (COPD), respiratory failure, dementia, acute or chronic kidney disease, and all interactions between covariates and time variable. Adjusted means for MPI, within each tertile of LOS, were also estimated. Post hoc pairwise comparisons between the estimated means at different LOS intervals, with respect to baseline, were investigated through suitable contrasts and p-values were adjusted for multiple comparisons, according to Hochberg's method. A p-value <0.05 was considered for statistical significance. All analyses were performed using SAS Release 9.3 (SAS Institute, Cary, NC).
Results
Study population
Patients' baseline characteristics, according to MPI score at admission, are reported in Table 1. At admission, 226 (23.6%) subjects had a low MPI-1 score, 321 (33.4%) had an intermediate MPI-2, and 413 (43.1%) had a high MPI-3. Subjects with higher MPI score at admission were older (p < 0.001), were more frequently women (p < 0.001), and had higher prevalence of heart failure, renal failure, pneumonia, COPD, and dementia compared with subjects who were included in the lower MPI groups. Moreover, functional, cognitive, nutritional, clinical, and social statuses, assessed using the MPI subscales, were also worse in subjects with higher MPI score at admission (all p < 0.001).
Table 1.
Baseline Clinical Characteristics of Patients, According to Multidimensional Prognostic Index Grades at Admission
| Variable | All subjects | MPI-low | MPI-intermediate | MPI-high | p-Value for trenda |
|---|---|---|---|---|---|
| n (%) | 960 (100%) | 226 (23.54%) | 321 (33.34%) | 413 (43.02%) | |
| MPI at admission, mean ± SD | 0.56 ± 0.24 | 0.23 ± 0.08 | 0.50 ± 0.09 | 0.79 ± 0.08 | <0.001 |
| Age (years), mean ± SD | 85.02 ± 6.77 | 81.78 ± 6.73 | 84.77 ± 6.01 | 86.98 ± 6.64 | <0.001 |
| Female, n (%) | 578 (60.33) | 98 (43.56) | 191 (59.69) | 289 (69.98) | <0.001 |
| BADL, median (Q1-Q3) | 1 (0–5) | 6 (5–6) | 3 (1–5) | 0 (0–1) | <0.001 |
| IADL, median (Q1-Q3) | 1 (0–5) | 7 (5–8) | 2 (1–4) | 0 (0–0) | <0.001 |
| SPMSQ (No. of errors), median (Q1–Q3) | 3 (1–9) | 0 (0–2) | 2 (0–4) | 10 (5.5–10) | <0.001 |
| Mininutrition assessment, mean ± SD | 17.44 ± 6.71 | 23.77 ± 4.46 | 19.38 ± 4.61 | 12.47 ± 5.27 | <0.001 |
| Exton–Smith scale, mean ± SD | 13.91 ± 4.54 | 18.73 ± 1.93 | 15.77 ± 2.74 | 9.82 ± 2.79 | <0.001 |
| CIRS comorbidity, mean ± SD | 4.53 ± 2.20 | 3.30 ± 1.99 | 4.22 ± 1.96 | 5.44 ± 2.09 | <0.001 |
| Number of drugs, mean ± SD | 6.49 ± 3.16 | 5.33 ± 2.98 | 6.53 ± 3.14 | 7.10 ± 3.10 | <0.001 |
| Cohabit status, n (%) | 0.056 | ||||
| In family or caregiver | 643 (67.19) | 184 (81.78) | 216 (67.50) | 243 (58.98) | |
| Nursing home | 144 (15.05) | 2 (0.89) | 15 (4.69) | 127 (30.83) | |
| Alone | 170 (17.76) | 39 (17.33) | 89 (27.81) | 42 (10.19) | |
| Length of stay (days), median (Q1-Q3) | 8 (6–13) | 7 (5–11) | 8 (6–12) | 9 (6–14) | <0.001 |
| CHD, n (%) | 102 (10.63) | 16 (7.08) | 39 (12.15) | 47 (11.38) | 0.141 |
| Heart failure, n (%) | 239 (24.90) | 38 (16.81) | 76 (23.68) | 125 (30.27) | <0.001 |
| Arrhythmia, n (%) | 174 (18.13) | 43 (19.03) | 53 (16.51) | 78 (18.89) | 0.907 |
| Ischemic stroke, n (%) | 97 (10.10) | 19 (8.41) | 30 (9.35) | 48 (11.62) | 0.172 |
| Pneumonia, n (%) | 145 (15.10) | 19 (8.41) | 40 (12.46) | 86 (20.82) | <0.001 |
| COPD, n (%) | 131 (13.65) | 35 (15.49) | 53 (16.51) | 43 (10.41) | 0.038 |
| Respiratory failure, n (%) | 60 (6.25) | 9 (3.98) | 27 (8.41) | 24 (5.81) | 0.577 |
| Pulmonary embolism, n (%) | 18 (1.88) | 3 (1.33) | 7 (2.18) | 8 (1.94) | 0.654 |
| Cancer, n (%) | 103 (10.73) | 25 (11.06) | 40 (12.46) | 38 (9.20) | 0.353 |
| Dementia, n (%) | 169 (17.60) | 12 (5.31) | 38 (11.84) | 119 (28.81) | <0.001 |
| Kidney failure, n (%) | 109 (11.35) | 19 (8.41) | 31 (9.66) | 59 (14.29) | 0.016 |
p-Values from ANOVA F test for trend on rank values and Mantel–Haenszel chi-square test for continuous and categorical variables, respectively.
BADL, basic activities of daily living; CIRS, cumulative illness rating scale; IADL, instrumental activities of daily living; MPI, Multidimensional Prognostic Index; SD, standard deviation; SPMSQ, Short Portable Mental Status Questionnaire.
MPI change during hospitalization
In Table 2 are reported the unadjusted means for MPI at hospital discharge and hospital admission along with estimated change during hospital stay. Results are stratified according to MPI group at hospital admission. Overall, MPI score tended to decline between admission and discharge from the hospital, suggesting a global health status improvement during hospital stay (delta-MPI −0.003 p = 0.708). Nevertheless, change in MPI score was different according to MPI group at hospital admission. Indeed, patients with high and intermediate MPI score had a decrease of MPI score at discharge (delta-MPI −0.026, p < 0.001, and delta-MPI −0.066, p = 0.569, respectively), whereas patients in better health status at hospital admission, the MPI-low group, experienced a significant increase in MPI score (delta-MPI 0.041, p < 0.001).
Table 2.
Unadjusted Sample Means for MPI at Admission, MPI at Discharge, and Difference Between MPI at Discharge and MPI at Admission (Delta-MPI), According to MPI at Admission Grades
| MPI at admission | ||||
|---|---|---|---|---|
| Variables, mean ± SD | All | MPI-low | MPI-intermediate | MPI-high |
| MPI at admission | 0.564 ± 0.240 | 0.230 ± 0.080 | 0.503 ± 0.090 | 0.794 ± 0.081 |
| MPI at discharge | 0.561 ± 0.232 | 0.271 ± 0.127 | 0.498 ± 0.129 | 0.769 ± 0.104 |
| Delta-MPI | −0.003 ± 0.101 | 0.041 ± 0.116 | −0.006 ± 0.099 | −0.026 ± 0.084 |
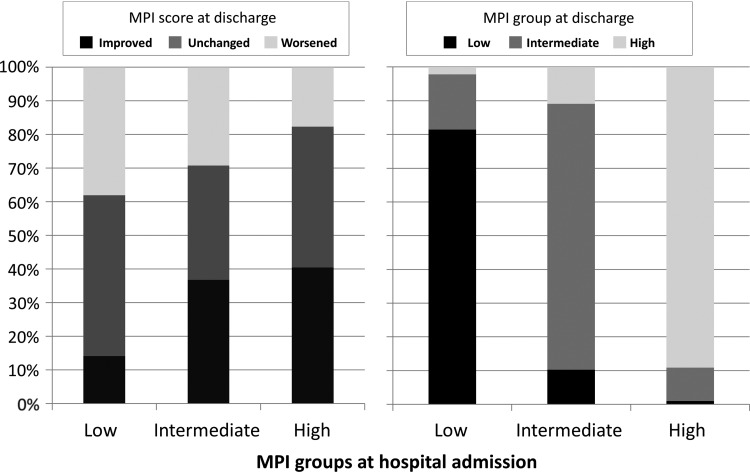
Overall, in almost 60% of the patients, MPI score at hospital discharge was different compared with the score at admission, but the likelihood of change and the direction of the change were strongly affected by the MPI level. As depicted in Figure 2 (left panel), among patients in the MPI-low group, only 14.2% improved the MPI during hospital stay, whereas among the intermediate and high groups, 36.8% and 40.4% had a better MPI score at the end of the hospitalization. Conversely, 38.1% of the low group had a worse MPI score at discharge compared with 29.3% and 17.7% in the intermediate and high MPI groups, respectively. Figure 2 (right panel) displays the distribution of patients in the three MPI groups at discharge as a function of MPI status at admission. Overall, 155 patients (16.1) changed MPI groups from admission to baseline, with patients in the MPI-intermediate group at baseline having the greater likelihood of change. Conversely, more than 80% of the patients of the low and high groups remained in the same category at hospital discharge.
FIG. 2.
Percent change in MPI score and MPI group at discharge according to MPI group at admission. Baseline MPI score range: MPI 1 (0.00–0.33), MPI 2 (0.34–0.66), and MPI 3 (0.67–1.0). MPI, Multidimensional Prognostic Index.
MPI change and length of hospital stay
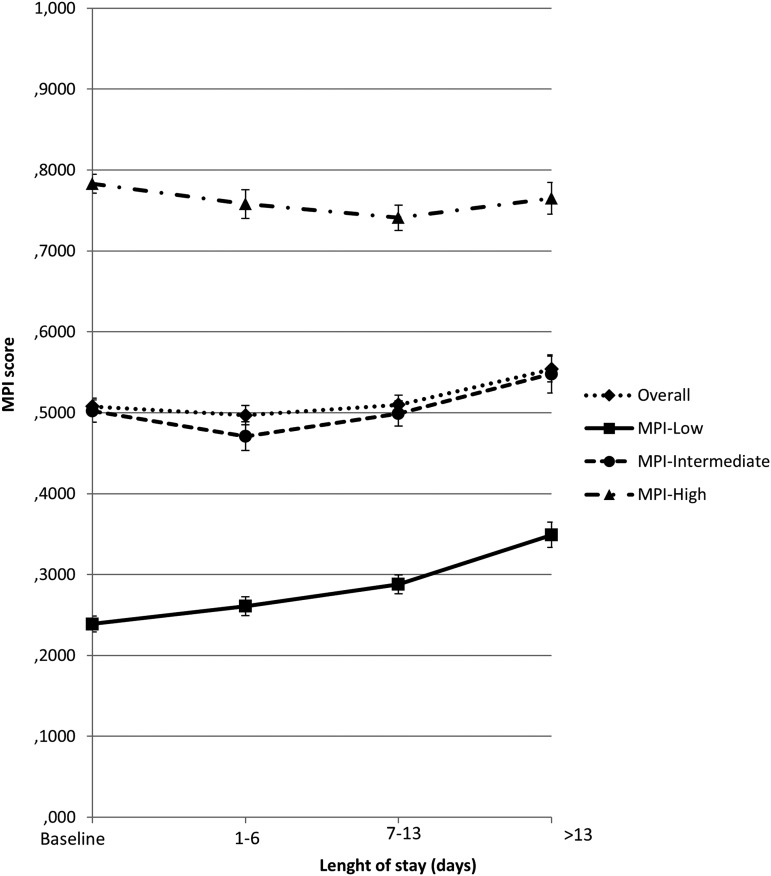
To further understand the evolution of MPI values from hospital admission to discharge, MPI score was investigated as a function of the LOS. Figure 3 displays MPI score according to tertiles of hospitalization length (i.e., 1–6, 7–13, and >13 days were the first, second, and third tertiles, respectively) and MPI group at admission. Overall, the evolution of MPI score had a curvilinear shape because it decreased for patients with short hospitalization (1–6 days) and then tended to increase for those with longer LOS. Again, the relationship between MPI score at discharge and LOS was substantially different according to MPI status at baseline.
FIG. 3.
Plots of adjusted means for MPI score at different length of hospital stay intervals, according to MPI group at hospital admission. Baseline MPI score range: MPI 1 (0.00–0.33), MPI 2 (0.34–0.66), and MPI 3 (0.67–1.0).
The sickest patients (MPI-high group at admission), compared with the MPI admission value, had a better score up to the second tertile of hospitalization length, whereas patients with lower MPI value at admission (the healthiest group) experienced a linear increase in MPI score as the LOS increased. The results were confirmed in a multivariable longitudinal linear model for repeated measurements, adjusting for multiple potential confounding factors, including age, sex, heart failure, arrhythmia, pneumonia, COPD, respiratory failure, dementia, acute or chronic kidney disease, and MPI score at admission (Table 3).
Table 3.
Estimated Adjusted Means for Multidimensional Prognostic Index Score from Multivariable Longitudinal Linear Model and Pairwise Comparisons
| Length of stay categories | Adjusted means (SE) | p-Valuea | |
|---|---|---|---|
| MPI adjusted means | |||
| Overall | Baseline | 0.508 (0.005) | <0.001 |
| 1–6 days | 0.497 (0.006) | ||
| 7–13 days | 0.510 (0.006) | ||
| >13 days | 0.554 (0.008) | ||
| MPI-low grade | Baseline | 0.239 (0.008) | <0.001 |
| 1–6 days | 0.261 (0.010) | ||
| 7–13 days | 0.288 (0.010) | ||
| >13 days | 0.349 (0.018) | ||
| MPI-intermediate | Baseline | 0.502 (0.007) | <0.001 |
| 1–6 days | 0.471 (0.009) | ||
| 7–13 days | 0.499 (0.008) | ||
| >13 days | 0.548 (0.012) | ||
| MPI-high | Baseline | 0.783 (0.006) | <0.001 |
| 1–6 days | 0.758 (0.009) | ||
| 7–13 days | 0.741 (0.008) | ||
| >13 days | 0.765 (0.010) | ||
| Pairwise comparisons | |||
| Overall | 1–6 days vs. Baseline | −0.011 (0.004) | 0.024 |
| 7–13 days vs. Baseline | 0.002 (0.005) | 0.739 | |
| >13 days vs. Baseline | 0.046 (0.008) | <0.001 | |
| MPI-low | 1–6 days vs. Baseline | 0.023 (0.008) | 0.024 |
| 7–13 days vs. Baseline | 0.050 (0.010) | <0.001 | |
| >13 days vs. Baseline | 0.110 (0.018) | <0.001 | |
| MPI-intermediate | 1–6 days vs. Baseline | −0.031 (0.007) | <0.001 |
| 7–13 days vs. Baseline | −0.003 (0.008) | 0.739 | |
| >13 days vs. Baseline | 0.047 (0.013) | 0.002 | |
| MPI-high | 1–6 days vs. Baseline | −0.025 (0.007) | 0.001 |
| 7–13 days vs. Baseline | −0.042 (0.007) | <0.001 | |
| >13 days vs. Baseline | −0.018 (0.010) | 0.282 | |
Multivariable longitudinal model included MPI at admission grades, length of stay (tertiles), age, sex, heart failure, arrhythmia, pneumonia, COPD, respiratory failure, dementia, and acute or chronic kidney disease.
p-Values for pairwise comparisons were adjusted following Hochberg's method.
COPD, chronic obstructive pulmonary disease.
Discussion
This study investigated modification of the MPI score, calculated using a CGA-based validated algorithm, in a sample of older Italian patients consecutively admitted for acute illness or relapse of chronic disease to acute geriatric wards. More than 60% of the patient at discharge had an MPI score significantly different from the score they had at hospital admission with the likelihood and the direction of MPI change during hospital stay varying according to MPI score and health status at baseline. Nevertheless, among patients with intermediate MPI score at hospital admission, almost 65% of the patients had a different MPI score at discharge, suggesting that MPI is not only a reliable and accurate clinical prognostic tool but it also has good sensitivity to capture the modification of the patient clinical condition over hospital stay.
Furthermore, MPI score modification was significantly related to the LOS and patients with the longer hospitalization experienced greater increase in MPI score at discharge. These results were based on a large multicenter study without selective exclusion criteria, including patients with multiple different medical conditions, therefore providing a good external validity and generalizability of the findings.
MPI has been consistently related to short17 and long-term mortality risk after hospital discharge in patients with a broad spectrum of specific diseases, including, but not limited to, pneumonia,12 dementia,13 congestive heart failure,14 and kidney failure.15 The results of the present work extend those of previous studies of this group, providing new insight into the potential clinical application of MPI assessment. Indeed, according to the results of this study, MPI besides being a reliable prognostic index for clinical decision-making for older adults,26 might be used to standardly track the clinical course of older patients and to monitor the treatment effects over time.
The MPI score, based on assessment of multiple determinants of the health status of older people, is likely to capture the integrated and synergistic negative effect of aging, comorbidity, disease severity, malnutrition, and cognition, therefore providing a synthetic, but sensitive, instrument that can be easily applied in everyday clinical practice.
The availability of a sensitive and reliable global health status indicator would be particularly useful for the care of the ill frail patient, in which, even when the specific disease is promptly and properly cured, hospitalization can be paralleled by an often irreversible decline in functional status and a change in quality of life.2 On the other hand, only 16% of the sample changed MPI group at discharge compared with baseline (Fig. 2, right panel), suggesting that the majority of the patients experienced only small variation of the MPI score during the hospitalization. The clinical meaning of small MPI change has not been determined so far and therefore it should be investigated in future longitudinal studies.
The relationship between LOS and change in clinical status during hospitalization has been rarely investigated and most of the published studies were focused on functional recovery only.27,28 Overall, these studies reported an inverse linear association of LOS and the likelihood of functional recovery at hospital discharge. Our analyses suggest a curvilinear relationship between LOS and change in MPI score during hospitalization and a different shape of the association as a function of baseline clinical status.
Indeed, for patients in the MPI-Low group, those with better clinical status at baseline, MPI tended to increase regardless of the LOS, whereas for patients in the intermediate and high MPI groups, a shorter LOS was associated with a significant decline in MPI score (better clinical status), but a longer LOS was associated with a relevant increase in MPI score. The results were independent of a number of potential confounders, including baseline MPI score, and therefore although a causal relationship between LOS and MPI score increase cannot be established, our findings reinforce the potential hazards of prolonged hospitalization for older frail patients.29
Some limitation should be considered interpreting these findings. The direction of MPI score change during hospitalization was strongly related to MPI value at baseline, with patients with low MPI being more likely to worsen and those with high MPI being more likely to improve, suggesting the phenomenon of regression toward the mean as a potential explanation of the observed results.30 Although multivariate analyses were adjusted for baseline MPI score,31 we cannot rule out the hypothesis that our results might be, at least partially, affected by this statistical issue.
MPI assessment needs a complete CGA assessment in an acute setting; it is possible that it is a complex bedside index to routinely use in elderly patients, especially in those with poor compliance and severe cognitive impairment. Our analysis did not include sensible indicators of disease severity (including, but not limited to, ejection fraction, pneumonia severity score, and clinical dementia rating scale) that might have allowed for a better disease characterization of the patients; nevertheless, previous studies conducted in patients with selected disease suggested that MPI prognostic power is better if compared with disease-specific severity indexes.12,14 Finally, the results of this study should be confirmed and validated in a different and, possibly, more representative population.
In conclusion, this study demonstrates that MPI, a well-established prognostic instrument, is also sensitive to change and might be used to objectively track and monitor the clinical evolution of acutely ill geriatric patients admitted to the hospital. Although the clinical impact of MPI change during hospitalization should be formally demonstrated, these results support the concept that sequential MPI assessment during hospital stay might help clinicians during the decision-making process, leading to a better quality of care provided.
Acknowledgments
MPI-triveneto study group investigators were as follows: Geriatric Unit, Azienda ULSS 3, Bassano del Grappa (VI), Italy: Luigi Marinangeli, MD, and Marco Mosele, MD; Geriatric Unit, Azienda ULSS 15, Camposampiero (PD), Italy: Giuseppe Soldà, MD, and Elena Del Giudice, MD; Geriatric Unit, Azienda ULSS 14, Chioggia (VE), Italy: Antonella Battuanello, MD, and Pietro Fabris, MD; Geriatric Unit, Azienda ULSS 7, Conegliano (TV), Italy: Sergio Peruzza, MD, and Giovanna Tomasi, MD; Geriatric Unit, Azienda ULSS 13, Dolo (VE), Italy: Alberto Cester, MD, and Pietro Bonometto, MD; Internal Medicine Unit, Azienda Ospedaliera Universitaria, Ferrara, Italy: Giovanni Zuliani, MD, and Gloria Brombo, MD; Geriatric Unit, Azienda ULSS 21, Legnago (VR), Italy: Alfredo Zanatta, MD, and Roberto Filippin, MD; Geriatric Unit, Azienda ULSS 12, Mestre (VE), Italy: Roberto Brugiolo, MD, and Anna Zucchero, MD; Geriatric Unit, Azienda ULSS 17, Monselice (PD), Italy: Lucio Conforto, MD, and Annalisa Timpini, MD; Geriatric Unit, Azienda ULSS 8, Montebelluna (TV), Italy: Emanuele Rizzo, MD, and Elena Faccioli, MD; Geriatric Unit, Azienda ULSS 16, Padova, Italy: Alberto Pilotto, MD, and Ramona Chiarini, PsyD; Geriatric Clinic, Azienda Ospedaliera, Padova, Italy: Enzo Manzato, MD, and Valter Giantin, MD; Geriatric Unit, Azienda Provinciale per i Sevizi Sanitari, Rovereto, Italy: Renzo Girardello, MD, and Sergio Minervini, MD; Geriatric Unit, Azienda ULSS 18, Rovigo, Italy: Pierluigi Dal Santo, MD, and Giuseppe Burattin, MD; Geriatric Unit, Azienda Provinciale per i Sevizi Sanitari, Trento, Italy: Gabriele Noro, MD, and Fabio Massaro, MD; Geriatric Unit, Azienda ULSS 9, Treviso, Italy: Massimo Calabrò, MD; Geriatric Unit, Azienda Ospedaliera Universitaria, Trieste, Italy: Gabriele Toigo, MD, and Giuseppe Castiglia, MD; Geriatric Clinic, Azienda Ospedaliera Universitaria Integrata, Verona, Italy: Mauro Zamboni, MD; Geriatric 3° Unit, Azienda Ospedaliera Universitaria Integrata, Verona, Italy: Vincenzo Di Francesco, MD, and Luca Pellizzari, MD; Geriatric Unit, Azienda ULSS 6, Vicenza, Italy: Paolo Chioatto, MD, and Maria Gulino, MD; and Unit of Biostatistics, Scientific Institute for Research and Care, Casa Sollievo della Sofferenza, San Giovanni Rotondo, Italy: Fabio Pellegrini, BioStat, and Massimiliano Coppetti, BioStat.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Gillick MR, Serrell NA, Gillick LS. Adverse consequences of hospitalization in the elderly. Soc Sci Med 1982;16:1033–1038 [DOI] [PubMed] [Google Scholar]
- 2.Creditor MC. Hazards of hospitalization of the elderly. Ann Intern Med 1993;118:219–223 [DOI] [PubMed] [Google Scholar]
- 3.Lakhan P, Jones M, Wilson A, Courtney M, Hirdes J, Gray LC. A prospective cohort study of geriatric syndromes among older medical patients admitted to acute care hospitals. J Am Geriatr Soc 2011;59:2001–2008 [DOI] [PubMed] [Google Scholar]
- 4.Fortinsky RH, Covinsky KE, Palmer RM, Landefeld CS. Effects of functional status changes before and during hospitalization on nursing home admission of older adults. J Gerontol A Biol Sci Med Sci 1999;54:M521–M526 [DOI] [PubMed] [Google Scholar]
- 5.Brown CJ, Friedkin RJ, Inouye SK. Prevalence and outcomes of low mobility in hospitalized older patients. J Am Geriatr Soc 2004;52:1263–1270 [DOI] [PubMed] [Google Scholar]
- 6.Gill TM. The central role of prognosis in clinical decision making. JAMA 2012;307:199–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Volpato S, Onder G, Cavalieri M, et al. Characteristics of nondisabled older patients developing new disability associated with medical illnesses and hospitalization. J Gen Intern Med 2007;22:668–674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McCusker J, Kakuma R, Abrahamowicz M. Predictors of functional decline in hospitalized elderly patients: A systematic review. J Gerontol A Biol Sci Med Sci 2002;57:M569–M577 [DOI] [PubMed] [Google Scholar]
- 9.De Saint-Hubert M, Schoevaerdts D, Cornette P, D'Hoore W, Boland B, Swine C. Predicting functional adverse outcomes in hospitalized older patients: A systematic review of screening tools. J Nutr Health Aging 2010;14:394–399 [DOI] [PubMed] [Google Scholar]
- 10.Pilotto A, Ferrucci L, Franceschi M, et al. Development and validation of a Multidimensional Prognostic Index for one-year mortality from comprehensive geriatric assessment in hospitalized older patients. Rejuvenation Res 2008;11:151–161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pilotto A, Rengo F, Marchionni N, et al. Comparing the prognostic accuracy for all-cause mortality of frailty instruments: A multicentre 1-year follow-up in hospitalized older patients. PLoS One 2012;7:e29090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pilotto A, Addante F, Ferrucci L, et al. The multidimensional prognostic index predicts short- and long-term mortality in hospitalized geriatric patients with pneumonia. J Gerontol A Biol Sci Med Sci 2009;64:880–887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pilotto A, Sancarlo D, Panza F, et al. The Multidimensional Prognostic Index (MPI), based on a comprehensive geriatric assessment predicts short- and long-term mortality in hospitalized older patients with dementia. J Alzheimers Dis 2009;18:191–199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pilotto A, Addante F, Franceschi M, et al. Multidimensional Prognostic Index based on a comprehensive geriatric assessment predicts short-term mortality in older patients with heart failure. Circ Heart Fail 2010;3:14–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pilotto A, Sancarlo D, Aucella F, et al. Addition of the multidimensional prognostic index to the estimated glomerular filtration rate improves prediction of long-term all-cause mortality in older patients with chronic kidney disease. Rejuvenation Res 2012;15:82–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sancarlo D, Pilotto A, Panza F, et al. A Multidimensional Prognostic Index (MPI) based on a comprehensive geriatric assessment predicts short- and long-term all-cause mortality in older hospitalized patients with transient ischemic attack. J Neurol 2012;259:670–678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Volpato S, Bazzano S, Fontana A, Ferrucci L, Pilotto A; on behalf of the MPI-TriVeneto Study Group. Multidimensional Prognostic Index Predicts Mortality and Length of Stay During Hospitalization in the Older Patients: A Multicenter Prospective Study. J Gerontol A Biol Sci Med Sci 2015;70:325–331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Katz S, Downs TD, Cash HR, et al. Progress in the development of an index of ADL. Gerontologist 1970;10:20–30 [DOI] [PubMed] [Google Scholar]
- 19.Lawton MP, Brody EM. Assessment of older people: Self-maintaining and instrumental activities of daily living. Gerontologist 1969;9:179–186 [PubMed] [Google Scholar]
- 20.Pfeiffer E. A short portable mental status questionnaire for the assessment of organic brain deficit in elderly patients. J Am Geriatr Soc 1975;23:433–441 [DOI] [PubMed] [Google Scholar]
- 21.Linn B, Linn M, Gurel L. The cumulative illness rating scale. J Am Geriatr Soc 1968;16:622–626 [DOI] [PubMed] [Google Scholar]
- 22.Guigoz Y, Vellas B. The mini nutritional assessment (MNA) for grading the nutritional state of elderly patients: Presentation of the MNA, history and validation. Nestle Nutr Workshop Ser Clin Perform Programme 1999;1:3–11 [DOI] [PubMed] [Google Scholar]
- 23.Bliss MR, McLaren R, Exton-Smith AN. Mattresses for preventing pressure sores in geriatric patients. Mon Bull Minist Health Public Health Lab Serv 1966;25:238–268 [PubMed] [Google Scholar]
- 24.Singer JD, Willett JB. Applied Longitudinal Data Analysis: Modeling Change and Event Occurrence. New York: Oxford University Press, 2003 [Google Scholar]
- 25.Diggle PJ, Liang KY, Zeger SL. Analysis of Longitudinal Data. Oxford: Oxford University Press, 1994 [Google Scholar]
- 26.Yourman LC, Lee SJ, Schonberg MA, Widera EW, Smith AK. Prognostic indices for older adults: A systematic review. JAMA 2012;307:182–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen YM, Chuang YW, Liao SC, Lin CS, Yang SH, Tang YJ, Tsai JJ, Lan JL, Chen DY. Predictors of functional recovery (FR) for elderly hospitalized patients in a geriatric evaluation and management unit (GEMU) in Taiwan. Arch Gerontol Geriatr 2010;50 Suppl 1:S1–S15 [DOI] [PubMed] [Google Scholar]
- 28.Palleschi L, De Alfieri W, Salani B, Fimognari FL, Marsilii A, Pierantozzi A, Di Cioccio L, Zuccaro SM. Functional recovery of elderly patients hospitalized in geriatric and general medicine units. The PROgetto DImissioni in GEriatria Study. J Am Geriatr Soc 2011;59:193–199 [DOI] [PubMed] [Google Scholar]
- 29.Anpalahan M, Gibson SJ. Geriatric syndromes as predictors of adverse outcomes of hospitalization. Intern Med J 2008;38:16–23 [DOI] [PubMed] [Google Scholar]
- 30.Bland JM, Altman DG. Some example of regression towards the mean. BMJ 1994;309:780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barnett AG, van der Pols JC, Dobson AJ. Regression to the mean: What it is and how to deal with it. Int J Epidemiol 2005;34:215–220 [DOI] [PubMed] [Google Scholar]