Abstract
Background:
The aim of this study was to clarify the influence of hepatic fibrosis on metachronous liver-specific recurrence in colorectal cancer (CRC) patients who underwent colorectal surgery with curative intent. Non-alcoholic steatohepatitis (NASH) is closely associated with hepatic fibrosis (HF). The number of patients who suffer from NASH is increasing because of the consumption of high-calorie diets. It remains unclear how much of an impact NASH and HF have on the development of liver metastasis in CRC.
Methods:
Patients who underwent curative surgical resection for CRC between 2000 and 2011 were included in this study. We evaluated the progression of HF by the non-alcoholic fatty liver disease fibrosis score (NFS) based on preoperative blood test results, age, body mass index, and diabetes mellitus. Patients were grouped according to high (fibrotic liver; FL) or low (normal liver; NL) NFS. The influence of HF on hepatic recurrence was assessed by survival analyses.
Results:
A total of 953 CRC patients were enrolled, comprising 293 in stage I, 327 in stage II, and 333 in stage III. The patients included were categorised as FL (77) or NL (876). The hepatic recurrence rates were 5.3% in the NL group and 10.4% in the FL group (P=0.02), whereas the overall recurrence rates were 16.0% in the NL group and 20.7% in the FL group (P=0.03). The 5-year liver-specific recurrence-free survival rate in the FL group was significantly poorer than that in the NL group (FL 89.1%, 95% confidence interval (CI) 78.4–94.7 vs NL 96.0%, 95% CI 94.3–97.2, log-rank test P<0.01). Multivariate analysis demonstrated that HF significantly promoted liver-specific recurrence compared with NL (HR=2.98, 95% CI 1.23–7.21; P=0.02).
Conclusion:
HF is a valuable prognostic factor for hepatic recurrence after curative surgical resection of CRC.
Keywords: fibrotic liver, liver metastasis, colorectal cancer, NAFLD fibrosis score, hepatic fibrosis, liver-specific recurrence
Non-alcoholic fatty liver disease (NAFLD) and non-alcoholic steatohepatitis (NASH) are liver diseases that occur in individuals who drink little or no alcohol. These diseases have become common in recent years and affect up to 25% of the United States population (Povero et al, 2014). NAFLD is caused by the accumulation of excessive fat in hepatocytes regardless of alcohol intake. The more severe form of NAFLD is NASH. NASH causes hepatocytes to swell and become damaged, leading to cirrhosis in adults. Obesity, in particular, is considered key to explaining NASH/NAFLD, taking into account the complex obesity-related causal web that involves hypertension, insulin resistance, and hyperlipidaemia.
The liver is the most common site for metastases derived from colorectal cancer (CRC). Between 10 and 20% of patients with colorectal adenocarcinoma have hepatic metastasis at the time of presentation, while another 20–25% of patients will go on to develop metastasis during the course of their illness (Adson et al, 1984; Bismuth et al, 1996; Berney et al, 1998; Fong et al, 1999). Given the rise in global obesity and NAFLD/NASH, the association between liver metastasis and NAFLD/NASH is considered to be a major area of interest regarding the development of liver metastasis from CRCs. However, controversy remains in relation to the influence of NAFLD/NASH on the development of liver metastasis. Animal models have revealed that NASH induced by a high-fat diet is positively associated with liver metastasis in CRC (Cox et al, 2013). However, a retrospective case–control study demonstrated that patients with cirrhosis or viral hepatitis infection developed liver metastasis less frequently compared with patients without these diseases (Augustin et al, 2013; Murono et al, 2013). Given the medical costs associated with the number of patients suffering from NAFLD/NASH, it is important to clarify the clinical association between liver metastasis and NAFLD/NASH.
The NAFLD fibrosis score (NFS) has recently been established to enable physicians to diagnose NAFLD/NASH more conveniently (Angulo et al, 2007). The NFS is estimated using a combination of clinical features and routine laboratory investigations, which has helped gain its acceptance among clinicians.
The objectives of this study are to clarify the impact of hepatic fibrosis (HF) diagnosed via the NFS on postoperative liver-specific recurrence in terms of the incidence, size, or distribution of liver metastasis among CRC patients.
Materials and Methods
Patients
This was a single-institution retrospective review of patients who underwent curative surgical resection for colon or rectal cancer between 2000 and 2011 in Keio University Hospital. Our inclusion criteria for this analysis consisted of (1) patients with histologically confirmed colon cancer and (2) a patient age >18 years. Our exclusion criteria were (1) carcinoma of the appendix, (2) stage IV disease, (3) inappropriate data for calculating the NFS preoperatively and (4) death within 30 days postoperatively.
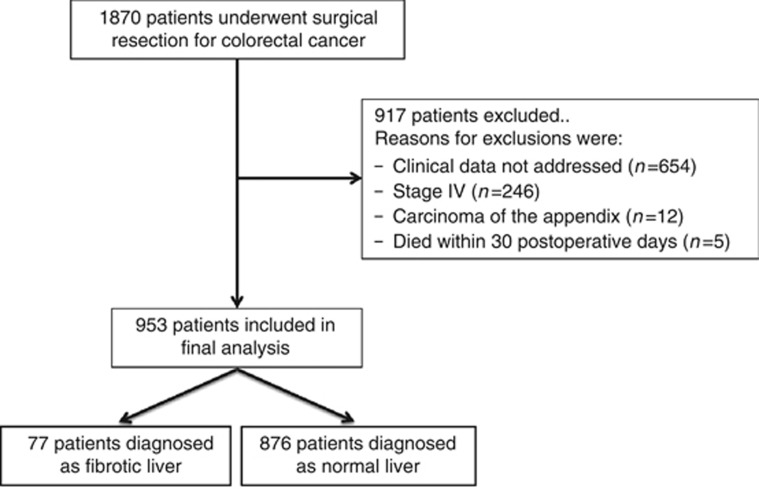
Out of a total of 1870 patients identified, 917 were excluded based on our inclusion and exclusion criteria (Figure 1). The remaining 953 patients met our criteria and were evaluated in this study.
Figure 1.
Flow diagram of the retrospective analysis with adequate data.
Data characteristics
Demographic, clinical, operative, and pathological data were obtained from hospital records. Clinical and laboratory data were collected before the operation. The laboratory evaluation included routine liver biochemistry (alanine aminotransferase (ALT), aspartate aminotransferase (Bismuth et al, 1996), and albumin levels), a complete blood count, and fasting glucose level. The presence of diabetes mellitus (DM; a fasting glucose level of 126 mg dl−1 or treatment with antidiabetic drugs) and obesity (a body mass index (BMI) >25 kg m−2) were also recorded. Tumour site was classified as the right-sided colon (from the caecum to the transverse colon), left-sided colon (from the descending to rectosigmoid colon), or rectum.
Calculation of NAFLD fibrosis score
To confirm the presence of a fibrotic liver, the NFS was applied in this analysis. The score is calculated as follows: NFS=−1.675+0.037 × age (years)+0.094 × BMI (kg m−2)+1.13 × IFG (impaired fasting glucose)/diabetes (yes=1, no=0)+0.99 × AST/ALT ratio−0.013 × platelet count (× 109/l)−0.66 × albumin level (g dl−1). Those patients with a NFS higher than 0.676 were diagnosed as having a fibrotic liver, as reported previously (Angulo et al, 2007). Patients were grouped according to high (fibrotic liver; FL) or low (normal liver; NL) NFS.
Statistical methods
The correlations of demographics with clinical and pathological data in the two groups are presented. Continuous variables are presented as means±s.d. Categorical variables are shown as the number of cases and percentages. Comparisons for continuous variables were performed using the Mann–Whitney U-test and, for binary variables, using the χ2-test or Fisher's exact test, where appropriate. Actuarial outcomes were compared using Kaplan–Meier curves and multivariate Cox proportional hazards regression. A log-rank test was used to determine any significant differences between curves. Patterns of recurrence consist of several distinct recurrence events attributed exclusively to one event, defined as a ‘competing risks situation'. Recurrences were therefore grouped as either liver-specific or extrahepatic. The cumulative incidence was estimated using each type of recurrence as a competing risk (liver-specific vs extrahepatic). The competing risks regression model defined by Fine and Gray (Zhang et al, 2011) was applied. To determine the potential effect modification by age, sex, BMI, hepatic virus, and DM, an interaction analysis was performed by adding the respective categorical variable product terms individually to the maximal model. All statistical tests were two-sided, and the level of significance was set at P=0.05. In all analyses, death before an event of interest was treated as a censoring event. All other statistical analyses were performed using Stata 12.1 (Stata Corporation, College Station, TX, USA).
Results
Characteristics of the CRC patients with or without HF by NAFLD fibrosis score
A total of 953 patients were included in this study. The mean NFS was determined to be −1.32±1.55. Of the total number of patients, 77 (8.1%) were diagnosed as having FL. The association between the demographic features and FL is shown in Table 1. FL was more frequently found in elderly patients with a poor performance status (P<0.01), DM (P<0.01), and the hepatic virus (P=0.01).
Table 1. Characteristics of the CRC patients.
| Factor | Number | Fibrotic liver | Normal liver | P-value |
|---|---|---|---|---|
| Total | 953 | 77 | 876 | |
| Age | ||||
| Mean±s.d. | 75.3±9.33 | 64.9±11.7 | <0.01 | |
| Sex | ||||
| Male | 566 | 45 (8.0%) | 521 (92.0%) | 0.9 |
| Female | 387 | 32 (8.3%) | 355 (91.7%) | |
| Location | ||||
| Right-sided colon | 336 | 31 (9.2%) | 305 (90.8%) | 0.58 |
| Left-sided colon | 407 | 29 (7.1%) | 378 (92.9%) | |
| Rectum | 210 | 17 (8.1) | 193 (91.9%) | |
| Lymphatic invasion (+) | 583 | 52 (67.53%) | 531 (91.08%) | 0.23 |
| Vascular invasion (+) | 593 | 50 (64.9%) | 543 (62.0%) | 0.61 |
| Stage | ||||
| I | 293 | 17 (5.8%) | 276 (94.2%) | 0.07 |
| II | 327 | 33 (10.0%) | 294 (90.0%) | |
| III | 333 | 27 (8.1%) | 306 (91.9%) | |
| Diabetes mellitus | 178 | 34 (44.2%) | 144 (16.44%) | <0.01 |
| Performance status | ||||
| 0, 1 | 884 | 64 (7.2%) | 820 (92.8%) | <0.01 |
| 2, 3, 4 | 69 | 13 (18.8%) | 56 (81.2%) | |
| Obesity (BMI >25) | ||||
| Absent | 743 | 56 (7.5%) | 687 (92.5%) | 0.25 |
| Present | 210 | 21 (10.0%) | 189 (90.0%) | |
| Hepatic virus | ||||
| Absent | 908 | 69 (7.6%) | 839 (92.4%) | 0.01 |
| Present | 45 | 8 (17.8%) | 37 (82.2%) | |
| Adjuvant chemotherapy | ||||
| No | 577 | 49 (8.5%) | 528 (91.5%) | 0.56 |
| Yes | 376 | 28 (7.4%) | 348 (92.6%) |
Abbreviations: BMI=body mass index; CRC=colorectal cancer.
Outcome of the patients with CRC and HF
The mean follow-up period was 51.2±32.9 months. Recurrence was observed in 156 (10.3%) of 953 patients. There were 38 hepatic recurrences, 37 lung recurrences, 28 local recurrences, 9 instances of peritoneal dissemination, and 44 multiple organ recurrences. In patients with multiple organ recurrences, 16 had hepatic recurrences. Thus, a total of 54 patients (5.7%) had hepatic recurrences. Hepatic recurrences were observed in 46 (5.3%) of 876 patients in the NL group and in 8 (10.4%) of 77 in the FL group, indicating a significant difference (P=0.02). However, no significant differences were found in other types of recurrences, such as lung, local, peritoneum, and others.
Overall recurrence
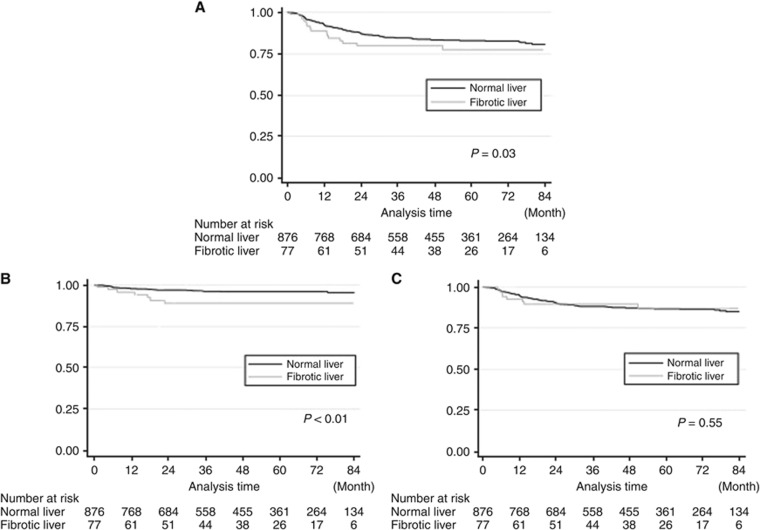
The overall recurrence-free survival (RFS) rates are shown in Figure 2A. Patients in the FL group had a significantly worse survival rate (P=0.03). We performed univariate and multivariate analyses to evaluate the significance of HF as an independent prognostic marker. HF was found to have a significant influence on the overall RFS.
Figure 2.
Kaplan–Meier curve of RFS between the FL and NL groups by the site of recurrence.(A) Recurrence-free survival curve. (B) Hepatic metastasis-free survival curve. (C) Extrahepatic metastasis-free survival curve. (A) Patients in the FL group had a significantly worse survival rate (log-rank P=0.03). (B) In hepatic metastasis, the 5-year RFS rate in the FL group was significantly poorer than that in the NL group (FL 89.1% vs NL 96.0%, log-rank P<0.01). (C) Patients in the FL group had a worse 5-year extrahepatic RFS rate (5-year hepatic RFS rate; FL 87.1% 95% CI 75.4–93.5 vs NL 86.5% 95% CI 83.8–88.7), but the difference did not reach statistical significance (log-rank P=0.55).
Site-specific recurrence
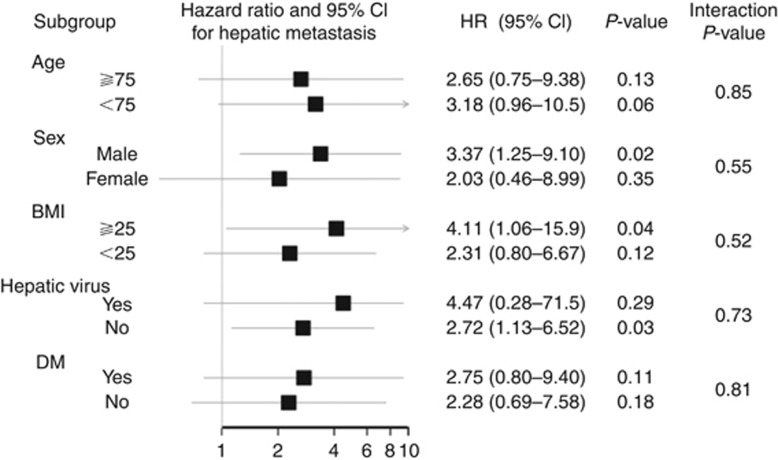
Hepatic recurrences were observed in 54 patients. The hepatic and extrahepatic RFS rates are shown in Figure 2B and C. The overall 5-year hepatic RFS rate was 95.5%, and there was a significant difference between the two groups (FL 89.1%, 95% confidence interval (CI) 78.4–94.7 vs NL 96.0%, 95% CI 94.3–97.2, P<0.01). In contrast, the extrahepatic RFS rates did not differ between the two groups. The 5-year extrahepatic RFS rate in the FL group was equivalent of that in the NL group (FL 87.1%, 95% CI 75.4–93.5 vs NL 86.5%, 95% CI 83.8–88.7, P=0.55). Univariate and multivariate analyses of the prognostic factors for liver-specific recurrence are presented in Table 2. These analyses demonstrated that HF was a significant factor for liver-specific recurrence (HR 2.98, 95% CI 1.23–7.21, P=0.02). The unadjusted HRs for hepatic RFS for these subgroups suggested that the patients' backgrounds might have some influence (Figure 3). From the interaction analysis, there was no effect modification by age, sex, BMI, hepatic virus, or DM, with overall P-values of 0.85, 0.55, 0.52, 0.73, and 0.81 for the respective product terms. The maximum size of the metastasis did not differ significantly between the two groups (mean diameter: FL 34.8±35.8 mm vs NL 24.6±14.2 mm, P=0.69). Also, the number of metastases did not differ significantly (mean number: FL 1.5±0.9 vs NL 4.3±7.9, P=0.38); neither did the distribution of these metastases (bilateral hepatic lobe metastasis: FL 14.3% vs NL 38.7%, P=0.19).
Table 2. Risk factors for any metastasis and for any hepatic metastasis.
|
Any metastasis |
Hepatic metastasis |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Univariate analysis |
Multivariate analysis |
Univariate analysis |
Multivariate analysis |
|||||||||
| Validate | HR | 95% CI | P-value | HR | 95% CI | P-value | HR | 95% CI | P-value | HR | 95% CI | P-value |
| Age >75 | 1.36 | 0.91–2.04 | 0.13 | 1.24 | 0.85–1.80 | 0.26 | 1.21 | 0.59–2.50 | 0.60 | 0.89 | 0.40–1.96 | 0.77 |
| Sex | 0.92 | 0.66–1.27 | 0.60 | 0.85 | 0.61–1.17 | 0.31 | 0.95 | 0.50–1.83 | 0.89 | 0.88 | 0.46–1.69 | 0.70 |
| Body mass index >25 | 0.57 | 0.35–0.95 | 0.03 | 1.17 | 0.57–2.40 | 0.68 | ||||||
| AST >35 | 1.83 | 1.07–3.15 | 0.03 | 0.33 | 0.46–2.42 | 0.28 | ||||||
| ALT >35 | 1.62 | 0.97–2.71 | 0.07 | 1.79 | 0.75–4.29 | 0.19 | ||||||
| Platelet count >100 000 | 1.79 | 0.25–12.8 | 0.56 | 0.58 | 0.80–4.24 | 0.59 | ||||||
| CEA >5.0 ng ml−1 | 1.90 | 1.31–2.75 | <0.01 | 1.72 | 0.89–3.33 | 0.11 | ||||||
| Diabetes mellitus | 1.11 | 0.74–1.65 | 0.62 | 1.78 | 0.88–3.59 | 0.11 | ||||||
| Hepatic virus | 1.07 | 0.53–2.19 | 0.84 | 1.09 | 0.26–4.54 | 0.90 | ||||||
| Hepatic fibrosis | 1.76 | 1.05–2.97 | 0.03 | 1.30 | 0.75–2.25 | 0.34 | 2.84 | 1.25–6.44 | 0.01 | 2.87 | 1.17–7.02 | 0.02 |
| Location | ||||||||||||
| Right-sided colon | Ref | — | — | Ref | — | — | ||||||
| Left-sided colon | 0.93 | 0.64–1.35 | 0.71 | 1.05 | 0.52–2.11 | 0.89 | ||||||
| Rectum | 1.37 | 0.92–2.05 | 0.16 | 0.73 | 0.28–1.91 | 0.52 | ||||||
| Vascular invasion | 3.42 | 2.25–5.22 | <0.01 | 1.86 | 1.21–2.87 | <0.01 | 4.43 | 1.72–11.3 | <0.01 | 2.81 | 1.06–7.43 | 0.04 |
| Stage | ||||||||||||
| I | Ref | — | — | Ref | — | — | Ref | — | — | Ref | — | — |
| II | 7.62 | 3.02–19.2 | <0.01 | 7 | 2.75–17.8 | <0.01 | 3.80 | 1.07–13.5 | 0.04 | 2.75 | 0.76–9.98 | 0.13 |
| III | 19.4 | 7.92–47.7 | <0.01 | 17.9 | 7.21–44.7 | <0.01 | 8.02 | 2.41–26.7 | <0.01 | 5.44 | 1.57–18.9 | <0.01 |
| PS 0, 1/2, 3 and 4 | 0.93 | 0.29–2.98 | 0.90 | 0.44 | 0.06–3.18 | 0.41 | ||||||
Abbreviations: ALT=alanine aminotransferase; AST=aspartate aminotransferase; CEA=carcinoembryonic antigen; CI=confidence interval; HR=hazard ratio; PS=performance status; Ref=reference.
Figure 3.
Forest plot of the subgroup analysis.Forest plot that compares the incidence of colorectal metastasis in FL to the incidence of colorectal metastasis in NL in the subgroup analysis. There was no effect modification evident between the subgroups.
Overall survival
Patients in the FL group had a significantly poorer survival rate (5-year OS rate FL 79.8% vs NL 92.2%, P<0.01). In the FL group, six (7.8%) patients died of causes other than cancer. In the NL group, 28 (3.2%) patients died of causes other than cancer. There were no significant differences between the two groups (P=0.16).
Competing-risks regression analysis
We then performed a competing-risks regression analysis to evaluate the influence of HF on metastasis from CRC (Table 3). HF was determined to be a significant risk factor for liver metastasis (HR 3.44, 95% CI 1.41–8.42, P<0.01) but was not associated with extrahepatic metastasis (HR 1.32, 95% CI 0.35–4.94, P=0.68).
Table 3. Competing-risks regression.
|
Any metastasis |
Hepatic metastasis |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Univariate analysis |
Multivariate analysis |
Univariate analysis |
Multivariate analysis |
|||||||||
| Validate | HR | 95% CI | P-value | HR | 95% CI | P-value | HR | 95% CI | P-value | HR | 95% CI | P-value |
| Age >75 | 1.35 | 0.90–2.01 | 0.15 | 1.39 | 0.91–2.13 | 0.13 | 1.19 | 0.58–2.44 | 0.64 | 0.89 | 0.41–1.97 | 0.78 |
| Sex | 0.70 | 0.35–1.38 | 0.30 | 0.82 | 0.56–1.20 | 0.31 | 0.70 | 0.35–1.38 | 0.30 | 0.85 | 0.44–1.62 | 0.62 |
| Body mass index >25 | 0.58 | 0.35–0.96 | 0.03 | 1.20 | 0.58–2.45 | 0.62 | ||||||
| AST >35 | 1.86 | 1.08–3.21 | 0.03 | 0.31 | 0.04–2.29 | 0.25 | ||||||
| ALT >35 | 1.59 | 0.95–2.66 | 0.08 | 1.72 | 0.72–4.11 | 0.22 | ||||||
| Platelet count >100 000 | 1.8 | 0.24–13.5 | 0.57 | 0.58 | 0.08–4.07 | 0.59 | ||||||
| CEA >5.0 ng ml−1 | 1.87 | 1.29–2.71 | <0.01 | 1.64 | 0.85–3.16 | 0.14 | ||||||
| Diabetes mellitus | 0.83 | 0.51–1.35 | 0.46 | 1.82 | 0.90–3.67 | 0.10 | ||||||
| Hepatic virus | 1.07 | 0.48–2.41 | 0.86 | 1.11 | 0.27–4.57 | 0.88 | ||||||
| Hepatic fibrosis | 1.57 | 0.88–2.81 | 0.13 | 0.87 | 0.42–1.82 | 0.72 | 1.07 | 0.33–3.47 | 0.92 | 2.86 | 0.98–12.8 | 0.02 |
| Location | ||||||||||||
| Right-sided colon | Ref | — | — | Ref | — | — | ||||||
| Left-sided colon | 0.86 | 0.55–1.33 | 0.49 | 1.05 | 0.52–2.10 | 0.90 | ||||||
| Rectum | 1.68 | 1.08–2.62 | 0.02 | 0.70 | 0.27–1.82 | 0.46 | ||||||
| Stage | ||||||||||||
| I | Ref | — | — | Ref | — | — | Ref | — | — | Ref | — | — |
| II | 11.9 | 3.68–38.7 | <0.01 | 15.1 | 3.63–62.8 | <0.01 | 11.9 | 3.68–38.7 | <0.01 | 3.54 | 0.98–12.8 | 0.05 |
| III | 26.2 | 8.25–83.1 | <0.01 | 43.6 | 10.7–177.9 | <0.01 | 26.2 | 8.25–83.1 | <0.01 | 7.03 | 2.12–23.3 | <0.01 |
| PS 0, 1/2, 3 and 4 | 1.55 | 0.81–2.99 | 0.19 | 0.42 | 0.06–3.08 | 0.39 | ||||||
Abbreviations: ALT=alanine aminotransferase; AST=aspartate aminotransferase; CEA=carcinoembryonic antigen; CI=confidence interval; HR=hazard ratio; PS=performance status; Ref=reference.
Discussion
To the best of our knowledge, this is the first study to report on the prognostic significance of HF categorised by the NFS (>0.676) following curative resection of primary colorectal tumours. The findings of this study demonstrated that patients in the FL group had a four-fold increased risk of liver metastasis compared with those in the NL group, whilst HF had no influence on the development of extrahepatic recurrence. Competing risk regression also demonstrated that HF is an independent risk factor for liver metastasis only, suggesting that tumour cells can metastasise under the fibrotic environment caused by NAFLD. These findings suggest involvement of HF in the development of liver metastasis and might offer a valuable insight for future investigations.
There are several known prognostic factors for liver metastasis, comprising demographic features and clinical risk scores. Histopathological findings such as mucinous histology, tumour budding, and extramural venous invasion are the most well-known and widely used prognostic factors for CRC patients (Edge and Compton, 2010; Kim et al, 2013; Rogers et al, 2014). Although these were valuable predictive factors in terms of the prediction of recurrence patterns, they were not able to predict organ-specific recurrence. Our findings revealed that the NFS obtained from only six metabolic and inflammatory variables could specifically predict liver metastasis, compared with conventional predictive factors. Further investigations are required to evaluate the interaction between tumour cells and steatohepatitis.
The findings of this study have provided new insight into the role of organ-specific microenvironments in the development of metastasis. Several studies have assessed the relationship between fibrosis and metastasis. In a murine model, suppression of hepatic fibrosis was shown to reduce the development of hepatic metastasis, which indicates that excessive deposition of connective tissue matrix in the liver might accelerate invasion and proliferation of cancer cells. Hepatic stellate cells (HSC) also reportedly have an important role in establishing hepatic fibrosis by releasing cytokines, such as tumour growth factor β and hepatocyte growth factor, which are necessary for hepatocyte regeneration (Matsusue et al, 2009). Given that cytokines can enhance the invasiveness of cancer cells, crosstalk between HSCs and colon cancer cells could be an alternative mechanism for liver metastasis formation (Shen et al, 2014). Although these studies are still at an early stage, they may lead to the development of treatments for liver metastatic inhibition via inhibition of local inflammation and cytokine excretion.
In contrast to the findings of this study, two other studies reported conflicting results. One such study used liver-to-spleen ratio (LSR) attenuation values in CT and demonstrated that hepatic metastases derived from CRC occur less frequently in patients with hepatic steatosis (Murono et al, 2013). Considering that several previous studies have demonstrated that the NFS is better than the LSR in terms of diagnosing HF, the results obtained using the LSR might therefore underestimate the influence of HF. It has been reported that colorectal hepatic metastases rarely occur in patients with a chronic hepatitis viral infection (Wang et al, 2012); however, in such patients, activation of the immune system might protect from the development of liver metastasis.
There were some limitations to the present study. First, this was a retrospective analysis at a single institution, and therefore a potential risk of selection bias existed. The validation of these results is thus required by a multicentre study. Second, the association (proved pathologically) between liver metastasis and HF remains unclear. Pathological findings are useful to provide robust evidence in terms of the immunological and histological aspects. However, our patients had disease of stages I/II/III. It is ethically difficult to obtain liver tissue samples from such patients. Moreover, transabdominal real-time elastography, which is a new method for non-invasive staging of liver fibrosis, might be useful as an alternative procedure. Finally, there are still many unanswered questions regarding the significance of a change in the NFS. If a reduction in the NFS is associated with the prevention of liver metastasis, regulation of the NFS could be important.
Conclusion
In this study, we found that the NFS is a valuable prognostic factor for liver metastasis in CRC patients. The findings of this study offer numerous suggestions for crosstalk between tumour cells and the organ microenvironment. These data suggest that poorer survival from colorectal cancer is an additional cause of excess mortality in those with hepatic fibrosis due to NASH.
The authors declare no conflict of interest.
Footnotes
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 4.0 Unported License.
References
- Adson MA, van Heerden JA, Adson MH, Wagner JS, Ilstrup DM (1984) Resection of hepatic metastases from colorectal cancer. Arch Surg 119(6): 647–651. [DOI] [PubMed] [Google Scholar]
- Angulo P, Hui JM, Marchesini G, Bugianesi E, George J, Farrell GC, Day CP (2007) The NAFLD fibrosis score: a noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology 45(4): 846–854. [DOI] [PubMed] [Google Scholar]
- Augustin G, Bruketa T, Korolija D, Milosevic M (2013) Lower incidence of hepatic metastases of colorectal cancer in patients with chronic liver diseases: meta-analysis. Hepatogastroenterology 60(125): 1164–1168. [DOI] [PubMed] [Google Scholar]
- Berney T, Mentha G, Roth AD, Morel P (1998) Results of surgical resection of liver metastases from non-colorectal primaries. Br J Surg 85(10): 1423–1427. [DOI] [PubMed] [Google Scholar]
- Bismuth H, Adam R, Levi F, Farabos C, Waechter F, Castaing D, Engerran L (1996) Resection of nonresectable liver metastases from colorectal cancer after neoadjuvant chemotherapy. Ann Surg 224(4): 509–520, discussion 520–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox TR, Bird D, Baker AM, Barker HE, Ho MW, Lang G, Erler JT (2013) LOX-mediated collagen crosslinking is responsible for fibrosis-enhanced metastasis. Cancer Res 73(6): 1721–1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edge SB, Compton CC (2010) The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol 17(6): 1471–1474. [DOI] [PubMed] [Google Scholar]
- Fong Y, Fortner J, Sun RL, Brennan MF, Blumgart LH (1999) Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg 230(3): 309–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SH, Shin SJ, Lee KY, Kim H, Kim TI, Kang DR, Ahn JB (2013) Prognostic value of mucinous histology depends on microsatellite instability status in patients with stage III colon cancer treated with adjuvant FOLFOX chemotherapy: a retrospective cohort study. Ann Surg Oncol 20(11): 3407–3413. [DOI] [PubMed] [Google Scholar]
- Matsusue R, Kubo H, Hisamori S, Okoshi K, Takagi H, Hida K, Sakai Y (2009) Hepatic stellate cells promote liver metastasis of colon cancer cells by the action of SDF-1/CXCR4 axis. Ann Surg Oncol 16(9): 2645–2653. [DOI] [PubMed] [Google Scholar]
- Murono K, Kitayama J, Tsuno NH, Nozawa H, Kawai K, Sunami E, Watanabe T (2013) Hepatic steatosis is associated with lower incidence of liver metastasis from colorectal cancer. Int J Colorectal Dis 28(8): 1065–1072. [DOI] [PubMed] [Google Scholar]
- Povero D, Eguchi A, Li HY, Johnson CD, Papouchado BG, Wree A, Feldstein AE (2014) Circulating extracellular vesicles with specific proteome and liver mcroRNAs are potential biomarkers for liver injury in experimental fatty liver disease. Plos One 9(12): e113651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers AC, Gibbons D, Hanly AM, Hyland JM, O'Connell PR, Winter DC, Sheahan K (2014) Prognostic significance of tumor budding in rectal cancer biopsies before neoadjuvant therapy. Mod Pathol 27(1): 156–162. [DOI] [PubMed] [Google Scholar]
- Shen A, Chen H, Chen Y, Lin J, Lin W, Liu L, Peng J (2014) Pien Tze Huang overcomes multidrug resistance and epithelial-mesenchymal transition in human colorectal carcinoma cells via suppression of TGF-beta pathway. Evid Based Complement Alternat Med 2014: 679436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang FS, Shao ZG, Zhang JL, Liu YF (2012) Colorectal liver metastases rarely occur in patients with chronic hepatitis virus infection. Hepatogastroenterology 59(117): 1390–1392. [DOI] [PubMed] [Google Scholar]
- Zhang X, Zhang MJ, Fine J (2011) A proportional hazards regression model for the subdistribution with right-censored and left-truncated competing risks data. Stat Med 30(16): 1933–1951. [DOI] [PMC free article] [PubMed] [Google Scholar]