Abstract
Background:
Accurate cause of death assignment is crucial for prostate cancer epidemiology and trials reporting prostate cancer-specific mortality outcomes.
Methods:
We compared death certificate information with independent cause of death evaluation by an expert committee within a prostate cancer trial (2002–2015).
Results:
Of 1236 deaths assessed, expert committee evaluation attributed 523 (42%) to prostate cancer, agreeing with death certificate cause of death in 1134 cases (92%, 95% CI: 90%, 93%). The sensitivity of death certificates in identifying prostate cancer deaths as classified by the committee was 91% (95% CI: 89%, 94%); specificity was 92% (95% CI: 90%, 94%). Sensitivity and specificity were lower where death occurred within 1 year of diagnosis, and where there was another primary cancer diagnosis.
Conclusions:
UK death certificates accurately identify cause of death in men with prostate cancer, supporting their use in routine statistics. Possible differential misattribution by trial arm supports independent evaluation in randomised trials.
Keywords: cluster randomised controlled trial, screening, prostate cancer, prostate cancer mortality, cause of death, death certification, sensitivity, specificity
Prostate cancer is the second commonest cause of cancer death in UK men (UK National Screening Committee, 2015). Death certificates are used in routine mortality statistics, large-scale epidemiological studies and randomised controlled trials. However, prostate cancer can be misattributed as the underlying cause of death on death certificates in men diagnosed with prostate cancer (Feuer et al, 1999). A review of US medical records (Albertsen et al, 2000) suggested that 29% of men with prostate cancer as the underlying cause of death on death certificates had died of some other condition (Albertsen et al, 2000).
The possibility of differential attribution bias in trials, where the primary end point is prostate cancer-specific mortality, is also a concern (Black et al, 2002). All-cause mortality is least open to bias (Black et al, 2002), but because prostate cancer death is relatively uncommon (UK National Screening Committee, 2015), all-cause mortality is less sensitive to the effects of screening.
The possibility of attribution bias has led us and others (Miller et al, 2000; de Koning et al, 2003; Miller et al, 2015) to conclude that assignment of the underlying cause of death in prostate cancer trials must be confirmed by an independent expert (CoDE) committee. We have compared the underlying cause of death determined by an independent CoDE committee, with the underlying cause of death listed on official death certificates in UK men with prostate cancer participating in a UK-wide trial (Lane et al, 2014; Turner et al, 2014).
Methods
Follow-up and identification of a prostate cancer-related event
All 413 000 men enrolled in the Cluster randomised trial of PSA testing for prostate cancer (CAP) trial have been traced and flagged for vital status follow-up at the Health and Social Care Information Centre (Turner et al, 2014). Blinded to death certificate and underlying cause of death, detailed information was obtained from the medical records of all men with a potential prostate cancer death (see Supplementary Table 1 for the triggers used to review a potential prostate cancer death), and used to generate a short structured clinical vignette (Williams et al, 2015); (see Supplementary Material 1).
Determination of cause of death
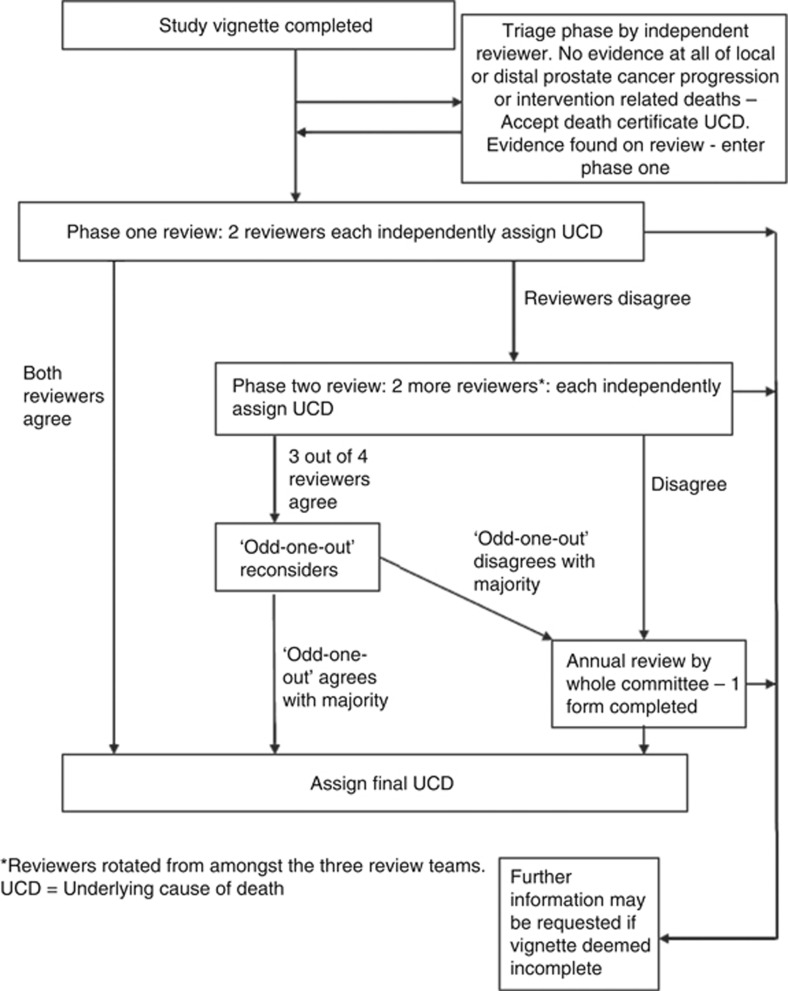
Members of an international CoDE committee reviewed the vignettes. They completed a questionnaire that when followed in sequence, and using detailed definitions adapted from the European Randomised Study of Screening for Prostate Cancer trial (de Koning et al, 2003) acted as an algorithm for assigning cause of death into the following categories: definite, probable, possible, unlikely or definitely not prostate cancer, and definite or probable intervention-related mortality (Supplementary Material 2). The committee was divided into three teams, each comprising four consultants from the following specialties: pathology, palliative care, urology and cancer surgery (see Figure 1 and Supplementary Material 1). The death certificate underlying cause of death was accepted where a review was not triggered (i.e., in the absence of a potential prostate cancer death, these were recorded as due to other causes).
Figure 1.
Process for evaluating cause of death.
Analysis
We examined the agreement between prostate cancer assigned as the underlying cause of death on the death certificate with prostate cancer (definite, probable or intervention-related) assigned as the cause of death after expert review. We calculated ‘sensitivity' as the proportion of confirmed prostate cancer deaths as assigned by the expert review process (denominator), which were listed as an underlying cause of death of prostate cancer on the death certificate (numerator) (Box 1). We calculated ‘specificity' as proportion of confirmed non-prostate cancer deaths as above. Each of sensitivity and specificity are accompanied by exact binomial (Clopper–Pearson) confidence intervals (Clopper and Pearson, 1934). We investigated whether sensitivity and specificity varied by age-group (splitting age at death into three groups of approximately equal size), the interval between date of diagnosis and date of death, and the presence of another cancer diagnosis. All parameters were calculated with their respective 95% confidence intervals.
Box 1.
Calculation of sensitivity and specificity
PCa=prostate cancer
CoDE=cause of death evaluation
Ethics
Ethical approval was provided by Trent Research Ethics Committee (MREC/01/4/025; MREC/03/4/093; 05/MRE04/78) and the Confidentiality Advisory Group (PIAG 4–09 (k)/2003; PIAG 1-05(f)/2006).
Results
Over 50 000 deaths were notified to the CAP investigators by the Health and Social Care Information Centre between 2002 and 2015. Of these, 2069 men had died of a potential prostate cancer-related death, and the underlying cause of death has been established for 1236 (60%) of these men to date.
Table 1 shows the number of deaths assigned as prostate cancer or other cause on the death certificates compared with the expert review process. Of a total of 1236 potential prostate cancer-related deaths, the independent CoDE committee attributed 523 (42%) to prostate cancer and 713 (58%) to other causes. The corresponding cause of death categories based on death certificates were 535 (43%) and 701 (57%), respectively. The expert committee agreed with death certificate derived underlying cause of death (prostate cancer or other) in 1134 cases (92% agreement, 95% CI: 90, 93%).
Table 1. Sensitivity and specificity of prostate cancer as an underlying cause of death on the death certificate vs prostate cancer assigned as the underlying cause of death after expert review of clinical vignettes, stratified by age at death, time between diagnosis and death, and presence or absence of another primary cancer diagnosis (n=1236).
| Variable | N | Sensitivity, % (95% CI); N | Specificity, % (95% CI); N |
|---|---|---|---|
| Total | 1236 | 91 (89, 94); N=523 | 92 (90, 94); N=713 |
|
Age (years) | |||
| <65 | 287 | 93 (87, 97); N=119 | 92 (86, 95); N=168 |
| 65–70 | 365 | 91 (85, 94); N=169 | 92 (88, 96); N=196 |
| >70 | 584 | 91 (87, 94); N=235 | 92 (89, 95); N=349 |
|
Years between diagnosis and death | |||
| Not notified via cancer registry | 216a | 100 (54, 100); N=6 | 99 (97–100); N=210 |
| <1 | 231 | 87 (80, 93); N=117 | 78 (69, 85); N=114 |
| 1–3 | 390 | 93 (89, 96); N=249 | 87 (80, 92); N=141 |
| >3 | 399 | 92 (87, 96); N=151 | 96 (92, 98); N=248 |
|
Other primary cancer presentb | |||
| Yes | 369 | 77 (65, 86); N=65 | 89 (85, 92); N=304 |
| No | 867 | 93 (91, 96); N=458 | 94 (91, 96); N=409 |
Sixty-six% were carcinomatosis; also includes PCa on death certificate only.
In addition to PCa.
Eight per cent of deaths categorised as due to other causes after review of the case vignettes had been assigned as prostate cancer on death certificates (death certificate specificity: 92% 95% CI: 90%, 94%). On the other hand, 9% of deaths classified as due to prostate cancer by the reviewers were assigned to other causes on the death certificates (death certificate sensitivity: 91% 95% CI: 89%, 94%).
For men who died within 1 year of their diagnosis of prostate cancer, the death certificates had a sensitivity of 78% (95% CI: 69%, 85%) and specificity of 87% (95% CI: 80%, 93%); for men who died between 1 and 3 years of diagnosis, specificity was 87% (95% CI: 80%, 92%) and sensitivity was 93% (95% CI: 89%, 96%). The presence of another cancer diagnosis, either notified by Health and Social Care Information Centre or present on the death certificate, was also associated with a lower sensitivity (77% 95% CI: 65%, 86%) and specificity (89% 95% CI: 85%, 91%). The age at death had little impact on sensitivity or specificity. Three of the 1236 deaths were categorised as intervention-related deaths by the committee.
Discussion
These data suggest that relying on underlying cause of death abstracted from official UK death certificates rather than an independent expert committee would result in some misattribution. Specifically, 9% of deaths assigned as being due to prostate cancer by the CoDE committee were recorded on death certificates as deaths from other causes (false negatives), and 8% of deaths considered on the death certificate to be due to prostate cancer were assigned to other causes (false positives) by the expert review. The impact of age was minimal, suggesting the use of UK death certificates could provide a relatively accurate means of evaluating population trends in prostate cancer mortality. However, where there was a death within 1 year of diagnosis of prostate cancer, both false positives (22%) and false negatives (13%) increased. This could reflect a tendency for competing causes of death to be less frequently considered by doctors completing death certificates when a prostate cancer diagnosis has only recently been made. The presence of another primary cancer, either on the death certificate or diagnosed as alive, also resulted in increased false positives (23%) and false negatives (11%). This could be because the clinical picture is unclear in these cases.
These results are based only on those deaths that were triggered for in-depth review by the expert committee because they were potential prostate cancer deaths. We did not review the other 49 000 deaths where there was no evidence of prostate cancer ever being diagnosed or where there was no evidence of other conditions that could have indicated a potential prostate cancer death, such as bone cancer (conceptualised as a potential misclassified bony metastasis). If all these other 49 000 deaths are correctly assumed not to be due to prostate cancer, this will have resulted in near perfect specificity for all deaths.
Similar level of agreement between death certification and expert review were observed in the USA (Albertsen et al, 2000), Sweden (Godtman et al, 2011) and Finland (Makinen et al, 2008). Common reasons for misclassification were cardiovascular or cancer co-morbidities (Albertsen et al, 2000). In a recent study (Miller et al, 2015), agreement between death certificate and death review committee was >90%, but death certificated causes of death missed treatment-related deaths and the misattribution was differential by trial arm.
The study's strength was that it was based on large trials, we identified intervention-related deaths, and we successfully masked the trial arm from the expert committee (Williams et al, 2015), even though this was reported to be difficult in another trial (Barry et al, 2013). Limitations are that the results may not be generalisable beyond the cohorts included in the trials, and the assumption that CoDE results were near perfect in accuracy.
UK death certificates provide a relatively accurate means for evaluating cause of death that would be acceptable for routine public health monitoring and large-scale epidemiological studies. In the context of randomised controlled trials, the potential for even a small amount of misattribution bias that is differential by trial arm means that an independent cause of death evaluation is likely to be necessary to provide unbiased outcome data.
Acknowledgments
We acknowledge the contribution of the CAP trial group. Investigators: RMM (Lead PI), JLD (PI), DEN (PI), FCH (PI), ELT (trial Co-ordinator), CM (statistician), JACS (statistician) and SN (health economist), JAL. Research staff: EMH, SYN, NJW, LD (data manager), EW (data manager), Joanna Thorn (health economist), CFD, LJH, Mari-Anne Rowlands and Lindsey Bell. Management committee: ELT (Chair), RMM, JLD, CM, JACS, SN, YBS, JAL, SEO, PB and SE. Trial steering committee: MB (Chair), PA, Tracy Roberts, MCR, JA, David Dearnaley, AZ, Fritz Schröder, Tim Peters, Peter Holding, Teresa Lennon, Sue Bonnington, Malcolm Mason, JO, RMM, JLD, DEN, FCH, ELT and JAL. Data monitoring committee: Lars Holmberg (Chair), Robert Pickard, Simon Thompson and Usha Menon. Cause of Death Committee: PA (Chair), CMR, JMF, JO, MCR, JA, MB, AZ, Amit Bahl and AK. Administrative staff: Marta Tazewell and Genevieve Hatton-Brown. We wish to extend our thanks to Pete Shiarly for the development of bespoke databases. We also wish to acknowledge the contribution of all members of the ProtecT study research groups. Thanks are extended to the Cancer Registries and staff at the Health and Social Care Information Centre. We would also like to thank all the men who participated in this study. The CAP trial is funded by Cancer Research UK and the UK Department of Health (C11043/A4286, C18281/A8145, C18281/A11326 and C18281/A15064). The ProtecT trial is funded by the UK National Institute for Health Research (NIHR) and Health Technology Assessment Programme (projects 96/20/06, 96/20/99). The views and opinions expressed herein are our own and do not necessarily reflect those of the Department of Health. We acknowledge the tremendous contribution of all the ProtecT study participants, investigators, researchers, data monitoring committee and trial steering committee (Chair: Michael Baum). We acknowledge the support from the Oxford NIHR Biomedical Research Centre through the Surgical Innovation and Evaluation Theme, and the Surgical Interventional Trials Unit, and Cancer Research UK through the Oxford Cancer Research Centre. Professors Donovan, Hamdy and Neal are NIHR Senior Investigators. CAP is sponsored by the University of Bristol and is registered at Current Controlled Trials (ISRCTN92187251). ProtecT trial is sponsored by the University of Oxford and is registered at Current Controlled Trials (ISRCTN20141297) and Clinical Trials.Gov (NCT02044172).
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on British Journal of Cancer website (http://www.nature.com/bjc)
Supplementary Material
References
- Albertsen PC, Walters S, Hanley JA (2000) A comparison of cause of death determination in men previously diagnosed with prostate cancer who died in 1985 or 1995. J Urol 163: 519–523. [PubMed] [Google Scholar]
- Barry MJ, Andriole GL, Culkin DJ, Fox SH, Jones KM, Carlyle MH, Wilt TJ (2013) Ascertaining cause of death among men in the prostate cancer intervention versus observation trial. Clin Trials 10: 907–914. [DOI] [PubMed] [Google Scholar]
- Black WC, Haggstrom DA, Welch HG (2002) All-cause mortality in randomized trials of cancer screening. J Natl Cancer Inst 94: 167–173. [DOI] [PubMed] [Google Scholar]
- Clopper CJ, Pearson ES (1934) The use of confidence or fiducial limits illustrated in the case of the binomial. Biometrika 26: 404–413. [Google Scholar]
- de Koning HJ, Blom J, Merkelbach JW, Raaijmakers R, Verhaegen H, Van VP, Nelen V, Coebergh JW, Hermans A, Ciatto S, Makinen T (2003) Determining the cause of death in randomized screening trial(s) for prostate cancer. BJU Int 92(Suppl 2): 71–78. [DOI] [PubMed] [Google Scholar]
- Feuer EJ, Merrill RM, Hankey BF (1999) Cancer surveillance series: interpreting trends in prostate cancer—part II: Cause of death misclassification and the recent rise and fall in prostate cancer mortality. J Natl Cancer Inst 91: 1025–1032. [DOI] [PubMed] [Google Scholar]
- Godtman R, Holmberg E, Stranne J, Hugosson J (2011) High accuracy of Swedish death certificates in men participating in screening for prostate cancer: a comparative study of official death certificates with a cause of death committee using a standardized algorithm. Scand J Urol Nephrol 45: 226–232. [DOI] [PubMed] [Google Scholar]
- Lane JA, Donovan JL, Davis M, Walsh E, Dedman D, Down L, Turner EL, Mason MD, Metcalfe C, Peters TJ, Martin RM, Neal DE, Hamdy FC ProtecT study group (2014) Active monitoring, radical prostatectomy, or radiotherapy for localised prostate cancer: study design and diagnostic and baseline results of the ProtecT randomised phase 3 trial. Lancet Oncol 15: 1109–1118. [DOI] [PubMed] [Google Scholar]
- Makinen T, Karhunen P, Aro J, Lahtela J, Maattanen L, Auvinen A (2008) Assessment of causes of death in a prostate cancer screening trial. Int J Cancer 122: 413–417. [DOI] [PubMed] [Google Scholar]
- Miller AB, Feld R, Fontana R, Gohagan JK, Jatoi I, Lawrence W Jr, Miller A, Prorok PC, Rajput A, Sherman M, Welch G, Wright P, Yurgalevitch S, Albertsen P (2015) Changes in and impact of the death review process in the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial. Rev Recent Clin Trials 10: 206–211. [DOI] [PubMed] [Google Scholar]
- Miller AB, Yurgalevitch S, Weissfeld JL (2000) Death review process in the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial. Control Clin Trials 21: 400S–406S. [DOI] [PubMed] [Google Scholar]
- Turner EL, Metcalfe C, Donovan JL, Noble S, Sterne JA, Lane JA, Avery KN, Down L, Walsh E, Davis M, Ben-Shlomo Y, Oliver SE, Evans S, Brindle P, Williams NJ, Hughes LJ, Hill EM, Davies C, Ng SY, Neal DE, Hamdy FC, Martin RM CAP trial group (2014) Design and preliminary recruitment results of the Cluster randomised triAl of PSA testing for Prostate cancer (CAP). Br Jf Cancer 110: 2829–2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UK National Screening Committee (2015) Screening for Prostate Cancer Review 2014 Update: Review Against Programme Appraisal Criteria for the UKNSC.
- Williams NJ, Hill EM, Ng SY, Martin RM, Metcalfe C, Donovan JL, Evans S, Hughes LJ, Davies CF, Hamdy FC, Neal DE, Turner EL (2015) Standardisation of information submitted to an endpoint committee for cause of death assignment in a cancer screening trial—lessons learnt from CAP (Cluster randomised triAl of PSA testing for prostate cancer). BMC Med Res Methodol 15: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.