Abstract
Embryophyte genomes typically encode multiple 13-lipoxygenases (13-LOXs) that initiate the synthesis of wound-inducible mediators called jasmonates. Little is known about how the activities of these different LOX genes are coordinated. We found that the four 13-LOX genes in Arabidopsis thaliana have different basal expression patterns. LOX2 expression was strong in soft aerial tissues, but was excluded both within and proximal to maturing veins. LOX3 was expressed most strongly in circumfasicular parenchyma. LOX4 was expressed in phloem-associated cells, in contrast to LOX6, which is expressed in xylem contact cells. To investigate how the activities of these genes are coordinated after wounding, we carried out gene expression analyses in 13-lox mutants. This revealed a two-tiered, paired hierarchy in which LOX6, and to a lesser extent LOX2, control most of the early-phase of jasmonate response gene expression. Jasmonates precursors produced by these two LOXs in wounded leaves are converted to active jasmonates that regulate LOX3 and LOX4 gene expression. Together with LOX2 and LOX6, and working downstream of them, LOX3 and LOX4 contribute to jasmonate synthesis that leads to the expression of the defense gene VEGETATIVE STORAGE PROTEIN2 (VSP2). LOX3 and LOX4 were also found to contribute to defense against the generalist herbivore Spodoptera littoralis. Our results reveal that 13-LOX genes are organised in a regulatory network, and the data herein raise the possibility that other genomes may encode LOXs that act as pairs.
Keywords: jasmonic acid, jasmonate, oxylipin, eicosanoid, wounding, defense, herbivore
1. Introduction
Lipoxygenases (LOXs) function to produce lipid mediators that operate in a broad range of processes, many of which are related to defense in animals [1] and in plants [2]. The Arabidopsis thaliana genome encodes six LOXs of which four are 13-LOXs, where “13” refers to the carbon atom in polyunsaturated 18-carbon fatty acids that are preferentially oxygenated by the LOX. 13-LOXs incorporate molecular oxygen into α-linolenic acid to produce its 13(S)-hydroperoxide [3], a molecule that is transformed into jasmonates which regulate wound-induced defense gene expression [4,5]. To complete jasmonate synthesis, fatty acid hydroperoxides formed through LOX action are dehydrated and cyclized to form the intermediate 12-oxo-phytodienoic acid (OPDA) in reactions catalysed by allene oxide synthase (AOS) and auxiliary proteins [6,7]. Further transformations of OPDA then result in the production of jasmonic acid (JA) and its biologically active derivatives, chief among which is jasmonyl-isoleucine (JA-Ile) [8].
Jasmonates (and/or their immediate precursors) produced in response to wounding do not stay where they are formed and can be transported efficiently within tissues. Following wounding, jasmonates/jasmonate precursors produced via LOX6 action in the vasculature of Arabidopsis leaves are highly mobile and move radially outwards from veins into the mesophyll [9]. In addition to jasmonate mobility, many 13-LOXs are themselves jasmonate-inducible. Therefore, in theory, jasmonates produced via the activity of any 13-LOX could be dispersed to different cell types capable of expressing other LOXs that also make jasmonate precursors. This raises an obvious question: how is the activity of the four 13-LOX genes in Arabidopsis coordinated?
The roles of 13-LOXs in jasmonate-controlled defense responses have been studied in numerous plants, including, but not restricted to potato [10], wild tobacco [11], tomato [12], rice [13], and maize [14], as well as in Arabidopsis, a plant in which systematic LOX gene mutagenesis has been employed [15]. Intriguingly, while all four 13-LOXs encoded in the A. thaliana genome contribute to the synthesis of jasmonic acid [15], they each appear to have somewhat different functions in physically damaged leaves—the subject of the present work. For example, in addition to the initiation of JA synthesis in wounded leaves [16,17], LOX2 also plays a minor role in JA synthesis in undamaged leaves distal to wounds [18]. Furthermore, close to the site of damage, LOX2-derived hydroperoxides are also channelled into the synthesis of arabidopsides, galactolipids that carry one or more esterified OPDA or dinor-OPDA molecules [17,19,20,21]. Consistent with arabidopsides being defensive secondary metabolites, plants lacking LOX2 were more susceptible to the lepidopteran herbivore Spodoptera littoralis than is the wild type [17].
LOX6 also plays a role in leaf defense. The experiments that revealed this role involved the genetic removal of each of the three other 13-LOXs through producing a lox2 lox3 lox4 triple mutant. In this plant, LOX6 functioning alone was capable of maintaining the defense of emerging leaves and shoot apical tissues in Arabidopsis rosettes [15]. Interestingly, the relative impact of LOX6 in early wound-stimulated jasmonate production in leaves increases with the distance from damage sites. That is, LOX6 was necessary for most of the rapid distal expression of the regulatory gene JASMONATE ZIM-DOMAIN 10 (JAZ10) when the rosette was wounded [15], making this LOX of particular relevance in studies of long distance wound signalling.
Finally, the LOX3/LOX4 pair contributes approximately 20% of the total JA pool that accumulates in leaves in the first three minutes after wounding [15], however, no roles for these two LOXs in leaves are known. Here, we investigated the relative contributions of LOX2, LOX3, LOX4, and LOX6 to each other’s expression, as well as to the expression of a defense gene. We then used herbivory assays to investigate LOX3 and LOX4 function in leaves. Our results revealed a lipoxygenase network that operates to coordinate jasmonate synthesis and defense responses in wounded leaves.
2. Results and Discussion
2.1. 13-LOX Expression Patterns in Unwounded Rosettes
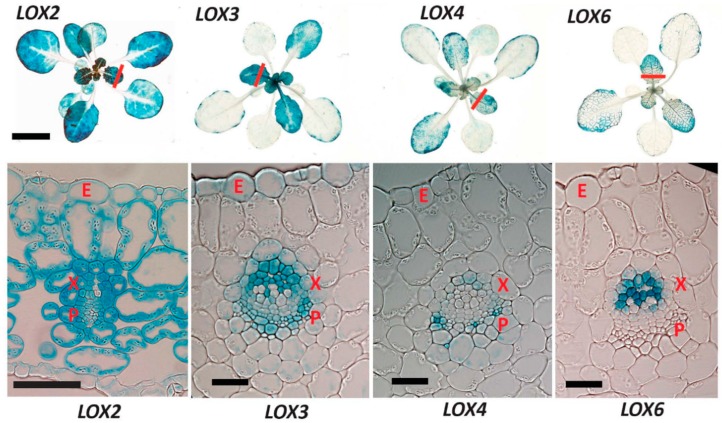
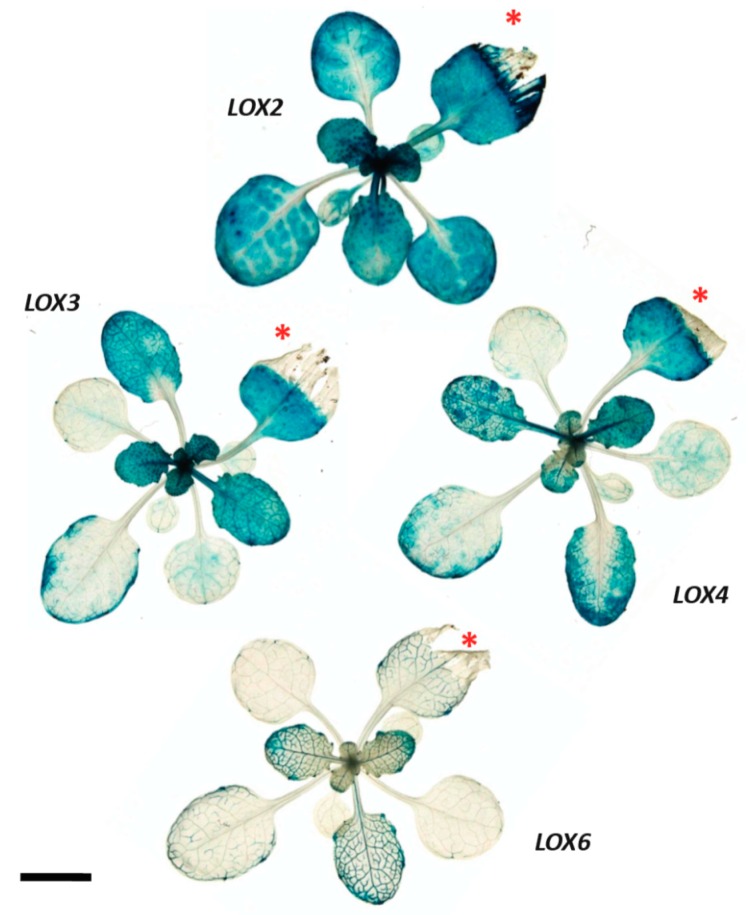
The expression patterns of Arabidopsis LOXs have been examined at the seedling stage [22], but equivalent data for leaves were lacking. Is each 13-LOX expressed in a different leaf cell type? To characterize basal 13-LOX gene activity in unwounded leaves, each promoter was fused to a secretable β-glucuronidase (GUS) reporter gene. LOX6, principally expressed in xylem contact cells [9,15], served as a comparison with other 13-LOXs, as shown in Figure 1. GUS staining in younger leaves was stronger than in older leaves for all 13-LOX promoters, and sections of younger leaves were compared. LOX2 had the only promoter among the four that was widely active in most tissues except in and near maturing veins. Because of this, transversal sections for visualizing LOX2 reporter expression were cut nearer the leaf tip than for the other reporters (red bars in Figure 1). LOX2 protein is readily detectable in leaves [23] and LOX2 expression was strong in mesophyll cells, bundle sheaths, and leaf-tip vasculature—but only at a distance from maturing veins. LOX3 activity was perivascular and strongest in the xylem region, with weaker expression in the mesophyll. LOX4 promoter activity was strongest in small cells in the phloem region. These might be companion cells, although their identity was not verified. Phloem is a known region of JA synthesis enzyme localisation [24]. We noted that LOX2 expression was almost a “mirror image” of the LOX3, LOX4, and LOX6. The expression of these three LOXs (unlike for LOX2) was strong in the maturing vasculature of expanding leaves.
Figure 1.
13-lipoxygenases (13-LOX) promoter-driven β-glucuronidase (GUS) activity in 3.5 week-old plants. Upper images: rosettes. Scale bar = 1 cm. Red bars indicate approximate section locations shown in lower images. E = epidermal cell; X = xylem region; P = phloem region. Scale bars for sections = 30 µm. Note that the LOX2 section was cut nearer the leaf tip than the other LOX sections.
Notably, all 13-LOXs are expressed in vascular tissues with only basal LOX2 expression excluded from maturing veins. LOX3 and LOX6 expression is strongest near the xylem. LOX4 is the only 13-LOX to show basal expression almost exclusively in the phloem region. In terms of cellular space covered by promoter activities, LOX4 and LOX6 in unwounded leaves display a relatively small basal promoter activity domain, whereas the other two 13-LOXs (LOX2 and LOX3) have more extensive basal activity domains (Figure 1). GUS staining in the rosettes of wounded plants is shown in the Appendix (Figure A1).
2.2. LOX2 and LOX6 Regulate LOX3 and LOX4 Expression
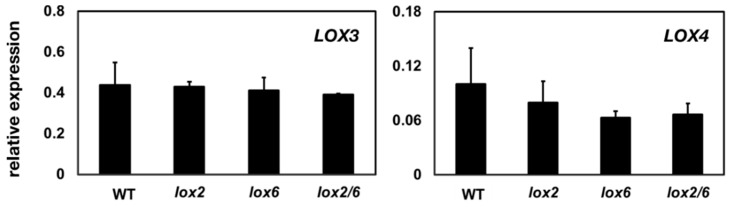
LOX6 transcripts are not wound-inducible in roots [25] or leaves [9,15]. LOX2 expression was investigated in the lox6 single mutant and the lox3 lox4 double mutant. LOX2 remained wound-inducible in each of the genetic backgrounds, however, in the absence of the functional LOX6 gene, there was weakly reduced LOX2 expression following wounding (Figure 2). LOX6 activity therefore contributes to LOX2 gene expression in wounded leaves. The possibility that there are compensatory effects whereby above-WT activity of a particular 13-LOX gene is stimulated by mutation of one or more of its homologues was tested. Figure A2 shows that basal LOX3 or LOX4 expression was not affected in unwounded leaves in the lox2- and lox6-containing backgrounds.
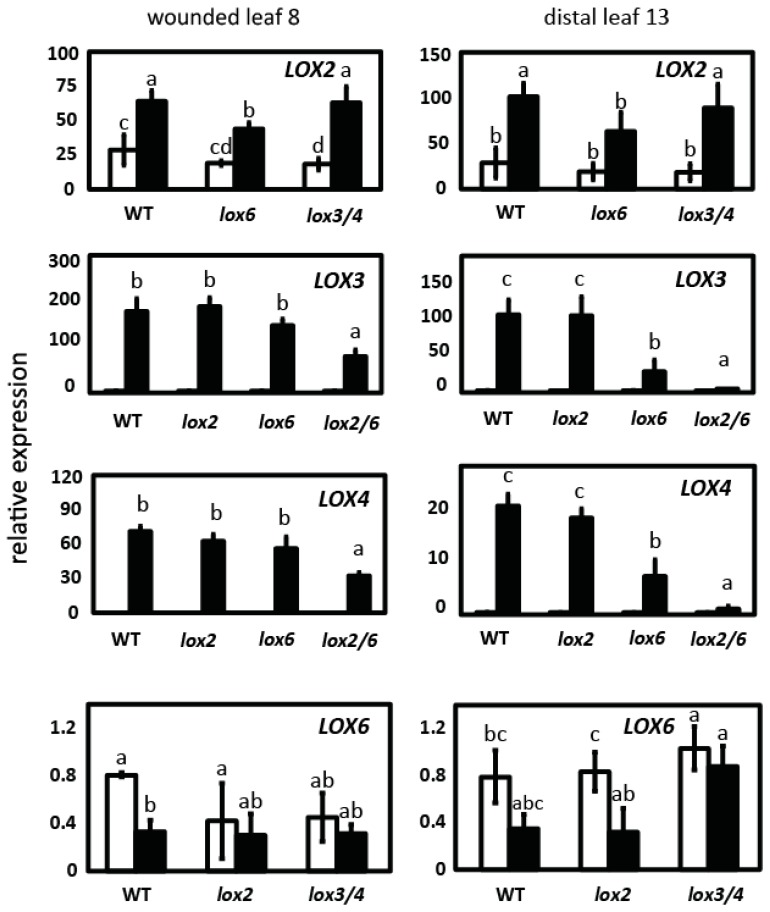
Figure 2.
13-LOX gene expression in different 13-lox mutant backgrounds. LOX2 expression analysed in WT, in lox6A and in lox3B lox4A double mutants. LOX3 and LOX4 expression was analyzed in WT, lox2-1, and lox6A single mutants, and the lox2-1 lox6A double mutant. LOX6 expression was analyzed in lox2-1 and the lox3B lox4A double mutant. Leaves 8 (wounded) and 13 (distal) were snap-frozen before (unfilled bars) and 1 h after the wounding (filled bars). Data are from three (controls) and three to four (wounded) biological replicates (±SD). Letters (a, b, and c) refer to significant differences (ANOVA and t-test; p < 0.05).
LOX3 and LOX4 transcript levels in the WT and both lox2 and lox6 single mutants were similar in the wounded leaf, while LOX6 was found to be required for full, wound-induced LOX3 and LOX4 transcript levels in the distal leaf (Figure 2). In the wounded leaf, the double mutant lox2 lox6 displayed 2-fold lower inductions of LOX3 and LOX4 transcripts compared to the WT. In the distal leaf, LOX3 and LOX4 gene expression was reduced by 97.2% and 96.3%, respectively, in the lox2 lox6 double mutant relative to the WT. In the distal leaf, the lox6 single mutant displayed an approximately 20-fold induction of LOX3 transcripts, but in lox2 these transcripts were induced to a far higher level (approximately 100-fold)—that is to a level similar to that in the WT. Similarly, a strong effect on distal LOX4 transcript accumulation was observed in lox6 compared to lox2. LOX6 was not wound-inducible in the WT or in lox mutant backgrounds (Figure 2). The low transcript levels and associated large error bars for this LOX limit the interpretation of this result.
2.3. 13-LOXs Contribute Differentially to Inducible VSP2 Defense Gene Expression
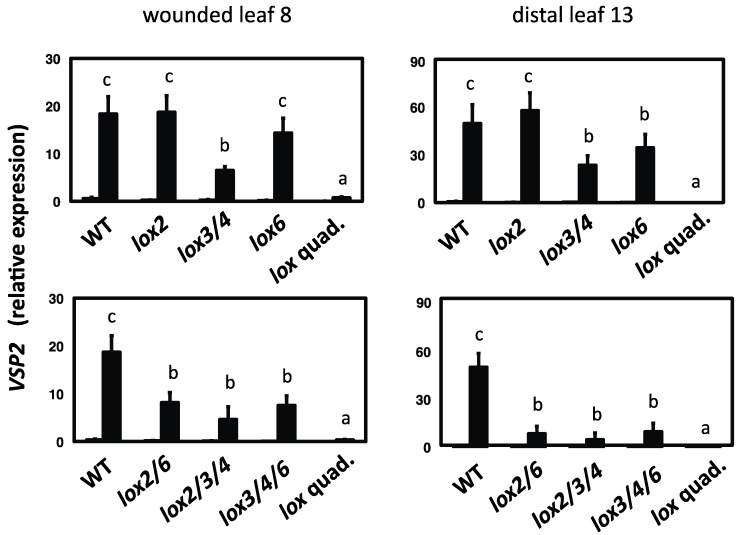
The expression of the jasmonate-regulated defense gene (VEGETATIVE STORAGE PROTEIN2; VSP2) was then investigated. VSP2 is expressed at maximal levels several hours after wounding leaves [26] so a 4 h timepoint after wounding was chosen for initial experiments. VSP2 transcript levels after wounding were reduced relative to the WT in the wounded leaf of the double mutant lox3 lox4, while in the distal leaf their levels were reduced in both the lox3 lox4 double mutants and the lox6 single mutant (Figure 3). Consistent with a role of all 13-LOXs in controlling VSP2 expression through jasmonate production, the lox2 lox6 double mutant, and the lox2 lox3 lox4 and lox3 lox4 lox6 triple mutants were unable to reach WT VSP2 levels in either the wounded leaf or the distal undamaged leaf. Figure 3 shows that VSP2 expression was significantly reduced in the lox2 lox6 double mutant, whereas the lox6 mutation alone had little effect on VSP2 transcript levels.
Figure 3.
Wound-induced VSP2 expression in 13-lox mutants. Leaves 8 (wounded) and 13 (distal) were snap-frozen at 4 h after wounding (filled bars) or harvested at the same timepoint from unwounded plants (unfilled bars). Data from three (unwounded controls) to four (wounded samples) biological replicates (±SD). Letters (a, b, and c) refer to significant differences (ANOVA and t-test; p < 0.05). VSP2 transcript levels in unwounded plants is low and statistics are shown for wounded treatments only. WT = wild type; lox2 = lox2-1; lox3/4 = the lox3B lox4A double mutant; lox6 = lox6A; lox2/6 = the lox2-1 lox6A double mutant; lox2/3/4 = lox2-1 lox3B lox4A triple mutant; lox3/4/6 = lox3B lox4A lox6A triple mutant; lox quad. = lox2-1 lox3B lox4A lox6A quadruple mutant.
2.4. Comparison of LOX3, LOX4 and VSP2 Expression in the WT and the lox2 lox6 Double Mutant
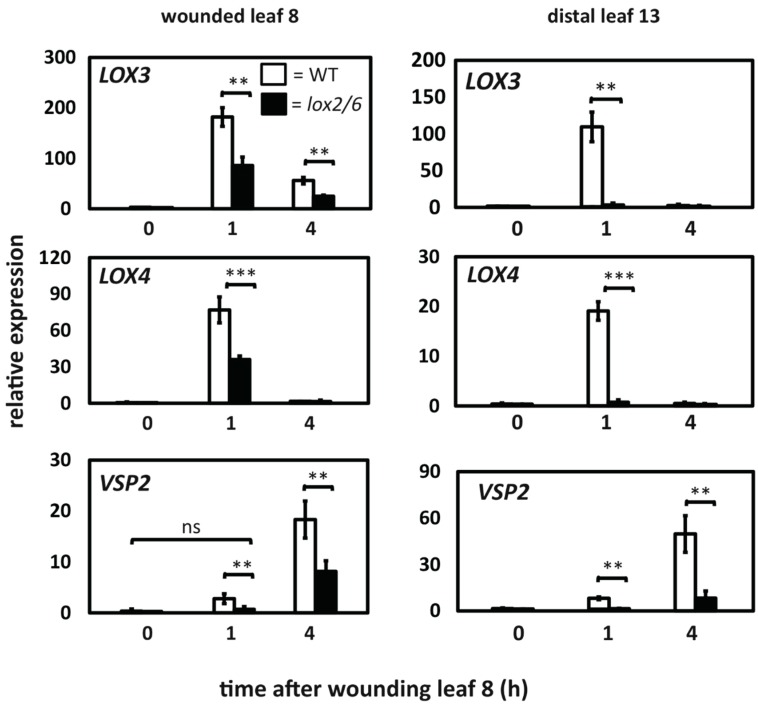
To gain insights into the temporal control of gene expression we compared the wound induction of LOX3, LOX4, and VSP2 transcripts in the WT and the lox2 lox6 double mutant. As shown in Figure 4, LOX3 and LOX4 transcript levels were upregulated rapidly (within 1 h) in the wounded leaf and in the distal leaf of the WT. However, LOX3 and LOX4 transcript accumulation in response to wounding was reduced in the lox2 lox6 double mutant. The possibility that the lox2 lox6 mutations might cause increased expression of LOX3 and LOX4 is, given the results in Figure 2, considered unlikely. Over the experimental period, LOX3 and LOX4 transcript levels in the distal leaf of lox2 lox6 never reached WT levels. This is consistent with the LOX2/LOX6 pair acting upstream of the LOX3/LOX4 pair and contributing to their expression. Using an identical timeframe we observed that VSP2 transcripts accumulated with different kinetics in the WT and the lox2 lox6 double mutant. The wound-induced expression of VSP2 was reduced in the lox2 lox6 double mutant (i.e., LOX2 and LOX6 are essential for full VSP2 gene expression). This led us to test the roles of the LOX3/LOX4 gene pair in herbivory assays.
Figure 4.
Wound-inducible LOX3, LOX4, and VSP2 expression in WT (open bars) and the lox2-1 lox6A double mutant (black bars). Leaves 8 (wounded) and 13 (distal) were snap-frozen before and after wounding. Data are from three to four biological replicates (±SD). Data from a 2h timepoint also included in the original experiment are not shown. Asterisks refer to data significantly different from WT (ns: not significant; *, p < 0.05; **, p < 0.01, and ***, p < 0.001; t-test).
2.5. All 13-LOXs Act in Defense against a Lepidopteran Herbivore
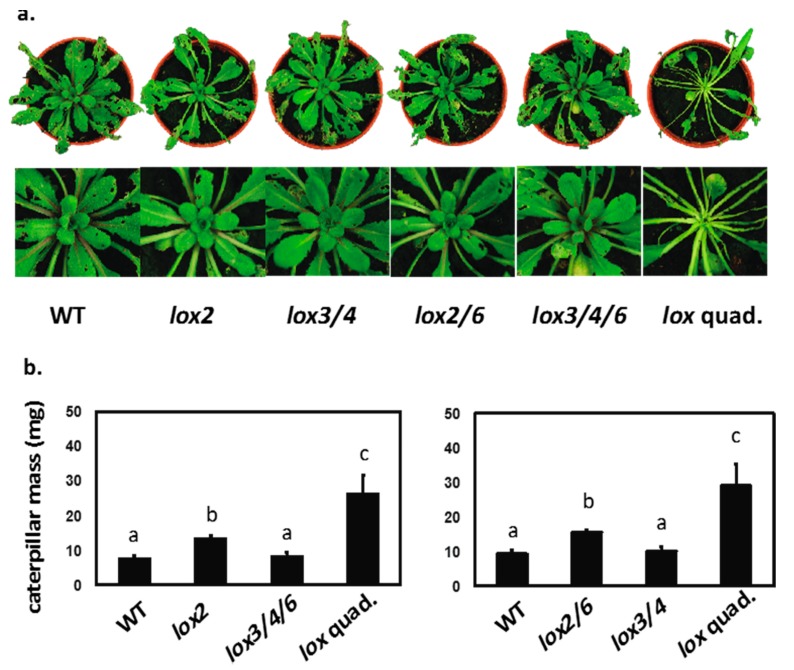
The lox2 lox3 lox4 lox6 quadruple mutant is known to display greatly reduced resistance to larvae of Spodoptera littoralis relative to the WT [15]. This genotype was used as a control to investigate the roles of LOX2, LOX3, and LOX4 in defense. In all plants—except the quadruple mutant, where the center of the rosette was attacked—damage inflicted by S. littoralis was restricted to expanded leaves (Figure 5a). Larvae also grew fast on the lox2-1 single mutant (Figure 5b) as described in Glauser et al. [17]. To further investigate LOX2 function we used the LOX2 “mirror” mutant (i.e., the triple mutant lox3 lox4 lox6 that retains functional LOX2 as its only 13-LOX). As judged by measuring insect weight gain, LOX2 on its own in the triple mutant mediated near-WT-levels of defense (Figure 5a,b). Since redundancy between LOX3 and LOX4 has been observed in early responses to leaf wounding [15], larval growth was examined on a lox3 lox4 double mutant and on the “mirror” mutant lox2 lox6 that retains functional LOX3 and LOX4 as its only 13-LOXs. Caterpillar growth was found to be similar to that on the WT for both the lox3 lox4 double and lox3 lox4 lox6 triple mutants. However, the insects gained 1.7 times more weight than they did on the WT when feeding on the lox2 lox6 double mutant, but this weight gain was less than that seen on the quadruple mutant (3.1 times more weight gain than on the WT). That is, the presence of functional LOX3 and LOX4 in the lox2 lox6 double mutant actively reduced insect weight gain.
Figure 5.
Contributions of 13-LOXs to defense against a chewing herbivore. (a) Damage to rosettes inflicted by Spodoptera littoralis larvae. WT = wild type; lox3/4 = the lox3B lox4A double mutant; lox2/6 = the lox2-1 lox6A double mutant; lox3/4/6 = lox3B lox4A lox6A triple mutant; lox quad. = lox2-1 lox3B lox4A lox6A quadruple mutant; (b) Larval mass after feeding. Insects were harvested at 11d. Letters (a, b, and c) refer to significant differences (ANOVA and t-test; p < 0.05).
In summary, while LOX2 and LOX6 are known to make different contributions to leaf defense [9,15], the new results show that LOX3 and LOX4 also help protect the rosette.
2.6. Functional Pairs of 13-LOXs in Leaf Defense
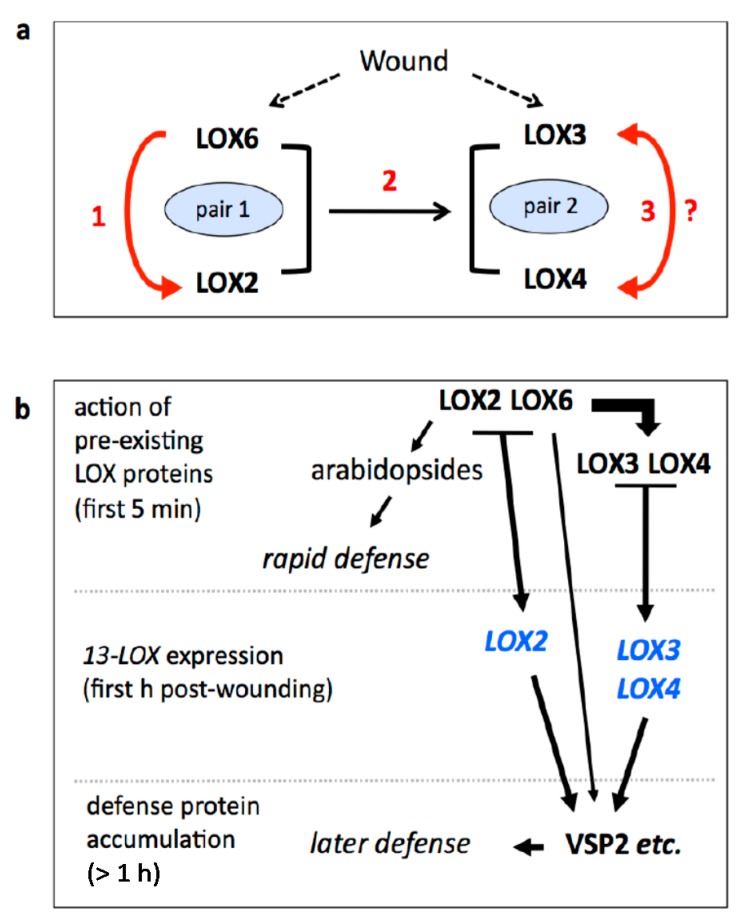
An outcome of the present work was the finding that 13-LOX activities appear to function in an organised regulatory network. Specifically, we provide evidence consistent with LOX2 and LOX6 functioning upstream of LOX3 and LOX4. This is shown in Figure 6.
Figure 6.
Figure 6. 13-LOX activities in wounded Arabidopsis leaves. (a) 13-LOXs interact through jasmonate production. 1. LOX6 participates in the production of jasmonate that upregulates LOX2 expression (from Figure 2). 2. LOX6 and LOX2 together produce jasmonates that enhance LOX3 and LOX4 expression (from Figure 2). 3. Still hypothetical, LOX3 and LOX4 may enhance each other’s expression through jasmonate production; (b) 13-LOX roles in the wound response. Preformed LOX2 rapidly produces arabidopsides, secondary compounds implicated in direct defense [17]. LOX6 and LOX2 produce jasmonates necessary for early gene expression (e.g., JAZ10; 9,15 and LOX3/4 expression; Figure 2 and Figure 4). LOX3 and LOX4 produce jasmonates that ensure correct late-phase VSP2 gene expression (from Figure 3 and Figure 4). Proteins, black; transcripts, blue.
While we suggest that LOX2/LOX6 and LOX3/LOX4 act in pairs in Arabidopsis, it is important to note that each enzyme has some distinct roles. LOX2 activity in the Columbia (Col) accession is dedicated in large part to the rapid production of arabidopsides in and near wounds [17]. LOX2 is closely related to a Nicotiana attenuata LOX that produces substrates for 2(E)-hexenal synthesis via HPL activity [27]. However, the HPL (HYDROPEROXIDE LYASE) gene, which uses 13-LOX products for the synthesis of volatile aldehydes, is mutated in Col accessions—and Col plants produce little or no 2(E)-hexenal upon wounding [28]—so it is possible that LOX2 would also produce this volatile in accessions with a functional HPL gene. Moreover, LOX2 expression seems to be optimally placed for the defense of soft tissues: it would be difficult for a chewing herbivore to avoid damaging cells in its broad basal expression domain. As shown both previously [9] and herein, LOX2 also contributes to the production of biologically active jasmonates in leaves distal to wounds, and these jasmonates stimulate LOX3, LOX4, and VSP2 expression.
LOX6 produces the precursors of biologically active jasmonates, and it is possible that this enzyme builds pre-formed OPDA pools that are mobilized on wounding [25]. The relative level of jasmonate originating from LOX6 activity increases at a distance from a wound [15], and LOX6 plays a more powerful role than LOX2 in this respect. LOX6, like LOX2, contributes to VSP2 gene expression. Finally, lox6 mutants are more drought-sensitive than the WT, hinting at other roles for this LOX [25]. LOX3 and LOX4 proteins also contribute to jasmonate synthesis that then leads to increased LOX3, LOX4, and VSP2 expression. Alone (i.e., in the absence of LOX6 and LOX2) the expression of the LOX3/LOX4 pair is barely activated in leaves distal to wounds (note that it is possible that long-distance wound signals activate pre-formed LOX3 and LOX4 enzymes). However, LOX3 transcript levels increase after mechano-stimulation [29], and LOX3 protein is jasmonate-inducible in the vicinity of wounds [30]. Under the conditions used herein, LOX3 and LOX4 do not strongly activate LOX2 and LOX6 gene expression (Figure 2), therefore arrow 2 in Figure 6a is unidirectional. Our studies have not determined the relative contributions of LOX3 and LOX4 to leaf defense, however, differential activities for these LOXs in leaves are possible [31]. Work remains to further refine the models shown in Figure 6. Additionally, the LOX network we propose is likely to extend beyond 13-LOXs and there is already evidence for interaction between 13- and 9-LOX pathways in rice (e.g., [32]). In Arabidopsis, transcripts of LOX1, a 9-lipoxygenase, are strongly jasmonate-inducible [33], raising the possibility that the 13-LOX network encompasses 9-LOXs.
Lastly, each of the four Arabidopsis 13-LOXs should provide unique insights into signalling mechanisms that operate to initiate jasmonate synthesis. Recently, it was proposed that stress-responsive signal pathways involving coupled glutamate receptor-like/lipoxygenase (GLR/LOX) modules may exist [34]. If so, each GLR-LOX pathway might have unique characteristics.
3. Experimental Section
3.1. Plant Material and Growth Conditions
A. thaliana (L.) Heynh. T-DNA insertion mutants in the Columbia background were obtained from the European Arabidopsis Stock Center (NASC): LOX3 (At1g17420; lox3B = SALK_147830), LOX4 (At1g72520; lox4A = SALK_071732), and LOX6 (At1g67560; lox6A = SALK_138907). The lox2-1 (At3g45140) mutant, double, triple, and quadruple mutants all based on the lox2-1, lox3B, lox4A, and lox6A alleles have been described previously [15,17,35]. The aos mutant was from [36]. Plants were grown individually in 7 cm diameter pots at 21 °C, 10 h light d−1 (100 μmol·s−1·m−2) photoperiod, and 70% humidity. Leaves of five-week-old plants were numbered from oldest to youngest. All wounding experiments (crushing 50% of leaf 8 with metal forceps) were performed between 12 a.m. and 16.30 p.m. Leaves were snap-frozen in liquid N2 and stored at −80 °C before extractions for RT-qPCR.
3.2. Insect Feeding Assays
Eleven pots, each with a 4.5 week-old plant, were isolated in eleven plexiglass boxes. Newly hatched Spodoptera littoralis (Boisduval; Noctuidae; Lepidoptera) caterpillars were placed on each plant (four larvae per pot). Larvae were harvested after 11 days. The weight of larvae from one box per number of recovered larvae was considered as one biological replicate [15].
3.3. Gene Expression
RT-qPCR was performed as described in [15] with a PCR program detailed in [30]. Data were standardized to the UBC21 ubiquitin conjugase reference gene. The following primers were used: UBC21 (At5g25760): 5′-CAGTCTGTGTGTAGAGCTATCATAGCAT-3’, 5′-AGAAGATTCCCTGAGTCGCAGTT-3’. VSP2 (At5g24770): 5’-CATCATAGAGCTCGGGATTGAACCC-3’, 5’-AGATGCTTCCAGTAGGTCACGC-3’. LOX2: 5’-ATTACGGTAGAAGACTACGCACAAC-3’, 5’-GTAATTTAAGCTCTACCCCCTTGAG-3’. LOX3: 5’-AACACAACCACATGGTCTTAAACTC-3’, 5’-GGAGCTCAGAGTCTGTTTTGATAAG-3’. LOX4: 5’-ATAGAACGAGTCACGTCTTATCCAC-3’, 5’-CATAAACAAACGGTTCGTCTCTAAC-3’.
3.4. GUS Staining and Light Microscopy
Promoter/GUS fusion plants for LOX2 (At3g45140), LOX3 (At1g17420), and LOX4 (At1g72520) were from ([9]; see supplemental material in that paper for LOX3pro:GUS and LOX4pro:GUS). The LOX6pro:GUS fusion plants were from [15]. 26 individual T1 plants from each construct were stained and at least six selected to produce T2 independent lines from which at least six for each independent line were stained for in-depth analysis. For staining and embedding [37], 3.5 week-old rosettes were prefixed in acetone:water (90:10, v:v) for 45 min on ice, washed twice in 50 mM sodium phosphate buffer (pH 7.2), and vacuum-infiltrated for 10 min (for rosette staining) or 2 h (for leaf sectioning), then left 16 h at 37 °C in the dark in 10 mM Na2EDTA; 50 mM sodium phosphate buffer, pH 7.2; 2 mM (for rosettes) or 3 mM (for thin sections) K4Fe(CN)6; 2 mM (for rosettes) or 3 mM (for thin sections) K3Fe(CN)6; 0.1% (v/v) Triton X-100; 0.6 mg·mL−1 5-bromo-4-chloro-3-indolyl-β-d-glucuronide (X-Gluc). Rosettes were then either transferred to ethanol: water (70:30, v/v) for photography or fixed (glutaraldehyde:formaldehyde:50 mM sodium phosphate (pH 7.2) buffer = 2:5:43, v:v:v). For embedding, stained leaves were dehydrated in an ethanol gradient (10%, 30%, 50%, 70%, 90%, and twice absolute) for 30 min. Embedding in Technovit 7100 resin (Haslab GmbH, Ostermundigen, Switzerland) was carried out according to the manufacturer’s instructions. Sections (5 μm) were made with a RM2255 microtome (Leica, Muttenz, Switzerland) using disposable Leica TC-65 blades.
4. Conclusions
Our results highlight the fact that three of the four 13-LOXs in Arabidopsis are expressed primarily in the vasculature. The exception, LOX2, is expressed in soft tissues; this LOX has only low basal expression in mature veins. Although they have different roles in the wound response, all four 13-LOXs operate together in a regulatory network. This network can be seen as being comprised of two pairs of LOXs; the first pair (LOX6 and LOX2) produces precursors of jasmonates in response to wounding. This helps to control the expression of the second LOX pair: LOX3 and LOX4, and the enzymes encoded by these genes also contribute to jasmonate synthesis. We raise the possibility that other plant genomes may encode pairs of LOXs, and that these may also act hierarchically to control each other’s expression.
Acknowledgments
We thank A.-S. Fiorucci and I. Acosta for valuable comments, N. Geldner and E. Dohmann for plasmids, C. Bonnet and P. Reymond for insect eggs, and S. Stolz for excellent technical help. Supported by Swiss National Science Foundation grants 31003A-138235 (to E.E.F.) and 200020-146200 (to J.-L.W. and E.E.F.).
Appendix
Figure A1.
Expression of LOX promoter::GUS reporters in wounded rosettes. Plants were wounded on a single leaf (orange asterisk) and harvested 6 h later prior to staining for GUS activity. Scale bar = 1 cm.
Figure A2.
Basal LOX3 and LOX4 expression is not affected in the lox2 and lox6 backgrounds. Samples were from leaf 8 of five-week-old plants. LOX3 and LOX4 transcript levels are relative to those of UBC21.
Author Contributions
A.C. and A.L. performed experiments; A.C., A.L., J.L.W. and E.E.F. designed research; E.E.F. wrote the paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Haeggstrom J.Z., Funk C.D. Lipoxygenase and leukotriene pathways: Biochemistry, biology, and roles in disease. Chem. Rev. 2011;111:5866–5898. doi: 10.1021/cr200246d. [DOI] [PubMed] [Google Scholar]
- 2.Andreou A., Feussner I. Lipoxygenases—Structure and reaction mechanism. Phytochemistry. 2009;70:1504–1510. doi: 10.1016/j.phytochem.2009.05.008. [DOI] [PubMed] [Google Scholar]
- 3.Bannenberg G., Martinez M., Hamberg M., Castresana C. Diversity of the enzymatic activity in the lipoxygenase gene family of Arabidopsis thaliana. Lipids. 2009;44:85–95. doi: 10.1007/s11745-008-3245-7. [DOI] [PubMed] [Google Scholar]
- 4.Browse J. Jasmonate passes muster: A receptor and targets for the defense hormone. Ann. Rev. Plant Biol. 2009;60:183–205. doi: 10.1146/annurev.arplant.043008.092007. [DOI] [PubMed] [Google Scholar]
- 5.Wasternack C., Hause B. Jasmonates: Biosynthesis, perception, signal transduction and action in plant stress response, growth and development. An update to the 2007 review in Annals of Botany. Ann. Bot. 2013;111:1021–1058. doi: 10.1093/aob/mct067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dong-Sun L., Nioche P., Hanberg M., Raman C.S. Structural insights into the evolutionary paths of oxylipin biosynthetic enzymes. Nature. 2008;455:363–368. doi: 10.1038/nature07307. [DOI] [PubMed] [Google Scholar]
- 7.Schaller A., Stintzi A. Enzymes in jasmonate biosynthesis—Structure, function, regulation. Phytochemistry. 2009;70:1532–1538. doi: 10.1016/j.phytochem.2009.07.032. [DOI] [PubMed] [Google Scholar]
- 8.Fonseca S., Chini A., Hamberg M., Adie B., Porzel A., Kramell R., Miersch O., Wasternack C., Solano R. (+)-7-iso-Jasmonoyl-l-isoleucine is the endogenous bioactive jasmonate. Nat. Chem. Biol. 2009;5:344–350. doi: 10.1038/nchembio.161. [DOI] [PubMed] [Google Scholar]
- 9.Gasperini D., Chauvin A., Acosta I.F., Kurenda A., Wolfender J.-L., Stolz S., Chételat A., Farmer E.E. Axial and radial oxylipin transport. Plant Physiol. 2015 doi: 10.1104/pp.15.01104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Royo J., Leon J., Vancanneyt G., Albar J.P., Rosahi S., Ortego F., Castanera P., Sanchez-Serrano J.J. Antisense-mediated depletion of a potato lipoxygenase reduces wound induction of proteinase inhibitors and increases weight gain of insect pests. Proc. Natl. Acad. Sci. USA. 1999;96:1146–1151. doi: 10.1073/pnas.96.3.1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Halitschke R., Baldwin I.T. Antisense LOX expression increases herbivore performance by decreasing defense responses and inhibiting growth-related transcriptional reorganization in Nicotiana attenuata. Plant J. 2003;36:794–807. doi: 10.1046/j.1365-313X.2003.01921.x. [DOI] [PubMed] [Google Scholar]
- 12.Yan L., Zhai Q., Wei J., Li S., Wang B., Huang T., Du M., Sun J., Kang L., Li C.-B., et al. Role of tomato lipoxygenase D in wound-induced jasmonate biosynthesis and plant immunity to insect herbivores. PLoS Genet. 2013:16. doi: 10.1371/journal.pgen.1003964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou G., Qi J., Ren N., Cheng J., Erb M., Mao B., Lou Y. Silencing OsHI-LOX makes rice more susceptible to chewing herbivores, but enhances resistance to a phloem feeder. Plant J. 2009;60:638–648. doi: 10.1111/j.1365-313X.2009.03988.x. [DOI] [PubMed] [Google Scholar]
- 14.Christensen S.A., Nemchenko A., Borrego E., Murray I., Sobhy I.S., Bosak L., DeBlasio S., Erb M., Robert C.A.M., Vaughn K.A., et al. The maize lipoxygenase, ZmLOX10, mediates green leaf volatile, jasmonate and herbivore-induced plant volatile production for defense against insect attack. Plant J. 2013;74:59–73. doi: 10.1111/tpj.12101. [DOI] [PubMed] [Google Scholar]
- 15.Chauvin A., Caldelari D., Wolfender J.-L., Farmer E.E. Four 13-lipoxygenases contribute to rapid jasmonate synthesis in wounded Arabidopsis thaliana leaves: A role for lipoxygenase 6 in responses to long-distance wound signals. New Phytol. 2013;197:566–575. doi: 10.1111/nph.12029. [DOI] [PubMed] [Google Scholar]
- 16.Bell E., Creelman R.A., Mullet J.E. A chloroplast lipoxygenase is required for wound-induced jasmonic acid accumulation in Arabidopsis. Proc. Natl. Acad. Sci. USA. 1995;92:8675–8679. doi: 10.1073/pnas.92.19.8675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Glauser G., Dubugnon L., Mousavi S.A.R., Rudaz S., Wolfender J.-L., Farmer E.E. Velocity estimates for signal propagation leading to systemic jasmonic acid accumulation in wounded Arabidopsis. J. Biol. Chem. 2009;284:34506–34513. doi: 10.1074/jbc.M109.061432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Glauser G., Grata E., Dubugnon L., Rudaz S., Farmer E.E., Wolfender J.-L. Spatial and temporal dynamics of jasmonate synthesis and accumulation in Arabidopsis in response to wounding. J. Biol. Chem. 2008;283:16400–16407. doi: 10.1074/jbc.M801760200. [DOI] [PubMed] [Google Scholar]
- 19.Seltmann M.A., Stingl N.E., Lautenschlaeger J.K., Krischke M., Mueller M.J., Berger S. Differential impact of lipoxygenase 2 and jasmonates on natural and stress-induced senescence in Arabidopsis. Plant Physiol. 2010;152:1940–1950. doi: 10.1104/pp.110.153114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zoeller M., Stingl N., Krischke M., Fekete A., Walker F., Berger S., Mueller M.J. Lipid profiling of the Arabidopsis hypersensitive response reveals specific lipid peroxidation and fragmentation processes: Biogenesis of pimelic and azelaic acid. Plant Physiol. 2012;160:365–378. doi: 10.1104/pp.112.202846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nilsson A.K., Fahlberg P., Ellerstrom M., Andersson M.X. Oxo-phytodienoic acid (OPDA) is formed on fatty acids esterified to galactolipids after tissue disruption in Arabidopsis thaliana. FEBS Lett. 2012;586:2483–2487. doi: 10.1016/j.febslet.2012.06.010. [DOI] [PubMed] [Google Scholar]
- 22.Vellosillo T., Martinez M., Lopez M.A., Vicente J., Cascon T., Dolan L., Hamberg M., Castresana C. Oxylipins produced by the 9-lipoxygenase pathway in Arabidopsis regulate lateral root development and defense responses through a specific signaling cascade. Plant Cell. 2007;19:831–846. doi: 10.1105/tpc.106.046052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rodríguez V.M., Chételat A., Majcherczyk P., Farmer E.E. Chloroplastic phosphoadenosine phosphosulfate (PAPS) metabolism regulates basal levels of the prohormone jasmonic acid in Arabidopsis leaves. Plant Physiol. 2010;152:1335–1345. doi: 10.1104/pp.109.150474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hause B., Hause G., Kutter C., Miersch O., Wasternack C. Enzymes of jasmonate biosynthesis occur in tomato sieve elements. Plant Cell Physiol. 2003;44:643–648. doi: 10.1093/pcp/pcg072. [DOI] [PubMed] [Google Scholar]
- 25.Grebner W., Stingl N.E., Oenel A., Mueller M.J., Berger S. Lipoxygenase6-dependent oxylipin synthesis in roots is required for abiotic and biotic stress resistance of Arabidopsis. Plant Physiol. 2013;161:2159–2170. doi: 10.1104/pp.113.214544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Berger S., Mitchell-Olds T., Stotz H.U. Local and differential control of vegetative storage protein expression in response to herbivore damage in Arabidopsis thaliana. Physiol. Plant. 2002;114:85–91. doi: 10.1046/j.0031-9317.2001.1140112.x. [DOI] [PubMed] [Google Scholar]
- 27.Allmann S., Halitschke R., Schuurink R.C., Baldwin I.T. Oxylipin channelling in Nicotiana attenuata: Lipoxygenase 2 supplies substrates for green leaf volatile production. Plant Cell Environ. 2010;33:2028–2040. doi: 10.1111/j.1365-3040.2010.02203.x. [DOI] [PubMed] [Google Scholar]
- 28.Duan H., Huang M.-Y., Palacio K., Schuler M.A. Variations in CYP74B2 (Hydroperoxide lyase) gene expression differentially affect hexenal signaling in the Columbia and Landsberg erecta ecotypes of Arabidopsis. Plant Physiol. 2005;139:1529–1544. doi: 10.1104/pp.105.067249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chehab E.W., Yoa C., Henderson Z., Kim S., Braam J. Arabidopsis touch-induced morphogenesis is jasmonate-mediated and protects against pests. Curr. Biol. 2012;22:701–706. doi: 10.1016/j.cub.2012.02.061. [DOI] [PubMed] [Google Scholar]
- 30.Gfeller A., Bearenfaller K., Loscos J., Chetelat A., Baginsky S., Farmer E.E. Jasmonate controls polypeptide patterning in undamaged tissue in wounded Arabidopsis leaves. Plant Physiol. 2011;156:1797–1807. doi: 10.1104/pp.111.181008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ozalvo R., Cabrere J., Escobar C., Christensen S.A., Borrega E.J., Kolomiets M.V., Castresana C., Iberkleid I., Horowitz S.B. Two closely related members of Arabidopsis 13-lipoxygenases (13-LOXs), LOX3 and LOX4, reveal distinct functions in response to plant-parasitic nematode infection. Mol. Plant Pathol. 2014;15:319–332. doi: 10.1111/mpp.12094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhou G., Ren N., Qi J., Lu J., Xiang C., Ju H., Cheng J., Lou Y. The 9-lipoxygenase Osr9-LOX1 interacts with the 13-lipoxygenase-mediated pathway to regulate resistance to chewing and piercing-sucking herbivores in rice. Physiol. Plant. 2014;152:59–69. doi: 10.1111/ppl.12148. [DOI] [PubMed] [Google Scholar]
- 33.Melan M.A., Dong X., Endara M.E., Davis K.R., Ausubel F., Peterman T.K. An Arabidopsis thaliana lipoxygenase gene can be induced by pathogens, abscisic acid, and methyl jasmonate. Plant Physiol. 1993;1001:441–450. doi: 10.1104/pp.101.2.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Farmer E.E., Gasperini D., Acosta I. The squeeze cell hypothesis for the activation of jasmonate synthesis in response to wounding. New Phytol. 2014;204:282–288. doi: 10.1111/nph.12897. [DOI] [PubMed] [Google Scholar]
- 35.Caldelari D., Wang G., Farmer E.E., Dong X. Arabidopsis lox3 lox4 double mutants are male sterile and defective in global proliferative arrest. Plant Mol. Biol. 2011;75:25–33. doi: 10.1007/s11103-010-9701-9. [DOI] [PubMed] [Google Scholar]
- 36.Park J.H., Halitschlke R., Kim H.B., Baldwin I.T., Feldmann K.A., Feyereisen R. A knock-out mutation in allene oxide synthase results in male sterility and defective wound signal transduction in Arabidopsis due to a block in jasmonic acid biosynthesis. Plant J. 2002;31:1–12. doi: 10.1046/j.1365-313X.2002.01328.x. [DOI] [PubMed] [Google Scholar]
- 37.Scheres B., Wolkenfelt H., Terlouw M., Lawson E., Dean C., Weisbeek P. Embryonic Origin of the Arabidopsis Primary Root and Root-Meristem Initials. Development. 1994;120:2475–2487. [Google Scholar]