Abstract
tRNAs are the fundamental components of the translation machinery as they deliver amino acids to the ribosomes during protein synthesis. Beyond their essential function in translation, tRNAs also function in regulating gene expression, modulating apoptosis and several other biological processes. There are multiple layers of regulatory mechanisms in each step of tRNA biogenesis. For example, tRNA 3′ trailer processing is altered upon nutrient stress; tRNA modification is reprogrammed under various stresses; nuclear accumulation of tRNAs occurs upon nutrient deprivation; tRNA halves accumulate upon oxidative stress. Here we address how environmental stresses can affect nearly every step of tRNA biology and we describe the possible regulatory mechanisms that influence the function or expression of tRNAs under stress conditions.
Keywords: tRNA processing, tRNA subcellular dynamics, tRNA modification, tRNA fragments
1. Introduction
Transfer RNAs (tRNAs) serve their essential roles of delivering amino acids to the cytoplasmic protein synthesis machinery. To function as an adaptor during protein synthesis, the tRNA isoacceptor is charged with a cognate amino acid to form aminoacyl-tRNA (aa-tRNA). Beyond the essential role of translating the genetic code, tRNAs also function in other biological processes, such as signaling in the general amino acid control (GAAC) pathway, sensing the nutritional stress, serving as primers for retroviral replication, and regulation of apoptosis by directly binding to cytochrome C [1,2,3,4,5]. Aminoacylated tRNAs have also been implicated as substrates for non-ribosomal peptide bond formation, modification of phospholipids in the cell membrane, protein labeling for degradation, and antibiotic biosynthesis [5]. Therefore, tRNAs have emerging roles in adaptive translation, signaling dynamics, and regulating biological processes [6,7,8].
tRNA biogenesis involves multiple steps, many of which are conserved throughout eukaryotes. Eukaryotic tRNAs are transcribed by RNA polymerase III as precursor molecules (pre-tRNAs). The pre-tRNAs undergo an elaborate set of post-transcriptional steps to generate mature tRNAs. These steps include: removal of both the 5′ leader and 3′ trailer sequences, nucleotide addition to all 3′ ends and to a 5′ end of one tRNA, tRNAHis, removal of introns from transcripts transcribed by intron-containing genes, and addition of nucleotide modifications. In addition to the regulation of each step of tRNA biogenesis, cells also employ multiple tRNA quality control mechanisms to prevent inappropriate substrates being used in the protein synthesis [9]. Due to the importance of functional tRNA, alterations in the levels of tRNA transcripts and defects in tRNA processing and modifications result in several human diseases, including neuronal disorders [10], pontocerebellar hypoplasia [11], nonsyndromic X-linked intellectual disability (NSXLID) [12] and cancer [6,7,13,14,15,16].
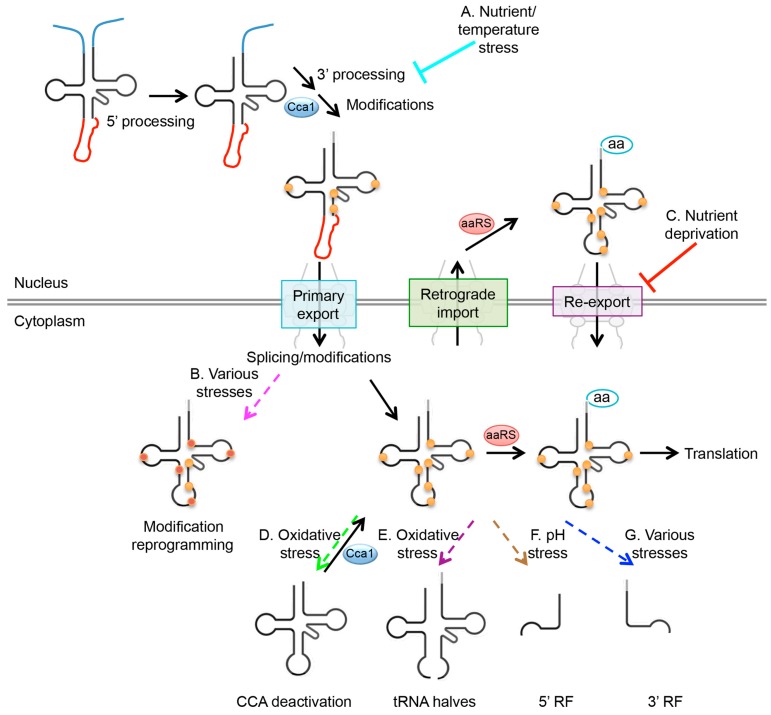
tRNAs are stable with half-lives estimated from ~9 h to up to days [16,17,18]. As tRNA biogenesis involves multiple steps, the expression and/or function of tRNA is likely to be tightly regulated by distinct pathways. Several reviews have discussed the modulation of tRNA levels and modifications in controlling translation [6,8,19]. Here we address the data supporting the notion that environmental stresses can influence nearly every step of tRNA biology. We focus on the regulation of tRNA processing, modification, subcellular dynamics, and fragmentation that are altered in response to stress conditions (Figure 1). This review focuses on the recent discoveries employing the yeast, Saccharomyces cerevisiae. In addition, similarities and differences of the regulatory mechanisms in budding yeast to those in other organisms are described if the information is available.
Figure 1.
Stress-induced regulation in tRNA biology. The canonical tRNA biogenesis pathway and subcellular traffic are indicated with solid black arrows. (A) Upon nutrient and/or temperature stress, 3′ trailer sequence processing is likely inhibited and thus aberrant pre-tRNAs accumulate (Cyan blunt-ending line); (B) tRNA modification is subject to change under certain cellular conditions (Magenta dotted arrow); (C) Upon nutrient deprivation, tRNA re-export step is likely inhibited by multiple mechanisms and thus tRNAs accumulate in the nucleus (Red blunt-ending line); (D) Upon oxidative stress, tRNAs are endonucleolytically cleaved within their 3′ CCA termini (Green dotted arrow). Oxidative stress-induced deactivation of the 3′ CCA termini is a dynamically reversible process; (E) When tRNAs are exposed to oxidative stress, heat shock, and UV irradiation, mature tRNAs are endonucleolytically cleaved in the anticodon loops, generating 5′ and 3′ tRNA halves (Purple dotted arrow); (F) The 5′ tRFs are derived from the 5′ parts of mature tRNAs and are formed by a cleavage in the D loop. 5′ tRF can be formed when cells are grown at high pH environment (Brown dotted arrow); (G) 3′ CCA tRFs correspond to 3′ parts of mature tRNAs containing processed 3′ CCA termini and are formed by cleavage at the T loop (Blue dotted arrow). Dicer, angiogenin, and other RNase A family members have been implicated in the generation of the 3′ tRF.
2. Pre-tRNA Processing
The majority of yeast pre-tRNA transcripts contain 5′ leader and 3′ trailer sequences [20,21]. Maturation of tRNAs in nearly all organisms begins with removal of the 5′ extension by the endonuclease RNase P which is located in the nucleolus in yeast [22]. In bacteria, Archaea, fungi and vertebrates, RNase P, is a ribonucleoprotein complex comprised of a single RNA and a variable number of protein subunits [23], but in plants mitochondrial and nuclear forms of RNase P are protein-only enzymes [24,25]. The RNA subunit is the catalytic component in bacteria which have one protein subunit [26,27] and in Archaea which have five protein components [28,29]. As the RNA component of human RNase P is catalytic but with very poor kinetics, the eukaryotic protein subunits have been proposed to play a supportive role in catalyzing the removal of the 5′ end of tRNA [30,31,32]. Yeast nucleolar RNase P consists of nine proteins (Pop1, Pop3-8, Rpp1, and Rpr2) and a single essential RNA (RPR1) [22]. The source of eukaryotic RNase P catalysis remains unclear since RNA components have substantial differences in regions important for stability and catalysis [30,31]. In addition to the canonical role in pre-tRNA processing, most of the proteins subunits of the yeast and human RNase P are shared with RNase MRP, involved in rRNA maturation [22,23,25].
3′ end maturation is far more complex than 5′ end processing of tRNA. Maturation of 3′ extensions from pre-tRNA requires both exo- and endo-nucleases [16,33,34]. Yeast Rex1 is a 3′ to 5′ exonuclease that participates in the processing of pre-tRNA trailers as well as in the processing of other RNAs such as 5S rRNA, 5.8S rRNA, and snRNAs [35,36,37]. RNase Z, yeast Trz1, is the endonuclease that participates in 3′ end processing for both mitochondrial and nuclear encoded tRNAs [4,34,38,39,40]. Recent studies show that the majority of tRNAs utilize both endonucleolytic cleavage and exonucleolytic trimming pathways, with a preference for the endonucleolytic cleavage [34]. Exonucleolytic digestion occurs to some degree in wild-type cells and not only when endonucleolytic cleavage is inhibited. However, 3′ end processing of pre-tRNAs with longer 3′ trailers depends to a greater extent on endonuclease, probably due to an inefficient exonucleolytic pathway, inhibited by secondary structures comprised within their longer 3′ extension [34]. The tRNA binding La protein (yeast Lhp1) is involved in pre-tRNA processing. Lhp1 that possesses chaperon activity stabilizes pre-tRNAs by directly binding to the oligo U within 3′ termini, which are generated upon Pol III termination [41,42]. Precursors with different 3′ ends display diverse affinities for La binding, which favors Trz1-mediated endonucleolytic cleavage and protects against exonucleolytic trimming [43]. It is proposed that 3′ oligo U length is a primary determinant of La binding with subsequent steps distinguished by 3′ endo- vs. exo-nuclease, chaperon activities and nuclear surveillance [35,40,44,45].
3. tRNA 3′ Processing Can Be Regulated by Stress
Recent studies suggest that in budding yeast 3′ processing of pre-tRNAs is specifically regulated by growth conditions [46]. Analyses of Northern blot and RNA sequencing show that upon shift to elevated temperatures and/or to glycerol-containing medium, aberrant pre-tRNAs accumulate in a tRNA-specific manner in yeast. Under heat stress elevated levels of aberrant tRNATyr with extended 3′ termini and processed 5′ ends are detected, suggesting that 3′ trailer removal of tRNATyr is inhibited (Figure 1A). Interestingly, stress-induced inhibition of pre-tRNAIleUAU processing results in accumulation of unprocessed pre-tRNAIleUAU at both 3′ and 5′ termini and part of these pre-tRNAs with longer 3′ trailers. As cells with deletion of REX1 accumulated 3′ extended pre-tRNAs even under normal conditions, yeast Rex1 nuclease appears to be limiting for 3′ end processing [46]. It is possible that Rex1 and other 3′ processing enzymes are regulated by cell growth conditions to influence 3′ processing of pre-tRNAs or the levels and stability of pre-tRNAs are altered upon stress. Although tRNA 3′ processing pathway is conserved, whether stress-induced inhibition of 3′ processing occurs in vertebrates requires further investigations.
Human La protein is regulated by growth conditions [47]. Under nutrient-rich conditions, La is phosphorylated and localized in the nucleoplasm where it can associate with pre-tRNAs [48,49]. In contrast, non-phosphorylated La distributes throughout the cells and it preferentially associated with 5′-terminal oligopyrimidine-containing mRNAs that control protein synthesis in the cytoplasm [49]. After induction of apoptosis by exposing cells to various types of DNA damage reagents, La is rapidly dephosphorylated by a protein phosphatase 2A-like activity and a subset of La is proteolytically cleaved in vivo [50]. Therefore, post-translational modification of La provides a mechanism to regulate tRNA processing.
4. tRNA Nucleotidyl Transferase in Stress Response
All tRNAs contain a 3′ terminal CCA sequence that is necessary for tRNA aminoacylation. In yeast and vertebrates, addition of 3′ CCA sequence is catalyzed by nucleotidyl transferase, while E. coli tRNAs are encoded with a CCA sequence. But E. coli also possesses the gene for the CCA adding enzyme, which functions in tRNA 3′ end repair [51,52]. Yeast tRNA nucleotidyl transferase is encoded by CCA1 [53]. CCA1 encodes multiple isoforms, Cca1-I, Cca1-II, and Cca1-III, which are generated by alternative transcriptional and distinct translational start sites. These isoforms are differently distributed among mitochondria, the cytoplasm, and the nucleoplasm [54]. Addition of 3′ CCA sequence is normally catalyzed by the nuclear pool of CCA adding enzyme, while the cytoplasmic pool functions in tRNA 3′ repair. It is thought that the mitochondrial form functions in both biogenesis and 3′ end repair of mitochondria encoded tRNAs.
Recent data for vertebrate cells have shown that upon oxidative stress the 3′ CCA sequence is removed by endonuclease angiogenin, thereby rapidly repressing translation. Upon removal of stress addition of 3′ CCA ends is restored by nucleotidyl transferase, allowing aminoacylation and translation to ensue [55]. Therefore, deactivation of CCA sequence of tRNA leads to a rapid and reversible regulation of translation repression upon stress. tRNA nucleotidyl transferase can generate 3′ CCACCA termini via extended polymerization on particular tRNAs that are hypomodified and/or possess aberrant tertiary conformations, and these CCACCA-containing tRNAs are subsequently targeted for degradation [56,57]. Therefore, tRNA nucleotidyl transferase is responsive to stress to regulate translation and control tRNA quality (Figure 1D).
5. tRNA Modifications
One of the remarkable features of tRNA from all organisms is that they are highly modified by numerous post-transcriptional steps. More than 100 different modifications are known in nature, ranging from single methylation to multiple step reactions [58]. Some modifications are restricted to Archaea, bacteria, or eukaryotes, but many are shared. Some modified bases are present in almost all tRNAs, such as dihydrouridine (D) and pseudouridine (Ψ), whereas others are present only on a single tRNA [59,60].
To generate complexity of highly modified tRNA isoacceptors, genomes encode a large number of enzymes responsible for catalyzing the correct modifications at the proper site of particular tRNAs. The majority of the genes that are responsible for modifications have been identified in S. cerevisiae, and a large number of genes for tRNA modification have been identified in other organisms and compiled at Modomics (http://modomics.genesilico.pl) [16].
It is now know that tRNA modifications serve numerous purposes, including translation fidelity, maintenance of proteome integrity, tRNA folding or stability, and tRNA discrimination. In general, many modifications in or around the anticodon loop affect translation or growth by regulating codon-anticodon interactions and reading frame maintenance. The deamination of adenosine (A) to inosine (I) at wobble position 34 of tRNA provides an example of tRNA modification affecting decoding. As A only base pairs with U, but I base pairs with U, C, and A, tRNAs with I at the wobble position have a broader codon-anticodon interaction capacity [61]. Modifications in the anticodon loop can also maintain the reading frames during translation. For example, mutation of the genes responsible for (yW) modification of tRNAPhe at position 37 results in increases in -1 frameshifting during translation [62].
Interestingly, modifications at wobble base can maintain proteome integrity. In eukaryotes, the wobble uridine (U34) base of tRNAs carries a 5-methoxycarbonylmethyl (mcm5) or 5-carbamoylmethyl (ncm5). Following mcm5U34 addition, base of tRNAGluUUC, tRNALysUUU, and tRNAGlnUUG is further decorated with a 2-thio group (s2) [63,64,65]. As loss of U34 modifications leads to ribosome pausing at their cognate codons in yeast and Caenorhabditis elegans, U34 modifications affect the translation rates at a subset of cognate codons. Cells lacking U34 modifications trigger proteotoxic stress and accumulate aggregates of endogenous proteins and therefore result in an imbalance in protein homeostasis [66]. In addition, an integrated analysis of proteome, transcriptome and gene-specific ribosome footprinting indicate that Trm9-catalyzed tRNA modifications at the wobble position, mcm5U and mcm5s2U, regulate global protein expression via codon-biased translation [67].
Modifications in the main body of the tRNA can affect tRNA folding or stability. For example, reduced levels of tRNASerCGA are observed in strains with a tRNASer mutation that also lack m5U54 or Ψ55 due to deletion of TRM2 or PUS4 [68] and reduced levels of tRNASerCGA and tRNASerUGA are detected at high temperature in strains lacking Um44 and ac4C12 due to deletion of TRM44 and TAN1 [69,70]. Although tRNAs are among the most stable RNA species in vivo, they can be degraded when the sequence or modifications of the tRNAs are altered. In addition, when cells possess mutations of multiple modifications genes, such as pus1Δ pus4Δ, trm4Δ trm8Δ, or trm44Δ tan1Δ synthetic lethality or temperature sensitive growth phenotype occurs [71,72]. The temperature sensitive growth is likely caused by tRNA instability [70,73], and turnover of unstable tRNA is mediated by the 5′ to 3′ rapid tRNA decay pathway (RTD) [72]. Thus, tRNA modifications are key determinants of tRNA stability.
Modifications at various positions specifically provide tRNA discrimination. For instance, tRNAiMet is modified at adenosine 64 (Ar(p)64) by Rit1 which only interacts with unique T stem-loop of tRNAiMet. Modified tRNAiMet does not interact with eEF1A so that it functions only at an initiating AUG codon [74]. However, the exact functions of many of other modifications are not yet known.
6. Reprogramming of tRNA Modification upon Stress
tRNA modification enzymes are misregulated in various human diseases, including cancer and neurological disorders, suggesting tRNA modification is subject to change under certain cellular conditions [7,47]. Several studies provide evidence that tRNA modifications are altered under elevated temperature and growth arrest conditions [75,76,77,78]. Recent high-throughput modification analyses by using a chromatography-coupled mass spectrometry platform have revealed that the levels of assessed tRNA modification are uniquely changed in response to H2O2, methyl methanesulfonate (MMS), arsenite, and hypochlorite [79] (Figure 1B). Chan et al. reported that the levels of 23 of the known 25 ribonucleoside modifications individually altered in response to each toxicant with signature changes in the hierarchical clustering analyses [47,79]. Pseudouridine, the most abundant RNA modification, is regulated by cell growth state and temperature as Ψ-seq profiles show that the levels of pseudouridine modification on mRNA and ncRNA vary upon environmental change [80,81]. Altered tRNA modifications may affect the stability of specific tRNAs, causing these tRNAs to be degraded or stabilized under certain cellular conditions. In addition, these stress-specific patterns of tRNA modification changes are linked to selective translation of codon-biased mRNAs for stress response proteins [19,47]. For example, in S. cerevisiae, the levels of 5-methylcytosine (m5C) modification by tRNA methyltransferase, Trm4, at the wobble C34 base in tRNALeuCAA increase upon exposure to oxidative stress. This altered modification allows selective translation of stress-related genes with over-represented Leu-UUG codons, including the ribosomal protein Rpl22a [82]. In contrast, the levels of m5C are largely unaffected upon exposure to other stresses, such as MMS, arsenite, and hypochlorite.
Changes in wobble base tRNA modification levels may influence codon usage patterns in specific transcripts, designated as modification tunable transcripts (MoTTs), which is codon specific regulation of translation [83]. Chan et al. demonstrated a coordinated translational stress response system involving stress-specific reprogramming of tRNA wobble modifications that leads to selective translation of codon-biased mRNAs representing different classes of critical response proteins [84]. Therefore, distinct stresses uniquely reprogram tRNA modifications to modulate certain transcripts and control translation, suggesting that alteration of tRNA modifications serves as a novel regulatory mechanism for translational control in stress response.
tRNA m5C methylatransferase Dnmt2 also has a prominent role in the stress response. Drosophila Dnmt2 mutants are significantly less viable under oxidative or heat stress conditions, and Dnmt2 relocalized to stress granules following heat shock. Substrate tRNAs in Dnmt2 mutants are more sensitive to stress-induced angiogenin-mediated cleavage, suggesting that Dnmt2-mediated tRNA methylation may play a role in protecting tRNAs from stress-induced cleavage [85]. In contrast, the Kluyveromyces lactis γ-toxin is a secreted endonuclease that inhibits growth of sensitive microbes, such as S. cerevisiae. γ-toxin cleaves tRNAs that possess mcm5s2U modification at the wobble position [86]. Therefore, tRNA anticodon loop modifications can influence tRNA endonucleolytic cleavage.
7. tRNA Subcellular Dynamics
tRNA subcellular trafficking was considered as a one-way movement, from the nucleus, the site of tRNA biogenesis, to the cytoplasm, the site of protein synthesis. However, it is now known that tRNA subcellular movement is bidirectional between the nucleus and the cytoplasm. Newly transcribed end-processed tRNAs move from the nucleus to the cytoplasm via primary nuclear export step. The cytoplasmic tRNAs travel back to the nucleus via retrograde nuclear import and the imported tRNAs then once again access the cytoplasm via the re-export step (Reviews: [4,9,16,87]). The tRNA retrograde pathway is conserved from yeast to vertebrates [88]. This retrograde pathway serves for at least four roles in the distinct cellular processes, including yW37 modification of yeast tRNATrp [89], delivery of retrotranscirbed HIV genomes to the nucleus [90], tRNA quality control [9,91], and efficient translation of particular mRNAs involved in methionine and arginine biosynthesis [92].
Movement of tRNAs between the nucleus and the cytoplasm proceeds via association of importin-β family members. In vertebrates, importin-β family member Exportin-t functions in tRNA nuclear export [93,94]. Homologs of exportin-t have been studied in budding yeast (Los1), fission yeast (Xpo-t), and plants (PAUSED) [95,96,97,98,99]. In vitro biochemical studies of the vertebrate exportin-t and crystallography structural studies of the fission yeast Xpo-t show that exportin-t preferentially binds to the appropriately structured tRNA backbone with mature tRNA 5′ and 3′ ends, although it has no preference for intron-containing or intron-less tRNAs [96,100,101,102]. In vivo biochemical analyses of the budding yeast Los1 show that both intron-containing pre-tRNAs and spliced tRNAs, regardless of whether they are aminoacylated, assembled into Los1-RanGTP complexes [103]. Consistent with in vivo analyses, structural studies show that S. pombe Xpo-t interacts with the 3′ end of tRNA but predict that Xpo-t is unlikely to distinguish between charged and uncharged tRNAs [96]. Taken together, all studied exportin-t homologs monitor the common features of tRNA backbone and do not distinguish between intron-containing pre-tRNAs and spliced tRNAs. Interestingly, Lund and Dahlberg show that kinetically end-processing occurs after intron-removal in the nucleus so that exportin-t likely rarely catches intron-containing pre-tRNAs for nuclear export [101]. Despite the fact that pre-tRNA splicing occurs in the nucleus in vertebrates whereas it occurs in the cytoplasm in yeast [104], exportin-t and its homologs are likely to function in both primary nuclear export and re-export of tRNAs.
The vertebrate importin-β family member Exportin-5 (Exp-5) has also been implicated in the nuclear export of tRNA. The genetics data suggested that the yeast Exp-5 homologue, Msn5, is dedicated in the tRNA re-export step for the tRNA encoded by intron-containing genes. In vivo biochemical analysis show that Msn5 preferentially assembles with RanGTP and spliced, aminoacylated tRNAs and the specificity of Msn5 for mature tRNAs appears to be due to export complex also assembling with the translation elongation factor, eEF1a. Therefore, Msn5 functions in tRNA nuclear re-export for tRNAs that are encoded by intron-containing genes [103,105].
Although Los1 (exportin-t) and Msn5 (exportin-5) both function in tRNA nuclear export, they cannot be the only nuclear exporters for tRNA as los1Δ msn5Δ double mutant cells are viable [105,106]. Recent genome-wide studies in yeast report that Crm1 (mammalian exportin-1) may play a role in tRNA nuclear export as cells harboring double mutations of CRM1 and LOS1 exhibit synthetic growth defects and mutation of CRM1 causes altered tRNA nuclear-cytoplasmic distribution [107]. Whether Crm1 directly or indirectly export tRNA to the cytoplasm requires further study.
The tRNA retrograde import process may occur via, at least, two parallel and independent mechanisms. One pathway appears to be mediated by β-importin family member, Mtr10, as cells with deletion of MTR10 failed to accumulate cytoplasmic tRNAs in the nucleus [105,108]. However, there is no in vivo biochemical evidence supporting the RanGTP or RanGDP-dependent interactions of Mtr10 with tRNAs [103]. Mtr10 may function indirectly in tRNA retrograde import. Another pathway appears to be Ran-independent and mediated by the heat shock protein, Ssa2 [109]. Takano et al. observed that Ssa2 binds to tRNAs that are poorly folded, suggesting that it has chaperone-like activity for RNA. Cells with deletion of SSA2 are unable to efficiently import tRNAs into the nucleus upon nutrient starvation, supporting the hypothesis that yeast Ssa2 functions as a carrier for import of cytoplasmic tRNAs into the nucleus [109].
8. tRNA Subcellular Dynamics in Response to Nutrient Availability
The tRNA retrograde pathway is responsive to loss of nutrients as cells accumulate tRNA in the nucleus upon amino acids (aa), glucose, or phosphate deprivation [108,109,110,111,112], while cells exhibit an even distribution of tRNA throughout the nucleus and cytoplasm under nutrient replete conditions. The subcellular distribution of tRNAs between the nucleus and the cytoplasm results from the balance among the rates of primary export, nuclear import, and re-export. Retrograde tRNA nuclear import is constitutive [105], thus implicating regulation of nutrient-dependent nuclear accumulation of cytoplasmic tRNAs at the re-export step (Figure 1C). There are multiple possible mechanisms by which tRNA subcellular distribution could be regulated, including nutrient-regulated tethering of tRNAs, as well as regulation of importin-β family members in response to nutrient conditions.
Recent studies have shown that tRNA nuclear re-export is subject to complex regulation as cells utilize distinct mechanisms to respond to aa vs. glucose availability [113]. Nuclear-cytoplasmic distributions of Msn5 and Los1 are altered upon glucose deprivation but not aa deprivation. Under fed conditions Los1 and Msn5 are primarily nuclear where they are able to interact with tRNAs and then export them to the cytoplasm. Upon glucose deprivation, Msn5, with kinetics identical to tRNA nuclear accumulation, becomes primarily cytoplasmic and is therefore unable to access nuclear tRNAs to deliver them to the cytoplasm. Redistribution of tRNA exportins upon glucose removal, likely due to collapse of the RanGTP gradient, provides an explanation for nuclear accumulation of imported cytoplasmic tRNAs [113]. Thus, glucose deprivation (but not aa deprivation) causes a rapid (10 min) collapse of the RanGTP gradient, causing tRNAs to accumulate in the nucleus.
Subcellular distributions of the nuclear-cytoplasmic shuttling proteins, Los1 and Msn5, are also affected by carbon source and other stress conditions [114,115,116]. In cells grown with a fermentable sugar as the carbon source, Los1 is located primarily in the nucleus. In contrast, in cells grown in a non-fermentable carbon source, or when the cells are exposed to DNA damaging agents, Los1 is primarily cytoplasmic separated from the tRNAs awaiting nuclear export [114,115,116]. The subcellular distribution of Msn5 is also affected by stress. Msn5 is concentrated in nuclei of unstressed cells, but it is located in the cytoplasm upon exposure to ethanol, heat, starvation, or severe oxidative stress [116,117,118]. Further investigation would be required to understand the crosstalk among tRNA subcellular distribution, the β-importin family, and other stresses.
Upon aa deprivation the subcellular distribution of Los1 and Msn5 resemble fed conditions, thereby implicating other mechanisms independent of the subcellular distribution of Los1 and Msn5 to account for how tRNA re-export responds to aa depletion. As tRNAs can be aminoacylated in the nucleus by the nuclear pool of aminoacyl-tRNA synthetases (aaRS), uncharged tRNA may accumulate in the nucleus when tRNA charging is defective or when cells are deprived for auxotrophic amino acids that they are unable to synthesize [101,105,110,112,119,120,121]. There are two separate studies support the notion that tRNA charging defects inhibit nuclear export of cognate tRNA. First, charging defects in methionyl-tRNA synthetase caused defective nuclear export of cognate tRNAMet, however, nuclear export of non-cognate tRNAIle and tRNATyr were not affected [121]. Second, depletion of yeast THG1, which is critical for changing of tRNAHis, results in uncharged tRNAHis [122]. thg1Δ cells accumulate tRNAHis but not tRNATyr in the nucleus [122]. Therefore, nuclear aminoacylation status of tRNA affects nuclear export of cognate tRNAs but not the export of non-cognate tRNAs that have been assessed, suggesting that nuclear aminoacylation status of tRNA could provide one regulatory mechanism for cognate tRNA nuclear re-export.
Nuclear accumulation of tRNA upon acute aa removal is rapid and reversible [112]. Upon aa deprivation tRNATyr, tRNALeu, tRNAIle, tRNAMet and tRNAHis accumulate in BY4741 cells [105,108,110,111,112]. Unlike vertebrates, yeasts are able to produce all 20 aa unless they harbor mutations in particular aa biosynthesis pathways. Upon aa deprivation BY4741 cells are able to produce prototrophic amino acids, such as Tyr and Ile, and thus tRNATyr and tRNAIle are charged [112]. However, these charged tRNAs accumulate in the nucleus when cells are depleted of all aa, suggesting that a separate mechanism sensitive to aa availability must play a role in tRNA subcellular dynamics. Ssa2 is a tRNA-binding protein whose deletion compromises nuclear accumulation of tRNAs upon nutrient starvation. Since it has been shown that there is a marginal but reproducible increase in the nuclear pool of Ssa2-FLAG upon aa starvation [109], it is possible that alteration of Ssa2 levels regulate tRNA nuclear import upon aa deprivation. Further investigations are necessary to determine whether other possible mechanisms are involved in regulating tRNA subcellular dynamics, such as aa signaling, alteration of modification status of β-importin or Ssa2, regulation of β-importin or Ssa2 interacting proteins, and tethering of components in the various subcellular compartments.
9. tRNA-Derived Fragment as a Regulator in Cellular Process
tRNAs can be cleaved into tRNA-derived ncRNAs in all domains of life and cleavage of tRNAs often occurs during stress conditions [6,123,124,125,126,127,128]. tRNA-derived ncRNAs can be broadly classified into two main groups: tRNA halves and tRNA-derived fragments (tRFs) [123,129]. tRNA halves, which are composed of 30–35 nucleotides derived from either the 5′ or 3′ part of mature tRNAs, are generated under various stress conditions (Figure 1E). When vertebrate tRNAs are exposed to oxidative stress, heat shock, and UV irradiation, angiogenin, a member of the RNase A family, is activated and endonucleolytically cleaves mature tRNAs [130]. Angiogenin may first cleave within the CCA terminus of tRNAs and subsequently cleaves in the anticodon loops of tRNAs, generating 5′ and 3′ tRNA halves [55]. Angiogenin is usually sequestered in the nucleolus or bound to its inhibitor Rnh1 under normal conditions, whereas it is activated and released into the cytoplasm upon exposed to stress. However, the regulation of how angiogenin releases from these cellular compartments is not clear.
In yeast, Rny1, a member of the RNase T2, is responsible for the generation of tRNA halves. Cleavage of yeast tRNA species occurs in response to oxidative stress, methionine starvation, extended growth, and heat, but not in cells undergoing UV stress and glucose starvation [123]. Under normal conditions, yeast Rny1 is localized in the vacuole. Upon exposed to oxidative stress, Rny1 is thought to be released into the cytoplasm, which provides a mechanism by which it could access tRNAs for cleavage [131].
The function of tRNA halves is poorly understood. Certain tRNA halves inhibit translation initiation by displacing the cap-binding complex eIF4F from capped mRNA and inducing the assembly of cytoplasmic stress granules [132,133]. tRNA halves can additionally inhibit stress-induced apoptosis by binding to cytochrome c, therefore protecting cells from apoptosis during osmotic stress [134].
tRNAs can be cleaved at other positions to yield a diverse set of additional tRNA-derived fragments. tRFs are shorter than tRNA halves, ranging from 13 to 32 nt in length (Figure 1F,G). In many organisms, various tRNA-derived ncRNAs are produced from mature tRNAs or their precursor transcripts. Based on their position of origin in pre-tRNAs or mature tRNAs, different types of tRFs are classified as 5′ tRFs, 3′ CCA tRFs, 3′ U tRFs, internal tRFs and 5′ leader-exon tRFs [5,135]. The 5′ tRFs are derived from the 5′ parts of mature tRNAs and are formed by a cleavage in the D loop. 3′ CCA tRFs correspond to 3′ parts of mature tRNAs containing 3′ CCA termini and are formed by cleavage at the T loop. Dicer, angiogenin, and other RNase A family members have been implicated in the generation of the 3′ tRF. The 3′ U tRFs are cleaved by RNaseZ from the 3′ leader sequence of precursor tRNAs (pre-tRNAs) that harbor a stretch of U residues at 3′ end, which is produced upon RNA polymerase III termination. The internal tRFs (itRFs) are derived from a combination of cleavages in the anticodon loop and either D loop or TΨC loop [136]. The 5′ leader-exon tRFs include the 5′ leader sequence of pre-tRNAs and the 5′ part of mature tRNAs [137]. However, the mechanism of formation of 5′ leader-exon tRNAs in not clear.
In higher eukaryotes, some of these tRNA derived tRNA fragments associated with Argonaute proteins and they have emerging roles in gene expression regulation as regulatory RNAs [14,138]. The stress-induced 5′-tRF derived from tRNAVal in the archaeon Haloferax volcanii directly binds to small ribosome subunits, and therefore tRF probably fine tunes the rate of protein synthesis to regulate gene expression upon stress conditions [139]. In the pumpkin (Cucurbita maxima), tRNA fragments that are found in the phloem sap efficiently inhibit translation in an unspecific manner in vitro, indicating that these tRNA fragments may interfere with ribosomal activity [140]. Therefore, accumulating data implicate that fragments as signaling molecules that modulate translation. Furthermore, a tRF derived from the 3′ trailer sequence of human pre-tRNASerUGA is known to promote cell proliferation [129], suggesting that some tRNA fragments may function beyond translation. Interestingly, recent studies show that paternal diet in mice can alter the levels and/or the modifications of tRNA fragments, mainly derived from 5′ ends of tRNAs, in mature sperm to regulate gene expression of metabolic pathways [141,142].
10. Perspectives
tRNA levels are highly regulated and tRNAs are subjected to many post-transcriptional regulatory mechanisms. In budding yeast, 3′ end processing of tRNA is specifically inhibited by certain stress and this stress-responsive 3′ end maturation of tRNA could contribute to fine-tune the levels of functional tRNA in response to cellular conditions. Various sources of stress dynamically shift the population of tRNA modification with unique signature for each type of stress. Discoveries in cell biology have shown that tRNA subcellular traffic is bidirectional between the nucleus and the cytoplasm. Distinct glucose and amino acid dependent mechanisms regulate dynamics of tRNA subcellular distribution upon nutrient deprivation. Production of tRNA fragments serve as intracellular signaling molecules for stress response. Therefore, accumulating data suggest an emerging role of tRNA in stress response and diseases. Although we highlight the possible mechanisms in modulating tRNA pools, the integrated view of how these mechanisms regulate tRNA subcellular dynamics, levels and modification remain unclear. Stress-induced alteration in tRNA modification may affect the function or levels of tRNA in translation or other tRNA-involved biological processes. Whether or how tRNA modification enzymes respond to specific stress to uniquely reprogram tRNA modification levels requires further investigations. Since tRNAs may function as critical regulators in cell proliferation and differentiation, further study of the involvement of tRNA and tRNA fragments in signaling pathways and stress response pathway will lead to new links between tRNA function and other global cellular response systems and will give us insight of how cells respond to the environment.
Acknowledgments
This work was supported by a grant from the NIH (GM27930) to Anita K. Hopper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Dever T.E., Hinnebusch A.G. GCN2 whets the appetite for amino acids. Mol. Cell. 2005;18:141–142. doi: 10.1016/j.molcel.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 2.Mei Y., Stonestrom A., Hou Y.M., Yang X. Apoptotic regulation and tRNA. Protein Cell. 2010;1:795–801. doi: 10.1007/s13238-010-0107-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Varshavsky A. The N-end rule pathway of protein degradation. Genes Cells. 1997;2:13–28. doi: 10.1046/j.1365-2443.1997.1020301.x. [DOI] [PubMed] [Google Scholar]
- 4.Hopper A.K. Transfer RNA post-transcriptional processing, turnover, and subcellular dynamics in the yeast Saccharomyces cerevisiae. Genetics. 2013;194:43–67. doi: 10.1534/genetics.112.147470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Raina M., Ibba M. tRNAs as regulators of biological processes. Front. Genet. 2014;5:171. doi: 10.3389/fgene.2014.00171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kirchner S., Ignatova Z. Emerging roles of tRNA in adaptive translation, signalling dynamics and disease. Nat. Rev. Genet. 2015;16:98–112. doi: 10.1038/nrg3861. [DOI] [PubMed] [Google Scholar]
- 7.Torres A.G., Batlle E., Ribas de Pouplana L. Role of tRNA modifications in human diseases. Trends Mol. Med. 2014;4914:10. doi: 10.1016/j.molmed.2014.01.008. [DOI] [PubMed] [Google Scholar]
- 8.Wilusz J.E. Controlling translation via modulation of tRNA levels. Wiley Interdiscip. Rev. RNA. 2015;6:453–470. doi: 10.1002/wrna.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hopper A.K., Huang H.Y. Quality control pathways for nucleus-encoded eukaryotic tRNA biosynthesis and subcellular trafficking. Mol. Cell Biol. 2015;35:2052–2058. doi: 10.1128/MCB.00131-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lemmens R., Moore M.J., Al-Chalabi A., Brown R.H., Jr., Robberecht W. RNA metabolism and the pathogenesis of motor neuron diseases. Trends Neurosci. 2010;33:249–258. doi: 10.1016/j.tins.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 11.Budde B.S., Namavar Y., Barth P.G., Poll-The B.T., Nurnberg G., Becker C., van Ruissen F., Weterman M.A., Fluiter K., te Beek E.T., et al. tRNA splicing endonuclease mutations cause pontocerebellar hypoplasia. Nat. Genet. 2008;40:1113–1118. doi: 10.1038/ng.204. [DOI] [PubMed] [Google Scholar]
- 12.Guy M.P., Shaw M., Weiner C.L., Hobson L., Stark Z., Rose K., Kalscheuer V.M., Gecz J., Phizicky E.M. Defects in tRNA anticodon loop 2′-O-methylation are implicated in nonsyndromic x-linked intellectual disability due to mutations in FTSJ1. Hum. Mutat. 2015;36:1176–1187. doi: 10.1002/humu.22897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pavon-Eternod M., Gomes S., Geslain R., Dai Q., Rosner M.R., Pan T. tRNA over-expression in breast cancer and functional consequences. Nucleic Acids Res. 2009;37:7268–7280. doi: 10.1093/nar/gkp787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maute R.L., Schneider C., Sumazin P., Holmes A., Califano A., Basso K., Dalla-Favera R. tRNA-derived microRNA modulates proliferation and the DNA damage response and is down-regulated in B cell lymphoma. Proc. Natl. Acad. Sci. USA. 2013;110:1404–1409. doi: 10.1073/pnas.1206761110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pavon-Eternod M., Gomes S., Rosner M.R., Pan T. Overexpression of initiator methionine tRNA leads to global reprogramming of tRNA expression and increased proliferation in human epithelial cells. RNA. 2013;19:461–466. doi: 10.1261/rna.037507.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Phizicky E.M., Hopper A.K. tRNA biology charges to the front. Genes Dev. 2010;24:1832–1860. doi: 10.1101/gad.1956510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gudipati R.K., Xu Z., Lebreton A., Seraphin B., Steinmetz L.M., Jacquier A., Libri D. Extensive degradation of RNA precursors by the exosome in wild-type cells. Mol. Cell. 2012;48:409–421. doi: 10.1016/j.molcel.2012.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Anderson J., Phan L., Cuesta R., Carlson B.A., Pak M., Asano K., Bjork G.R., Tamame M., Hinnebusch A.G. The essential Gcd10p-Gcd14p nuclear complex is required for 1-methyladenosine modification and maturation of initiator methionyl-tRNA. Genes Dev. 1998;12:3650–3662. doi: 10.1101/gad.12.23.3650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gu C., Begley T.J., Dedon P.C. tRNA modifications regulate translation during cellular stress. FEBS Lett. 2014;588:4287–4296. doi: 10.1016/j.febslet.2014.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O’Connor J.P., Peebles C.L. In vivo pre-tRNA processing in Saccharomyces cerevisiae. Mol. Cell Biol. 1991;11:425–439. doi: 10.1128/MCB.11.1.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hopper A.K., Phizicky E.M. tRNA transfers to the limelight. Genes Dev. 2003;17:162–180. doi: 10.1101/gad.1049103. [DOI] [PubMed] [Google Scholar]
- 22.Xiao S., Scott F., Fierke C.A., Engelke D.R. Eukaryotic ribonuclease P: A plurality of ribonucleoprotein enzymes. Annu. Rev. Biochem. 2002;71:165–189. doi: 10.1146/annurev.biochem.71.110601.135352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jarrous N., Gopalan V. Archaeal/eukaryal RNase P: Subunits, functions and RNA diversification. Nucleic Acids Res. 2010;38:7885–7894. doi: 10.1093/nar/gkq701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gutmann B., Gobert A., Giege P. Prorp proteins support RNase P activity in both organelles and the nucleus in Arabidopsis. Genes Dev. 2012;26:1022–1027. doi: 10.1101/gad.189514.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pinker F., Bonnard G., Gobert A., Gutmann B., Hammani K., Sauter C., Gegenheimer P.A., Giege P. Ppr proteins shed a new light on RNase P biology. RNA Biol. 2013;10:9. doi: 10.4161/rna.25273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guerrier-Takada C., Gardiner K., Marsh T., Pace N., Altman S. The RNA moiety of ribonuclease P is the catalytic subunit of the enzyme. Cell. 1983;35:849–857. doi: 10.1016/0092-8674(83)90117-4. [DOI] [PubMed] [Google Scholar]
- 27.Torres-Larios A., Swinger K.K., Krasilnikov A.S., Pan T., Mondragon A. Crystal structure of the RNA component of bacterial ribonuclease p. Nature. 2005;437:584–587. doi: 10.1038/nature04074. [DOI] [PubMed] [Google Scholar]
- 28.Pannucci J.A., Haas E.S., Hall T.A., Harris J.K., Brown J.W. RNase P RNAs from some archaea are catalytically active. Proc. Natl. Acad. Sci. USA. 1999;96:7803–7808. doi: 10.1073/pnas.96.14.7803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cho I.M., Lai L.B., Susanti D., Mukhopadhyay B., Gopalan V. Ribosomal protein L7Ae is a subunit of archaeal RNase P. Proc. Natl. Acad. Sci. USA. 2010;107:14573–14578. doi: 10.1073/pnas.1005556107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marquez S.M., Chen J.L., Evans D., Pace N.R. Structure and function of eukaryotic ribonuclease P RNA. Mol. Cell. 2006;24:445–456. doi: 10.1016/j.molcel.2006.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marquez S.M., Harris J.K., Kelley S.T., Brown J.W., Dawson S.C., Roberts E.C., Pace N.R. Structural implications of novel diversity in eucaryal RNase P RNA. RNA. 2005;11:739–751. doi: 10.1261/rna.7211705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kikovska E., Svard S.G., Kirsebom L.A. Eukaryotic RNase P RNA mediates cleavage in the absence of protein. Proc. Natl. Acad. Sci. USA. 2007;104:2062–2067. doi: 10.1073/pnas.0607326104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li Z., Deutscher M.P. Maturation pathways for E. coli tRNA precursors: A random multienzyme process in vivo. Cell. 1996;86:503–512. doi: 10.1016/S0092-8674(00)80123-3. [DOI] [PubMed] [Google Scholar]
- 34.Skowronek E., Grzechnik P., Spath B., Marchfelder A., Kufel J. tRNA 3′ processing in yeast involves tRNase Z, Rex1, and Rrp6. RNA. 2014;20:115–130. doi: 10.1261/rna.041467.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Copela L.A., Fernandez C.F., Sherrer R.L., Wolin S.L. Competition between the Rex1 exonuclease and the La protein affects both Trf4p-mediated RNA quality control and pre-tRNA maturation. RNA. 2008;14:1214–1227. doi: 10.1261/rna.1050408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ozanick S.G., Wang X., Costanzo M., Brost R.L., Boone C., Anderson J.T. Rex1p deficiency leads to accumulation of precursor initiator trnamet and polyadenylation of substrate RNAs in Saccharomyces cerevisiae. Nucleic Acids Res. 2009;37:298–308. doi: 10.1093/nar/gkn925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Van Hoof A., Staples R.R., Baker R.E., Parker R. Function of the Ski4p (Csl4p) and Ski7p proteins in 3′-to-5′ degradation of mRNA. Mol. Cell Biol. 2000;20:8230–8243. doi: 10.1128/MCB.20.21.8230-8243.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen Y., Beck A., Davenport C., Shattuck D., Tavtigian S.V. Characterization of Trz1, a yeast homolog of the human candidate prostate cancer susceptibility gene ELAC2 encoding tRNase Z. BMC Mol. Biol. 2005;6:12. doi: 10.1186/1471-2199-6-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Daoud R., Forget L., Lang B.F. Yeast mitochondrial RNase P, RNase Z and the RNA degradosome are part of a stable supercomplex. Nucleic Acids Res. 2012;40:1728–1736. doi: 10.1093/nar/gkr941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Maraia R.J., Lamichhane T.N. 3′ processing of eukaryotic precursor tRNAs. Wiley Interdiscip. Rev. RNA. 2011;2:362–375. doi: 10.1002/wrna.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lin-Marq N., Clarkson S.G. A yeast RNA binding protein that resembles the human autoantigen La. J. Mol. Biol. 1995;245:81–85. doi: 10.1006/jmbi.1994.0008. [DOI] [PubMed] [Google Scholar]
- 42.Stefano J.E. Purified lupus antigen La recognizes an oligouridylate stretch common to the 3′ termini of RNA polymerase iii transcripts. Cell. 1984;36:145–154. doi: 10.1016/0092-8674(84)90083-7. [DOI] [PubMed] [Google Scholar]
- 43.Yoo C.J., Wolin S.L. The yeast La protein is required for the 3′ endonucleolytic cleavage that matures tRNA precursors. Cell. 1997;89:393–402. doi: 10.1016/S0092-8674(00)80220-2. [DOI] [PubMed] [Google Scholar]
- 44.Chakshusmathi G., Kim S.D., Rubinson D.A., Wolin S.L. A La protein requirement for efficient pre-tRNA folding. EMBO J. 2003;22:6562–6572. doi: 10.1093/emboj/cdg625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Van Horn D.J., Yoo C.J., Xue D., Shi H., Wolin S.L. The La protein in schizosaccharomyces pombe: A conserved yet dispensable phosphoprotein that functions in tRNA maturation. RNA. 1997;3:1434–1443. [PMC free article] [PubMed] [Google Scholar]
- 46.Foretek D., Wu J., Hopper A.K., Boguta M. Control of Saccharomyces cerevisiae pre-tRNA processing by environmental conditions. RNA. 2016;22:339–349. doi: 10.1261/rna.054973.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Endres L., Dedon P.C., Begley T.J. Codon-biased translation can be regulated by wobble-base tRNA modification systems during cellular stress responses. RNA Biol. 2015;12:603–614. doi: 10.1080/15476286.2015.1031947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Intine R.V., Sakulich A.L., Koduru S.B., Huang Y., Pierstorff E., Goodier J.L., Phan L., Maraia R.J. Control of transfer RNA maturation by phosphorylation of the human La antigen on serine 366. Mol. Cell. 2000;6:339–348. doi: 10.1016/S1097-2765(00)00034-4. [DOI] [PubMed] [Google Scholar]
- 49.Intine R.V., Tenenbaum S.A., Sakulich A.L., Keene J.D., Maraia R.J. Differential phosphorylation and subcellular localization of La RNPs associated with precursor tRNAs and translation-related mRNAs. Mol. Cell. 2003;12:1301–1307. doi: 10.1016/S1097-2765(03)00429-5. [DOI] [PubMed] [Google Scholar]
- 50.Rutjes S.A., Utz P.J., van der Heijden A., Broekhuis C., van Venrooij W.J., Pruijn G.J. The La (SS-B) autoantigen, a key protein in RNA biogenesis, is dephosphorylated and cleaved early during apoptosis. Cell Death Differ. 1999;6:976–986. doi: 10.1038/sj.cdd.4400571. [DOI] [PubMed] [Google Scholar]
- 51.Reuven N.B., Deutscher M.P. Substitution of the 3' terminal adenosine residue of transfer RNA in vivo. Proc. Natl. Acad. Sci. USA. 1993;90:4350–4353. doi: 10.1073/pnas.90.10.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhu L., Deutscher M.P. tRNA nucleotidyltransferase is not essential for Escherichia coli viability. EMBO J. 1987;6:2473–2477. doi: 10.1002/j.1460-2075.1987.tb02528.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Aebi M., Kirchner G., Chen J.Y., Vijayraghavan U., Jacobson A., Martin N.C., Abelson J. Isolation of a temperature-sensitive mutant with an altered tRNA nucleotidyltransferase and cloning of the gene encoding tRNA nucleotidyltransferase in the yeast Saccharomyces cerevisiae. J. Biol. Chem. 1990;265:16216–16220. [PubMed] [Google Scholar]
- 54.Martin N.C., Hopper A.K. How single genes provide tRNA processing enzymes to mitochondria, nuclei and the cytosol. Biochimie. 1994;76:1161–1167. doi: 10.1016/0300-9084(94)90045-0. [DOI] [PubMed] [Google Scholar]
- 55.Czech A., Wende S., Morl M., Pan T., Ignatova Z. Reversible and rapid transfer-RNA deactivation as a mechanism of translational repression in stress. PLoS Genet. 2013;9:16. doi: 10.1371/journal.pgen.1003767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kuhn C.D., Wilusz J.E., Zheng Y., Beal P.A., Joshua-Tor L. On-enzyme refolding permits small RNA and tRNA surveillance by the CCA-adding enzyme. Cell. 2015;160:644–658. doi: 10.1016/j.cell.2015.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wilusz J.E., Whipple J.M., Phizicky E.M., Sharp P.A. tRNAs marked with CCACCA are targeted for degradation. Science. 2011;334:817–821. doi: 10.1126/science.1213671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Czerwoniec A., Dunin-Horkawicz S., Purta E., Kaminska K.H., Kasprzak J.M., Bujnicki J.M., Grosjean H., Rother K. Modomics: A database of RNA modification pathways. 2008 update. Nucleic Acids Res. 2009;37:D118–D121. doi: 10.1093/nar/gkn710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jackman J.E., Alfonzo J.D. Transfer RNA modifications: Nature’s combinatorial chemistry playground. Wiley Interdiscip. Rev. RNA. 2013;4:35–48. doi: 10.1002/wrna.1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.El Yacoubi B., Bailly M., de Crecy-Lagard V. Biosynthesis and function of posttranscriptional modifications of transfer RNAs. Annu. Rev. Genet. 2012;46:69–95. doi: 10.1146/annurev-genet-110711-155641. [DOI] [PubMed] [Google Scholar]
- 61.Gerber A.P., Keller W. An adenosine deaminase that generates inosine at the wobble position of tRNAs. Science. 1999;286:1146–1149. doi: 10.1126/science.286.5442.1146. [DOI] [PubMed] [Google Scholar]
- 62.Waas W.F., Druzina Z., Hanan M., Schimmel P. Role of a tRNA base modification and its precursors in frameshifting in eukaryotes. J. Biol. Chem. 2007;282:26026–26034. doi: 10.1074/jbc.M703391200. [DOI] [PubMed] [Google Scholar]
- 63.Leidel S., Pedrioli P.G., Bucher T., Brost R., Costanzo M., Schmidt A., Aebersold R., Boone C., Hofmann K., Peter M. Ubiquitin-related modifier Urm1 acts as a sulphur carrier in thiolation of eukaryotic transfer RNA. Nature. 2009;458:228–232. doi: 10.1038/nature07643. [DOI] [PubMed] [Google Scholar]
- 64.Nakai Y., Nakai M., Hayashi H. Thio-modification of yeast cytosolic tRNA requires a ubiquitin-related system that resembles bacterial sulfur transfer systems. J. Biol. Chem. 2008;283:27469–27476. doi: 10.1074/jbc.M804043200. [DOI] [PubMed] [Google Scholar]
- 65.Schlieker C.D., Van der Veen A.G., Damon J.R., Spooner E., Ploegh H.L. A functional proteomics approach links the ubiquitin-related modifier Urm1 to a tRNA modification pathway. Proc. Natl. Acad. Sci. USA. 2008;105:18255–18260. doi: 10.1073/pnas.0808756105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nedialkova D.D., Leidel S.A. Optimization of codon translation rates via tRNA modifications maintains proteome integrity. Cell. 2015;161:1606–1618. doi: 10.1016/j.cell.2015.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Deng W., Babu I.R., Su D., Yin S., Begley T.J., Dedon P.C. Trm9-catalyzed tRNA modifications regulate global protein expression by codon-biased translation. PLoS Genet. 2015;11:16. doi: 10.1371/journal.pgen.1005706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Johansson M.J., Bystrom A.S. Dual function of the tRNA(m(5)U54)methyltransferase in tRNA maturation. RNA. 2002;8:324–335. doi: 10.1017/S1355838202027851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chernyakov I., Whipple J.M., Kotelawala L., Grayhack E.J., Phizicky E.M. Degradation of several hypomodified mature tRNA species in Saccharomyces cerevisiae is mediated by Met22 and the 5′–3′ exonucleases Rat1 and Xrn1. Genes Dev. 2008;22:1369–1380. doi: 10.1101/gad.1654308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kotelawala L., Grayhack E.J., Phizicky E.M. Identification of yeast tRNA Um(44) 2′-O-methyltransferase (Trm44) and demonstration of a Trm44 role in sustaining levels of specific tRNA(Ser) species. RNA. 2008;14:158–169. doi: 10.1261/rna.811008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Grosshans H., Lecointe F., Grosjean H., Hurt E., Simos G. Pus1p-dependent tRNA pseudouridinylation becomes essential when tRNA biogenesis is compromised in yeast. J. Biol. Chem. 2001;276:46333–46339. doi: 10.1074/jbc.M107141200. [DOI] [PubMed] [Google Scholar]
- 72.Alexandrov A., Chernyakov I., Gu W., Hiley S.L., Hughes T.R., Grayhack E.J., Phizicky E.M. Rapid tRNA decay can result from lack of nonessential modifications. Mol. Cell. 2006;21:87–96. doi: 10.1016/j.molcel.2005.10.036. [DOI] [PubMed] [Google Scholar]
- 73.Dewe J.M., Whipple J.M., Chernyakov I., Jaramillo L.N., Phizicky E.M. The yeast rapid tRNA decay pathway competes with elongation factor 1a for substrate tRNAs and acts on tRNAs lacking one or more of several modifications. RNA. 2012;18:1886–1896. doi: 10.1261/rna.033654.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Shin B.S., Kim J.R., Walker S.E., Dong J., Lorsch J.R., Dever T.E. Initiation factor eIF2gamma promotes eIF2-GTP-Met-tRNAi(Met) ternary complex binding to the 40s ribosome. Nat. Struct. Mol. Biol. 2011;18:1227–1234. doi: 10.1038/nsmb.2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Han L., Kon Y., Phizicky E.M. Functional importance of Psi38 and Psi39 in distinct tRNAs, amplified for tRNAGln(UUG) by unexpected temperature sensitivity of the s2U modification in yeast. RNA. 2015;21:188–201. doi: 10.1261/rna.048173.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Preston M.A., D'Silva S., Kon Y., Phizicky E.M. tRNAHis 5-methylcytidine levels increase in response to several growth arrest conditions in Saccharomyces cerevisiae. RNA. 2013;19:243–256. doi: 10.1261/rna.035808.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Alings F., Sarin L.P., Fufezan C., Drexler H.C., Leidel S.A. An evolutionary approach uncovers a diverse response of tRNA 2-thiolation to elevated temperatures in yeast. RNA. 2015;21:202–212. doi: 10.1261/rna.048199.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Damon J.R., Pincus D., Ploegh H.L. tRNA thiolation links translation to stress responses in Saccharomyces cerevisiae. Mol. Biol. Cell. 2015;26:270–282. doi: 10.1091/mbc.E14-06-1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chan C.T., Dyavaiah M., DeMott M.S., Taghizadeh K., Dedon P.C., Begley T.J. A quantitative systems approach reveals dynamic control of tRNA modifications during cellular stress. PLoS Genet. 2010;6:16. doi: 10.1371/journal.pgen.1001247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Carlile T.M., Rojas-Duran M.F., Zinshteyn B., Shin H., Bartoli K.M., Gilbert W.V. Pseudouridine profiling reveals regulated mRNA pseudouridylation in yeast and human cells. Nature. 2014;515:143–146. doi: 10.1038/nature13802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Schwartz S., Bernstein D.A., Mumbach M.R., Jovanovic M., Herbst R.H., Leon-Ricardo B.X., Engreitz J.M., Guttman M., Satija R., Lander E.S., et al. Transcriptome-wide mapping reveals widespread dynamic-regulated pseudouridylation of ncRNA and mRNA. Cell. 2014;159:148–162. doi: 10.1016/j.cell.2014.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chan C.T., Pang Y.L., Deng W., Babu I.R., Dyavaiah M., Begley T.J., Dedon P.C. Reprogramming of tRNA modifications controls the oxidative stress response by codon-biased translation of proteins. Nat. Commun. 2012;3:937. doi: 10.1038/ncomms1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Dedon P.C., Begley T.J. A system of RNA modifications and biased codon use controls cellular stress response at the level of translation. Chem. Res. Toxicol. 2014;27:330–337. doi: 10.1021/tx400438d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chan C.T., Deng W., Li F., DeMott M.S., Babu I.R., Begley T.J., Dedon P.C. Highly predictive reprogramming of tRNA modifications is linked to selective expression of codon-biased genes. Chem. Res. Toxicol. 2015;28:978–988. doi: 10.1021/acs.chemrestox.5b00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Schaefer M., Pollex T., Hanna K., Tuorto F., Meusburger M., Helm M., Lyko F. RNA methylation by Dnmt2 protects transfer RNAs against stress-induced cleavage. Genes Dev. 2010;24:1590–1595. doi: 10.1101/gad.586710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Huang B., Lu J., Bystrom A.S. A genome-wide screen identifies genes required for formation of the wobble nucleoside 5-methoxycarbonylmethyl-2-thiouridine in Saccharomyces cerevisiae. RNA. 2008;14:2183–2194. doi: 10.1261/rna.1184108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hopper A.K., Shaheen H.H. A decade of surprises for tRNA nuclear-cytoplasmic dynamics. Trends Cell Biol. 2008;18:98–104. doi: 10.1016/j.tcb.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 88.Shaheen H.H., Horetsky R.L., Kimball S.R., Murthi A., Jefferson L.S., Hopper A.K. Retrograde nuclear accumulation of cytoplasmic tRNA in rat hepatoma cells in response to amino acid deprivation. Proc. Natl. Acad. Sci. USA. 2007;104:8845–8850. doi: 10.1073/pnas.0700765104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ohira T., Suzuki T. Retrograde nuclear import of tRNA precursors is required for modified base biogenesis in yeast. Proc. Natl. Acad. Sci. USA. 2011;108:10502–10507. doi: 10.1073/pnas.1105645108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zaitseva L., Myers R., Fassati A. tRNAs promote nuclear import of HIV-1 intracellular reverse transcription complexes. PLoS Biol. 2006;4:16. doi: 10.1371/journal.pbio.0040332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kramer E.B., Hopper A.K. Retrograde transfer RNA nuclear import provides a new level of tRNA quality control in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA. 2013;110:21042–21047. doi: 10.1073/pnas.1316579110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chu H.Y., Hopper A.K. Genome-wide investigation of the role of the tRNA nucleus-cytoplasm trafficking pathway in regulation of the yeast s. Cerevisiae transcriptome and proteome. Mol. Cell Biol. 2013;2013:26. doi: 10.1128/MCB.00785-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Arts G.J., Fornerod M., Mattaj I.W. Identification of a nuclear export receptor for tRNA. Curr. Biol. 1998;8:305–314. doi: 10.1016/S0960-9822(98)70130-7. [DOI] [PubMed] [Google Scholar]
- 94.Kutay U., Lipowsky G., Izaurralde E., Bischoff F.R., Schwarzmaier P., Hartmann E., Gorlich D. Identification of a tRNA-specific nuclear export receptor. Mol. Cell. 1998;1:359–369. doi: 10.1016/S1097-2765(00)80036-2. [DOI] [PubMed] [Google Scholar]
- 95.Hunter C.A., Aukerman M.J., Sun H., Fokina M., Poethig R.S. PAUSED encodes the Arabidopsis exportin-t ortholog. Plant Physiol. 2003;132:2135–2143. doi: 10.1104/pp.103.023309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Cook A.G., Fukuhara N., Jinek M., Conti E. Structures of the tRNA export factor in the nuclear and cytosolic states. Nature. 2009;461:60–65. doi: 10.1038/nature08394. [DOI] [PubMed] [Google Scholar]
- 97.Hellmuth K., Lau D.M., Bischoff F.R., Kunzler M., Hurt E., Simos G. Yeast Los1p has properties of an exportin-like nucleocytoplasmic transport factor for tRNA. Mol. Cell Biol. 1998;18:6374–6386. doi: 10.1128/MCB.18.11.6374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hopper A.K., Schultz L.D., Shapiro R.A. Processing of intervening sequences: A new yeast mutant which fails to excise intervening sequences from precursor tRNAs. Cell. 1980;19:741–751. doi: 10.1016/S0092-8674(80)80050-X. [DOI] [PubMed] [Google Scholar]
- 99.Sarkar S., Hopper A.K. tRNA nuclear export in Saccharomyces cerevisiae: In situ hybridization analysis. Mol. Biol. Cell. 1998;9:3041–3055. doi: 10.1091/mbc.9.11.3041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Arts G.J., Kuersten S., Romby P., Ehresmann B., Mattaj I.W. The role of exportin-t in selective nuclear export of mature tRNAs. EMBO J. 1998;17:7430–7441. doi: 10.1093/emboj/17.24.7430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lund E., Dahlberg J.E. Proofreading and aminoacylation of tRNAs before export from the nucleus. Science. 1998;282:2082–2085. doi: 10.1126/science.282.5396.2082. [DOI] [PubMed] [Google Scholar]
- 102.Lipowsky G., Bischoff F.R., Izaurralde E., Kutay U., Schafer S., Gross H.J., Beier H., Gorlich D. Coordination of tRNA nuclear export with processing of tRNA. RNA. 1999;5:539–549. doi: 10.1017/S1355838299982134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Huang H.Y., Hopper A.K. In vivo biochemical analyses reveal distinct roles of beta-importins and eEF1a in tRNA subcellular traffic. Genes Dev. 2015;29:772–783. doi: 10.1101/gad.258293.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yoshihisa T., Yunoki-Esaki K., Ohshima C., Tanaka N., Endo T. Possibility of cytoplasmic pre-tRNA splicing: The yeast tRNA splicing endonuclease mainly localizes on the mitochondria. Mol. Biol. Cell. 2003;14:3266–3279. doi: 10.1091/mbc.E02-11-0757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Murthi A., Shaheen H.H., Huang H.Y., Preston M.A., Lai T.P., Phizicky E.M., Hopper A.K. Regulation of tRNA bidirectional nuclear-cytoplasmic trafficking in Saccharomyces cerevisiae. Mol. Biol. Cell. 2010;21:639–649. doi: 10.1091/mbc.E09-07-0551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Takano A., Endo T., Yoshihisa T. tRNA actively shuttles between the nucleus and cytosol in yeast. Science. 2005;309:140–142. doi: 10.1126/science.1113346. [DOI] [PubMed] [Google Scholar]
- 107.Wu J., Bao A., Chatterjee K., Wan Y., Hopper A.K. Genome-wide screen uncovers novel pathways for tRNA processing and nuclear-cytoplasmic dynamics. Genes Dev. 2015;29:2633–2644. doi: 10.1101/gad.269803.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Shaheen H.H., Hopper A.K. Retrograde movement of tRNAs from the cytoplasm to the nucleus in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA. 2005;102:11290–11295. doi: 10.1073/pnas.0503836102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Takano A., Kajita T., Mochizuki M., Endo T., Yoshihisa T. Cytosolic Hsp70 and co-chaperones constitute a novel system for tRNA import into the nucleus. eLife. 2015;4 doi: 10.7554/eLife.04659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Grosshans H., Hurt E., Simos G. An aminoacylation-dependent nuclear tRNA export pathway in yeast. Genes Dev. 2000;14:830–840. [PMC free article] [PubMed] [Google Scholar]
- 111.Hurto R.L., Tong A.H., Boone C., Hopper A.K. Inorganic phosphate deprivation causes tRNA nuclear accumulation via retrograde transport in Saccharomyces cerevisiae. Genetics. 2007;176:841–852. doi: 10.1534/genetics.106.069732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Whitney M.L., Hurto R.L., Shaheen H.H., Hopper A.K. Rapid and reversible nuclear accumulation of cytoplasmic tRNA in response to nutrient availability. Mol. Biol. Cell. 2007;18:2678–2686. doi: 10.1091/mbc.E07-01-0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Huang H.Y., Hopper A.K. Separate responses of karyopherins to glucose and amino acid availability regulate nucleocytoplasmic transport. Mol. Biol. Cell. 2014;25:2840–2852. doi: 10.1091/mbc.E14-04-0948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ghavidel A., Kislinger T., Pogoutse O., Sopko R., Jurisica I., Emili A. Impaired tRNA nuclear export links DNA damage and cell-cycle checkpoint. Cell. 2007;131:915–926. doi: 10.1016/j.cell.2007.09.042. [DOI] [PubMed] [Google Scholar]
- 115.Karkusiewicz I., Turowski T.W., Graczyk D., Towpik J., Dhungel N., Hopper A.K., Boguta M. Maf1 protein, repressor of RNA polymerase III, indirectly affects tRNA processing. J. Biol. Chem. 2011;286:39478–39488. doi: 10.1074/jbc.M111.253310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Pierce J.B., van der Merwe G., Mangroo D. Protein kinase a is part of a mechanism that regulates nuclear reimport of the nuclear tRNA export receptors Los1p and Msn5p. Eukaryot. Cell. 2014;13:209–230. doi: 10.1128/EC.00214-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Quan X., Tsoulos P., Kuritzky A., Zhang R., Stochaj U. The carrier Msn5p/Kap142p promotes nuclear export of theHsp70 Ssa4p and relocates in response to stress. Mol. Microbiol. 2006;62:592–609. doi: 10.1111/j.1365-2958.2006.05395.x. [DOI] [PubMed] [Google Scholar]
- 118.Quan X., Yu J., Bussey H., Stochaj U. The localization of nuclear exporters of the importin-beta family is regulated by Snf1 kinase, nutrient supply and stress. Biochim. Biophys. Acta. 2007;1773:1052–1061. doi: 10.1016/j.bbamcr.2007.04.014. [DOI] [PubMed] [Google Scholar]
- 119.Feng W., Hopper A.K. A Los1p-independent pathway for nuclear export of intronless tRNAs in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA. 2002;99:5412–5417. doi: 10.1073/pnas.082682699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Azad A.K., Stanford D.R., Sarkar S., Hopper A.K. Role of nuclear pools of aminoacyl-tRNA synthetases in tRNA nuclear export. Mol. Biol. Cell. 2001;12:1381–1392. doi: 10.1091/mbc.12.5.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Sarkar S., Azad A.K., Hopper A.K. Nuclear tRNA aminoacylation and its role in nuclear export of endogenous tRNAs in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA. 1999;96:14366–14371. doi: 10.1073/pnas.96.25.14366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Gu W., Hurto R.L., Hopper A.K., Grayhack E.J., Phizicky E.M. Depletion of Saccharomyces cerevisiae tRNA(His) guanylyltransferase Thg1p leads to uncharged trnahis with additional m(5)C. Mol. Cell Biol. 2005;25:8191–8201. doi: 10.1128/MCB.25.18.8191-8201.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Thompson D.M., Lu C., Green P.J., Parker R. tRNA cleavage is a conserved response to oxidative stress in eukaryotes. RNA. 2008;14:2095–2103. doi: 10.1261/rna.1232808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kaufmann G. Anticodon nucleases. Trends Biochem. Sci. 2000;25:70–74. doi: 10.1016/S0968-0004(99)01525-X. [DOI] [PubMed] [Google Scholar]
- 125.Masaki H., Ogawa T. The modes of action of colicins E5 and D, and related cytotoxic tRNases. Biochimie. 2002;84:433–438. doi: 10.1016/S0300-9084(02)01425-6. [DOI] [PubMed] [Google Scholar]
- 126.Anderson P., Ivanov P. tRNA fragments in human health and disease. FEBS Lett. 2014;588:4297–4304. doi: 10.1016/j.febslet.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Gebetsberger J., Polacek N. Slicing tRNAs to boost functional ncRNA diversity. RNA Biol. 2013;10:1798–1806. doi: 10.4161/rna.27177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Thompson D.M., Parker R. Stressing out over tRNA cleavage. Cell. 2009;138:215–219. doi: 10.1016/j.cell.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 129.Lee Y.S., Shibata Y., Malhotra A., Dutta A. A novel class of small RNAs: tRNA-derived RNA fragments (tRFs) Genes Dev. 2009;23:2639–2649. doi: 10.1101/gad.1837609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Fu H., Feng J., Liu Q., Sun F., Tie Y., Zhu J., Xing R., Sun Z., Zheng X. Stress induces tRNA cleavage by angiogenin in mammalian cells. FEBS Lett. 2009;583:437–442. doi: 10.1016/j.febslet.2008.12.043. [DOI] [PubMed] [Google Scholar]
- 131.Thompson D.M., Parker R. The RNase Rny1p cleaves tRNAs and promotes cell death during oxidative stress in Saccharomyces cerevisiae. J. Cell Biol. 2009;185:43–50. doi: 10.1083/jcb.200811119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ivanov P., Emara M.M., Villen J., Gygi S.P., Anderson P. Angiogenin-induced tRNA fragments inhibit translation initiation. Mol. Cell. 2011;43:613–623. doi: 10.1016/j.molcel.2011.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ivanov P., O'Day E., Emara M.M., Wagner G., Lieberman J., Anderson P. G-quadruplex structures contribute to the neuroprotective effects of angiogenin-induced tRNA fragments. Proc. Natl. Acad. Sci. USA. 2014;111:18201–18206. doi: 10.1073/pnas.1407361111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Saikia M., Jobava R., Parisien M., Putnam A., Krokowski D., Gao X.H., Guan B.J., Yuan Y., Jankowsky E., Feng Z., et al. Angiogenin-cleaved tRNA halves interact with cytochrome c, protecting cells from apoptosis during osmotic stress. Mol. Cell Biol. 2014;34:2450–2463. doi: 10.1128/MCB.00136-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Sobala A., Hutvagner G. Transfer RNA-derived fragments: Origins, processing, and functions. Wiley Interdiscip. Rev. RNA. 2011;2:853–862. doi: 10.1002/wrna.96. [DOI] [PubMed] [Google Scholar]
- 136.Telonis A.G., Loher P., Honda S., Jing Y., Palazzo J., Kirino Y., Rigoutsos I. Dissecting tRNA-derived fragment complexities using personalized transcriptomes reveals novel fragment classes and unexpected dependencies. Oncotarget. 2015;6:24797–24822. doi: 10.18632/oncotarget.4695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Hanada T., Weitzer S., Mair B., Bernreuther C., Wainger B.J., Ichida J., Hanada R., Orthofer M., Cronin S.J., Komnenovic V., et al. Clp1 links tRNA metabolism to progressive motor-neuron loss. Nature. 2013;495:474–480. doi: 10.1038/nature11923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Zamudio J.R., Kelly T.J., Sharp P.A. Argonaute-bound small RNAs from promoter-proximal RNA polymerase II. Cell. 2014;156:920–934. doi: 10.1016/j.cell.2014.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Gebetsberger J., Zywicki M., Kunzi A., Polacek N. tRNA-derived fragments target the ribosome and function as regulatory non-coding RNA in Haloferax volcanii. Archaea. 2012;2012:260909. doi: 10.1155/2012/260909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Zhang S., Sun L., Kragler F. The phloem-delivered RNA pool contains small noncoding RNAs and interferes with translation. Plant Physiol. 2009;150:378–387. doi: 10.1104/pp.108.134767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Sharma U., Conine C.C., Shea J.M., Boskovic A., Derr A.G., Bing X.Y., Belleannee C., Kucukural A., Serra R.W., Sun F., et al. Biogenesis and function of tRNA fragments during sperm maturation and fertilization in mammals. Science. 2016;351:391–396. doi: 10.1126/science.aad6780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Chen Q., Yan M., Cao Z., Li X., Zhang Y., Shi J., Feng G.H., Peng H., Zhang X., Zhang Y., et al. Sperm tsRNAs contribute to intergenerational inheritance of an acquired metabolic disorder. Science. 2016;351:397–400. doi: 10.1126/science.aad7977. [DOI] [PubMed] [Google Scholar]