Abstract
Malfunction of cystic fibrosis transmembrane conductance regulator (CFTR), a member of the ABC protein superfamily that functions as an ATP-gated chloride channel, causes the lethal genetic disease, cystic fibrosis. This review focuses on the most recent findings on the gating mechanism of CFTR. Potential clinical relevance and implications to ABC transporter function are also discussed.
Ion channels and active transporters are integral membrane proteins ubiquitously expressed in every living cell. They allow trafficking and communications between the intracellular and extracellular environments that would be otherwise isolated by the plasma membrane. Traditionally, channels and transporters are thought to be completely different entities. For ion channels, it can be as simple as a tunnel-like structure embedded in the lipid bilayer with the addition of a regulatable gate to control the patency of the tunnel. On the other hand, an active transporter must be equipped with an energy-harvesting machine that utilizes some sorts of free energy input to drive the transport cycle in a preferred direction to translocate its cargos against a concentration gradient. Furthermore, it was generally believed that an active transporter must not form a channel-like conformation that grants access from both sides of the membrane; otherwise the cargo would flip through the concentration gradient and hence ruin all its efforts (30).
Despite these apparent differences in the mechanism of action, phylogenic analysis revealed several closely related ion channels and transporters clustered in two distinct families of membrane proteins: the CLC protein family and ATP binding cassette (ABC) protein superfamily (review in Ref.18). These surprising findings apparently break the long-held boundary between channels and transporters but at the same time open an unprecedented opportunity for us to get a glimpse of the evolutionary relationship between these two important classes of membrane proteins. Evidently, breakthroughs in the past two decades in solving high-resolution crystal structures of membrane proteins have also called for reexamining the similarities and differences between channels and transporters. For example, the crystal structure of an eukaryotic CLC transporter (28) clearly shows how a channel-like structure can actually effect the function of Cl−/H+ exchange (an example of so-called secondary active transporter).
On the other hand, ABC protein superfamily contains mostly primary active transporters that utilize ATP hydrolysis as the source of free energy to move substrates into (importers) or out of (exporters) the cell. Members of the ABC protein family carry out a broad spectrum of functions, including uptake of nutrients (25, 29), exporting metabolic wastes (33), regulating ion channel function (17), and enabling multidrug resistance in cancer cells (66). Among them, CFTR is a unique member in that, instead of functioning as an active transporter, it is a bona fide ion channel (11). Moreover, malfunction of CFTR constitutes the fundamental cause of a common lethal genetic disease, cystic fibrosis (64). Therefore, studying the structural mechanism of CFTR function is expected to not only elucidate the channel-transporter relationship but also bear significant clinical relevance. Extensive understanding in how pathogenic mutations cause dysfunction of CFTR and how these functional defects can be mitigated by small pharmaceutical reagents may serve as a foundation for developing new strategies in CF treatment (15, 67, 74, 77).
CFTR-An ATP-Gated Chloride Channel Evolved From Transporters
Like other members in the ABC protein superfamily, CFTR contains the four canonical domains: two transmembrane domains (TMDs) that form the ion-conductive pathway and two nucleotide binding domains (NBDs) where ATP binds. In addition to these four domains, CFTR also has a unique regulatory domain (R domain) that is not found in other ABC proteins. The R domain harbors multiple serine and threonine residues that can be phosphorylated by protein kinase A (PKA). NMR studies suggested that the R domain assumes a disordered structure, and its conformation and interdomain interactions change in accordance with the phosphorylation level (10). In its native form, the R domain is known to mainly inhibit channel activity, and this inhibition is released after phosphorylation by PKA, since removal of the R domain renders the CFTR channel phosphorylation independent while it mostly retains its ATP-dependent gating properties (12, 21). Since this review will be focused on how interactions of ATP with NBDs control opening/closing of the gate in TMDs (a step following phosphorylation of the R domain), interested readers are referred to more extensive reviews on R domain function (3, 31, 58).
By comparing the crystal structures of CFTR’s two NBDs (Ref. 49 and PDB no. 3GD7) with those in other ABC transporters (7, 26, 38, 40, 51, 82), one concludes that the overall architecture of the NBDs is well conserved during evolution. For CFTR as well as other ABC proteins, the NBD serves as an engine that harvests the free energy of ATP hydrolysis to drive the transport/gating cycle. Early functional studies provided compelling evidence for a role of ATP binding and hydrolysis in controlling CFTR gating (41, 76, 84), but the structural basis for gating motion is largely unknown until the seminal work by Vergani et al. (75). Inspired by the high-resolution structures of ABC proteins that show a characteristic “head-to-tail” dimer of their NBDs (7, 26, 38, 40, 51, 82) (FIGURE 1), Vergani et al. (75) took a novel bioinformatics approach to demonstrate that CFTR’s two NBDs also dimerize upon ATP binding. By comparing the sequences of >10,000 ABC proteins, they found that two residues co-evolve as a pair that likely forms a hydrogen bond at the NBD dimer interface. The specific interaction between these two residues in CFTR was elegantly confirmed by rigorous mutant cycle analysis. Detailed single-channel kinetic studies convincingly showed that the formation of a NBD dimer is a prerequisite for gate opening (75). The importance of NBD dimer formation in CFTR gating was reinforced by results showing robust ATP-independent channel activity upon cross linking CFTR’s two NBDs (53).
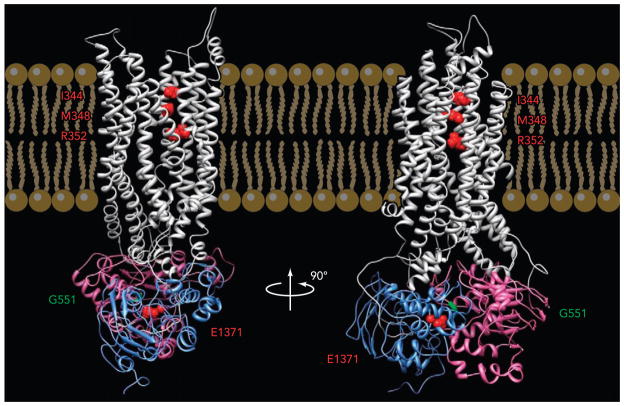
FIGURE 1. A modeled CFTR structure.
Side views of a homology model of CFTR based on the prokaryotic ABC exporter, Sav1866 (54). Gray, TMDs; purple, NBD1; blue, NBD2. Three residues in TM6 mentioned in this review (from top to bottom: I344, M348, R352) are labeled in red. G551 in NBD1 is labeled in green. E1371 in NBD2 is labeled in red (courtesy of Dr. David Dawson).
Unlike the NBDs that are well conserved among ABC proteins, the TMDs share little sequence homology. In fact, the number of transmembrane helixes per TMD also varies [e.g., 20 for BtuCD and HI1470/1 (40, 51, 59), 14 for maltose transporter (57)]. This is perhaps not surprising since the substrates for ABC transporters diverge enormously. Furthermore, in many ABC importers, the substrate is bound to an accessary protein before it is docked to the external end of the TMDs (26, 38, 40, 57), whereas the substrate of an exporter from the intracellular side can bind directly without the assistance of a partner protein. Such diversity thus makes it hard to predict the structure of CFTR’s TMDs based on the existing structures of ABC proteins (see Refs. 6, 56).
Although it is generally accepted that the two TMDs of CFTR form the ion conductive pathway, the pore architecture of CFTR remains poorly understood. The pore structure and its conformational change during gating are of particular interest since they might shed light on how a transporter can be morphed into an ion channel. Interestingly, although the topology of TMDs in ABC importers exhibits a great variety, all ABC exporters seem to preserve a “6 + 6” symmetry, with each TMD consisting of six transmembrane helices. The available crystal structures of ABC exporters also reveal that the last transmembrane helix from each TMD (TM6 and 12) lines the cargo translocation pathway (7, 26, 82). Although the exact molecular motion for a transport cycle is unclear, the “inward-facing” configuration of the apoform (7, 38, 59, 82) and the “outward-facing” conformation (26, 40, 51, 57, 82) of the ATP-bound form of ABC transporters have lent support to the long-held alternating access mechanism (62): an internal and an external gate open alternatively to enact an unidirectional flow of the substrate across the translocation pathway. For ABC exporters, which likely bear an evolutionary relationship with CFTR, the cargo from the intracellular side binds to the inward-facing TMDs of an apoprotein, wherein the internal gate is open but the external gate is closed. Subsequent ATP-induced NBD dimerization triggers the TMDs to flip to an outward facing configuration in which the internal gate is closed but the external gate is open, allowing the cargo to dissociate to the extracellular space. For some active transporters such as Na-K-ATPase, to prevent a “leakage” of the cargo, it is known that one gate closes before the opening of the other gate, creating an occluded state whose ion translocation pathway is not accessible from either side of the membrane (30). However, so far there is no evidence pointing to the existence of such an occluded state in the transport cycle for ABC transporters. Instead, it was proposed that each TMD/NBD complex moves synchronously as a rigid body during the transport cycle. Thus the closing of one gate and the opening of the other gate occurs simultaneously (44, 48).
Does CFTR employ a similar structural mechanism to enact the function of a gated pore? Recently, by using substituted cysteine accessibility method (SCAM), three groups have provided experimental evidence identifying indeed transmembrane helices (TM) 6 and 12 as pore lining for CFTR (8, 9, 27, 60) (cf. Refs. 56, 79). Interestingly, the pore-lining residues in both TM6 and TM12 are readily accessible to cytoplasmic applications of a large thiol reagent of 13 Å in width in the closed state but not in the open state (9), suggesting that the internal entrance of the pore is wider in the closed state but narrows down in the open state, a surprising observation for an ion channel, and yet is in agreement with the idea of a flip-flop motion in the TMDs during an opening/closing transition of CFTR.
The alternating access model for transporter function also dictates that two gates are required to function efficiently (30), whereas, as an ion channel, CFTR could work with just one gate. The popular “degenerated transporter” hypothesis states that the cytoplasmic gate of a primordial ABC exporter was removed during evolution, resulting in only one external gate to regulate ionic flow in CFTR’s ion permeation pathway (1, 18, 30). This hypothesis has been speculative until a recent study showing that residues deep inside the pore can be readily accessed by hydrophilic thiol reagents applied from the intracellular side of the membrane in both open and closed state (9), as if there does not exist an internal gate in the pore of CFTR (cf. Ref. 60).
Role of ATP Hydrolysis in CFTR Gating
Active transporters pump cargo against a concentration gradient. Such a process is not at equilibrium since the transport cycle is driven constantly in one direction by an input of free energy. For ABC transporters, ATP hydrolysis provides the energy source to drive such a transport cycle. On the other hand, gating of most ion channels is regarded as an equilibrium process. The notion that CFTR may exploit ATP hydrolysis to drive its conformational changes during gating transitions places this intriguing protein in a unique position in transport physiology.
The hydrolytic activity is known to be preserved in one of the two ATP binding sites in CFTR (68). Early research has also concluded that ATP hydrolysis plays a critical role in facilitating gate closure since the open time is drastically prolonged in hydrolysis-deficient mutant channels (13, 16, 76, 84). Therefore, it had been proposed that CFTR inherits the canonical machinery from ABC proteins, by which ATP hydrolysis evokes an irreversible conformational change that inevitably leads to gate closure. This idea is known as irreversible gating model, which is also supported by a previous report showing two distinct open states with asymmetrical gating transitions between them (34).
On the other hand, robust channel openings can still occur in hydrolytic-deficient mutants or WT channel gated by non-hydrolyzable ATP analogs (4, 13, 16, 35, 41, 71, 76, 84). As a result, an equilibrium model was proposed, suggesting that the free energy harvested from ATP hydrolysis simply shifts the thermodynamic equilibrium to favor the closed state, but the transition between the open and closed states is still at equilibrium. This is known as the reversible gating model that has been used as a framework for several publications (2, 4, 5).
This decade-long debate was tentatively settled recently as Csanady and colleagues (23) showed a bimodal distribution of the open time histogram with a paucity of short opening events, a critical piece of single-channel kinetic evidence supporting that closing of the gate involves at least one irreversible step presumably associated with ATP hydrolysis. This latest report effectively refutes the reversible model wherein the closing process is simply the backward reaction of the one-step opening process. Mathematical analysis (20) based on the irreversible gating scheme also disapproves the practice of using the rate equilibrium free-energy relationship (REFER) for interpreting hydrolysis-driven CFTR gating (5).
Fitting their open dwell time distributions with a simple four-state model, Csanady et al. (23) also concluded that the ATP hydrolysis cycle and the gating cycle are tightly coupled. This strict coupling hypothesis has been implicated in many previous studies. For example, it is known for nearly two decades that the open time is dramatically prolonged in hydrolysis-deficient CFTR mutants or in CFTR gated by non-hydrolysable ATP analog (16, 35, 41, 71). Moreover, the asymmetrical gating transitions demonstrated by Gunderson and Kopito (34) also imply a strict coupling between ATP hydrolysis and channel closure. Nonetheless, for hydrolysis-deficient mutants such as E1371S- and K1250A-CFTR, ATP is still capable of activating these channels and the open probability (Po) of these hydrolysis-deficient mutants is even higher than that of WT-CFTR, suggesting that, under an equilibrium condition when ATP hydrolysis is absent, CFTR can still function fairly well. So what exactly is the role of ATP hydrolysis in gating? Does it trigger an irreversible conformational change by redirecting the channel to a pathway that is fundamentally different from that in equilibrium gating? It seems hard to envisage this is the case since there is no evidence that the CFTR protein undergoes any covalent modifications during gating transitions. Alternatively, perhaps ATP hydrolysis simply offers a fast track that accelerates the closing process that is normally present even under an equilibrium condition. It has been difficult to tackle this sort of questions directly because ATP hydrolysis is “invisible” in electrophysiological recordings, but some recent technical developments described below bestow an opportunity to address these pertinent biophysical issues.
Fishing the “Invisible” Kinetic States
For decades, ion channel researchers have been empowered by two unparalleled advantages inherent in electrophysiological techniques, namely the acquisition of data at a single-molecule level and real-time recording with an exceptional temporal resolution. But the major limitation of such technique is that it only detects electrical signals coming from an open channel, and thus only the status of the gate is unveiled. Any other conformational changes during gating, as long as the conductance is not affected, will be overlooked. For example, electrophysiological recordings of CFTR currents only reveal channel opening and closing, whereas events involving ATP hydrolysis and conformational changes in the NBDs remain invisible. However, based on the theory that CFTR opens into a conformation wherein its two NBDs form a head-to-tail dimer with its ligand ATP sandwiched in between (42, 75), experiments can be designed to distinguish the status of NBDs because the accessibility of the ligand-binding sites depends on whether the two NBDs are associated or dissociated. Lately, by applying hydrolyzable or non-hydrolyzable ATP analogs at a given time for a given duration, two novel functional states with unequivocal information about the status of NBDs were identified (46, 71). New mechanistic insights have subsequently emerged.
It has been known for years that, upon applying non-hydrolyzable ATP analogs such as pyrophosphate (PPi) and adenylyl-imidodiphosphate (AMP-PNP) with ATP, opening bursts of WT-CFTR channels (~400 ms) are significantly prolonged to tens of seconds, as if the channels are locked in the open state (16, 35, 41). This locked-open state was interpeted as the result of PPi (or AMP-PNP) binding to NBD2 since a similar locked-open phenotype was seen with ATP as a ligand in mutations that abolish ATP hydrolysis in NBD2 (8, 13, 16, 76, 84).
Interestingly, when these non-hydrolyzable ATP analogs, e.g., PPi, are applied alone, their effects depend on the timing of the application. When applied seconds after ATP washout, they are still capable of lock-opening the CFTR channel with considerably high efficacy (71). However, such robust effect of PPi vanishes if the same concentration of PPi is applied minutes after ATP washout. These results suggest that ATP can somehow prime the CFTR channel for a better responsiveness to subsequent PPi application, as if the channels retain the “memory” of being activated by ATP. This time-dependent responsiveness of CFTR to non-hydrolyzable ATP analogs effectively distinguishes two different closed states: the C2 state, emerging soon after channel closure upon ATP washout, responds to PPi/AMP-PNP robustly, whereas the C1 state appears much later after ATP removal and responds to the same ligand poorly. Since the lifetime of the C2 state is shortened by mutations that presumably weaken ATP binding in ABP1 formed by the head subdomain of NBD1 and the tail subdomain of NBD2, Tsai et al. (71) proposed that the priming effect of ATP is due to ATP binding to ABP1. The fact that the C2 state is long-lasting then suggests a tight binding of ATP in ABP1.
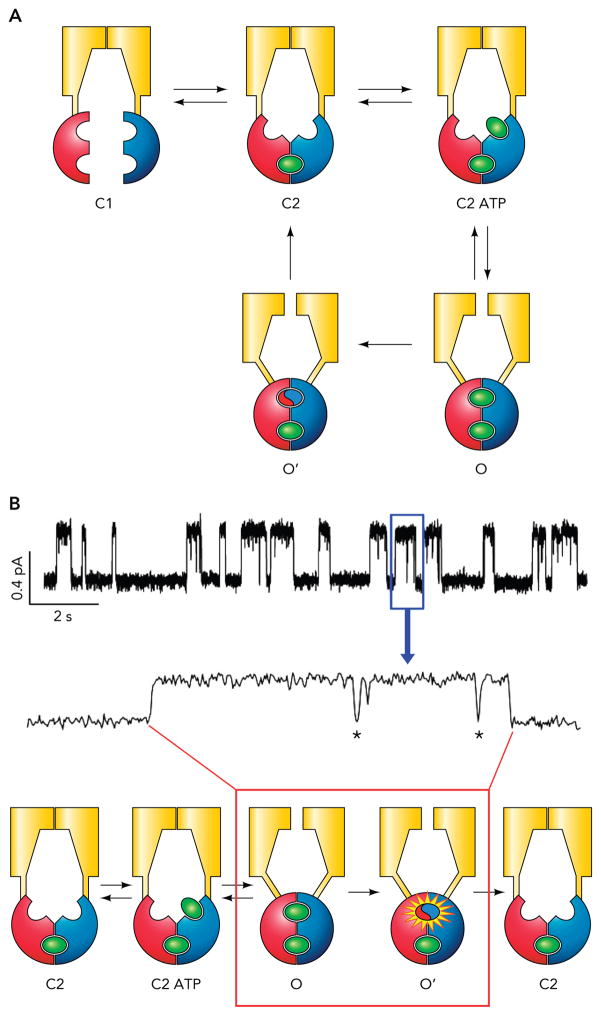
The configurations of these two closed states were further determined by ligand exchange experiments wherein ATP was switched rapidly to a high-affinity, hydrolyzable ATP analog, N6-phenyl-ethyl-ATP. At the single-channel level, the Po of WT-CFTR increases upon ligand swap in two steps (an immediate increase of the Po due to a shortening of the closed time and a delayed increase caused by a prolongation of the open time for tens of seconds), as if two bound ATP molecules are replaced with two distinct time courses (70). Since mutations in both the head subdomain of NBD1 and the tail subdomain (i.e., the signature sequence) of NBD2 affect the rate of the slower, second-phase ligand exchange, it is concluded that the tight binding of ATP is due to a stable engagement of these two subdomains that form ABP1. A simple model for CFTR gating was proposed from this series of work (FIGURE 2). In essence, after ATP hydrolysis takes place in ABP2 formed by the head subdomain of NBD2 and the tail subdomain of NBD1, the channel will first enter a closed state with a partially separated NBD dimer (the C2 state in FIGURE 2). Such partial dimer is extremely stable; it lasts for tens of seconds before the partial NBD dimer eventually dissociates (the C1 state in FIGURE 2). This model was also supported by a report employing an independent approach. By studying the energetic coupling between different residue pairs located around ABP1, Szollosi et al. (69) found that the coupling energy between these residue pairs does not change throughout the gating cycle. Since each pair tested includes one residue in the head of NBD1 and the other in the tail of NBD2, these results indicate that interaction of two NBDs around ABP1 remains static during gating. (69). Of note, like CFTR, many members in the ABC protein family contain only one catalysis-competent site (32, 39, 52, 72). For these ABC transporters with asymmetrical NBDs, hydrolysis of one ATP in the catalysis-competent site is sufficient to drive the transport cycle. Lately, a crystal structure of such ABC transporter revealed a partial dimeric NBD configuration (37). This structural study leads to a model of the transport cycle very similar to the one proposed by Tsai et al. (70), suggesting that CFTR is not an exception in the ABC protein superfamily; instead, mechanistic insights uncovered in CFTR research may be applicable to other members of ABC proteins. Taken one step further, for those ABC proteins with two catalysis-competent sites, one has to ask: Is hydrolysis of two ATP molecules required to drive a transport cycle? A recent molecular dynamic study indeed raised the possibility that hydrolysis of ATP in one of the two sites is sufficient to break the NBD dimer (36). Further studies are needed to verify this interesting hypothesis.
FIGURE 2. Traditional strict coupling model for CFTR gating.
A: the strict coupling model demands a one-to-one stoichiometry between the ATP hydrolysis cycle and the gating cycle. Notably, the rate of C2 → C1 transition is slow (~0.03 s−1); therefore, in the continuous presence of ATP, the channel rarely visits the C1 state. B: a cartoon that shows how the gating transitions described in A correspond to a WT-CFTR opening event. The C1 is not included for the reason mentioned above. Also note those briefly closed events known as flickers (marked by asterisk) within an opening burst.
Lately, another novel state was identified by taking advantage of a fast solution exchange system that allows ligand switches within milliseconds (4). Instead of applying PPi after complete ATP washout, ATP was replaced with PPi for different durations in an attempt to capture short-lived state(s) that emerges right after ATP hydrolysis. This experimental protocol indeed reveals two distinct populations of channels: the previously identified C2 state and a new transient state [named state X in (46)] that responds to PPi at a distinguishably faster rate. The fact that state X readily responds to PPi or AMP-PNP indicates that its NBDs must have been separated at least to the size of AMP-PNP and that the hydrolytic products in ABP2 have dissociated. By switching the ligand directly from ATP to PPi when the channel stays in an opening burst in recordings with one single CFTR, Jih et al. (46) were able to show that the channel can slip from the open state to a locked-open state without visiting the C2 closed state. Thus, contrary to what the strict coupling mode (FIGURE 2B) would predict, within an opening burst, there exists a state (i.e., state X) that can respond to a new ligand, hence indicating that ATP has been hydrolyzed and the hydrolytic products dissociated with a disengaged ABP2 in an opening burst.
If we assume that the state X is an open state, these results would imply that, when the NBD dimer separates, the gate has not closed yet. In other words, there would be a delay in signal transduction from the NBDs to the gate. If another ATP molecule kicks in at this juncture, binds to the vacant ABP2, and induces NBD dimer formation before the gate closes, then the channel would go through two ATP hydrolysis cycles in one opening burst, a violation of one-to-one stoichiometry between ATP consumption and an opening/closing cycle. However, to substantiate this provocative supposition, one needs to demonstrate a true open state with clear responsiveness to a new ligand because of the well known existence of short-lived closed events buried in an opening burst (marked in FIGURE 2B).
Removing the Invisibility Cloak
The idea that ATP hydrolysis provides the free energy source for conformational changes leading to gate closure means that gating of CFTR is not an equilibrium process. Since ATP hydrolysis happens within an opening burst, there must exist kinetic step(s) that violates microscopic reversibility. An excellent example of non-equilibrium gating was reported by Richard and Miller (63) for CLC-0 chloride channel. In this particular case, it is the chloride ion gradient that serves as the energy source to drive gating in a cyclic manner (cf. Ref. 50).
Similar asymmetrical gating transitions for CFTR were first reported by Gunderson and Kopito (34). In their heavily filtered WT-CFTR single-channel recordings, they found two open states that can be distinguished by their distinct conductance levels (named O1 and O2 by the authors). More importantly, the gating transition follows a preferred order: C → O1 → O2 → C ≧ C → O2 → O1 → C. Such time asymmetry in gating transitions indicates a violation of microscopic reversibility and requires an input of free energy, in this case from ATP hydrolysis, to drive the O1 → O2 transition. In theory, this report could set the stage to address the role of ATP hydrolysis in CFTR gating as each observed O1 → O2 transition reports hydrolysis of one ATP molecule (i.e., ATP hydrolysis becomes “visible” in single-channel recordings). However, several technical issues have prevented a wide application of this method. First, even after heavy filtering (10 Hz), the O1 and O2 states are still barely distinguishable (34, 43), which thus precludes more rigorous kinetic analysis. Second, clearly resolvable O1 and O2 states are not regularly found in the subsequent literatures. Third, despite carrying out experiments under similar conditions reported by Gunderson and Kopito (34) and Ishihara and Welsh (43), we did not find unambiguous O1 and O2 states in WT-CFTR (unpublished data).
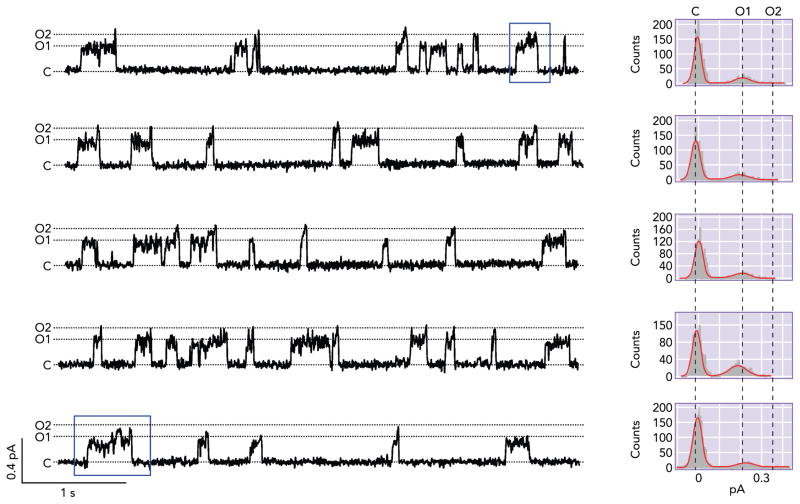
Fortuitously, this hydrolysis-dependent O1 →O2 transition was discerned in a CFTR mutant, R352C. Single-channel recordings of R352C-CFTR revealed two distinguishable conductance states: a smaller one (O1), about one-third of the WT conductance, and a larger one (O2), about one-half of the WT conductance (8). Interestingly, the transition between these two open states follows a certain pattern (47). When the channel opens from the closed state, it usually enters the smaller O1 state, whereas, when it closes, it usually departs from the larger O2 state. Within an opening burst, there is usually a transition from O1 to O2 state, but very few O2 to O1 transitions are seen (FIGURE 3A). Such asymmetrical gating pattern echoes the finding in Gunderson and Kopito (34). However, this mutant channel affords a much better temporal resolution because of an ~40% difference in the single-channel amplitude between the O1 and O2 states. The idea that the C → O1 → O2 → C preferred transition is driven by ATP hydrolysis is supported not only by the time asymmetry but also by the observation that the O1 →O2 transition seen in an opening burst is abolished when the hydrolysis-deficient mutation (E1371S) is engineered into the R352C background.
FIGURE 3. Unique gating features of R352C-CFTR.
Five representative traces from patches that contain only one R352C-CFTR in the presence of 2.75 mM ATP. R352C-CFTR shows two distinct levels of open-state conductance: the smaller O1 and the larger O2. When the channel opens, it usually opens to the smaller O1 state, whereas, when the channel closes, it usually departs from the larger O2 state. Within each opening burst, there often is one O1 → O2 transition, but one rarely finds events containing the O2 → O1 transition. The blue box shows an opening event that consists of more than one O1 → O2 transition (i.e., the channel going through the “re-entry” pathway described in FIGURE 4). This figure is adapted from Ref. 47 (from the Journal of General Physiology).
An even more intriguing observation made with the R352C mutant channel is that, occasionally, there are opening events that consist of more than one O1 →O2 transition (boxed in FIGURE 3). If the interpretation that O1 → O2 transition represents ATP hydrolysis holds, this would imply that hydrolysis of >1 ATP molecule can occur within one opening burst, i.e., a violation of one-to-one stoichiometry between the ATP hydrolysis cycle and the gating cycle. Furthermore, there is a higher frequency of such opening events that embody multiple rounds of ATP hydrolysis at higher [ATP], suggesting an ATP-dependent pathway that allows the channel in the post-hydrolytic O2 state to “re-enter” the pre-hydrolytic O1 state (O2 → O2ATP → O1 in FIGURE 4).
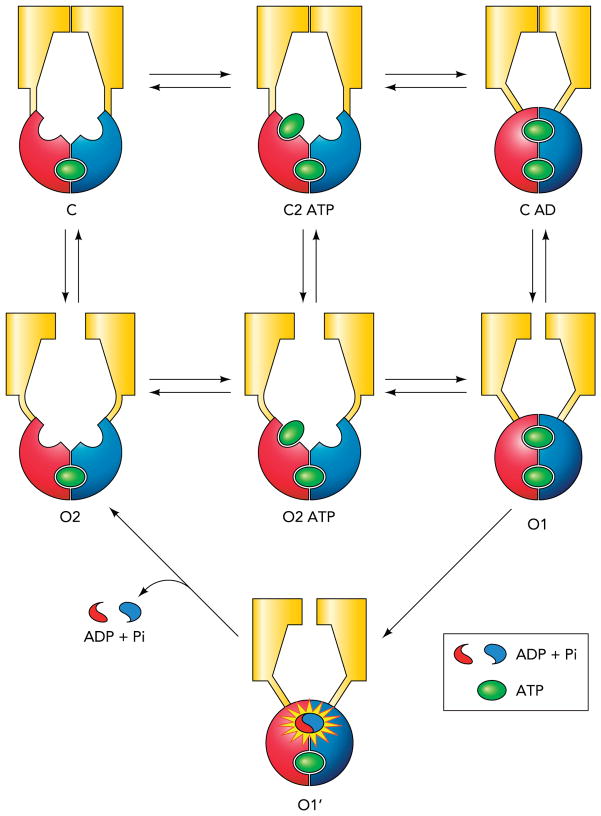
FIGURE 4. Energetic coupling model for CFTR gating.
The conformational changes in the TMDs and the NBDs are not strictly coupled, but the conformational change in one domain will facilitate the conformational change in the other domain. ATP hydrolysis, according to this model, simply provides a shortcut for the channel to escape from the absorbing state (O1).
The existence of the re-entry pathway also indicates that neither ATP hydrolysis nor the separation of the NBD dimer per se closes the gate. A new model that features an energetically coupled relationship between the NBDs and the TMDs is proposed to describe CFTR gating (FIGURE 4). The basic concept of the energetic coupling model follows the classic idea of allosteric modulation (54). The whole scheme is divided into three reversible steps: ligand binding/unbinding (two horizontal transitions on the left), NBD dimerization/separation (two horizontal transitions on the right), and opening/closing of the gate (three vertical transitions). This model states that both NBDs and TMDs hold a certain degree of autonomy, but the conformational change in one domain facilitates the conformational change in the other domain (i.e., dimerization of the NBDs facilitates the gate to open and vice versa). Thus, in the absence of ATP hydrolysis, e.g., in E1371S-CFTR, the channel works simply because ATP-induced dimerization of NBDs promotes gate opening and the Po is exceedingly high because the channel is trapped in the stable O1 state. For WT channels, ATP hydrolysis provides the free energy input to release the channel from the absorbing state.
To our knowledge, there are no published results that contradict this new model of CFTR gating. In fact, many of the puzzling data published previously may be explained by the model. For example, compared with classic ligand-gated ion channels such as the nicotinic acetylcholine receptor channel with a ligand-independent Po that is 107 times lower than ligand-dependent Po (45), CFTR is known to assume unusually high spontaneous, ATP-independent gating activity that is ~100 times lower than ATP-dependent activity (12). In fact, ATP-independent gating can be seen in a mutant whose NBD2 is removed (24, 78). Once the gate in TMDs is allowed to fluctuate between the open and closed conformations (i.e., C2 ↔ O2) without the obligatory motion of NBDs as the model depicts, some ATP-independent openings are expected. The model actually predicts that this constitutive, ATP-independent gating can be altered by maneuvers that perturb regions away from the NBDs. Indeed, Wang et al. (80, 81) found mutations in the regions presumably connecting TMDs and NBDs that can greatly enhance ATP-independent gating. Likewise in Bai et al. (8), chemical modifications of an engineered cysteine (I344C or M348C) in TM6 drastically increase ATP-independent activity to the level of ATP-dependent activity before modifications. Furthermore, the model also predicts that, for a maneuver that stabilizes the open state of spontaneous gating (i.e., the O2 state), the frequency of the re-entry events will increase in the presence of ATP so that the opening burst duration will be prolonged. However, unlike the hydrolysis-deficient mutants, in this case the prolongation of opening bursts depends on continuously fueling the channel with ATP. Results shown in Fig. 12B in Bai et al. (8) basically verify this prediction.
This new model could also potentially explain how pharmacological reagents work on CFTR gating. One reagent of particular interest is Kalydeco (or Vx-770), discovered by high-through drug screening (73). This FDA-approved drug for CF treatment (1, 61) has been shown to increase the open time of both WT- and G551D-CFTR (73, 83), a baffling result since, although WT channels are gated by ATP-induced NBD dimerization, the G551D mutation abolishes ATP responsiveness, presumably because introducing a negatively charged side chain at the signature sequence of NBD1 prevents NBD dimerization due to electrostatic repulsion (14). Here, the new gating model offers a testable hypothesis that Kalydeco works by promoting spontaneous ATP-independent gating (C2 ↔ O2 transitions), the part of the gating scheme likely preserved in G551D-CFTR. Future studies along this line will not only shed light on how this clinically important drug acts but also provide clues for rational design of new treatment for CF.
Implication of the Energetic Coupling Model in ABC Transporter Function
An immediate implication of the energetic coupling model on the gating motion of ABC proteins is that, at least for CFTR, the NBDs and the TMDs may not go through a rigid-body-like motion as previously proposed (44, 48). If not rigid body motion, what would be the alternatives? The data on R352C-CFTR support the notion that a partial separation of the NBD dimer does not necessarily close the gate in the TMDs, but the fact that the O1 and O2 states exhibit different single-channel amplitudes indicates that ATP hydrolysis and/or subsequent partial separation of NBDs must affect the conformation of the ion permeation pathway in TMDs. Although more studies are needed to reveal the exact motion of TMDs associated with ATP hydrolysis/NBD separation, one possible scenario is that partial separation of the NBD dimer drags parts of the TMDs that are in proximity to the NBD-TMD interface with it while the distal end of the TMDs remains relatively static. Such bending of the helices in the TMDs would result in a molecular strain, the relaxation of which subsequently causes gate closure. A similar idea was also proposed by Csanady et al. (22) for gate opening following NBD dimerization.
If similar conformational changes do occur in ABC transporters, the C AD and the O2 states in FIGURE 4 would represent an occluded state and a conductive state, respectively. Although the presence of an occluded state for a transporter can be accepted at ease since this conformation is thought to be a safeguard (30), the existence of a conductive state does present a problem. For a transporter to operate effectively, a conductive state wherein the cargo can flow through the translocation pathway freely is not allowed. However, this gold standard was broken when a structure of a prokaryotic CLC transporter indicates the existence of such a state (28). Interestingly, by limiting the rate of substrate flux in such a transient conductive state, this protein is still a fairly efficient transporter (28). Therefore, we speculate that even if the O2 state exists as a conductive state in ABC transporters, it may not be a disaster in terms of transport efficiency. Notably, nearly all substrates for ABC exporters are large organic molecules (62). Thus the pore in this conductive state may not be wide enough for the large substrates to slip through at a fast rate to jeopardize the efficiency of transport.
Another related issue concerning the efficiency of transport, from the perspective of energy consumption, is that the energetic coupling model predicts, on average, more than one ATP molecule consumed per gating cycle. For ion channels like CFTR, the energy utilization remains efficient as the additional ATP molecules hydrolyzed are reflected in an increase of the open time. However, when this idea is extrapolated to ABC transporters, futile ATP hydrolysis that does not contribute to cargo translocation becomes an inevitable conclusion. Surprising as it may seem, such slight imperfection in energy utilization can be found elsewhere. It has been shown that there is futile ATP hydrolysis in kinesin motors that is not coupled to a forward motion (19, 65). For CLC transporters, a deviation from perfect energy efficiency was predicted as well (28). It has even been proposed that such “molecular slips” may benefit the organism in some cases (55). Therefore, as long as it does not jeopardize the survival of the organism, a slight deviation from ideal energy efficiency is likely well tolerated. Of course, whether these provocative ideas out of recent biophysical work of an “atypical” ABC protein are applicable to the majority of ABC transporters remains to be seen. Nonetheless, a wealth of recent data seems to suggest that CFTR and at least the exporter members of the ABC transporters may simply be considered as two peas in a pod (see above for details). Therefore, the unique nature of CFTR as an ion channel grants a unique opportunity to tackle the mechanism of ABC proteins with unrivaled temporal resolution. It is our belief that by combining electrophysiological, structural, and biochemical data, a comprehensive understanding of how this important family of membrane proteins works is within our reach.
Conclusions
In just the past few years, significant progress was made in understanding the gating mechanism of CFTR. New silent states have been caught by using non-hydrolyzable ATP analogs as baits. A unique mutant channel that foretells ATP hydrolysis within an opening burst was discovered. A wealth of ensuing data points to a new idea for the gating mechanism of CFTR: during gating transitions, the motions in the NBDs and the TMDs are energetically coupled, contrary to the long-held view that each TMD/NBD complex moves concurrently as a rigid body. The energetic coupling model (FIGURE 4) predicts that both the NBDs and TMDs hold a certain degree of autonomy. The nonstrict coupling between the two domains allows ATP to capture the post-hydrolytic channel before the gate closes and reshuffle it back to the pre-hydrolytic open state (the re-entry pathway), creating a “molecular slip” that results in an non-integral stoichiometry between the ATP hydrolysis cycle and the gating cycle. As a member of the ABC protein superfamily, such a breakthrough in CFTR could bear a broader implication to the functional mechanism of ABC transporters at large. Besides satisfying scientific curiosity, the re-entry pathway also provides a new target for CFTR potentiators, since promoting such a pathway could increase the activity of CFTR by prolonging the open time. In fact, Kalydeco, a novel CF drug that significantly prolongs the open time of WT-CFTR as well as many disease-associated mutant CFTR, likely achieves its function by promoting the re-entry pathway. Future studies are expected to exploit this new mechanism of CFTR gating in designing strategies for CF treatment.
Acknowledgments
We thank Dr. David Dowson for providing the coordinate for the CFTR homology model used in FIGURE 1.
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant R01 DK-55835 and a grant (Hwang 11P0) from the Cystic Fibrosis Foundation to T.-C. Hwang.
Footnotes
No conflicts of interest, financial or otherwise, are declared by the author(s).
Author contributions: K.-Y.J. and T.-C.H. conception and design of research; K.-Y.J. performed experiments; K.-Y.J. analyzed data; K.-Y.J. and T.-C.H. interpreted results of experiments; K.-Y.J. prepared figures; K.-Y.J. and T.-C.H. drafted manuscript; K.-Y.J. and T.-C.H. edited and revised manuscript; T.-C.H. approved final version of manuscript.
References
- 1.Accurso FJ, Rowe SM, Clancy JP, Boyle MP, Dunitz JM, Durie PR, Sagel SD, Hornick DB, Konstan MW, Donaldson SH, Moss RB, Pilewski JM, Rubenstein RC, Uluer AZ, Aitken ML, Freedman SD, Rose LM, Mayer-Hamblett N, Dong Q, Zha J, Stone AJ, Olson ER, Ordonez CL, Campbell PW, Ashlock MA, Ramsey BW. Effect of VX-770 in persons with cystic fibrosis and the G551D-CFTR mutation. N Engl J Med. 2010;363:1991–2003. doi: 10.1056/NEJMoa0909825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aleksandrov AA, Aleksandrov L, Riordan JR. Nucleoside triphosphate pentose ring impact on CFTR gating and hydrolysis. FEBS Lett. 2002;518:183–188. doi: 10.1016/s0014-5793(02)02698-4. [DOI] [PubMed] [Google Scholar]
- 3.Aleksandrov AA, Aleksandrov LA, Riordan JR. CFTR (ABCC7) is a hydrolyzable-ligand-gated channel. Pflügers Arch. 2007;453:693–702. doi: 10.1007/s00424-006-0140-z. [DOI] [PubMed] [Google Scholar]
- 4.Aleksandrov AA, Chang X, Aleksandrov L, Riordan JR. The non-hydrolytic pathway of cystic fibrosis transmembrane conductance regulator ion channel gating. J Physiol. 2000;528:259–265. doi: 10.1111/j.1469-7793.2000.00259.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aleksandrov AA, Cui L, Riordan JR. Relationship between nucleotide binding and ion channel gating in cystic fibrosis transmembrane conductance regulator. J Physiol. 2009;587:2875–2886. doi: 10.1113/jphysiol.2009.170258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alexander C, Ivetac A, Liu X, Norimatsu Y, Serrano JR, Landstrom A, Sansom M, Dawson DC. Cystic fibrosis transmembrane conductance regulator: using differential reactivity toward channel-permeant and channel-impermeant thiol-reactive probes to test a molecular model for the pore. Biochemistry. 2009;48:10078–10088. doi: 10.1021/bi901314c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aller SG, Yu J, Ward A, Weng Y, Chittaboina S, Zhuo R, Harrell PM, Trinh YT, Zhang Q, Urbatsch IL, Chang G. Structure of P-glycoprotein reveals a molecular basis for poly-specific drug binding. Science. 2009;323:1718–1722. doi: 10.1126/science.1168750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bai Y, Li M, Hwang TC. Dual roles of the sixth transmembrane segment of the CFTR chloride channel in gating and permeation. J Gen Physiol. 2010;136:293–309. doi: 10.1085/jgp.201010480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bai Y, Li M, Hwang TC. Structural basis for the channel function of a degraded ABC transporter, CFTR (ABCC7) J Gen Physiol. 2011;138:495–507. doi: 10.1085/jgp.201110705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baker JM, Hudson RP, Kanelis V, Choy WY, Thibodeau PH, Thomas PJ, Forman-Kay JD. CFTR regulatory region interacts with NBD1 predominantly via multiple transient helices. Nature Struct Mol Biol. 2007;14:738–745. doi: 10.1038/nsmb1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bear CE, Li CH, Kartner N, Bridges RJ, Jensen TJ, Ramjeesingh M, Riordan JR. Purification and functional reconstitution of the cystic fibrosis transmembrane conductance regulator (CFTR) Cell. 1992;68:809–818. doi: 10.1016/0092-8674(92)90155-6. [DOI] [PubMed] [Google Scholar]
- 12.Bompadre SG, Ai T, Cho JH, Wang X, Sohma Y, Li M, Hwang TC. CFTR gating I: characterization of the ATP-dependent gating of a phosphorylation-independent CFTR channel (DeltaR-CFTR) J Gen Physiol. 2005;125:361–375. doi: 10.1085/jgp.200409227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bompadre SG, Cho JH, Wang X, Zou X, Sohma Y, Li M, Hwang TC. CFTR gating II: Effects of nucleotide binding on the stability of open states. J Gen Physiol. 2005;125:377–394. doi: 10.1085/jgp.200409228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bompadre SG, Sohma Y, Li M, Hwang TC. G551D and G1349D, two CF-associated mutations in the signature sequences of CFTR, exhibit distinct gating defects. J Gen Physiol. 2007;129:285–298. doi: 10.1085/jgp.200609667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cai ZW, Liu J, Li HY, Sheppard DN. Targeting F508del-CFTR to develop rational new therapies for cystic fibrosis. Acta Pharmacol Sin. 2011;32:693–701. doi: 10.1038/aps.2011.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carson MR, Travis SM, Welsh MJ. The two nucleotide-binding domains of cystic fibrosis transmembrane conductance regulator (CFTR) have distinct functions in controlling channel activity. J Biol Chem. 1995;270:1711–1717. doi: 10.1074/jbc.270.4.1711. [DOI] [PubMed] [Google Scholar]
- 17.Chan KW, Zhang H, Logothetis DE. N-terminal transmembrane domain of the SUR controls trafficking and gating of Kir6 channel subunits. EMBO J. 2003;22:3833–3843. doi: 10.1093/emboj/cdg376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen TY, Hwang TC. CLC-0 and CFTR: chloride channels evolved from transporters. Physiol Rev. 2008;88:351–387. doi: 10.1152/physrev.00058.2006. [DOI] [PubMed] [Google Scholar]
- 19.Crevel I, Carter N, Schliwa M, Cross R. Coupled chemical and mechanical reaction steps in a processive Neurospora kinesin. EMBO J. 1999;18:5863–5872. doi: 10.1093/emboj/18.21.5863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Csanady L. Application of rate-equilibrium free energy relationship analysis to nonequilibrium ion channel gating mechanisms. J Gen Physiol. 2009;134:129–136. doi: 10.1085/jgp.200910268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Csanady L, Chan KW, Seto-Young D, Kopsco DC, Nairn AC, Gadsby DC. Severed channels probe regulation of gating of cystic fibrosis transmembrane conductance regulator by its cytoplasmic domains. J Gen Physiol. 2000;116:477–500. doi: 10.1085/jgp.116.3.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Csanady L, Nairn AC, Gadsby DC. Thermodynamics of CFTR channel gating: a spreading conformational change initiates an irreversible gating cycle. J Gen Physiol. 2006;128:523–533. doi: 10.1085/jgp.200609558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Csanady L, Vergani P, Gadsby DC. Strict coupling between CFTR’s catalytic cycle and gating of its Cl− ion pore revealed by distributions of open channel burst durations. Proc Natl Acad Sci USA. 2010;107:1241–1246. doi: 10.1073/pnas.0911061107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cui L, Aleksandrov L, Chang XB, Hou YX, He L, Hegedus T, Gentzsch M, Aleksandrov A, Balch WE, Riordan JR. Domain interdependence in the biosynthetic assembly of CFTR. J Mol Biol. 2007;365:981–994. doi: 10.1016/j.jmb.2006.10.086. [DOI] [PubMed] [Google Scholar]
- 25.Davidson AL, Chen J. ATP-binding cassette transporters in bacteria. Ann Rev Biochem. 2004;73:241–268. doi: 10.1146/annurev.biochem.73.011303.073626. [DOI] [PubMed] [Google Scholar]
- 26.Dawson RJ, Locher KP. Structure of a bacterial multidrug ABC transporter. Nature. 2006;443:180–185. doi: 10.1038/nature05155. [DOI] [PubMed] [Google Scholar]
- 27.El Hiani Y, Linsdell P. Changes in accessibility of cytoplasmic substances to the pore associated with activation of the cystic fibrosis transmembrane conductance regulator chloride channel. J Biol Chem. 2010;285:32126–32140. doi: 10.1074/jbc.M110.113332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Feng L, Campbell EB, Hsiung Y, MacKinnon R. Structure of a eukaryotic CLC transporter defines an intermediate state in the transport cycle. Science. 2010;330:635–641. doi: 10.1126/science.1195230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ferenci T. The recognition of maltodextrins by Escherichia coli. Eur J Biochem. 1980;108:631–636. doi: 10.1111/j.1432-1033.1980.tb04758.x. [DOI] [PubMed] [Google Scholar]
- 30.Gadsby DC. Ion channels versus ion pumps: the principal difference, in principle. Nat Rev Mol Cell Biol. 2009;10:344–352. doi: 10.1038/nrm2668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gadsby DC, Vergani P, Csanady L. The ABC protein turned chloride channel whose failure causes cystic fibrosis. Nature. 2006;440:477–483. doi: 10.1038/nature04712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gao M, Cui HR, Loe DW, Grant CE, Almquist KC, Cole SP, Deeley RG. Comparison of the functional characteristics of the nucleotide binding domains of multidrug resistance protein 1. J Biol Chem. 2000;275:13098–13108. doi: 10.1074/jbc.275.17.13098. [DOI] [PubMed] [Google Scholar]
- 33.Gottesman MM, Ambudkar SV. Overview: ABC transporters and human disease. J Bioenerg Biomembr. 2001;33:453–458. doi: 10.1023/a:1012866803188. [DOI] [PubMed] [Google Scholar]
- 34.Gunderson KL, Kopito RR. Conformational states of CFTR associated with channel gating: the role ATP binding and hydrolysis. Cell. 1995;82:231–239. doi: 10.1016/0092-8674(95)90310-0. [DOI] [PubMed] [Google Scholar]
- 35.Gunderson KL, Kopito RR. Effects of pyrophosphate and nucleotide analogs suggest a role for ATP hydrolysis in cystic fibrosis transmembrane regulator channel gating. J Biol Chem. 1994;269:19349–19353. [PubMed] [Google Scholar]
- 36.Gyimesi G, Ramachandran S, Kota P, Dokholyan NV, Sarkadi B, Hegedus T. ATP hydrolysis at one of the two sites in ABC transporters initiates transport related conformational transitions. Biochim Biophys Acta. 2011;1808:2954–2964. doi: 10.1016/j.bbamem.2011.07.038. [DOI] [PubMed] [Google Scholar]
- 37.Hohl M, Briand C, Grutter MG, Seeger MA. Crystal structure of a heterodimeric ABC transporter in its inward-facing conformation. Nature Struct Mol Biol. 2012;19:395–402. doi: 10.1038/nsmb.2267. [DOI] [PubMed] [Google Scholar]
- 38.Hollenstein K, Frei DC, Locher KP. Structure of an ABC transporter in complex with its binding protein. Nature. 2007;446:213–216. doi: 10.1038/nature05626. [DOI] [PubMed] [Google Scholar]
- 39.Hou Y, Cui L, Riordan JR, Chang X. Allosteric interactions between the two non-equivalent nucleotide binding domains of multidrug resistance protein MRP1. J Biol Chem. 2000;275:20280–20287. doi: 10.1074/jbc.M001109200. [DOI] [PubMed] [Google Scholar]
- 40.Hvorup RN, Goetz BA, Niederer M, Hollenstein K, Perozo E, Locher KP. Asymmetry in the structure of the ABC transporter-binding protein complex BtuCD-BtuF. Science. 2007;317:1387–1390. doi: 10.1126/science.1145950. [DOI] [PubMed] [Google Scholar]
- 41.Hwang TC, Nagel G, Nairn AC, Gadsby DC. Regulation of the gating of cystic fibrosis transmembrane conductance regulator Cl channels by phosphorylation and ATP hydrolysis. Proc Natl Acad Sci USA. 1994;91:4698–4702. doi: 10.1073/pnas.91.11.4698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hwang TC, Sheppard DN. Gating of the CFTR Cl− channel by ATP-driven nucleotide-binding domain dimerisation. J Physiol. 2009;587:2151–2161. doi: 10.1113/jphysiol.2009.171595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ishihara H, Welsh MJ. Block by MOPS reveals a conformation change in the CFTR pore produced by ATP hydrolysis. Am J Physiol Cell Physiol. 1997;273:C1278–C1289. doi: 10.1152/ajpcell.1997.273.4.C1278. [DOI] [PubMed] [Google Scholar]
- 44.Ivetac A, Campbell JD, Sansom MS. Dynamics and function in a bacterial ABC transporter: simulation studies of the BtuCDF system and its components. Biochemistry. 2007;46:2767–2778. doi: 10.1021/bi0622571. [DOI] [PubMed] [Google Scholar]
- 45.Jackson MB. Kinetics of unliganded acetylcholine receptor channel gating. Biophys J. 1986;49:663–672. doi: 10.1016/S0006-3495(86)83693-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jih KY, Sohma Y, Li M, Hwang TC. Identification of a novel post-hydrolytic state in CFTR gating. J Gen Physiol. 2012;139:359–370. doi: 10.1085/jgp.201210789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jih KY, Sohma Y, Hwang TC. Nonintegral stoichiometry in CFTR gating revealed by a pore-lining mutation. J Gen Physiol. 2012;140:347–359. doi: 10.1085/jgp.201210834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Khare D, Oldham ML, Orelle C, Davidson AL, Chen J. Alternating access in maltose transporter mediated by rigid-body rotations. Mol Cell. 2009;33:528–536. doi: 10.1016/j.molcel.2009.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lewis HA, Buchanan SG, Burley SK, Conners K, Dickey M, Dorwart M, Fowler R, Gao X, Guggino WB, Hendrickson WA, Hunt JF, Kearins MC, Lorimer D, Maloney PC, Post KW, Rajashankar KR, Rutter ME, Sauder JM, Shriver S, Thibodeau PH, Thomas PJ, Zhang M, Zhao X, Emtage S. Structure of nucleotide-binding domain 1 of the cystic fibrosis transmembrane conductance regulator. EMBO J. 2004;23:282–293. doi: 10.1038/sj.emboj.7600040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lisal J, Maduke M. The ClC-0 chloride channel is a ‘broken’ Cl−/H+ antiporter. Nature Struct Mol Biol. 2008;15:805–810. doi: 10.1038/nsmb.1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Locher KP, Lee AT, Rees DC. The E. coli BtuCD structure: a framework for ABC transporter architecture and mechanism. Science. 2002;296:1091–1098. doi: 10.1126/science.1071142. [DOI] [PubMed] [Google Scholar]
- 52.Matsuo M, Kioka N, Amachi T, Ueda K. ATP binding properties of the nucleotide-binding folds of SUR1. J Biol Chem. 1999;274:37479–37482. doi: 10.1074/jbc.274.52.37479. [DOI] [PubMed] [Google Scholar]
- 53.Mense M, Vergani P, White DM, Altberg G, Nairn AC, Gadsby DC. In vivo phosphorylation of CFTR promotes formation of a nucleotide-binding domain heterodimer. EMBO J. 2006;25:4728– 4739. doi: 10.1038/sj.emboj.7601373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Monod J, Wyman J, Changeux JP. On the nature of allosteric transitions: a plausible model. J Mol Biol. 1965;12:88–118. doi: 10.1016/s0022-2836(65)80285-6. [DOI] [PubMed] [Google Scholar]
- 55.Nelson N, Sacher A, Nelson H. The significance of molecular slips in transport systems. Nature Rev Mol Cell Biol. 2002;3:876–881. doi: 10.1038/nrm955. [DOI] [PubMed] [Google Scholar]
- 56.Norimatsu Y, Ivetac A, Alexander C, Kirkham J, O’Donnell N, Dawson DC, Sansom MS. Cystic fibrosis transmembrane conductance regulator: a molecular model defines the architecture of the anion conduction path and locates a “bottleneck” in the pore. Biochemistry. 2012;51:2199–2212. doi: 10.1021/bi201888a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Oldham ML, Khare D, Quiocho FA, Davidson AL, Chen J. Crystal structure of a catalytic intermediate of the maltose transporter. Nature. 2007;450:515–521. doi: 10.1038/nature06264. [DOI] [PubMed] [Google Scholar]
- 58.Ostedgaard LS, Baldursson O, Welsh MJ. Regulation of the cystic fibrosis transmembrane conductance regulator Cl− channel by its R domain. J Biol Chem. 2001;276:7689–7692. doi: 10.1074/jbc.R100001200. [DOI] [PubMed] [Google Scholar]
- 59.Pinkett HW, Lee AT, Lum P, Locher KP, Rees DC. An inward-facing conformation of a putative metal-chelate-type ABC transporter. Science. 2007;315:373–377. doi: 10.1126/science.1133488. [DOI] [PubMed] [Google Scholar]
- 60.Qian F, El Hiani Y, Linsdell P. Functional arrangement of the 12th transmembrane region in the CFTR chloride channel pore based on functional investigation of a cysteine-less CFTR variant. Pflügers Arch. 2011;462:559–571. doi: 10.1007/s00424-011-0998-2. [DOI] [PubMed] [Google Scholar]
- 61.Ramsey BW, Davies J, McElvaney NG, Tullis E, Bell SC, Drevinek P, Griese M, McKone EF, Wainwright CE, Konstan MW, Moss R, Ratjen F, Sermet-Gaudelus I, Rowe SM, Dong Q, Rodriguez S, Yen K, Ordonez C, Elborn JS. A CFTR potentiator in patients with cystic fibrosis and the G551D mutation. N Engl J Med. 2011;365:1663–1672. doi: 10.1056/NEJMoa1105185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rees DC, Johnson E, Lewinson O. ABC transporters: the power to change. Nature Rev Mol Cell Biol. 2009;10:218–227. doi: 10.1038/nrm2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Richard EA, Miller C. Steady-state coupling of ion-channel conformations to a transmembrane ion gradient. Science. 1990;247:1208–1210. doi: 10.1126/science.2156338. [DOI] [PubMed] [Google Scholar]
- 64.Riordan JR, Rommens JM, Kerem B, Alon N, Rozmahel R, Grzelczak Z, Zielenski J, Lok S, Plavsic N, Chou JL, et al. Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science. 1989;245:1066–1073. doi: 10.1126/science.2475911. [DOI] [PubMed] [Google Scholar]
- 65.Schnitzer MJ, Visscher K, Block SM. Force production by single kinesin motors. Nature Cell Biol. 2000;2:718–723. doi: 10.1038/35036345. [DOI] [PubMed] [Google Scholar]
- 66.Sharom FJ. ABC multidrug transporters: structure, function and role in chemoresistance. Pharmacogenomics. 2008;9:105–127. doi: 10.2217/14622416.9.1.105. [DOI] [PubMed] [Google Scholar]
- 67.Sheppard DN. Cystic fibrosis: CFTR correctors to the rescue. Chem Biol. 2011;18:145–147. doi: 10.1016/j.chembiol.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 68.Stratford FL, Ramjeesingh M, Cheung JC, Huan LJ, Bear CE. The Walker B motif of the second nucleotide-binding domain (NBD2) of CFTR plays a key role in ATPase activity by the NBD1-NBD2 heterodimer. Biochem J. 2007;401:581–586. doi: 10.1042/BJ20060968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Szollosi A, Muallem DR, Csanady L, Vergani P. Mutant cycles at CFTR’s non-canonical ATP-binding site support little interface separation during gating. J Gen Physiol. 2011;137:549–562. doi: 10.1085/jgp.201110608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tsai MF, Li M, Hwang TC. Stable ATP binding mediated by a partial NBD dimer of the CFTR chloride channel. J Gen Physiol. 2010;135:399–414. doi: 10.1085/jgp.201010399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tsai MF, Shimizu H, Sohma Y, Li M, Hwang TC. State-dependent modulation of CFTR gating by pyrophosphate. J Gen Physiol. 2009;133:405–419. doi: 10.1085/jgp.200810186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ueda K, Komine J, Matsuo M, Seino S, Amachi T. Cooperative binding of ATP and MgADP in the sulfonylurea receptor is modulated by glibenclamide. Proc Natl Acad Sci USA. 1999;96:1268–1272. doi: 10.1073/pnas.96.4.1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Van Goor F, Hadida S, Grootenhuis PD, Burton B, Cao D, Neuberger T, Turnbull A, Singh A, Joubran J, Hazlewood A, Zhou J, McCartney J, Arumugam V, Decker C, Yang J, Young C, Olson ER, Wine JJ, Frizzell RA, Ashlock M, Negulescu P. Rescue of CF airway epithelial cell function in vitro by a CFTR potentiator, VX-770. Proc Natl Acad Sci USA. 2009;106:18825–18830. doi: 10.1073/pnas.0904709106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Van Goor F, Straley KS, Cao D, Gonzalez J, Hadida S, Hazlewood A, Joubran J, Knapp T, Makings LR, Miller M, Neuberger T, Olson E, Panchenko V, Rader J, Singh A, Stack JH, Tung R, Grootenhuis PD, Negulescu P. Rescue of Delta-F508-CFTR trafficking and gating in human cystic fibrosis airway primary cultures by small molecules. Am J Physiol Lung Cell Mol Physiol. 2006;290:L1117–L1130. doi: 10.1152/ajplung.00169.2005. [DOI] [PubMed] [Google Scholar]
- 75.Vergani P, Lockless SW, Nairn AC, Gadsby DC. CFTR channel opening by ATP-driven tight dimerization of its nucleotide-binding domains. Nature. 2005;433:876–880. doi: 10.1038/nature03313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Vergani P, Nairn AC, Gadsby DC. On the mechanism of MgATP-dependent gating of CFTR Cl− channels. J Gen Physiol. 2003;121:17–36. doi: 10.1085/jgp.20028673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Verkman AS, Lukacs GL, Galietta LJ. CFTR chloride channel drug discovery: inhibitors as antidiarrheals and activators for therapy of cystic fibrosis. Curr Pharmaceut Design. 2006;12:2235–2247. doi: 10.2174/138161206777585148. [DOI] [PubMed] [Google Scholar]
- 78.Wang W, Bernard K, Li G, Kirk KL. Curcumin opens cystic fibrosis transmembrane conductance regulator channels by a novel mechanism that requires neither ATP binding nor dimerization of the nucleotide-binding domains. J Biol Chem. 2007;282:4533–4544. doi: 10.1074/jbc.M609942200. [DOI] [PubMed] [Google Scholar]
- 79.Wang W, El Hiani Y, Linsdell P. Alignment of transmembrane regions in the cystic fibrosis transmembrane conductance regulator chloride channel pore. J Gen Physiol. 2011;138:165–178. doi: 10.1085/jgp.201110605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wang W, Okeyo GO, Tao B, Hong JS, Kirk KL. Thermally unstable gating of the most common cystic fibrosis mutant channel (DeltaF508): “rescue” by suppressor mutations in nucleotide binding domain 1 and by constitutive mutations in the cytosolic loops. J Biol Chem. 2011;286:41937–41948. doi: 10.1074/jbc.M111.296061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wang W, Wu J, Bernard K, Li G, Wang G, Bevensee MO, Kirk KL. ATP-independent CFTR channel gating and allosteric modulation by phosphorylation. Proc Natl Acad Sci USA. 2010;107:3888–3893. doi: 10.1073/pnas.0913001107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ward A, Reyes CL, Yu J, Roth CB, Chang G. Flexibility in the ABC transporter MsbA: alternating access with a twist. Proc Natl Acad Sci USA. 2007;104:19005–19010. doi: 10.1073/pnas.0709388104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yu H, Burton B, Huang CJ, Worley J, Cao D, Johnson JP, Jr, Urrutia A, Joubran J, Seepersaud S, Sussky K, Hoffman BJ, Van Goor F. Ivacaftor potentiation of multiple CFTR channels with gating mutations. J Cystic Fibros. 2012;11:237–245. doi: 10.1016/j.jcf.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 84.Zeltwanger S, Wang F, Wang GT, Gillis KD, Hwang TC. Gating of cystic fibrosis transmembrane conductance regulator chloride channels by adenosine triphosphate hydrolysis. Quantitative analysis of a cyclic gating scheme. J Gen Physiol. 1999;113:541–554. doi: 10.1085/jgp.113.4.541. [DOI] [PMC free article] [PubMed] [Google Scholar]