Abstract
Mitochondria are essential organelles for cell survival, programmed cell death, and autophagy. They undergo cycles of fission and fusion, which are subverted by infectious pathogens and altered in many human diseases. Mitochondrial fission is mediated by the dynamin‐related protein Drp1, but the precise mechanism of its action is not well understood. In the last and current issues of EMBO Reports, two new studies 1, 2 reveal that the filamentous septin GTPases interact directly with Drp1, promoting mitochondrial fission. Moreover, mitochondria were found to promote the assembly of septin filaments into cages around cytosolic Shigella flexneri bacteria 2, which are targeted for autophagy. Thus, septins emerge as integral components of the machinery of mitochondrial fission and may pose a novel link between mitochondria and autophagy.
Subject Categories: Autophagy & Cell Death; Membrane & Intracellular Transport; Microbiology, Virology & Host Pathogen Interaction
Mitochondria are essential for cellular health and homeostasis as they produce energy in the form of ATP, activate apoptosis, and regulate autophagy. They are dynamic organelles that divide, fuse, and undergo cytoskeleton‐dependent transport. Maintaining a physiological balance between mitochondrial fission and fusion is important for cell survival. To date, a growing number of human diseases is attributed to alterations in mitochondria morphology and dynamics 3. Moreover, infectious pathogens subvert mitochondrial dynamics in order to evade innate immunity responses and/or to promote cell‐to‐cell spreading by inducing apoptosis 4.
Mitochondrial fusion and fission are mediated by proteins of the dynamin family of GTPases, which oligomerize into helical filaments that wrap around membranes. Mitochondrial fission requires the dynamin‐like protein Drp1. Recent studies show that actomyosin filaments assemble on mitochondrial membranes, promoting membrane constriction as well as Drp1 recruitment and oligomerization 5. Mechanistically, however, the roles of actomyosin and Drp1 in mitochondrial fission remain poorly understood.
Similar to the evolutionarily related dynamins, septins are oligomerizing GTPases that assemble into higher order filaments and rings 6. Septins associate with actomyosin and cell membranes. Interestingly, septins also form cage‐like structures around the gram‐negative pathogenic bacteria Shigella flexneri, restricting their intracellular mobility and priming them for destruction by autophagy 7.
In two new studies published in the previous and current issues of EMBO Reports 1, 2, septins emerge as integral components of the Drp1‐mediated mechanism of mitochondrial fission (Fig 1A). Both studies report that mitochondria become longer after septin knockdown. This effect was specific to mitochondria as ER, Golgi complex, peroxisomes, and the percentage of ER areas that transverse mitochondria were unchanged 1. Pagliuso et al 1 show that elongated mitochondria form not because of defective mitochondrial fusion, but rather due to a decrease in mitochondrial fission.
Figure 1. Septins in mitochondrial fission and bacterial autophagy.
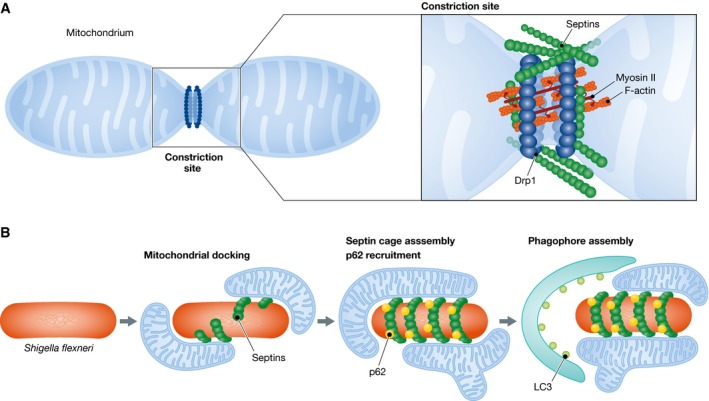
(A) Mitochondrial fission involves actomyosin and is mediated by the dynamin‐related protein Drp1. Septins interact directly with Drp1, promoting Drp1 localization to the mitochondrial fission site. (B) After cell invasion and exit from the phagocytic vacuole, Shigella flexneri bacteria are encaged by septin filaments, which are required for the recruitment of autophagic markers such as p62 and LC3. Mitochondrial membranes promote the assembly of septin cages around Shigella and hence may link mitochondria to the autophagy machinery.
Imaging by super‐resolution light and electron microscopy showed that septins localize at sites of mitochondrial membrane constriction 1, 2. In both studies, cytoplasmic septin filaments appear to intersect mitochondrial fission sites. However, septin puncta and bulge‐like structures are also seen to accumulate at incipient sites of mitochondrial fission in living cells 1. Interestingly, septins localize to approximately a third of mitochondrial constriction sites 1, suggesting that the role of septins in mitochondrial fission is either spatially and temporally restricted or potentially septins are involved in mechanisms different from Drp1‐mediated fission.
Septins and Drp1 appear to function in the same pathway as simultaneous depletion of septins and Drp1 did not result in an additive phenotype 2. Co‐immunoprecipitations and pull‐down assays with recombinant proteins showed that Drp1 interacts directly with SEPT2 1. Moreover, this interaction was enhanced and diminished, respectively, upon treatment with a mitochondrial toxin that increases fission and an inhibitor of the Drp1 GTPase activity. Drp1 and septins colocalize at sites of mitochondrial fission, and septin knockdown diminishes the mitochondrial levels of Drp1, but the reverse was not observed. Septin depletion did not affect the phosphorylation of serine 616 of Drp1, which is required for mitochondrial fission 2. However, targeting SEPT7 to mitochondria increased the recruitment of Drp1 2. Taken together, these data indicate that septins are involved in the recruitment of Drp1.
Because septins interact with actomyosin, which was recently shown to promote the oligomerization of Drp1, Pagliuso et al tested whether septins affect the mitochondrial recruitment of Drp1 via actomyosin. Depolymerization of actin with cytochalasin D did not affect the interaction of SEPT2 with Drp1 1, and Sirianni et al 2 showed that actin depolymerization enhances mitochondrial colocalization with SEPT7 rings. Septin depletion had no effect on the mitochondrial localization of myosin II or the levels of phosphorylated myosin II 1. Similarly, the mitochondrial localization of the phosphorylated regulatory myosin light chain and the actin‐binding proteins Arp2/3, cortactin, and cofilin were reportedly unchanged 1. Thus, septins appear to function independently of actin, but more studies are needed to explore the relationship between actomyosin, septins, and Drp1 during mitochondrial fission.
Given that septins assemble into cages around Shigella bacteria 7, which are known to increase mitochondrial fragmentation, Sirianni et al investigated whether septin cages associate with mitochondria (Fig 1B). Using SEPT6 as bait, shotgun proteomics revealed that mitochondrial protein hits increased by twofold in Shigella‐infected cells 2. Imaging experiments showed that Shigella‐surrounding septin cages were in close association with mitochondria. Inhibition of mitochondrial fission, which results in longer mitochondria, increased the number of septin cages, while abrogation of mitochondrial fusion had the opposite effect. Elegant time‐lapse imaging showed that mitochondria nucleate the assembly of septin filaments that cage Shigella. Mitochondria membranes, therefore, promote the assembly of septin cages around Shigella, which in turn may counteract septin cage assembly by inducing the fragmentation of mitochondria 2.
As the precise role of septins in the mechanism of mitochondrial fission remains unclear, numerous new questions arise. How are septins recruited to incipient sites of mitochondrial fission? Do septins play an active role in the constriction and reorganization of the double mitochondrial membrane? How do septin filaments interface with and/or affect the filamentous oligomers of Drp1? Do cytoplasmic septin filaments or septins associated with tubular endomembranes (e.g. ER, endosomes) induce fission upon encountering mitochondrial membranes? Answers to these questions could lead to new insights into the spatial and temporal regulation of the mitochondrial fusion/fission cycle.
Future studies will also be necessary to elucidate any functional links between septins, mitochondria, and autophagy. In Shigella‐infected cells, septins are required for the recruitment of autophagic markers to cytosolic bacteria, and septins now appear to bridge the autophagy apparatus to bacteria‐bound mitochondria. As mitochondria membranes promote the assembly of septins, it is plausible that septins function in mitophagy and possibly the assembly of the phagophore, the initial membrane of the autophagosome, by interacting with mitochondria or other endomembranes. Thus, further studies of septins in bacterial autophagy could shed more light on the molecular mechanisms of autophagy and its functional interplay with mitochondria.
See also: A Sirianni et al (July 2016) and A Pagliuso et al (June 2016)
References
- 1. Pagliuso A, Tham TN, Stevens JK et al (2016) EMBO Rep 17: 858–873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sirianni A, Krokowski S, Lobato‐Marquez D et al (2016) EMBO Rep 17: 1029–1043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nunnari J, Suomalainen A (2012) Cell 148: 1145–1159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Escoll P, Mondino S, Rolando M et al (2016) Nat Rev Microbiol 14: 5–19 [DOI] [PubMed] [Google Scholar]
- 5. Hatch AL, Gurel PS, Higgs HN (2014) J Cell Sci 127: 4549–4560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mostowy S, Cossart P (2012) Nat Rev Mol Cell Biol 13: 183–194 [DOI] [PubMed] [Google Scholar]
- 7. Mostowy S, Bonazzi M, Hamon MA et al (2010) Cell Host Microbe 8: 433–444 [DOI] [PubMed] [Google Scholar]


