Abstract
Up to 40% of youth with autism spectrum disorder (ASD) also suffer from anxiety, and this comorbidity is linked with significant functional impairment. However, the mechanisms of this overlap are poorly understood. We investigated the interplay between ASD traits and anxiety during reward processing, known to be affected in ASD, in a community sample of 1472 adolescents (mean age=14.4 years) who performed a modified monetary incentive delay task as part of the Imagen project. Blood-oxygen-level dependent (BOLD) responses to reward anticipation and feedback were compared using a 2x2 analysis of variance test (ASD traits: low/high; anxiety symptoms: low/high), controlling for plausible covariates. In addition, we used a longitudinal design to assess whether neural responses during reward processing predicted anxiety at 2-year follow-up. High ASD traits were associated with reduced BOLD responses in dorsal prefrontal regions during reward anticipation and negative feedback. Participants with high anxiety symptoms showed increased lateral prefrontal responses during anticipation, but decreased responses following feedback. Interaction effects revealed that youth with combined ASD traits and anxiety, relative to other youth, showed high right insula activation when anticipating reward, and low right-sided caudate, putamen, medial and lateral prefrontal activations during negative feedback (all clusters PFWE<0.05). BOLD activation patterns in the right dorsal cingulate and right medial frontal gyrus predicted new-onset anxiety in participants with high but not low ASD traits. Our results reveal both quantitatively enhanced and qualitatively distinct neural correlates underlying the comorbidity between ASD traits and anxiety. Specific neural responses during reward processing may represent a risk factor for developing anxiety in ASD youth.
Introduction
Anxiety is common in youth with autism spectrum disorder (ASD)1, 2, 3, 4, 5 and in young people with sub-diagnostic autistic traits.6, 7 Comorbid anxiety causes significant functional impairment in young people with ASD8, 9 and impacts on the quality of life of their families.10 However, the mechanisms of this association are poorly understood. While considerable research has examined the neural correlates of anxiety in adolescents, few studies have examined these correlates in children with symptoms of ASD. Here we investigate whether aberrations in reward processing underlie the co-occurrence of ASD traits and anxiety and whether they predict the new onset of anxiety in youth with ASD traits.
Reward processing has been proposed to be central to ASD,11, 12 with aberrant processing of primary,13 social14, 15 and monetary rewards16 reported in children and young people with ASD. Furthermore, studies in youth with ASD have reported associations between brain activations during reward processing and ASD traits such as social communication difficulties17 and restricted and repetitive behaviors.18 Some15, 19 but not all studies16 have suggested that the extent to which reward processing in youth with ASD differs from typically developing controls may depend on reward type.
Surprisingly, however, the question whether these reward aberrations are inherent to ASD symptoms or related to disorders that co-occur with ASD remains unanswered. This is a key question considering that less than 10% of children with ASD are free of any concomitant disorders according to some studies.20, 21 Anxiety disorders and behavioral difficulties are consistently identified as the most common comorbidities in youth with ASD.9, 22 These comorbid disorders are associated with aberrant reward processing in their own right, and therefore could influence reward processing in youth with ASD. Young people with anxiety show disrupted frontostriatal activation when anticipating reward23, 24, 25 and when receiving reward feedback.26 Youth with behavioral difficulties, in particular oppositional defiant disorder (ODD) symptoms and irritability, often show aberrant responses when rewards fail to appear, perhaps due to the frustrative nature of negative outcomes.27, 28
The aim of the present study is to use functional magnetic resonance imaging (fMRI) to disentangle the interplay of ASD traits and anxiety during reward processing. To enable the investigation of these factors, we use a large, community-based sample of adolescents with high vs low levels of ASD traits and anxiety symptoms, who completed a widely used, modified monetary incentive delay (MID) fMRI reward task. Our aim is to distinguish between two important models of comorbidity. One model assumes that neural correlates of comorbidity simply reflect the co-occurrence of neural mechanisms seen with each ASD traits and anxiety separately.29, 30 In the other model, the neural correlates of the comorbidity are unique, that is, not seen with any of the two disorders. This distinction is crucial from an etiological and clinical perspective, as finding unique correlates would suggest that the comorbidity might represent a separate nosological process, what has been termed a ‘third independent disorder'.31 In addition, our aim is to establish the potential predictive value of neural correlates of comorbidity. Using a longitudinal design, we examine whether neural correlates found in people with concurrent ASD traits−anxiety comorbidity can be used to predict the likelihood of new-onset anxiety in those with high ASD traits only. We achieve this aim in two steps.
First, we test the independent influence of each ASD traits and anxiety on brain correlates of reward processing, and also examine possible interaction effects between ASD traits and anxiety. The latter is particularly important in order to assess whether combined ASD traits and anxiety is associated with distinct etiological mechanisms.30
Second, we assess whether the brain activations found in our cross-sectional interaction analyses represent a biomarker that also predicts successive comorbidity32 between ASD traits and anxiety, that is, whether such brain activations predict the new onset of anxiety in those with high ASD traits. To achieve this, we run regression models with anxiety at 2-year follow-up as the outcome, and brain activations (relevant to the comorbid group) as the predictor of interest, separately for participants with low vs high ASD traits, controlling for baseline anxiety. To capture major elements of reward processing, we enquire about two key stages: reward anticipation and reward feedback. The former is often associated with heightened frontostriatal activation in youth with anxiety symptoms,23, 24, 25, 33 whereas negative feedback is likely to elicit frustration, possibly related to irritability/ODD symptoms that are common in ASD1, 5 but also in anxiety.34 For completeness, we also examine positive reward feedback, since youth with ASD tend to show reduced responsiveness to rewards in fMRI studies.35, 36 By using a community sample we avoid the risk of referral bias typical of clinical or convenience samples, a pertinent issue when investigating comorbidity,29 although the extent to which our results translate to youth who meet the diagnostic criteria for ASD remains to be tested. We also follow the emerging evidence that ASD traits, but also the mechanisms underlying them, fall on a continuum within the general population.37, 38
Materials and methods
Participants
Data were obtained from the Imagen database established across eight sites in France, UK, Ireland and Germany, which includes 2223 adolescents recruited in schools. We used data from the first (age around 14 years) and second waves (age around 16 years) of Imagen. Recruitment and assessment procedures were described in detail previously.39 All local ethics research committees approved the study. Written informed consent was obtained from a parent or guardian, and verbal assent was obtained from the adolescent. Any adolescents with IQ<70 were excluded from this study. After quality control for neuroimaging and behavioral tests, final sample sizes were 1472 for reward anticipation, 1601 for negative feedback and 1726 for positive feedback. Differences in sample sizes across conditions are due to some fMRI contrasts being non-estimable for some participants at the first-level analysis stage. Calculation of the optimum number of participants needed for an fMRI study is difficult to perform a priori. Conventional calculations to compute statistical power for a given effect size are not applicable in imaging, primarily because the MR signal in each voxel has a large degree of spatial and temporal variability. Previous studies assessing the reliability of fMRI group-level analyses have suggested that a sample size of n=20−24 is sufficient to obtain good statistical power and accurate activation maps.40, 41, 42
Measures
IQ was estimated with the Wechsler Intelligence Scale for Children—Fourth Edition, WISC-IV,43 in wave 1 and entered into Psytools (Delosis, London), an online computer platform. Two standardized indices were calculated from the WISC subtests applied during neuropsychological testing for Imagen: Verbal Comprehension (derived from Vocabulary and Similarities subtests) and Perceptual Reasoning (derived from Block Design and Matrix Reasoning subtests).
ASD traits were assessed in wave 1 using the ASD section from the Development and Well-Being Assessment (DAWBA;44 www.dawba.info), a parent-reported, self-administered structured diagnostic interview with 15 questions about social difficulties, 14 questions about restricted, repetitive behaviors and interests, and three questions about language development to assess DSM-IV-defined ASD symptoms. The diagnostic algorithm derived from the DAWBA ASD module shows strong agreement with that from the Autism Diagnostic Interview—Revised45, 46 and has a high predictive value for ASD diagnoses in community settings.47 In line with the newest characterization of ASD,48 we classified participants as having ‘high' levels of ASD traits if their parents/carers reported three or more symptoms on the social difficulties subscale and three or more symptoms on the restricted and repetitive behaviors subscale of the DAWBA ASD section. Based on this criterion close to 5% of the sample had high ASD traits (Table 1), consistent with the prevalence of clinically relevant autistic traits reported in previous population-based studies.49
Table 1. Demographic characteristics of the sample (mean±s.d. or frequency data, showing results for participants with scans available for the reward anticipation condition).
| Low ASD traits | High ASD traits | |
|---|---|---|
| Baseline | ||
| n | 1402 | 70 |
| Male gender | 657 (46.9%) | 47 (67.1%)** |
| Age in years | 14.4±0.4 | 14.4±0.4 |
| WISC Verbal | 112.0±14.7 | 108.1±15.1* |
| WISC Reasoning | 108.1±13.9 | 108.8±14.4 |
| ASD symptoms (DAWBA) | ||
| Total | 0.3±1.6 | 17.4±5.5*** |
| Social difficulties | 0.2±1.2 | 9.9±4.1*** |
| Repetitive behaviors | 0.1±0.5 | 6.8±3.7*** |
| Language development | 0.1±0.3 | 0.6±0.9*** |
| Continuous psychopathology (SDQ) | ||
| Emotional symptoms | 1.8±1.9 | 3.5±2.9*** |
| Conduct problems | 1.6±1.5 | 2.9±2.2*** |
| Hyperactivity | 2.8±2.2 | 4.5±2.9*** |
| Impact | 0.6±1.3 | 2.2±2.4*** |
| Diagnostic categories | ||
| Any anxiety | 326 (23.3%) | 30 (42.9%)*** |
| Depression | 44 (3.1%) | 10 (14.3%)*** |
| ODD | 483 (34.5%) | 43 (61.4%)*** |
| 2-year follow-up | ||
| n | 1019 | 50 |
| Male gender | 465 (45.7%) | 35 (70.0%)** |
| SDQ | ||
| Emotional symptoms | 1.6±1.9 | 2.9±2.9** |
| Conduct problems | 1.4±1.4 | 2.0±1.9* |
| Hyperactivity | 2.2±2.0 | 3.6±2.5*** |
| Impact | 0.5±1.4 | 1.3±2.1* |
| Diagnostic categories | ||
| Any anxiety | 196 (19.3%) | 17 (34.0%)* |
| Depression | 36 (3.5%) | 6 (12.0%)* |
| ODD | 295 (29.0%) | 23 (46.0%)* |
Abbreviations: ASD, autism spectrum disorder; DAWBA, Development and Well-Being Assessment; ODD, oppositional defiant disorder; SDQ, Strengths and Difficulties Questionnaire.
*P<0.05; **P<0.01; ***P<0.001 (t-test or chi-square).
Anxiety, ODD and depression prevalences were estimated based on the established and widely used DAWBA computer algorithm,50, 51 which indicates the probability of receiving a DSM-IV-defined diagnosis based on answers provided during the interview. DAWBA algorithm band 2 or above was chosen as a cut-off for relevant psychiatric symptoms. Participants were classified as having ‘any anxiety' if they scored at band 2 or above for at least one DSM-IV anxiety disorder; this algorithm identified 24% of the sample (see Table 1).
Emotional symptoms were assessed using the parent-reported emotional symptoms subscale from the Strengths and Difficulties Questionnaire (SDQ).52 The scale includes three questions about anxiety, one question about somatic symptoms and one about low mood. A score of 5 and above indicates substantial risk of clinically significant emotional problems52 and was used as a cut-off. We used emotional symptoms instead of ‘any anxiety' in confirmatory analyses (see below) to ensure that our findings were not measure-specific.
Additional relevant symptoms of hyperactivity, conduct problems and functional impairment were assessed using the parent-reported SDQ.52
Modified MID task
In wave 1, the participants performed a modified version of the well-established MID task53, 54 to study neural responses to reward. The task has three conditions: reward anticipation (anticipation of large win versus anticipation of no win), receipt of negative feedback (feedback of missed large win versus feedback of missed no win) and positive feedback (feedback of hit large win versus feedback of hit no win). A detailed description of the task is provided in Supplementary Appendix 1.
Magnetic resonance imaging data acquisition
Structural and functional magnetic resonance imaging (MRI) data were acquired at eight Imagen assessment sites with 3-T MRI scanners of different manufacturers (Siemens, Munich, Germany; Philips, Best, The Netherlands; General Electric, Chalfont, St Giles, UK; Bruker, Ettlingen, Germany). The scanning variables were chosen to be compatible with all scanners. The same scanning protocol was used at all sites. In brief, high-resolution T1-weighted three-dimensional structural images were acquired for anatomical localization and co-registration with the functional time series. fMRI blood-oxygen-level dependent (BOLD) images were acquired with a gradient-echo, echo-planar imaging sequence. For the MID task, 300 volumes were acquired for each subject. Each volume consisted of 40 slices aligned to the anterior commissure−posterior commissure line (2.4 mm slice thickness, 1mm gap) acquired in a descending order. The echo time was optimized (echo time=30 m, repetition time=2200 ms) to provide reliable imaging of subcortical areas. See Supplementary Appendix 1 for detailed description of the fMRI data acquisition.
Statistical analyses
Behavioral performance
We first tested whether participants were motivated by the potential of winning a reward. As a proxy of task engagement, we compared mean response accuracy in ‘no win' and ‘big win' trials using a paired-samples t-test (two-tailed), separately for participants with low and high ASD traits.
Imaging analyses
Imaging analyses were performed using the Statistical Parametric Mapping suite (SPM 8, Functional Imaging Laboratory, University College London, London, UK; www.fil.ion.ucl.ac.uk/spm). Analyses were performed at an a priori voxel threshold of P<0.01 and cluster threshold of P<0.05 with family-wise error (FWE) correction. Gender, handedness, site of scanning, WISC Verbal Comprehension and WISC Reasoning were included as covariates in all analyses, in line with previous studies.35, 55, 56 All findings are reported at whole-brain level. (NB: While SPM does not provide estimates of variance for between-groups brain activation data, we performed Levene's tests for equality of variances on extracted region-of-interest data (ROI; significant regions from whole-brain ANOVAs were extracted), which confirmed statistically equal variances between the groups.)
Effects of ASD traits and anxiety on reward processing
We ran a 2 × 2 ANOVA (ASD traits: low vs high; any anxiety: low (DAWBA bands 0/1) vs high (band 2 or above)) to test for main effects of ASD traits and anxiety, and an ASD-by-anxiety interaction, separately for reward anticipation, negative and positive feedback conditions. Where an ASD-by-anxiety interaction was found, we ran follow-up t-tests to assess between-groups differences in brain activations.
Covariates
We repeated the above ANOVAs twice, first adding ODD and then depression into the model as potential confounders.
Effects of emotional problems
To ensure that our main findings were not measure-specific, we ran confirmatory ANOVAs and t-tests using the SDQ emotional symptoms subscale (cut-off at 5) instead of DAWBA-defined ‘any anxiety'.
Longitudinal predictions
Our second aim was to assess whether the brain activations found in cross-sectional interaction analyses above are (a) a phenotypic manifestation of the co-occurrence between ASD traits and anxiety, or (b) whether they represent a biomarker that predicts not only cross-sectional, but also successive comorbidity between ASD traits and anxiety. To test this we conducted logistic regression analyses with anxiety (low vs high) at 2-year follow-up as the outcome, and brain activations found in our cross-sectional interaction effects as predictors of interest. Analyses were run in Stata 11,57 and the ROIs were extracted from the contrast maps for a given reward condition, using MarsBaR toolbox in SPM based on the WFUPickAtlas toolbox definitions.58 To ensure that the ROIs predicted new onset of anxiety, baseline anxiety status was added to the model as an additional predictor. We also added baseline ASD traits (continuous variable) to the model, to ensure that the predictions were not driven by the severity of ASD-specific impairments. To assess the specificity of brain predictions, regressions were run separately within low and high ASD trait groups. If an ROI predicted new-onset anxiety in one group but not the other, a regression model was run across the whole sample with an interaction term between ROI activations and ASD traits (low vs high) to identify the strength of a possible interaction. These analyses were not corrected for multiple comparisons since the ROIs were pre-defined based on our cross-sectional results.
Results
Participant characteristics
As expected, participants with high ASD traits scored significantly higher on all subscales of the DAWBA ASD section compared to those with low ASD traits (Table 1) and had a higher proportion of males. While the groups did not differ in age or performance IQ, youth with high ASD traits scored lower on verbal comprehension. Participants with high ASD traits scored higher on hyperactivity, emotional and conduct problems, and showed higher functional impairment. Those with high ASD traits were more likely to display symptoms of anxiety, depression and ODD. Sample characteristics were similar for the other two reward conditions (Supplementary Tables S1A-C).
Behavioral performance
Paired-samples t-tests revealed that participants with high and low ASD traits both responded more accurately in ‘big win' compared to ‘no win' trials (high ASD traits: 65.1% vs 57.9%, t(69)=3.90, P<0.001; low ASD traits: 67.4% vs 53.8%, t(1401)=28.49, P<0.001), suggesting that both groups were motivated by the potential of winning a reward. The increase in response accuracy between ‘no win' and ‘big win' trials was higher for those with low vs high ASD traits, t(1470)=2.97, P=0.003, d=0.39.
Reward anticipation
We first conducted an ANOVA to test the effects of ASD traits and anxiety on brain activations during reward anticipation.
Main effects
We found a main effect of ASD-trait severity and a main effect of anxiety (Table 2). Participants with high ASD traits (n=70) showed lower BOLD responses in the right superior frontal gyrus (SFG) extending to the anterior and midcingulate regions relative to youth with low ASD traits (n=1402). Participants with anxiety symptoms displayed increased activation in the right middle frontal and middle temporal gyri (MFG and MTG), irrespective of ASD-trait severity.
Table 2. The effects of ASD traits and anxiety on BOLD responses during reward anticipation.
|
Peak MNI coordinates |
|||||||
|---|---|---|---|---|---|---|---|
| Region | Brodmann area | Cluster size (voxels) | x | y | z | Z | P (FWE) |
| Interaction: ASD traits x anxiety | |||||||
| R middle and superior temporal gyri, R insula | 21/22/13 | 283 | 45 | −16 | −11 | 3.97 | 0.001 |
| 57 | −37 | −8 | 3.80 | ||||
| 63 | −16 | −11 | 3.71 | ||||
| High<low ASD traits | |||||||
| R superior and medial frontal gyri extending bilaterally to the dACC and MCC | 9/24/32 | 936 | −18 | 17 | 37 | 4.88 | <0.001 |
| 18 | 17 | 49 | 4.72 | ||||
| 9 | 50 | 37 | 4.58 | ||||
| Any anxiety>no anxiety | |||||||
| R middle frontal gyrus | 8 | 254 | 39 | 23 | 43 | 4.41 | 0.002 |
| 21 | 59 | 37 | 3.89 | ||||
| 42 | 8 | 49 | 3.66 | ||||
| R middle temporal gyrus | 21 | 209 | 45 | −16 | −11 | 3.98 | 0.008 |
| 60 | −16 | −5 | 3.67 | ||||
| 57 | −37 | −11 | 3.47 | ||||
| ASDANX>ASDONLY | |||||||
| R middle temporal gyrus | 21 | 257 | 57 | −37 | −8 | 4.56 | <0.001 |
| 63 | −13 | −2 | 4.31 | ||||
| 69 | −37 | −2 | 3.90 | ||||
| R middle frontal gyrus | 8 | 251 | 42 | 23 | 43 | 4.27 | <0.001 |
| 42 | 11 | 52 | 3.71 | ||||
| 42 | 32 | 43 | 3.45 | ||||
| L insula, L inferior frontal gyrus | 13/45/47 | 162 | −42 | 17 | −11 | 4.27 | 0.011 |
| −36 | 17 | 1 | 3.43 | ||||
| −45 | 17 | 7 | 3.36 | ||||
| L inferior parietal lobule | 40 | 124 | −57 | −43 | 55 | 3.86 | 0.047 |
| −48 | −61 | 52 | 3.33 | ||||
| −66 | −37 | 37 | 3.11 | ||||
| L superior and middle temporal gyri | 39/22/40 | 246 | −60 | −67 | 7 | 3.71 | 0.001 |
| −51 | −82 | 7 | 3.70 | ||||
| −51 | −79 | 22 | 3.68 | ||||
| R inferior parietal lobule | 40 | 256 | 51 | −52 | 55 | 3.60 | <0.001 |
| 60 | −34 | 55 | 3.49 | ||||
| 48 | −34 | 64 | 3.41 | ||||
| ASDANX>ANXONLY | |||||||
| L insula, L inferior frontal gyrus | 47 | 186 | −42 | 17 | −8 | 4.68 | 0.017 |
| −36 | 14 | 4 | 3.65 | ||||
| −54 | 20 | 1 | 3.57 | ||||
| L and R cuneus and calcarine | 18/17 | 222 | −12 | −82 | 10 | 4.30 | 0.006 |
| 12 | −85 | 10 | 3.77 | ||||
| −6 | −76 | 22 | 3.38 | ||||
Abbreviations: ANXONLY, high anxiety and low ASD traits; ASD, autism spectrum disorder; ASDANX, high ASD traits and anxiety; ASDONLY, high ASD traits, low anxiety; BOLD, blood-oxygen-level dependent; dACC, dorsal anterior cingulate cortex; FWE, family-wise error correction; L, left hemisphere; MCC, middle cingulate cortex; MNI, Montreal Neurological Institute; R, right hemisphere.
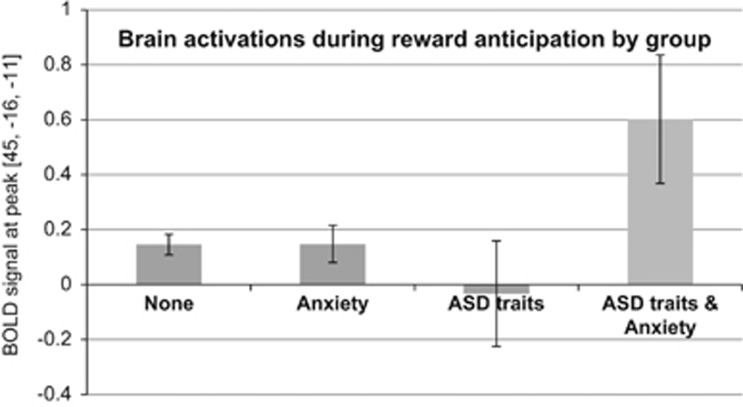
Interaction
We also found an interaction between ASD traits and anxiety in a cluster encompassing right MTG, superior temporal gyrus and insula (Table 2). Figure 1 illustrates the interaction, showing significantly increased activation at the cluster's peak in ASDANX relative to all other groups. Follow-up t-tests revealed that the ASDANX (n=30) group showed significantly increased brain activation relative to ASDONLY (n=40) in the left insula and left inferior frontal gyrus (IFG), right MFG, as well as bilateral inferior parietal lobule and temporal areas. ASDANX also displayed increased activation in the left insula, left IFG and posterior brain regions relative to ANXONLY (n=326).
Figure 1.
Interaction between autism spectrum disorder (ASD) traits and anxiety. Showing mean blood-oxygen-level dependent (BOLD) responses during reward anticipation and 95% confidence intervals at the cluster peak [45, -16, -11] located in the middle temporal gyrus. A similar pattern of results emerged for a peak in the right insula.
Covariates
All results remained significant after controlling for the effects of ODD, and we found two additional clusters in the ASD-by-anxiety interaction (ASDANX>all other groups in the left IFG, insula and MFG). After controlling for the effects of depression, all results remained significant, except for one cluster in the ASDANX>ASDONLY comparison (activations in the left insula and left IFG no longer significant).
Emotional problems
The results were broadly consistent with anxiety analyses and are described in detail in Supplementary Table S2A.
Negative feedback
Main effects
We found a main effect of ASD-trait severity and a main effect of anxiety (Table 3). Participants with high ASD traits (n=78) showed lower activation in the right superior and medial frontal gyri relative to youth with low ASD traits (n=1523). Irrespective of ASD-trait severity, participants with anxiety symptoms (n=396) showed decreased activation in the following regions following negative feedback: bilateral caudate, bilateral IFG, right SFG, left MFG, left inferior parietal lobule and left MTG.
Table 3. The effects of ASD traits and anxiety on BOLD responses during negative reward feedback.
|
Peak MNI coordinates |
|||||||
|---|---|---|---|---|---|---|---|
| Region | Brodmann area | Cluster size (voxels) | x | y | z | Z | P (FWE) |
| Interaction: ASD traits x anxiety | |||||||
| R superior and medial frontal gyri | 10 | 223 | 21 | 59 | 7 | 4.95 | 0.003 |
| 27 | 59 | 1 | 4.84 | ||||
| 18 | 47 | 22 | 4.34 | ||||
| R caudate, R putamen, R middle and inferior frontal gyri | 10 | 301 | 21 | 23 | 1 | 4.73 | <0.001 |
| 27 | 38 | −5 | 4.46 | ||||
| 21 | 23 | 13 | 3.76 | ||||
| L middle temporal gyrus | 183 | −51 | −76 | 13 | 4.05 | 0.010 | |
| −57 | −70 | 10 | 3.87 | ||||
| −24 | −85 | 16 | 3.36 | ||||
| High<Low ASD traits | |||||||
| R superior and medial frontal gyri | 10 | 137 | 21 | 44 | 1 | 4.33 | 0.046 |
| 18 | 53 | 13 | 4.06 | ||||
| 30 | 56 | −2 | 3.71 | ||||
| Any anxiety<no anxiety | |||||||
| R superior frontal gyrus extending to R medial frontal gyrus | 10 | 145 | 21 | 59 | 7 | 5.08 | 0.034 |
| 21 | 62 | 19 | 3.62 | ||||
| 6 | 65 | 28 | 2.75 | ||||
| R caudate, R inferior frontal gyrus | 449 | 18 | 47 | 22 | 4.79 | <0.001 | |
| 21 | 23 | 1 | 4.67 | ||||
| 27 | 38 | −5 | 4.29 | ||||
| L inferior parietal lobule | 184 | −36 | −46 | 28 | 4.62 | 0.009 | |
| −24 | −58 | 25 | 4.53 | ||||
| −24 | −52 | 34 | 3.87 | ||||
| L middle temporal gyrus | 182 | −60 | −70 | 7 | 4.12 | 0.010 | |
| −51 | −76 | 13 | 3.85 | ||||
| −51 | −67 | 13 | 3.33 | ||||
| L middle and inferior frontal gyrus | 193 | −48 | 29 | −5 | 4.04 | 0.007 | |
| −30 | 44 | 22 | 3.97 | ||||
| −30 | 41 | 14 | 3.72 | ||||
| L caudate extending to the subgenual ACC | 24/25 | 144 | −9 | 23 | −5 | 3.71 | 0.036 |
| −18 | 23 | 4 | 3.67 | ||||
| −12 | 14 | 4 | 3.41 | ||||
| ASDANX<ASDONLY | |||||||
| R middle and inferior frontal gyri (extending to the midcingulate/ACC) | 46/32 | 143 | 27 | 32 | 31 | 4.30 | 0.015 |
| 33 | 38 | 16 | 4.12 | ||||
| 42 | 35 | 13 | 3.66 | ||||
| L inferior and middle frontal gyri | 46 | 136 | −48 | 29 | −2 | 4.20 | 0.020 |
| −36 | 26 | 13 | 3.39 | ||||
| −45 | 32 | 19 | 3.38 | ||||
| L rolandic operculum / precentral gyrus, L inferior frontal gyrus | 22 | 120 | −63 | 5 | 4 | 4.00 | 0.040 |
| −54 | 2 | 7 | 3.65 | ||||
| −57 | 14 | 7 | 3.55 | ||||
| L and R lateral ventricles, corpus callosum (extending to L caudate) | 179 | 9 | −10 | 22 | 3.60 | 0.004 | |
| −6 | −19 | 25 | 3.35 | ||||
| −1 | 25 | 3.35 | |||||
| ASDANX<ANXONLY | |||||||
| R putamen and R caudate extending to subcallosal gyrus / gyrus rectus, R superior and medial frontal gyri | 34/13/10 | 472 | 27 | 59 | 1 | 5.33 | <0.001 |
| 15 | 56 | 7 | 4.70 | ||||
| 18 | 20 | 1 | 3.89 | ||||
| L putamen and L caudate, L middle frontal gyrus | 47 | 227 | −30 | 44 | −8 | 4.17 | 0.003 |
| −12 | 11 | 1 | 4.00 | ||||
| −21 | 23 | 1 | 3.88 | ||||
Abbreviations: ACC, anterior cingulate cortex; ASD, autism spectrum disorder; ANXONLY, high anxiety and low ASD traits; ASDANX, high ASD traits and anxiety; ASDONLY, high ASD traits and low anxiety; BOLD, blood-oxygen-level dependent; FWE, family-wise error correction; L, left hemisphere; MNI, Montreal Neurological Institute; R, right hemisphere.
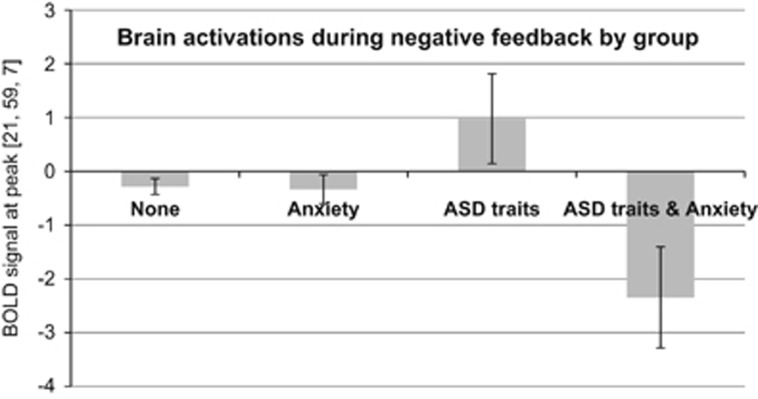
Interaction
We also found an interaction between ASD traits and anxiety severity in the right caudate and putamen, prefrontal regions and left MTG. While anxiety did not affect brain activations in these regions in youth with low ASD traits, young people with ASD traits and anxiety showed markedly lower activations compared to ASDONLY (Figure 2). Follow-up t-tests revealed that ASDANX (n=35) had significantly decreased brain activation in bilateral MFG and IFG extending to the anterior cingulate, left precentral gyrus, and corpus callosum extending to the left caudate compared to ASDONLY (n=43). ASDANX also displayed decreased activation in bilateral caudate and putamen relative to ANXONLY (n=361).
Figure 2.
Interaction between autism spectrum disorder (ASD) traits and anxiety. Showing mean blood-oxygen-level dependent (BOLD) responses during negative reward feedback and 95% confidence intervals at cluster 1 peak [21, 59, 7] in the right superior frontal gyrus. The same pattern of results emerged for the two other clusters with peaks in the right caudate and left middle temporal gyrus, and middle frontal gyrus.
Covariates
After controlling for the effects of ODD, the ASD-by-anxiety interaction remained significant, and we found an additional cluster encompassing the left caudate and left IFG. The main effect of anxiety remained significant in three out of six previously found clusters (the following clusters lost significance after controlling for ODD: right SFG k=120, PFWE=0.084; left MFG and IFG k=114, PFWE=0.104; left caudate and sgACC k=121, PFWE=0.081). The main effect of ASD was just below threshold for significance (frontal cluster k=131, PFWE=0.056). The ASDANX< ANXONLY t-test remained significant, but 3/4 clusters in the ASDANX<ASDONLY comparison lost significance (right MFG and IFG k=105, PFWE=0.084; left MFG and IFG k=109, PFWE=0.070; left IFG/rolandic operculum k=79, PFWE=0.260).
All results remained significant after controlling for the effects of depression, except for one cluster in the ASDANX<ASDONLY comparison (activation in the left rolandic operculum no longer significant, k=107, PFWE=0.084). We also found two additional clusters encompassing the left IFG, left caudate and left putamen in the ASD-by-anxiety interaction.
Emotional problems
The results were broadly consistent with anxiety analyses and are described in detail in Supplementary Table S2B.
Positive feedback
Main effects
Across the whole sample, we found a main effect of ASD-trait severity. Youth with high ASD traits (n=81) displayed increased activation in the bilateral thalamus and pallidum, as well as right putamen, compared to youth with low ASD traits (n=1645; Table 4). This finding remained significant after controlling for the effects of ODD, but lost significance after controlling for the effects of depression (right-sided cluster PFWE=0.061, k=148; left-sided cluster PFWE=0.671, k=65). No main effect of anxiety was found.
Table 4. The effects of ASD traits and anxiety on brain activation patterns following positive feedback.
|
Peak MNI coordinates |
|||||||
|---|---|---|---|---|---|---|---|
| Region | Brodmann area | Cluster size (voxels) | x | y | z | Z | P (FWE) |
| High>low ASD traits | |||||||
| R thalamus, R putamen, R globus pallidus | 163 | 30 | −4 | 4 | 5.03 | 0.039 | |
| 24 | −10 | 4 | 4.78 | ||||
| 21 | −22 | 7 | 3.74 | ||||
| L thalamus, L globus pallidus, midbrain, extending to L hippocampus | 185 | 15 | −16 | −5 | 4.23 | 0.021 | |
| −15 | −7 | 1 | 4.21 | ||||
| −9 | 22 | 1 | 3.66 | ||||
Abbreviations: ASD, autism spectrum disorder; FWE, family-wise error correction; L, left hemisphere; MNI, Montreal Neurological Institute; R, right hemisphere.
Interaction
We did not find a significant interaction.
Emotional problems
We did not find a main effect of ASD traits in our analyses with SDQ emotional subscale. Instead, we found a main effect of emotional problems in the left middle occipital gyrus (Supplementary Table S2C). No interaction was found.
Longitudinal predictions
Finally, we investigated whether brain correlates of reward processing found in our interaction analyses, and relevant to the comorbid group, represent a mechanism underlying successive comorbidity between ASD traits and anxiety.
Reward anticipation
The following ROI predictors were tested (all right-sided): MTG, insula, Brodmann area (BA) 32 (dorsal cingulate), caudate, thalamus and medial frontal gyrus (medFG, defined using the ‘frontal_sup_medial' mask from the automatic anatomical labelling atlas in WFUPickAtlas).
High ASD traits: In participants with high ASD traits, increased activations in the right medFG and right BA 32 during reward anticipation were associated with a higher likelihood of anxiety at follow-up, with baseline anxiety and ASD traits included in the model (ORmedFGright=62.33, 95% CI [1.46−2668.54], P=0.031; ORBA32right=33.22, 95% CI [1.47−750.36], P=0.028; Supplementary Table S3A).
Low ASD traits: None of the ROIs significantly predicted new-onset anxiety in participants with low ASD traits.
Interaction effects across the whole sample: The interaction term between ASD traits and right medFG was a significant predictor of new-onset anxiety at follow-up (OR=17.34, 95% CI (1.45−207.03), P=0.024). Likewise, the interaction between ASD traits and right BA 32 significantly predicted future anxiety (OR=15.75, 95% CI (1.32−187.48), P=0.029).
No significant results were found for the remaining ROIs.
Negative feedback
None of the ROIs we tested (right-sided: medFG, MFG, caudate, putamen, BA 32; left MTG) predicted dichotomous anxiety status at follow-up in either ASD traits group.
Discussion
We found independent effects of ASD traits and anxiety on neural correlates of reward processing. We also found interaction effects whereby youth with combined ASD traits and anxiety showed distinctively high right MTG and insula activation when anticipating reward, and low prefrontal activation during negative feedback. Moreover, in participants with ASD traits, brain activation patterns during reward anticipation predicted new onset of anxiety 2 years later.
ASD traits
During both reward anticipation and negative feedback, we observed attenuated BOLD activation in prefrontal regions in participants with high compared to low ASD traits. When anticipating reward, participants with high ASD traits showed reduced activation in dorsal ACC and right dorsal prefrontal cortex (PFC) (BA 9), regions involved in working memory, cognitive salience detection and monitoring of reward-based behavioral responses.59, 60, 61 Participants with ASD traits may attach less salience to rewards, consistent with previous studies showing reduced reward sensitivity in ASD youth.17, 36 During negative feedback, youth with ASD traits showed reduced activation in the right medial PFC compared to those with low ASD traits. Previous studies in healthy adults showed that while obtaining an expected reward is associated with an increase in medial PFC activation, reward omission leads to decreased activation in this region.62, 63 This functional modulation was proposed to reflect medial PFC's role in tracking rewarding outcomes. In this context, our results may indicate that participants with high ASD traits find the lack of expected reward relatively more punishing or aversive64 than those with low ASD traits. Combined with increased activation in reward-sensitive structures (putamen, thalamus, pallidum)65 in participants with high ASD traits following positive feedback, our results suggest that inadequate salience detection during reward anticipation may have led to exaggerated responses to both positive and negative reward outcomes.
Anxiety
Participants with anxiety showed increased activation in the right MFG during reward anticipation, but decreased right MFG activation following negative feedback, compared to participants without anxiety. MFG is part of the lateral PFC,66 a region implicated in cognitive control via inhibition of prepotent behavioral responses.67, 68, 69 Increased activation in right MFG during anticipation suggests that participants with higher anxiety symptoms required more cognitive effort to maintain stimulus−reward representations active when faced with competing events.68 This is consistent with previous studies where anxious adolescents showed more emotional interference70 and heightened concern about making errors23 during reward processing compared to controls. However, we did not find the expected pattern of enhanced striatal activation during the anticipation of reward, which occurs specifically in social anxiety disorder.71, 72 This could relate to the low rate of social anxiety in our sample. Conversely to reward anticipation, following negative reward feedback, anxious participants displayed reduced activation in the lateral PFC (right MFG and SFG, bilateral IFG) and bilateral caudate.
Interaction effects
The key aim of this study was to examine the interplay of ASD traits and anxiety symptoms during reward processing. We explored interaction effects to test whether the co-occurrence of ASD traits and anxiety was associated with a quantitative change in, or a qualitatively unique pattern of, reward-related brain activations. We found that youth with combined ASD traits and anxiety showed a unique pattern of high right insula activation during reward anticipation, as well as increased right MTG activation during anticipation and markedly low right-sided caudate, putamen, medial and lateral PFC activation during negative feedback. These effects remained significant after controlling for the effects of possible confounders (depression and ODD symptoms). Interestingly, insula hyperactivation was not observed in any of the main effects above, suggesting that youth with ASD traits and anxiety may display a qualitatively different pattern of neural activations during reward anticipation. The insula is implicated in ‘aversion-related' reward processing59, 73 particularly in anticipating and predicting salience of aversive events,74, 75, 76, 77 as well as in interoceptive processing.78, 79, 80 Interestingly, interoceptive prediction errors have been proposed to play a role in mood and anxiety disorders78, 81 and theory of mind.82 Future studies should test directly whether the distinct pattern of activations observed during reward anticipation in our ‘combined' group is related to interoceptive prediction errors, a possible etiological mechanism underlying the comorbidity between ASD and anxiety. Reduced right-sided caudate, putamen and medial PFC (BA 10) activation during negative feedback suggests that participants in the combined group may have found not receiving the reward more aversive than other participants, similarly to reward anticipation. We also found reduced lateral PFC activation in the combined group following negative feedback. Interestingly, some but not all activation patterns that characterize the interaction effect were also found in the main effect of ASD traits (BA 10) and anxiety (right lateral PFC and right caudate), suggesting shared and unique neural substrates of negative reward feedback in youth with combined ASD traits and anxiety.
Longitudinal findings
In participants with high but not low ASD traits, increased right medFG and right dorsal cingulate activations during reward anticipation were associated with increased likelihood of anxiety symptoms 2 years later. Predictions were significant after controlling for baseline anxiety, showing that MRI can predict new onset of anxiety problems.
Clinical implications
Our findings suggest that the presence of combined ASD traits and anxiety is associated with both a quantitatively potentiated neural response to negative reward feedback (interaction showing a further reduction in prefrontal activations found in main effects) as well as emergence of qualitatively different neural correlates during reward anticipation (activation in the right insula found exclusively in the interaction). This suggests that shared and distinct etiological mechanisms might be involved in the comorbidity between ASD and anxiety, and, if replicated, carries important clinical implications. If the co-occurrence of anxiety in ASD is underpinned by a distinct pathophysiological mechanism, the comorbid group may need to be recognized as a distinct nosological category and be researched in its own right. Moreover, it is possible that medication response in this group is also different. We know already from ADHD literature that the effectiveness of methylphenidate is reduced in some youth with ASD83 and in those with comorbid ADHD and anxiety.84, 85, 86 In addition, a specific biomarker of anxiety in ASD could aid differential diagnosis in cases where comorbid anxiety may be phenomenologically indistinguishable from ASD.87
Second, although MRI findings predicted only a small portion of the variance in new onset of anxiety at follow-up, brain activations were a significant predictor that could be used in establishing useful biomarkers of anxiety risk in youth with ASD traits. Our design strengthens the implication that the pattern of right-sided medFG and dorsal cingulate activations during reward processing is not merely a marker of anxiety, but may reflect an underlying mechanism by which young people with ASD traits become anxious. Ultimately, finding an MRI biomarker of anxiety in ASD has a potential of guiding treatment interventions and measuring treatment response, which is especially useful in cases where the value of clinical interview is limited due to social communication difficulties.5
Third, recent evidence suggests that disrupted processing of reward may lead to decision-making problems.88 Future studies should investigate whether reward-processing deficits can explain the presence of executive function deficits in ASD,89 and explore the role of comorbid anxiety in the process.
Limitations
Although we investigated both anticipation and feedback phases of reward processing, task learning was performed outside of the scanner; therefore it was not possible to study the neural correlates of stimulus−reward learning. Second, due to sample size limitations we did not distinguish between different types of anxiety disorders. Future studies should test whether the differential impact of specific types of anxiety on reward processing, seen in typically developing youth,90 holds in youth with ASD traits. Third, while our a priori choice of a cluster-forming threshold of P<0.01 at the voxel level is in line with previous reward-processing fMRI studies in adolescents that used the same91 or more lenient92 voxel-wise thresholds, and a large sample size is likely to limit the rate of false positives,41 it is still important that our results are replicated in an independent sample. Finally, the relative contribution of anxiety and ASD-specific difficulties to reward processing in youth with a clinical diagnosis of ASD remains to be studied.
In conclusion, over and above the independent effects of ASD traits and anxiety, we found qualitatively distinct and quantitatively potentiated neural correlates of reward processing in youth with combined anxiety and ASD traits. Future studies should assess whether the apparent co-occurrence of ASD and anxiety is associated with distinct etiological mechanisms.
Acknowledgments
This work received support from the following sources: the European Union-funded FP6 Integrated Project IMAGEN (reinforcement-related behaviour in normal brain function and psychopathology) (LSHM-CT- 2007-037286), the FP7 projects IMAGEMEND (602450; IMAging GEnetics for MENtal Disorders), AGGRESSOTYPE (602805) and MATRICS (603016), the Innovative Medicine Initiative Project EU-AIMS (115300-2), the Medical Research Council Grants 'Developmental pathways into adolescent substance abuse' (93558) and Consortium on Vulnerability to Externalizing Disorders and Addictions (c-VEDA) (MR/N000390/1), the Swedish funding agencies VR, FORTE and FORMAS, the Medical Research Council and the Wellcome Trust (Behavioural and Clinical Neuroscience Institute, University of Cambridge, Cambridge, UK), the National Institute for Health Research (NIHR) Biomedical Research Centre at South London and Maudsley NHS Foundation Trust and King's College London, the Bundesministeriumfür Bildung und Forschung (BMBF grants 01GS08152; 01EV0711; eMED SysAlc01ZX1311A; Forschungsnetz AERIAL) and the Deutsche Forschungsgemeinschaft (DFG grants SM 80/7-1, SM 80/7-2, SFB 940/1). Further support was provided by grants from ANR (project AF12-NEUR0008-01-WM2NA, and ANR-12-SAMA-0004), the Fondation de France, the Fondation pour la Recherche Médicale, the Mission Interministérielle de Lutte-contre-les-Drogues-et-les-Conduites-Addictives (MILDECA), the Assistance-Publique-Hôpitaux-de-Paris and INSERM (interface grant), Paris Sud University IDEX 2012, the National Institutes of Health, USA (Axon, Testosterone and Mental Health during Adolescence; RO1 MH085772-01A1), and by NIH Consortium grant U54 EB020403, supported by a cross-NIH alliance that funds Big Data to Knowledge Centres of Excellence. Dr Stringaris gratefully acknowledges funding from the Wellcome Trust.
DISCLAIMER
The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health.
Footnotes
Supplementary Information accompanies the paper on the Translational Psychiatry website (http://www.nature.com/tp)
Dr Banaschewski has served as an advisor or consultant to Bristol-Myers Squibb, Desitin Arzneimittel, Eli Lilly, Medice, Novartis, Pfizer, Shire, UCB and Vifor Pharma; he has received conference attendance support, conference support or speaking fees from Eli Lilly, Janssen McNeil, Medice, Novartis, Shire and UCB; and he is involved in clinical trials conducted by Eli Lilly, Novartis and Shire; the present work is unrelated to these relationships. Dr Gallinat has received research funding from the German Federal Ministry of Education and Research, AstraZeneca, Eli Lilly, Janssen-Cilag and Bristol-Myers Squibb; he has received speaking fees from AstraZeneca, Janssen-Cilag and Bristol-Myers Squibb. Dr Goodman is the owner of Youthinmind, Ltd., which provides no-cost and low-cost software and websites related to the Development and Well-Being Assessment and the Strengths and Difficulties Questionnaire. Ms Mikita received a one-off honorarium from John Wiley & Sons. Dr Stringaris has received grant or research support from the Guy's & St Thomas' Charity, University College London for a joint project with Johnson & Johnson, the Wellcome Trust and the National Institute for Health Research; he also receives royalties from Cambridge University Press and Oxford University Press. The remaining authors declare no conflict of interest.
Supplementary Material
References
- Mandy W, Roughan L, Skuse D. Three dimensions of oppositionality in autism spectrum disorder. J Abnorm Child Psychol 2014; 42: 291–300. [DOI] [PubMed] [Google Scholar]
- Simonoff E, Jones CR, Pickles A, Happé F, Baird G, Charman T. Severe mood problems in adolescents with autism spectrum disorder. J Child Psychol Psychiatry 2012; 53: 1157–1166. [DOI] [PubMed] [Google Scholar]
- Mazefsky CA, Conner CM, Oswald DP. Association between depression and anxiety in high-functioning children with autism spectrum disorders and maternal mood symptoms. Autism Res 2010; 3: 120–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Steensel FJA, Bogels SM, Perrin S. Anxiety disorders in children and adolescents with autistic spectrum disorders: a meta-analysis. Clin Child Fam Psychol Rev 2011; 14: 302–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikita N, Hollocks MJ, Papadopoulos AS, Aslani A, Harrison S, Leibenluft E et al. Irritability in boys with autism spectrum disorders: an investigation of physiological reactivity. J Child Psychol Psychiatry 2015; 56: 1118–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallett V, Ronald A, Rijsdijk F, Happe F. Disentangling the associations between autistic-like and internalizing traits: a community based twin study. J Abnorm Child Psychol 2012; 40: 815–827. [DOI] [PubMed] [Google Scholar]
- Hallett V, Ronald A, Rijsdijk F, Happe F. Association of autistic-like and internalizing traits during childhood: a longitudinal twin study. Am J Psychiatry 2010; 167: 809–817. [DOI] [PubMed] [Google Scholar]
- Mattila ML, Hurtig T, Haapsamo H, Jussila K, Kuusikko-Gauffin S, Kielinen M et al. Comorbid psychiatric disorders associated with Asperger syndrome/high-functioning autism: a community- and clinic-based study. J Autism Dev Disord 2010; 40: 1080–1093. [DOI] [PubMed] [Google Scholar]
- Simonoff E, Pickles A, Charman T, Chandler S, Loucas T, Baird G. Psychiatric disorders in children with autism spectrum disorders: prevalence, comorbidity, and associated factors in a population-derived sample. J Am Acad Child Adolesc Psychiatry 2008; 47: 921–929. [DOI] [PubMed] [Google Scholar]
- Kim JA, Szatmari P, Bryson SE, Streiner DL, Wilson FJ. The prevalence of anxiety and mood problems among children with autism and Asperger syndrome. Autism 2000; 4: 117–132. [Google Scholar]
- Dichter GS, Adolphs R. Reward processing in autism: a thematic series. J Neurodev Disord 2012; 4: 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaigg SB. The interplay between emotion and cognition in autism spectrum disorder: implications for developmental theory. Front Integr Neurosci 2012; 6: 113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cascio CJ, Foss-Feig JH, Heacock JL, Newsom CR, Cowan RL, Benningfield MM et al. Response of neural reward regions to food cues in autism spectrum disorders. J Neurodev Disord 2012; 4: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demurie E, Roeyers H, Baeyens D, Sonuga-Barke E. Common alterations in sensitivity to type but not amount of reward in ADHD and autism spectrum disorders. J Child Psychol Psychiatry 2011; 52: 1164–1173. [DOI] [PubMed] [Google Scholar]
- Delmonte S, Balsters JH, McGrath J, Fitzgerald J, Brennan S, Fagan AJ et al. Social and monetary reward processing in autism spectrum disorders. Mol Autism 2012; 3: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohls G, Schulte-Ruther M, Nehrkorn B, Muller K, Fink GR, Kamp-Becker I et al. Reward system dysfunction in autism spectrum disorders. Soc Cogn Affect Neurosci 2013; 8: 565–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damiano CR, Cockrell DC, Dunlap K, Hanna EK, Miller S, Bizzell J et al. Neural mechanisms of negative reinforcement in children and adolescents with autism spectrum disorders. J Neurodev Disord 2015; 7: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cascio CJ, Foss-Feig JH, Heacock J, Schauder KB, Loring WA, Rogers BP et al. Affective neural response to restricted interests in autism spectrum disorders. J Child Psychol Psychiatry 2014; 55: 162–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPartland JC, Crowley MJ, Perszyk DR, Mukerji CE, Naples AJ, Wu J et al. Preserved reward outcome processing in ASD as revealed by event-related potentials. J Neurodev Disord 2012; 4: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundstrom S, Reichenberg A, Melke J, Rastam M, Kerekes N, Lichtenstein P et al. Autism spectrum disorders and coexisting disorders in a nationwide Swedish twin study. J Child Psychol Psychiatry 2015; 56: 702–710. [DOI] [PubMed] [Google Scholar]
- Salazar F, Baird G, Chandler S, Tseng E, O'Sullivan T, Howlin P et al. Co-occurring psychiatric disorders in preschool and elementary school-aged children with autism spectrum disorder. J Autism Dev Disord 2015; 45: 2283–2294. [DOI] [PubMed] [Google Scholar]
- Leyfer OT, Folstein SE, Bacalman S, Davis NO, Dinh E, Morgan J et al. Comorbid psychiatric disorders in children with autism: interview development and rates of disorders. J Autism Dev Disord 2006; 36: 849–861. [DOI] [PubMed] [Google Scholar]
- Guyer AE, Nelson EE, Perez-Edgar K, Hardin MG, Roberson-Nay R, Monk CS et al. Striatal functional alteration in adolescents characterized by early childhood behavioral inhibition. J Neurosci 2006; 26: 6399–6405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Haim Y, Fox NA, Benson B, Guyer AE, Williams A, Nelson EE et al. Neural correlates of reward processing in adolescents with a history of inhibited temperament. Psychol Sci 2009; 20: 1009–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyer AE, Choate VR, Detloff A, Benson B, Nelson EE, Perez-Edgar K et al. Striatal functional alteration during incentive anticipation in pediatric anxiety disorders. Am J Psychiatry 2012; 169: 205–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helfinstein SM, Benson B, Perez-Edgar K, Bar-Haim Y, Detloff A, Pine DS et al. Striatal responses to negative monetary outcomes differ between temperamentally inhibited and non-inhibited adolescents. Neuropsychologia 2011; 49: 479–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjork JM, Chen G, Smith AR, Hommer DW. Incentive-elicited mesolimbic activation and externalizing symptomatology in adolescents. J Child Psychol Psychiatry 2010; 51: 827–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deveney CM, Connolly ME, Haring CT, Bones BL, Reynolds RC, Kim P et al. Neural mechanisms of frustration in chronically irritable children. Am J Psychiatry 2013; 170: 1186–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caron C, Rutter M. Comorbidity in child psychopathology: concepts, issues and research strategies. J Child Psychol Psychiatry 1991; 32: 1063–1080. [DOI] [PubMed] [Google Scholar]
- Banaschewski T, Neale BM, Rothenberger A, Roessner V. Comorbidity of tic disorders & ADHD: conceptual and methodological considerations. Eur Child Adolesc Psychiatry 2007; 16(Suppl 1): 5–14. [DOI] [PubMed] [Google Scholar]
- Neale MC, Kendler KS. Models of comorbidity for multifactorial disorders Am J Hum Genet 1995; 57: 935. [PMC free article] [PubMed] [Google Scholar]
- Angold A, Costello EJ, Erkanli A. Comorbidity. J Child Psychol Psychiatry Allied Disciplines 1999; 40: 57–87. [PubMed] [Google Scholar]
- Pine DS. Research review: a neuroscience framework for pediatric anxiety disorders. J Child Psychol Psychiatry 2007; 48: 631–648. [DOI] [PubMed] [Google Scholar]
- Stoddard J, Stringaris A, Brotman M, Montville D, Pine D, Leibenluft E. Irritability in child and adolescent anxiety disorders. Depression Anxiety 2014; 31: 566–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delmonte S, Balsters JH, McGrath J, Fitzgerald J, Brennan S, Fagan AJ et al. Social and monetary reward processing in autism spectrum disorders. Mol Autism: E 2012; 3: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohls G, Schulte-Ruther M, Nehrkorn B, Muller K, Fink GR, Kamp-Becker I et al. Reward system dysfunction in autism spectrum disorders. Soc Cogn Affect Neurosci 2013; 8: 565–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanken LM, Mous SE, Ghassabian A, Muetzel RL, Schoemaker NK, El Marroun H et al. Cortical morphology in 6- to 10-year old children with autistic traits: a population-based neuroimaging study. Am J Psychiatry 2015; 172: 479–486. [DOI] [PubMed] [Google Scholar]
- Constantino JN, Todd RD. Autistic traits in the general population: a twin study. Arch Gen Psychiatry 2003; 60: 524–530. [DOI] [PubMed] [Google Scholar]
- Schumann G, Loth E, Banaschewski T, Barbot A, Barker G, Büchel C et al. The IMAGEN study: reinforcement-related behaviour in normal brain function and psychopathology. Mol Psychiatry 2010; 15: 1128–1139. [DOI] [PubMed] [Google Scholar]
- Thirion B, Pinel P, Meriaux S, Roche A, Dehaene S, Poline JB. Analysis of a large fMRI cohort: statistical and methodological issues for group analyses. Neuroimage 2007; 35: 105–120. [DOI] [PubMed] [Google Scholar]
- Murphy K, Garavan H. An empirical investigation into the number of subjects required for an event-related fMRI study. Neuroimage 2004; 22: 879–885. [DOI] [PubMed] [Google Scholar]
- Desmond JE, Glover GH. Estimating sample size in functional MRI (fMRI) neuroimaging studies: statistical power analyses. J Neurosci Methods 2002; 118: 115–128. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Intelligence Scale for Children, 4th ed. (WISC-IV) Technical and Interpretive Manual. The Psychological Corporation: San Antonio, TX, USA, 2003. [Google Scholar]
- Goodman R, Ford T, Richards H, Gatward R, Meltzer H. The Development and Well-Being Assessment: Description and initial validation of an integrated assessment of child and adolescent psychopathology. J Child Psychol Psychiatry 2000; 41: 645–655. [PubMed] [Google Scholar]
- Lord C, Rutter M, Couteur A. Autism Diagnostic Interview-Revised: A revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord 1994; 24: 659–685. [DOI] [PubMed] [Google Scholar]
- Colvert E, Tick B, McEwen F, Stewart C, Curran SR, Woodhouse E et al. Heritability of autism spectrum disorder in a UK population-based twin sample. JAMA Psychiatry 2015; 72: 415–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen FS, Stewart CS, Colvert E, Woodhouse E, Curran S, Gillan N et al. Diagnosing autism spectrum disorder in community settings using the Development and Well-Being Assessment: validation in a UK population-based twin sample. J Child Psychol Psychiatry 2016; 57: 161–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- APADiagnostic and Statistical Manual of Mental Disorders, 5th edn. American Psychiatric Publishing: Arlington, VA, 2013. [Google Scholar]
- Kothari R, Skuse D, Wakefield J, Micali N. Gender differences in the relationship between social communication and emotion recognition. J Am Acad Child Adolesc Psychiatry 2013; 52: 1148–1157 e1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman R, Ford T, Richards H, Gatward R, Meltzer H. The Development and Well-Being Assessment: description and initial validation of an integrated assessment of child and adolescent psychopathology. J Child Psychol Psychiatry 2000; 41: 645–655. [PubMed] [Google Scholar]
- Goodman A, Heiervang E, Collishaw S, Goodman R. The 'DAWBA bands' as an ordered-categorical measure of child mental health: description and validation in British and Norwegian samples. Soc Psychiatry Psychiatr Epidemiol 2011; 46: 521–532. [DOI] [PubMed] [Google Scholar]
- Goodman R. Psychometric properties of the strengths and difficulties questionnaire. J Am Acad Child Adolesc Psychiatry 2001; 40: 1337. [DOI] [PubMed] [Google Scholar]
- Knutson B, Adams CM, Fong GW, Hommer D. Anticipation of increasing monetary reward selectively recruits nucleus accumbens. J Neurosci 2001; 21: 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stringaris A, Vidal-Ribas Belil P, Artiges E, Lemaitre H, Gollier-Briant F, Wolke S et al. The brain's response to reward anticipation and depression in adolescence: dimensionality, specificity and longitudinal predictions in a community-based sample. Am J Psychiatry 2015; 172: 1215–1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiss AL, Abrams MT, Singer HS, Ross JL, Denckla MB. Brain development, gender and IQ in children. A volumetric imaging study. Brain 1996; 119(Pt 5): 1763–1774. [DOI] [PubMed] [Google Scholar]
- Graham S, Jiang J, Manning V, Nejad AB, Zhisheng K, Salleh SR et al. IQ-related fMRI differences during cognitive set shifting. Cerebral Cortex 2010; 20: 641–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- StataCorpStata Statistical Software: Release 11. StataCorp LP: College Station, TX, USA, 2009. [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage 2003; 19: 1233–1239. [DOI] [PubMed] [Google Scholar]
- Richards JM, Plate RC, Ernst M. A systematic review of fMRI reward paradigms used in studies of adolescents vs. adults: the impact of task design and implications for understanding neurodevelopment. Neurosci Biobehav Rev 2013; 37: 976–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallis JD, Miller EK. Neuronal activity in primate dorsolateral and orbital prefrontal cortex during performance of a reward preference task. Eur J Neurosci 2003; 18: 2069–2081. [DOI] [PubMed] [Google Scholar]
- Ridderinkhof KR, van den Wildenberg WP, Segalowitz SJ, Carter CS. Neurocognitive mechanisms of cognitive control: the role of prefrontal cortex in action selection, response inhibition, performance monitoring, and reward-based learning. Brain Cogn 2004; 56: 129–140. [DOI] [PubMed] [Google Scholar]
- Knutson B, Fong GW, Adams CM, Varner JL, Hommer D. Dissociation of reward anticipation and outcome with event-related fMRI. Neuroreport 2001; 12: 3683–3687. [DOI] [PubMed] [Google Scholar]
- Knutson B, Fong GW, Bennett SM, Adams CM, Hommer D. A region of mesial prefrontal cortex tracks monetarily rewarding outcomes: characterization with rapid event-related fMRI. Neuroimage 2003; 18: 263–272. [DOI] [PubMed] [Google Scholar]
- Tom SM, Fox CR, Trepel C, Poldrack RA. The neural basis of loss aversion in decision-making under risk. Science 2007; 315: 515–518. [DOI] [PubMed] [Google Scholar]
- Haber SN, Knutson B. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology 2010; 35: 4–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridderinkhof KR, van den Wildenberg WP, Segalowitz SJ, Carter CS. Neurocognitive mechanisms of cognitive control: the role of prefrontal cortex in action selection, response inhibition, performance monitoring, and reward-based learning. Brain Cogn 2004; 56: 129–140. [DOI] [PubMed] [Google Scholar]
- Stringaris A. Emotion, emotion regulation and disorder: conceptual issues for clinicians and neuroscientists. In: Thapar A, Pine D, Leckman J, Scott S, Snowling M, Taylor E (eds). Rutter's Child and Adolescent Psychiatry, 6th edn. Wiley-Blackwell: Chichester, UK, 2015.
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci 2001; 24: 167–202. [DOI] [PubMed] [Google Scholar]
- Burle B, Vidal F, Tandonnet C, Hasbroucq T. Physiological evidence for response inhibition in choice reaction time tasks. Brain Cogn 2004; 56: 153–164. [DOI] [PubMed] [Google Scholar]
- Jazbec S, McClure E, Hardin M, Pine DS, Ernst M. Cognitive control under contingencies in anxious and depressed adolescents: an antisaccade task. Biol Psychiatry 2005; 58: 632–639. [DOI] [PubMed] [Google Scholar]
- Guyer AE, Choate VR, Pine DS, Nelson EE. Neural circuitry underlying affective response to peer feedback in adolescence. Soc Cogn Affect Neurosci 2012; 7: 81–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes EE, Christopher May J, Siegle GJ, Ladouceur CD, Ryan ND, Carter CS et al. Reward-related decision-making in pediatric major depressive disorder: an fMRI study. J Child Psychol Psychiatry 2006; 47: 1031–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst M, Fudge JL. A developmental neurobiological model of motivated behavior: anatomy, connectivity and ontogeny of the triadic nodes. Neurosci Biobehav Rev 2009; 33: 367–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loewenstein GF, Weber EU, Hsee CK, Welch N. Risk as feelings. Psychol Bull 2001; 127: 267–286. [DOI] [PubMed] [Google Scholar]
- Chua P, Krams M, Toni I, Passingham R, Dolan R. A functional anatomy of anticipatory anxiety. Neuroimage 1999; 9(6 Pt 1): 563–571. [DOI] [PubMed] [Google Scholar]
- Ploghaus A, Tracey I, Gati JS, Clare S, Menon RS, Matthews PM et al. Dissociating pain from its anticipation in the human brain. Science 1999; 284: 1979–1981. [DOI] [PubMed] [Google Scholar]
- Wittmann A, Schlagenhauf F, Guhn A, Lueken U, Gaehlsdorf C, Stoy M et al. Anticipating agoraphobic situations: the neural correlates of panic disorder with agoraphobia. Psychol Med 2014; 44: 2385–2396. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Stein MB. An insular view of anxiety. Biol Psychiatry 2006; 60: 383–387. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Wiens S, Rotshtein P, Ohman A, Dolan RJ. Neural systems supporting interoceptive awareness. Nat Neurosci 2004; 7: 189–195. [DOI] [PubMed] [Google Scholar]
- Seth AK, Suzuki K, Critchley HD. An interoceptive predictive coding model of conscious presence. Front Psychol 2011; 2: 395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett LF, Simmons WK. Interoceptive predictions in the brain. Nat Rev Neurosci 2015; 16: 419–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ondobaka S, Kilner J, Friston K. The role of interoceptive inference in theory of mind. Brain Cogn (in press). [DOI] [PMC free article] [PubMed]
- Simonoff E, Taylor E, Baird G, Bernard S, Chadwick O, Liang H et al. Randomized controlled double-blind trial of optimal dose methylphenidate in children and adolescents with severe attention deficit hyperactivity disorder and intellectual disability. J Child Psychol Psychiatry 2013; 54: 527–535. [DOI] [PubMed] [Google Scholar]
- Pliszka SR. Effect of anxiety on cognition, behavior, and stimulant response in ADHD. J Am Acad Child Adolesc Psychiatry 1989; 28: 882–887. [DOI] [PubMed] [Google Scholar]
- Taylor E, Schachar R, Thorley G, Wieselberg HM, Everitt B, Rutter M. Which boys respond to stimulant medication? A controlled trial of methylphenidate in boys with disruptive behaviour. Psychol Med 1987; 17: 121–143. [DOI] [PubMed] [Google Scholar]
- Moshe K, Karni A, Tirosh E. Anxiety and methylphenidate in attention deficit hyperactivity disorder: a double-blind placebo-drug trial. Attention Deficit Hyperactivity Disord 2012; 4: 153–158. [DOI] [PubMed] [Google Scholar]
- Hartley SL, Sikora DM. Which DSM-IV-TR criteria best differentiate high-functioning autism spectrum disorder from ADHD and anxiety disorders in older children? Autism 2009; 13: 485–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonuga-Barke EJ, Cortese S, Fairchild G, Stringaris A. Annual Research Review: Transdiagnostic neuroscience of child and adolescent mental disorders - differentiating decision making in attention-deficit/hyperactivity disorder, conduct disorder, depression, and anxiety. J Child Psychol Psychiatry 2015; 57: 321–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Happé F, Booth R, Charlton R, Hughes C. Executive function deficits in autism spectrum disorders and attention-deficit/hyperactivity disorder: examining profiles across domains and ages. Brain Cogn 2006; 61: 25–39. [DOI] [PubMed] [Google Scholar]
- Kessel EM, Kujawa A, Hajcak Proudfit G, Klein DN. Neural reactivity to monetary rewards and losses differentiates social from generalized anxiety in children. J Child Psychol Psychiatry 2015; 56: 792–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes EE, Ryan ND, Phillips ML, Manuck SB, Worthman CM, Moyles DL et al. Healthy Adolescents' Neural Response to Reward: associations with puberty, positive affect, and depressive symptoms. J Am Acad Child Adolesc Psychiatry 2010; 49: 162–172 e165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotlib IH, Hamilton J, Cooney RE, Singh MK, Henry ML, Joormann J. Neural processing of reward and loss in girls at risk for major depression. Arch Gen Psychiatry 2010; 67: 380–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.