Abstract
A diet rich in vegetables has been associated with a reduced risk of many diseases related to aging and modern lifestyle. Over the past several decades, many researches have pointed out the direct relation between the intake of bioactive compounds present in tomato and a reduced risk of suffering different types of cancer. These bioactive constituents comprise phytochemicals such as carotenoids and polyphenols. The direct intake of these chemoprotective molecules seems to show higher efficiencies when they are ingested in its natural biological matrix than when they are ingested isolated or in dietary supplements. Consequently, there is a growing trend for improvement of the contents of these bioactive compounds in foods. The control of growing environment and processing conditions can ensure the maximum potential accumulation or moderate the loss of bioactive compounds, but the best results are obtained developing new varieties via plant breeding. The modification of single steps of metabolic pathways or their regulation via conventional breeding or genetic engineering has offered excellent results in crops such as tomato. In this review, we analyse the potential of tomato as source of the bioactive constituents with cancer-preventive properties and the result of modern breeding programs as a strategy to increase the levels of these compounds in the diet.
Keywords: breeding, functional food, lycopene, β-carotene, flavonoid, anthocyanin
1. Introduction
Traditionally, the concept of quality in vegetables has been related with their external appearance. The main requirements of food industries and markets were focused on the demand of new varieties with higher uniformity, brighter colours and enhanced shelf life as essential characteristics. Following these market requirements, traditional breeding programs focused their attention in these external features together with higher yields and disease resistance, neglecting other important aspects such as flavour or functional characteristics. Over the past decades, flavour was reconsidered in breeding programs due to the continuous complaints of consumers about the loss of traditional organoleptic characteristics of vegetables [1]. More recently, following this lead, health benefits of food have also started to be valued [2]. Nowadays, consumers are aware of the functional characteristics of agricultural food products, and more consumers choose foods considering their healthy characteristics. Although it is not clear whether marketing or for health functionality is spurring this interest, or vice versa, there is an increasing attention in the development of new phytochemical-rich vegetable varieties via breeding programs [3].
It is a well-known fact that fruits and vegetables are an important source for the daily intake of healthy constituents to the diet like minerals (calcium, phosphorous, magnesium and other minor minerals), water-soluble vitamins (B and C), fat-soluble vitamins (A, E and K) and a wide variety of phytochemicals [4]. Among phytochemicals, we can find bioactive molecules capable to protect against diseases acting as free radical scavengers or antimicrobial agents. The most renowned phytochemicals from vegetables are polyphenols, carotenoids, organo-sulfur and seleno-compounds. Their presence in certain plants would, in part, justify the epidemiologic evidence of a protective role of diets rich in fruits, vegetables, legumes and whole grains [5].
The traditional Mediterranean diet would be an example of how a diet characterized by a high consumption of foods of plant origin, relatively low consumption of red meat and high consumption of olive oil results in reduced occurrence of cancer [6], coronary heart disease and other diet-related chronic diseases [7]. Among the components of the Mediterranean diet, fruits and vegetables outstand in their preventive role. In Western countries, such as in the United States, a goal of at least 9 to 13 servings a day of fruits and vegetables is promoted, though the real consumption does not reach half that Figure [8]. This goal has proved to be quite difficult to reach, as studies developed in the late 90s and early 2000s pointed out [9]. Increasing awareness of the need for consumption of these foods and a complete educational model starting at nursery schools would be required.
Although the bioactive constituents of fruits and vegetables may be delivered via dietary supplements, scientific evidences seem to point out that their direct intake in their natural matrix has more health benefits than if those chemoprotective molecules are ingested isolated or in dietary supplements [10]. In that line, some authors pointed out that whole tomato consumption is more effective than lycopene isolated for the prevention and the development of progression of prostate cancer in rats [11]. According to this, more researches suggested that the synergetic effects of these molecules with other phytochemicals presents in vegetables may enhance their health benefits [12,13]. Thus, a diet rich in vegetables is the best way to obtain a wide variety of phytochemicals [8]. It should be considered, though that in the case of liposoluble compounds the diet should be complemented with oils. It would be the case of carotenoids. Most fruits and vegetables rich in carotenes are low in lipids. Therefore, in order to promote their absorption, it would be necessary to promote the joint intake of lipids. For example, the addition of avocado to salads has proved to improve the absorption of tomato carotenoids [14] or in the case of cooked tomato, the use of olive oil in the cooking process would also greatly increase the absorption of lycopene [15].
We should be aware of the difficulty of clearly establishing the protective role of a certain compound in a complex diet over the development of chronic diseases, which are affected by many other risk factors. In this context, some studies have questioned the relation between the ingestion of vitamin C or carotenoids and a reduction in the risk of suffering cancer [16,17] while other studies continue to establish this relation [18,19]. Apart from the difficulty of establishing the role of a single component of a complex diet certain considerations such as the measurement errors in the dietary intake of fruit and vegetables may also attenuate the relationships observed in epidemiological studies [20].
In this complex context, there is an increasing interest in the development of new vegetable varieties with increased levels of bioactive components [3], in order to promote their protective role in well balanced diets such as the Mediterranean. Although, it is necessary to continue on research of the effects of phytochemicals on health to understand the mechanism of action and the associations of these molecules in cancer-risk prevention, the development of long-term breeding programs should be started to promote the benefits of vegetable consumption.
The high level of consumption and the high economic value of tomato production has spurred the scientific and breeding efforts in this species. Although tomato does not outstand for its high concentration in bioactive compounds, the high levels of tomato intake both fresh and processed, position tomato as one of the main sources of chemoprotective compounds to diet [21], including vitamin C. Although tomato cultivars with increased vitamin C contents have been developed, vitamin C content is rather unstable, as it is highly dependent on the environment (especially via genotype x environment interactions) and it is quickly used as antioxidant under stress conditions. Thus, its management in breeding programs can be quite complicated [22]. Apart from vitamin C, the main bioactive compounds present in tomato (Solanum lycopersicum L.) are carotenoids and polyphenols. Here we review the chemoprotective characteristics of these last tomato bioactive compounds, their biosynthesis and the achievements in breeding programs targeted to increase their contents.
2. Accumulation of Bioactive Compounds in Tomato
In tomato, carotenoids are synthesized in the leaves, flowers and fruits. In the leaf tissues, carotenoids act as photoprotectors [23], being lutein the main carotenoid present meanwhile the presence of the xanthopylls violaxanthin and neoxanthin confer the characteristic yellow colouration to flowers [24]. In ripe tomato fruits, lycopene is the main carotenoid that can be found and it causes its red colouration (Table 1).
Table 1.
Typical composition (mg 100 g−1 fresh weight) in tomato ripe fruits of carotenoids and polyphenols, (adapted from [24,25,26,27]).
| Carotenoid | Concentration | Polyphenol | Concentration |
|---|---|---|---|
| Lycopene | 7.8–18.1 | Naringenin chalcone | 0.9–18.2 |
| Phytoene | 1.0–2.9 | Rutin | 0.5–4.5 |
| Phytofluene | 0.2–1.6 | Quercetin | 0.7–4.4 |
| β-Carotene | 0.1–1.2 | Chlorogenic acid | 1.4–3.3 |
| γ-Carotene | 0.05–0.3 | Caffeic Acid | 0.1–1.3 |
| δ-Carotene | 0–0.2 | Naringenin | 0–1.3 |
| Lutein | 0.09 | Kaempferol-3-rutinoside | 0–0.8 |
| Neurosporene | 0–0.03 | p-Coumaric acid | 0–0.6 |
| α-Carotene | 0–0.002 | Ferulic acid | 0.2–0.5 |
| Neoxanthin | - | Kaempferol | 0–0.2 |
| Violaxanthin | - | Myricetin | - |
| Anteraxanthin | - | Cyanidin | - |
| Zeaxanthin | - | Pelargonidin | - |
| Delphinidin | - |
The contents of carotenoids, as well as other chemoprotective substances are highly conditioned by the genotype and environmental conditions (reviewed by Tiwari and Cummings [28]). Considering this variability, lycopene concentrations from standard tomato cultivars range from 7.8 to 18.1 mg 100 g−1 fresh weight (fw) (Table 1). Other colourless intermediates from the carotenoid biosynthetic pathway may be found in tomatoes. This is the case of phytoene and phytofluene with concentrations around 2.9 and 1.6 mg 100 g−1 fw, respectively. The second main coloured carotenoid present in tomato is β-carotene, responsible for orangey colours. Its concentration is much lower, up to 1.2 mg 100 g−1 fw (Table 1). Apart from these major carotenoids, lesser amounts of γ-carotene, δ-carotene, lutein, neurosporene, α-carotene and other carotenoids can also be found in tomatoes [24,29] (Table 1).
Carotenoid distribution in the fruit is not regular. Lycopene can be found at higher concentration in the pericarp if compared with the locules, meanwhile β-carotene concentration is higher in the locules compared with the pericarp [25]. Moreover, lycopene concentration varies during the ripening process. Initially it starts to be present in the locules at the breaker stage, and then its concentration rises during the ripening process [30].
Polyphenols are present in tomato at lower concentrations (Table 1). These powerful antioxidants can be divided into different groups according to their core structure. Main tomato polyphenols are hydroxycinnamic acids, flavanones, flavonols, and anthocyanins. In addition, flavonol glycosides like rutin and kaempferol-3-rutinoside are also present in tomato fruits.
Naringenin chalcone is the main polyphenol found in tomato with concentrations up to 18.2 mg 100 g−1 fw [27]. The flavanone naringenin is present at lower concentrations, up to 1.3 mg 100 g−1 fw [26]. Quercetin is the main flavonol and one of the most important flavonoids from tomato. Its content varies from 0.7 to 4.4 mg 100 g−1 fw [26] in different tomato types. It can also be found in its glycosylated form as rutin, with concentrations up to 4.5 mg 100 g−1 fw [27]. The accumulation of rutin gives to the tomato peel its typical yellow colour. Chlorogenic acid is the main polyphenol from hydroxycinnamic acid family; its concentration ranges between 1.4 and 3.3 mg 100 g−1 fw [27]. Other flavonols such as kaempferol and myricetin are found in small quantities or traces in cultivated tomato, though they are present in related wild species [26,31,32].
The accumulation of flavonoids in tomato is tissue specific and develops at specific stages. For example, naringenin chalcone accumulates almost specifically in the peel simultaneously with the accumulation of carotenoids and degradation of chlorophylls, peaking up in overripe peels [33]. In fact, the flavonoid pathway is not active in the flesh due to the lack of expression of the genes involved in the flavonoid pathway [34]. Flavonol accumulation (mainly quercetin and kaempferol glucosides) is also almost (98%) restricted to the peel [35].
Usually the production of anthocyanins in tomato is restricted to vegetative tissues, such as the hypocotyl. Under certain conditions, including high irradiance and low temperature, they can accumulate in the stem and leaves. The main anthocyanins in tomato are derived from delphinidin, malvidin and petunidin [36,37].
Apart from an evident genotype and environmental effect on the accumulation of these bioactive compounds, in processed food their contents will also depend on the processing method, which may help to liberate compounds or may degrade them (reviewed by Nicoli et al. [38]). The nutritional value of tomatoes may be increased after thermal processing, as described by Dewanto et al. [39]. Their results pointed out that lycopene concentration is increased as it may be released from its natural matrix during the heat process. Dewanto et al. [39] also evaluated phenolic and total flavonoid content of heat-processed tomatoes, revealing non-significant changes in their content after the thermal process. Other authors have found a reduction of polyphenol content as a consequence of cooking. In this sense, Crozier et al. [40] showed an 82% of loss of quercetin content if tomatoes are boiled, a 65% loss if they are microwaved and 35% if they are fried. On the other hand, carotenoid concentration remains constant if tomatoes are boiled or even it can be more concentrated in tomato paste [41].
The results on the retention of polyphenols in tomato processed products are variable. Muir et al. [33] found that 65% of flavonols present in high-flavonol tomatoes were retained in processed tomato paste, while Stewart et al. [35] found that tomato flavonols like quercetin, can resist common processing methods and therefore they can be found in tomato-derived products such as tomato juice or tomato puree which are particularly rich these compounds. Obviating the relative differences in these studies, it seems clear that health benefits of processed tomato products may be similar or even higher than raw tomatoes, and make tomato-derived products a perfect alternative choice as functional food.
3. Chemoprotective Characteristics of Tomato Bioactive Compounds
3.1. Carotenoids
During the last several decades, many studies highlight the beneficial effects of the intake of tomato and tomato-derived products with a lower cancer risk (reviewed by Giovannucci [42]) and mainly with lower prostate cancer risk [43,44,45,46].
Considering the intake of tomato products, results from a 6-year follow-up cohort study by Mills et al. [47] pointed out a lesser prostate cancer risk associated to tomato consumption among other vegetables. In agreement to that, a meta-analysis carried out by Etminan et al. [45] highlighted a lower prostate cancer relative risk (RR) related to a high consumption of raw tomatoes (RR = 0.89, 95% CI) and specially cooked-tomato products (RR = 0.81, 95% CI). Giovannucci et al. [44] also reported a lower relative risk (RR = 0.77, 95% CI) from developing prostate cancer associated with the consumption of two servings of tomato sauce per week. In this study, a continuation of the Health Professionals Follow-Up Study (HPFS), the authors concluded that considering the moderate association found, it could be missed in a small studies or in those with substantial errors in measurement or based on a single dietary assessment. In a continuation of this study, Wu et al. [48] found a significant inverse association between higher plasma lycopene levels and lower risk of prostate cancer, though it was restricted to older men (age > 65) without a family history of prostate cancer (highest vs. lowest quintile Odds Ratio, OR = 0.43; 95% CI). At that point, few studies considered lycopene levels in the plasma. With a smaller sample, Lu et al. [49] found significant inverse associations between prostate cancer and lycopene plasma concentration (OR = 0.17, 95% CI). In this case, mean plasma lycopene level for the upper quartile was 401 mmol L−1. On the other hand, Kristal et al. [50] in the Prostate Cancer Prevention Trial (1683 cases and 1751 controls) analysed plasma lycopene levels (≥0.47 mg L−1 in the highest quartile) and found that prediagnostic serum lycopene concentration was not associated with the risk of total, low-, or high-grade prostate cancer incidence.
With the same cohort of the HPFS, in 2014 Zu et al. [51] pooled the results of 49,898 male health professionals from 1986 to 2010 and found a positive relationship between dietary lycopene intake and plasma lycopene levels, with mean plasma lycopene levels of 934.5 mol L−1 in the highest quintile of lycopene intake (n = 1200), and concluded that higher lycopene intake was inversely associated with total prostate cancer and more strongly with lethal prostate cancer. In fact, higher lycopene intake was associated with biomarkers in the cancer indicative of less angiogenic potential, thus probably reducing aggressive potential of cancer. The authors suggested that lycopene may inhibit angiogenesis of prostate cancer cells by regulating vascular endothelial growth factor, taking in consideration the results obtained by Yang et al. [52] in mice.
On the other hand, Kirsh et al. [53] in the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial including 29,361 men during 4 years found no reason to support the hypothesis that higher tomato product consumption protects from prostate cancer. Indeed, the relation of lycopene intake and cancer has been controversial. As an example, in 2007 the US Food and Drug Administration concluded that there was limited evidence supporting an association between tomato consumption and reduced risk of prostate cancer [16]. But almost simultaneously, the World Cancer Research Fund and the American Institute for Cancer Research [54] concluded that foods containing lycopene may have a positive effect against prostate cancer. Interestingly, Zu et al. [51] suggested that lycopene intake would primarily affect prostate cancer progression.
The role of lycopene supplements in the evolution of cancer has also been analysed in clinical trials. In 2001 Kuckuk et al. [55] evaluated the impact of oral intake of lycopene (15 mg twice daily, during 3 weeks) in the evolution of patients with prostate cancer. In the intervention group, plasma prostate-specific antigen (PSA) levels decreased by 18% while in the control group they increased by 14%, and considering all biomarkers the authors concluded that lycopene supplementation may decrease the growth of prostate cancer, though the sample was too small to be conclusive.
Ansari and Gupta [56] found a 96% reduction of PSA levels after a 6-month supplementation of 2 mg lycopene twice daily in orchidectomy patients in comparison with patients only with surgical treatment (up to 90%). This study highlighted that the reduction turned to be more marked after two years of treatment (99% and 96% respectively). In other study with located prostate cancer patients, the serum PSA levels as well as leukocyte oxidative and prostate oxidative DNA damages were reduced after lycopene supplementation (30 mg lycopene per day during 3 weeks) before their radical prostatectomy [57]. Vaishampayan et al. [58] found that lycopene supplementation alone (30 mg day−1 in two doses) or combined with soy isoflavones (40 mg day-1 in two doses) had activity in prostate cancer patients with PSA relapse disease and may delay progression of both hormone-refractory and hormone-sensitive prostate cancer. Although the number of patients was limited (37 and 33 in each group). Other works, also highlighted the effects of lycopene delaying a high-grade prostate intraepithelial neoplastia (HGPIN) from developing into occult prostate cancer [59].
On the contrary, other authors have found no effect of lycopene supplementation and prostate cancer prevention or evolution. Regarding cancer evolution, Jatoi et al. [60] found no relationship between lycopene supplementation (30 mg day−1 during 16 months) and the progression of prostate cancer in 46 men with androgen-independent prostate cancer. Clark et al. [61] tested wider dose ranges of lycopene supplementation (15–120 mg day−1) in 36 patients with biochemical relapse of prostate cancer, and found no stabilization of PSA levels as a consequence of the treatment. They also found that plasma lycopene levels were similar for the 15–90 mg day−1 doses when the concentration reached a plateau after three months of supplementation. Bunker et al. [62] studied the evolution of 81 men with high-grade prostatic intraepithelial neoplasia, atypical foci or repeated non-cancerous biopsies and found no effect of lycopene supplementation (30 mg day−1) after the first month of treatment. But the same authors suggested that lowering of serum PSA may not be an appropriate endpoint for the long-term studies on the effect of lycopene supplementation for reducing prostate cancer initiation or progression.
Prostate cancer preventing properties of other tomato carotenoids have also been questioned in other works. In that sense, Neuhouser et al. [63] revealed in the Beta-Carotene and Retinol Efficacy Trial (CARET) that the supplementation with β-carotene (30 mg day−1) and retinol (25,000 IU retinyl palmitate day−1) had no significant effect on total prostate cancer incidence. In fact, it could be negative, as they found that supplemented individuals had higher relative risk (RR = 1.52, 95% CI) of developing an aggressive prostate cancer (Gleason score 7–10) that was reduced in the post-intervention period (RR = 0.75, 95% CI). CARET only included smokers, thus this results may not apply to non-smokers.
Apart from prostate cancer, the incidence of other cancers has been also associated with tomato carotenoids. In 2007, the World Cancer Research Fund and the American Institute for Cancer Research [54] concluded that apart from the positive effects of lycopene containing foods on prostate cancer, foods containing carotenoids probably protected against cancers of the mouth, pharynx, and larynx, and also lung cancer, and specifically, those containing β-carotene would probably protect against oesophageal cancer. In this sense, the meta-analysis performed by Ge et al. [64] confirmed that higher intake of β-carotene, α-carotene, lycopene, β-cryptoxanthin, lutein, and zeaxanthin reduced oesophageal cancer risk with pooled ORs of 0.58, 0.81, 0.75, 0.80, and 0.71 (95% CI), respectively.
Regarding lung cancer, in 1996 Ziegler et al. [65] concluded that β-carotene may have a role in the prevention of lung cancer, but the dosage should be carefully studied, as lung cancer incidence and mortality were increased in studies with β-carotene supplementation with male smokers. In fact, results from Alpha-Tocopherol Beta-Carotene (ATBC) study revealed higher lung cancer incidence in participants who received β-carotene supplementation, 20 mg day−1 during 8 years [66], especially among smokers [67]. Similarly, the CARET study highlighted that β-carotene supplementation had an adverse effect on lung cancer incidence and lung cancer mortality in smokers and workers exposed to asbestos [68]. It has been proposed that this procarcinogenic effect may be explained by the activity of β-carotene-oxidized products produced as a consequence of the free radical-rich environment in the lungs of cigarette smokers [69]. Wright et al. [70] suggested that the increased risk of lung cancer would be related to aberrant cell growth, while Mondul et al. [71] found that the metabolomic profiles of response to β-carotene supplementation pointed to the induction of cytochrome p450 enzymes CYP1A2 and CYP2E1. As several medications used for cardiovascular diseases are metabolized by CYP1A2, an interaction between the supplemented β-carotene and prescribed medication may have resulted in the increased mortality found in the ATBC study, though a dysregulated glycemic control could also have contributed. These findings stressed the importance to increase our knowledge on the possible interactions between dietary supplements and other pharmacological agents. Nevertheless, the fact that supplementation at high doses may have adverse effects, does not exclude a positive effect at dietary levels [72]. Other works have found no effect of β-carotene supplementation and lung cancer incidence [73].
Controversy also applies in the case of breast cancer. Eliassen et al. [74] carried out a pooled analysis of eight cohort studies where serum carotenoids and breast cancer risk were evaluated. This study pointed out a reduced relative risk of suffering breast cancer among women with higher plasma carotenoids. Among tomato carotenoids, the authors found a lower relative risk for lycopene (RR = 0.78, 95% CI) followed by β-carotene (RR = 0.83, 95% CI). Pantavos et al. [75] analysing dietary intake of antioxidants in 3209 women found that low intake of α-carotene and β-carotene was associated with a higher risk of breast cancer among smokers (Hazard Ratio, HR = 2.48, 95% CI and HR = 2.31, 95% CI for α- and β-carotene, respectively). On the other hand, Greenlee et al. [76] studying the Life After Cancer Epidemiology (LACE) cohort (2264 women diagnosed of breast cancer) concluded that frequent use of combination carotenoids was associated with increased risk of death from breast cancer (HR = 2.07, 95% CI) and all-cause mortality (HR = 1.75, 95% CI).
Results from the Linxian Cancer Prevention Study highlighted a significant reduction in cancer mortality (RR = 0.87, 95% CI), especially from stomach cancer (RR = 0.79, 95% CI) after β-carotene, vitamin E and selenium supplementation [77]. But a recent meta-analysis [78] concluded that although data from case-control studies suggested a protective role of α- and β-carotene against gastric cancer, there were inconsistencies compared with cohort studies, and no conclusive evidence was found for this suggestion.
The mechanism of action of carotenoids in cancer prevention was extensively reviewed by Tanaka et al. [72] and Trejo-Solís et al. [79]. Antioxidant capacity of these compounds may justify part of this protection. Carotenoids exert strong antioxidant capacity because they contain many double-conjugated bonds. In that sense, lycopene can act as antioxidant because its ability to trap the 1O2 twice as effective as that of β-carotene. Moreover, another antioxidant potential of lycopene is to react with free radicals. The elimination of these Reactive Oxygen Species (ROS) is important in the cancer chemoprevention since they can facilitate the carcinogenesis-related processes by means of the oxidation of cellular biomolecules. In addition, lycopene may exert its effect though the regulation of the antioxidant response element (reviewed by Trejo-Solís et al. [79]).
But apart from their antioxidant capacity, other mechanisms contribute to cancer prevention, including immune modulation, hormone and growth factor signaling, regulatory mechanisms of cell cycle progression, cell differentiation and apoptosis. In fact, the existence of this variety of mechanisms has led to propose that the initial effect of carotenoids should involve modulation of transcription (reviewed by Tanaka et al. [72]).
3.2. Polyphenols
Plant polyphenols have been reported to interfere with the initiation, promotion and progression of cancer [80]. In fact, polyphenols exert numerous effects on tumorigenic cell transformation, on tumour cells in vitro and in vivo, and may interact with conventional anti-tumour therapies [81]. There exist different polyphenol families and they differ in their chemoprotective capabilities. For example, among 68 polyphenols, the order of their potency to suppress in vitro the human liver cancer cells resulted to be chalcones > flavones > chromones > isoflavones > flavanones > coumarins [82].
The effect of the different classes of polyphenols on cancer risk incidence has been evaluated in several case-control studies [83,84,85,86]. In that line, Bosetti et al. [85] evaluated the intake of different families of polyphenols and their effect on women breast cancer incidence in a large case-control study carried out in Italy during 4 years. A reduced risk of suffering breast cancer was related with a high dietary intake of flavonols (>29.9 mg day−1) (highest vs. lowest quintile OR = 0.80, 95% CI) and flavones (>0.6 mg day−1) (OR = 0.81, 95% CI), but no significant associations were found for other tomato polyphenols such as flavanones (>62.2 mg day−1) (OR = 0.95, 95% CI) and anthocyanidins (>20.5 mg day−1) (OR = 1.09, 95% CI). Specifically, a diet rich in quercetin (4.7 mg day−1) have also been associated with a lower breast cancer risk in women (RR = 0.62, 95% CI) [87]. On the other hand, another case-control study carried out in Greece evaluating fruit and vegetable consumption found no associations between dietary intake of flavonols, flavanones, flavan-3-ols, isoflavones or anthocyandins and breast cancer risk; however, a statistically lower risk (OR = 0.87, 95% CI) were found for flavone intake (0.5 mg day−1) [84].
Phenolic compounds also seem to protect against colorectal cancer. In this case, a large case-control study carried out by Rossi et al. [86] in Italy showed that diets with a higher intake of flavonols (>28.5 mg day−1) (OR = 0.64, 95% CI) and anthocyanidins (>31.7 mg day−1) (OR = 0.67, 95% CI) among other phenolic compounds were associated with a lower risk of suffering colorectal cancer. However, the same study found no associations between total flavonoids and flavanones and colorectal cancer. Other studies went further and tested the dose-response activity of quercetin. In that line, Cruz-Correa et al. [88] found that a joint supplementation of 20 mg quercetin together with 480 mg curcumin thrice per day prior colectomy were capable to decrease the number and size (60.4% and 50.9% from baseline respectively) of ileal and rectal adenomas in patients with familial adenomatous polyposis (FAP).
Flavonols also would exert a protective effect against stomach cancer. García-Closas et al. [83] evaluating food habits in Spanish participants found a lower risk of suffering stomach cancer in participants with higher dietary intakes of quercetin (>8.5 mg day−1) (OR = 0.62, 95% CI). Other flavonols like kaempferol and myricetin were also evaluated in this work, but the authors found that only kaempferol (dietary intakes higher than 1.3 mg day−1) also had protective effects (OR = 0.48, 95% CI). Some studies though have discarded a significant protective affect against these types of cancer [87].
The chemopreventive effects of polyphenols against lung cancer have also been proven (reviewed by Neuhouser [89]). Among them, the results from the Finnish Mobile Clinic Health Examination Survey pointed that these chemoprotective effects of dietary flavonoid intake (3.51 mg day−1) (RR = 0.54, 95% CI) may be mainly attributed to quercetin [90]. Knekt et al. [87] evaluated the dietary intakes of participants in basis of flavonoid concentration in the same study, reporting a lower incidence of total cancer associated with higher intakes of quercetin (RR = 0.77, 95% CI), especially for lung cancer in men (3.9 mg day−1) (RR = 0.42, 95% CI). The cohort study carried out by Hirvonen et al. [91] also revealed an inverse relationship between the joint dietary intake of flavones and flavonols (16.3 mg day−1) and lung cancer risk (RR = 0.56, 95% CI) in male smokers from the ATBC study.
Regarding liver cancer, Lagiou et al. [92] also found a chemoprotective effect of dietary flavone intake (>1.16 mg day−1) and hepatocellular carcinoma (OR = 0.41, 95% CI) for patients without hepatitis. An inverse association was also found between dietary consumption of flavan-3-ol (>66.3 mg day−1), anthocyanidins (>152.7 mg day−1) and total flavonoids (>358.1 mg day−1) and cholangiocarcinoma (though only few cases were available for the study).
Anti-cancer properties of other tomato polyphenols such as chlorogenic and caffeic acids have been less studied in patients; however there are many studies where they have been tested using cellular models (reviewed by Weng and Yen [93]). In that line, Yang et al. [94] found that chlorogenic acid may induce apoptosis in vitro in human leukemia U937 cells by the reduction of the levels of mitochondrial membrane potential and increasing the activation of caspase-3-pathways. The effect of caffeic acid on fibrosarcoma HT-1080 cell line was evaluated by Rajendra-Prasad et al. [95]. Their results pointed out that caffeic acid treatments can lower the cell viability of fibrosarcoma cells. Other tomato phenolic compounds like naringenin present anti-cancer properties in vitro. In that line, some works pointed out the anti-proliferative effects of naringenin in different cancer cell lines like HT29 colon cancer cells [96] or MCF-7 breast cancer cells [97]. Moreover, combinations of different polyphenols have been also evaluated revealing that naringenin and quercetin are effective inhibitors of the MDA-MB-435 human breast carcinoma cell line [98].
The ability of phenolic compounds as reactive oxygen scavengers is the main motivator of its antioxidant properties. Among the main polyphenols present in vegetables, the action of quercetin in in vivo studies seems to be related with the inhibition and induction of survival and death signalling pathways respectively in liver cancer cells and its strong antioxidant activity and consequent prevention of ROS-induced DNA mutations in critical genes for cell cycle control [99]. In addition to ROS quenching, the antioxidant protective effect of flavonoids may be related to their modulating activity of several detoxifying enzymes like lipoxygenase, cyclooxygenase, inducible nitric oxide synthase, monooxygenase, xanthine oxidase and NADH oxidase (reviewed by Gibellini et al. [100]). Moreover, quercetin also can act in the chromatin remodelling and thus interfering with epigenetic alterations which are important in cancer progression. The effect of polyphenols on phase-I and -II enzymes can also modulate procarcinogenic metabolism. The modulation of NF-κB molecular pathway is another mechanism of polyphenol chemoprevention properties. In that sense, caffeic acid would inhibit growth and metastasis of HCC through modulation of expression of proteins involved mainly in NF-κB molecular pathway. Anyway, more research should be done in this field due to the vast number of potential antioxidant compounds from the polyphenol family.
4. Breeding Strategies for the Improvement of Carotenoid and Polyphenol Content in Tomato
Improvement of functional quality is a complex task because the accumulation of any antioxidant compound is the result of complex metabolic processes. Consequently, the increase in the available information on antioxidant biosynthetic pathways (enzymes involved and regulation mechanisms) and the identification of mutant genotypes with beneficial pathway alterations for the antioxidant accumulation is essential to obtain higher precision and better results in the development of breeding programs. Different breeding strategies can be followed with this purpose, and they all depend on the existence of variability for the accumulation of bioactive compounds in the cultivated species or wild relatives. Additionally, a different approach can be followed via genetic engineering. Advances in the improvement of carotenoid and polyphenol composition of tomato are reviewed herein.
4.1. Carotenoids
In tomato, breeding for fruit colour was one of the first fruit quality objectives demanded by markets. As this feature is conferred by carotenoid pigments (mainly lycopene and β-carotene) with important antioxidant properties, the improvement of fruit colour has indirectly led to the improvement of tomato nutritional and functional value. This situation justifies that breeding for improved carotenoid content is far more advanced than any other bioactive compounds. The high colour variability present in genus Solanum section Lycopersicum allowed the first studies of diverse genetic variants (natural mutants) for this attribute (Table 2). Studies on tomato natural mutants for carotenoid accumulation and on tomato transgenic plants with different carotenoid biosynthetic pathway alterations has allowed a detailed knowledge of the complete tomato carotenoid biosynthesis pathway (Figure 1) and its regulation strategies.
Table 2.
Mutants used in tomato breeding related to carotenoid accumulation.
| Altered Activity | Mutant/Gene | Fruit Colour | Details |
|---|---|---|---|
| Single step of biosynthesis pathway | r (yellow flesh) | Yellow fruits | Null mutation of PSY-1 gene arrests carotenoid synthesis and the yellow colour is due to rutin accumulation |
| t (tangerine) | Orange fruits | Defective CRTISO enzyme. Prolycopene (orange colour) is accumulated | |
| og (old gold) and ogc (old gold crimson) | Intense red fruits | Frameshift mutations originating defective CYC-B Lycopene is accumulated at the expense of β-carotene | |
| B (Beta) | Orange fruits | Mutation in the promoter of CYC-B gene (increased transcription). β -carotene increases at the expense of lycopene | |
| Del (Delta) | Orange fruits | LCY-E transcription is increased, and more δ-carotene is produced at the expense of lycopene | |
| hp-3 (high pigment 3) | Intense red fruits | Defective ZE mutant. Biosynthesis of ABA is decreased, resulting in increased plastid division and higher accumulation of carotenoids | |
| Regulation | hp-1, hp-1w (high pigment 1) | Intense red fruits | Mutation of DD1 homolog. Altered light regulation. Increased total carotenoids, vitamin C and polyphenols |
| hp-2, hp-2j, hp-2dg (high pigment 2) | Intense red fruits | Mutation of TDET1. Altered light signal-transduction machinery. Increased total carotenoids, vitamin C and quercetin | |
| Ip (Intense pigment) | Intense red fruits | Promotion of phytochrome signal amplification. Increased soluble solids and total carotenoids |
Figure 1.
Carotenoid and xanthophyll biosynthesis pathway in tomato (adapted from; [24,110,112,122,123]). The most important products and steps in carotenoid biosynthesis in common red tomatoes are underlined and marked with thick arrows respectively. The three regulation mechanisms are indicated with 90° rotated boxes.
In common red tomatoes, from the start of the maturing process (breaker stage) a high increment in synthesis of the enzymes PSY [101], PDS [102] and CRTISO [103] occurs, which results in a high increment in the all-trans-lycopene synthesis (marked with thickness arrows in Figure 1). At the same time, a strong repression of the synthesis of lycopene cyclases (enzymes involved in the formation of 6C cyclic end groups) occurs [104]. Usually, in common red tomatoes, only lycopene-β-cyclases, LCY-B [102] and the chromoplast specific CYC-B [105] enzymes are present. The drastic diminution of the lycopene-β-cyclases entails that only a low amount of β-carotene synthesis at the expense of lycopene can be achieved (Figure 1). Consequently, the prominent carotenoid in mature fruits will be all-trans-lycopene, which confers to the ripe fruits its typical red colour. Several natural mutant genes with altered steps in this biosynthetic pathway have been identified (Table 2). As a result, altered fruit carotenoid profile and fruit colours are obtained [103,105,106,107]. In green tissues of the plant, the carotenoid biosynthesis pathway does not stop with lycopene accumulation, but it continues with the xanthophyll biosynthesis pathway (Figure 1) and neoxanthin would be the last product synthesized, deriving in the abscisic acid (ABA) synthesis pathway [24].
Regulation of the carotenoid biosynthesis process can be done at three different levels. The first level consists in the regulation of the initial amount of the precursor of the synthesis of carotenoids (pre-pathway substrate regulation, Figure 1), the isopentenyl diphosphate (IPP), which determines the total amount of carotenoids that can be synthesised. IPP may arise at some developmental stages partly from the citoplasmic mevalonic (MVA) pathway [108], but it is mainly synthesised through the methylerythritol-4-phosphate (MEP) pathway, apparently bound to the plastidic compartment [109]. The first enzyme of MEP pathway, 1-deoxy-D-xylulose-5-phosphate synthase (DXS), has been proved to catalyse the first regulatory step in carotenoid biosynthesis [110] and, consequently, highly influences the final amount of carotenoids. This pre-pathway substrate regulation also can be altered by a reduction of ABA synthesis which has as consequence an increase of plastid division enabling higher biosynthesis and accumulation of carotenoids [111]. This was observed in high pigment-3 (hp-3) mutants. These mutants coding a defective zeaxanthin epoxidase (ZE) enzyme (Xanthophyll biosynthesis pathway, Figure 1) which arrest ABA synthesis and, as explained above, increased carotenoid accumulation.
A second level of regulation consists in the hormonal growth regulation of the fruit ripening process (light independent regulation, Figure 1) which also plays a major role in the control of the described carotenoid synthesis pathway. During the ripening process, ethylene has a strong positive control of the increment in the mRNA levels for the lycopene-producing enzymes phytoene synthase (PSY) and phytoene desaturase (PDS), at the same time, the mRNA levels of the genes for the lycopene β- and ε-cyclases diminish and completely disappear [104]. This explains that mutations affecting ethylene synthesis or perception, such as long life mutations rin, nor, alç..., not only delay the normal ripening process also result in altered carotenoid content [112].
Finally, there is a third level of regulation in which fruit localized phytochromes also regulate the extent of carotenoid accumulation [113]. Phytochrome response to light presence enables carotenoid synthesis that stops in darkness, thus conditioning day-cyclical biosynthesis periods (light-dependent regulation box, Figure 1). Several natural mutants present alterations in the normal phytochrome regulation with enhanced global carotenoid content (Table 2): the mutation high pigment-1 (hp-1), and the allelic hp-1w results in the plant acting as perceiving continuously the light [114]. The mutation high pigment-2 (hp-2), and the allelic hp-2j and hp-2dg [115], affects the photomorphogenesis regulatory gene TDET1, and also affects the light signal-transduction machinery [116]. Another mutant, Intense pigment (Ip) is implicated in a promotion of phytochrome signal amplification [117,118].
All the breeding strategies targeted to improve carotenoid content in tomato try to achieve a gene combination enabling a higher accumulation of one or more carotenoids in a desired genotype with good agronomic performance. Two main strategies can be used to do it: one is the use of germplasm with a high potential to accumulate one or more antioxidants as a gene donor in order to transfer them by conventional breeding programs and the other the use of advanced biotechnology to transfer foreign genes (genetic engineering) to allow a beneficial biosynthesis alteration of the desired antioxidant compound.
Both strategies can be complementary, and in fact genetic engineering can be used to avoid the negative side effects of certain genes changing their promotor (reviewed by Cebolla-Cornejo et al. [112]). Nevertheless, the level of public scepticism in certain regions (e.g., Europe) hinders the commercialization of transgenic varieties [3] and commercial approaches are right now based in conventional breeding programs.
In summary, the first approach involved the exploitation of natural diversity present in the genus (mainly genotypes with natural gene mutations identified in many studies and described before) as their use as donor parents in conventional breeding programs following different hybridization strategies and selection generations. Following this approach, some of the more successful fresh tomato cultivars developed carrying the Beta, B, gene with its modifier Beta-modifier, moB (Figure 1) have been Caro Red [119] and Caro Rich [120] and the processing tomato cultivar Caro beta [121]. These cultivars show orange fruits with β-carotene contents up to 5 mg 100 g−1 fw (roughly up to 10-fold the normal content in standard red cultivars). Unfortunately, these orange fruited cultivars with high β-carotene content have not been commercially successful, as consumers seem to prefer red tomato fruits.
Other approaches focused in the increase of lycopene content have obtained improved cultivars using mutants old gold (og) and old gold crimson (ogc) which inhibits the synthesis of β-carotene by cyclisation of lycopene resulting in higher lycopene contents (up to 30% of normal content) and lower β-carotene accumulations [122]. Tomato cultivars of the crimson type have been successfully commercialized due to their intense red pigmentation even when the fruits are not completely ripe. One of the problems of the use of mutants affecting single steps of the biosynthesis pathway is that the increase in the level of one carotenoid is obtained at the expense of another, thus little effect can be expected for total carotenoid content. Thus, mutants affecting the regulation of the pathway would have a more dramatic effect. In fact, better results have been obtained with cultivars carrying both crimson and high pigment genes (hp-1 or hp-2 alleles), as increments in the lycopene content up to 3- to 4-fold of common cultivars has been obtained [124]. Although, some deleterious effects on seed germination, plant vigour and yield have been associated with the high pigment mutants. The best expectations where deposited in the use of Intense pigment (Ip) mutant gene. This gene allows carotenoid accumulation similar to those of hp genes, but with lower deleterious effects and it is dominant (commercial hybrid development more interesting than with recessive genes as hp mutants) [125].
Regarding the second breeding strategy, the high advances in the knowledge of the carotenoid biosynthesis (metabolic pathway, precursors and regulation mechanisms) allowed the use of this information to obtain several experimental transgenic tomato lines with modified genes controlling some biosynthetic steeps. Most of this works try to emulate the carotenoid biosynthetic performance of some of the natural mutants identified, and some of them also try to avoid undesirable side-effects found in these mutants.
One approach used has been the modification of isoprenoid precursor’s pathway (pre-pathway substrate regulation) intervening in the both mevalonate (MVA; [109]) and methylerythritol-4-phosphate (MEP; [126]) pathways (Figure 1) trying to increase the total amount of carotenoids increasing the levels of the precursor. Only transformation with gene encoding 1-deoxy-D-xylulose-5-phosphate synthase (DXS) from Escherichia coli to increase MEP pathway showed interesting results (2.2 times higher β-carotene content).
The most explored approach has been placed in the development of transgenic lines with altered expression of the most important enzymes in the carotenoid synthetic pathway with the objective to alter the carotenoid profile and to increase the content of certain of interesting carotenoids. The focus was put on the enzymes phytoene synthase [127,128], phytoene desaturase [129] and mainly on lycopene cyclases [105,130,131,132,133,134] due to their role in the regulation of the whole pathway and its role in the partition of the main carotenoids lycopene and β-carotene. Several strategies have been used: constitutive or selective expression of foreign genes from several species and overexpression or repression of target genes by several mechanisms, but results obtained were contradictory. To avoid these problems, in recent years, a more refined strategy targeted to obtain a better expression of transgenes using more selective and specific promoters have been explored. In this sense, the characterization of the tomato PDS promoter [135], as well as S. habrochaites lycopene β-cyclase (CYC-B) promoter [136] bring information to enhance the use of new transgenes. Other efforts were targeted to use promoters which allows a selective expression of transgenes in fruit tissues and adequate developmental stages as occurs with fruit specific promoters such as ethylene response genes E8 and E4m polygalacturonase and lipoxygenase, mainly acting in the late-ripening stage or the LA22CD07 and LesAffx.6852.1.Sl at in green and red-ripening fruits [137].
Finally, the third approach used is based on the modification of some of the regulatory mechanisms of the carotenoid biosynthesis pathway. In this sense, some transgenic experimental lines tried to emulate the performance of high pigments mutants hp-1 [114] and hp-2 [138]. In this last case, the strategy used involved the suppression of photomorphogenesis regulatory gene TDET1 in fruits (using fruit specific promoters combined with a RNA interference approach based in inverted repeat constructs) to reduce negative collateral vegetative effects, and gave interesting results. Other works were focused in the development of transgenic experimental lines simulating performance of hp-3 mutants. In this sense, the down-regulation of ABA synthesis during ripening using an RNAi construct of the SINCED1 gene driven by the fruit specific promoter E8 [139,140], caused a reduction in ABA concentration, an increase of ethylene production and resulted in an increment in lycopene and β-carotene contents.
4.2. Polyphenols
The polyphenol content of tomatoes has gained importance during the last decade. Consequently, the achievements of breeding efforts still lag behind those obtained for carotenoids. Nevertheless, quite a lot of information is available regarding polyphenol biosynthesis in this crop. In fact, the polyphenol metabolic pathway has also been ascertained. Several transcription factors related with the regulation of polyphenol biosynthesis have been identified, but a lot of information regarding the spatial accumulation of polyphenols in the fruit and how it can be reverted is still required.
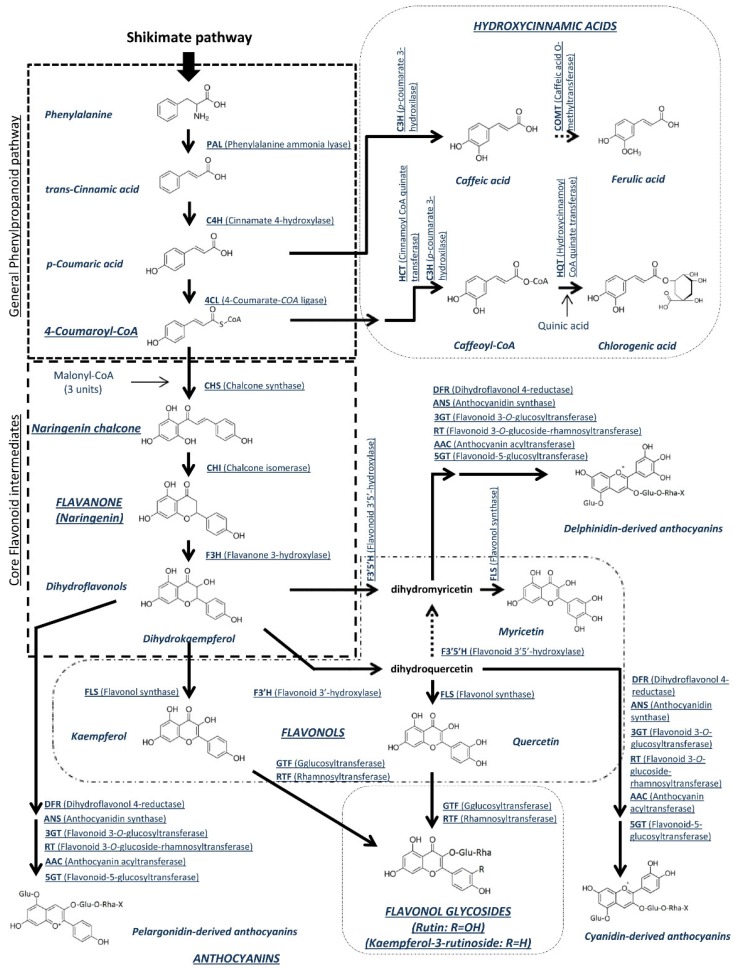
The phenylpropanoid biosynthetic pathway is the first step in the polyphenol biosynthesis and it uses the amino acid phenylalanine from the shikimate pathway as initial substrate (Figure 2). This biosynthetic pathway is common for two of the main classes of tomato polyphenols: hydroxycinnamic acids and flavonoids. The first step involves the conversion of phenylalanine into trans-cinnamic acid using the enzyme phenylalanine ammonia lyase (PAL). The enzyme cinnamate 4-hydroxylase (C4H) catalyses the conversion of the resulting product into p-coumaric acid. At this point, the flavonoid biosynthetic pathway continues with the conversion of p-coumaric acid into 4-coumaroyl-CoA as a result of the action of 4-coumarate-CoA ligase (4CL) [141].
Figure 2.
Polyphenol biosynthesis pathway in tomato (adapted from [141,142,145,146,147,148]. Broken arrows indicate common pathways in plants, which would probably apply to tomato. Solid lines confirmed in tomato.
Meanwhile the hydroxycinnamic acid pathway continues with the transformation of p-coumaric acid into caffeic acid catalysed by the p-coumarate 3-hydroxylase (C3H) [142]. Chlorogenic acid, one of the main polyphenols present in tomato [26], is formed from caffeoyl-CoA, which is transesterificated with quinic acid by hydroxycinnamoyl-Coenzyme A:quinate hydroxycinnamoyl transferase, HQT [143]. Caffeoyl-CoA would be obtained from 4-coumaroyl-CoA in three steps involving the successive activities of cinnamoyl CoA shikimate/quinate transferase (HCT), p-coumaroyl ester 3-hydroxylase (C3H) and HCT [144]. Finally, the third main hydroxycinnamic acid in tomato, ferulic acid, would be obtained from caffeic acid with the enzyme caffeic acid O-methyltransferase (COMT) [142].
On the other hand, the core flavonoid biosynthetic pathway starts with the conversion of the resulting 4-coumaroyl-CoA from the phenylpropanoid pathway into the yellow-coloured naringenin chalcone [141]. This key reaction is performed by the enzyme chalcone synthase (CHS) which begins with the condensation of one molecule of 4-coumaroyl-CoA with three molecules of malonyl-CoA. In most plants, including tomato, chalcones are not the end-product of the pathway. The enzyme chalcone isomerase (CHI) isomerizes naringenin chalcone into the flavanone naringenin. Finally, the core flavonoid intermediates pathway finishes with the formation of the dihydroflavonol dihydrokaempferol as a result of the action of the flavanone 3-hydroxylase (F3H). From this central intermediate, the flavonoid biosynthetic pathway diverges into several side branches, each resulting in a different class of flavonoid [141].
One of the most important classes of flavonoids in tomato are flavonols [26,149], which are synthesised from dihydrokaempferol. The three more important in tomato are kaempferol, quercetin, and myricetin. The three are formed from the corresponding dihydroflavonol, and have dihydrokaempferol as a starting point.
Flavonol synthase (FLS) catalyses the direct conversion of dihydrokaempferol into kaempferol. On the other hand, with dihydrokaempferol as substrate, the enzymes flavonoid 3’-hydroxylase (F3’H) and FLS would produce respectively dihydroquercetin and quercetin [147]. Quercetin is especially important in tomato, as it is the base for the formation of rutin. This quercetin glycoside is quite abundant in tomato [32], and it is obtained from quercetin by the action of the enzymes glucosyltransferase (GTF) and rhamnosyltransferase (RTF) [146].
The third flavonol, myricetin, would be derived in from dihydroquercetin in two steps. The first one catalysed by flavonoid 3’5’-hydroxylase, F3’5’H [145], would produce dihydromyricentin which would be converted into myricetin by FLS. Moreover, myricetin could also be directly obtained from dihydrokaempferol by means of the action of F3’5’H and FLS [145,146,147].
Anthocyanins are not naturally accumulated in the tomato fruit, but as it will be shown, interspecific crosses can restore this pathway in the fruit. Their synthesis would start from the three commented dihydroflavonols. Dihydroflavonol 4-reductase (DFR) would catalyse the conversion of dihydroflavonols into flavan-3,4-diols, which would be then transformed into anthocyanidins by anthocyanidin synthase (ANS). The final step would require the addition of sugars to form anthocyanins, which are anthocyanidin glycosides [141,144]. The main anthocyanins present in tomato would be derived from three anthocyanidins: delphidin, that would be synthesised following this scheme from dihydromyricetin, cyaniding from dihydroquercetin and pelargonidin from dihydrokaempferol. Other anthocyanidins would be derived from these. For example, delphinidin can be methylated on its 3’ hydroxyl group to form petunidin or on both its 3’ and 5’ hydroxyl groups to form malvidin [150].
As in the case of carotenoids, several mutants have been identified regarding polyphenol accumulation (Table 3), and those more used in breeding programs are related to the regulation of the pathway. It seems that CHI plays a central role in the rate determining step in the production of flavonols [151]. In fact, its over-expression increases dramatically the levels of flavonols at the expense of naringenin chalcone [33]. Ballester et al. [152] suggested that upon ripening an increase in the expression CHS expression and a coordinated decrease in the expression of CHI would result in the accumulation of naringenin chalcone and in a limitation of flavonol contents. Previously, Willits et al. [153] suggested that the lack of expression of CHI in the peel of the fruit, probably caused by a mutation in a fruit specific promoter would explain the high levels of naringenin chalcone in this tissue. The authors also assumed that cultivated tomato would have lost the expression of CHI in the peel in an early step of the domestication process.
Table 3.
Mutants used in tomato breeding related to polyphenol accumulation.
| Altered activity | Mutant/gene | Fruit colour | Details |
|---|---|---|---|
| Regulation | Y (yellow) | Pink fruits | Mutation of SlMYB12, transcription factor involved in the regulation of phenylpropanoid/flavonoid pathway. Lack of accumulation of naringenin chalcone in fruit peel |
| Aft (Anthocyanin fruit) | Purple fruits (external) | Probable regulation of flavonoid/anthocyanidin synthesis in fruits. Increased levels of flavonols, delphinidin, malvidin and petunidin | |
| Abg (Aubergine) | Purple fruits (external) | Possible allele of Aft. | |
| atv (atroviolacea) | Light purple fruits (external) | Possible role in the phytochrome phyB1 high irradiance response pathway. Strong accumulation of anthocyanins in vegetative parts. Small effect on fruit, but it has synergic effects with Aft | |
| hp-1, hp-1w (high pigment 1) | Intense red fruits | Mutation of DD1 homolog. Altered light regulation. Increased total carotenoids, vitamin C and polyphenols | |
| hp-2, hp-2j, hp-2dg (high pigment 2) | Intense red fruits | Mutation of TDET1. Altered light signal-transduction machinery. Increased total carotenoids, vitamin C and quercetin |
Naringenin chalcone is one of the prominent polyphenols in tomato. Its accumulation in the peel gives a yellow colour that in combination with red flesh results in an external red colour of the fruit. Several tomato landraces are characterized by an external pink colour, resulting from the lack of accumulation of naringenin chalcone and a consequent transparent peel. The evaluation of introgression lines of Solanum chmielewskii in tomato enabled the identification of the gene responsible for this mutation (yellow, y), initially described in 1925 [154]. This gene encodes SlMYB12, a transcription factor involved in the regulation of the phenylpropanoid and/or flavonoid pathway [152].
The expression of the Arabidopsis form AtMYB12 in tomato induced primary and secondary metabolism, binding directly to the promoters of different genes encoding enzymes of the primary metabolism such as 3-deoxy-D-arabino-heptulosonate 7-phosphate synthase, DAHPS, and plastidial enolase, ENO [155]. DAHPS is a key determinant if the flow of the shikimate pathway. A first step needed for the accumulation of phenylalanine, a substrate necessary for the flavonoid pathway. Additionally, it would also bind to promoters of genes related enzymes participating in the flavonoid pathway (PAL5A, PAL5C, PAL5D, CHS1 and F3H). As result a dramatic increase in flavonol and hydroxycinnamates is observed, reaching up to 10% of fruit dry weight.
Pandey et al. [156] also expressed constitutively AtMYB15, obtaining similar results. In this case, 305 unigenes were upregulated in the fruit tissue and 419 downregulated. Specifically, several enzymes of the flavonoid pathway were upregulated, especially CHS (300-fold enhanced expression in the fruit) and FLS (300-fold enhanced expression in the fruit). Additionally, genes involved in ethylene biosynthesis and signalling, ABA, auxin and Ga signalling were also modulated. Primary metabolism was also altered, and a differential regulation of genes involved in aromatic amino acid biosynthesis and carbohydrate metabolism was observed. Probably this modulation may be related to the elevated demand of C-source to support the enhanced biosynthesis of polyphenols.
Recent transcriptome analysis has identified additional transcription factors involved in the regulation of the pathway [157]. At least 20 transcription factors would correlate with expression of genes participating in the flavonoid biosynthesis pathway. As expected, SlMYB12 is included in this set. Other examples include LIM, which is highly correlated with the expression of genes involved in both the biosynthesis of ascorbic acid and flavonoids, and other MYB and bHLH genes.
The expression of endogenous genes encoding key enzymes of the flavonoid pathway (PAL, CHS, CHI, F3H and FLS) in tomato pericarp and collumela tissues has been found to be under detection limits [146]. This lack of expression would explain the low amounts of flavonoids found in these tissues. The expression of these genes can be achieved. For example, an overall increase in flavonol accumulation in the whole fruit was achieved with the expression Lc and C1 transcription factors from maize [151]. In this case, a clear over-expression of genes encoding CHS and F3H was observed, suggesting that the expression of these two genes would be necessary to improve flavonoid accumulation in tomato flesh. Other studies confirmed that the expression of the gene encoding FLS would also be required alter all [34]. In fact, the joint over-expression of genes encoding CHS and FLS would be required to pull the carbon flux towards the accumulation of flavonols [146].
Regarding the light regulation of the pathway, Giuntini et al. [158] showed that UV-B radiation differentially affects the expression of genes of the flavonoid pathway and results in altered content of flavonoids and hydroxycinnamic acids. The conventional cultivar Esperanza showed higher levels of naringenin chalcone, quercetin and rutin and of sinapic, caffeic, ferulic and p-coumaric acids in the flesh of UV-B shielded fruits at the red ripe stage, while in the high lycopene cultivar DRW5981 limited effects were observed at this stage, especially for flavonoid content. In the conventional cultivar Esperanza, the expression of genes encoding CHS and CHI expression was higher and that corresponding to F3H and F3’H was reduced in UV-B shielded fruits. In both cultivars, carotenoid accumulation followed the same response of flavonoid accumulation. In the high lycopene cultivar DRW5981 several genes of the flavonoid pathway were upregulated in the fruit flesh with the exception of CHI. As a result a dramatic increase in naringenin chalcone and a modest increase in quercetin were observed.
As commented in the carotenoid section, the high pigment (hp) tomato mutants also affect the light regulation of flavonoid synthesis. This overproduction of bioactive compounds is also associated with increased plastid biogenesis and therefore plastid-accumulating metabolites would be expected (reviewed by Azari et al. [159]).
The increase in the accumulation of flavonoids in conventional breeding programs, excluding genetic engineering has offered limited results. In fact, the analysis of different tomato germplasm for flavonol accumulation offered limited levels of variation (up to 10-fold). Quercetin in hydrolysed peel extracts varied between 6.3 and 64.9 μg g−1 fw, but the highest levels only represented a 2.5-fold increase compared to a standard commercial variety [34]. Similar levels of variation have been found by other authors, with the highest levels been found in the smaller cherry tomato fruits originating from sunny climates [35]. The limited variation found in the cultivated tomato suggested that it would be difficult to improve flavonol accumulation via conventional breeding.
Nevertheless, the use of the primary gene pool has enabled the identification and use of wild species from the Solanum section lycopersicum as sources of variation. Following this approach, tomato flavonoid content has been increased using the wild tomato species Solanum pennellii Correl, in order to restore the flavonoid pathway in fruit flesh. With this objective, germplasm expressing chalcone isomerase in the flesh was selected and used in the development of hybrids with the cultivated species with higher levels of quercetin diglycoside [153].
Something similar happened with the improvement of anthocyanins in tomato. In fact, in the cultivated species anthocyanins are not produced in the fruit, but as a result of interspecific crossed in breeding programs, several mutants with anthocyanin accumulation in the fruit have been identified. The three mutants that can lead to increased anthocyanin content in the peel of the fruit are Anthocyanin fruit (Aft) Aubergine (abg) and atroviolacea (atv).
The Anthocyanin fruit, Aft, mutant was identified in crosses with Solanum chilense Dunal. This gene is located in chromosome 10 and its presence in tomato leads to increased levels of delphinidin, malvidin and petunidin, as well as higher levels of the flavonols quercetin, 3.6-fold, and kaempferol, 2.7-fold [37].
Sapir et al. [37] proved that Aft fruits not only showed high levels of anthocyanins in the skin and outer pericarp of the fruit, but also of the flavonols quercetin and kaempferol. They also showed that Aft is encoded by a single locus on chromosome 10 fully associated with Anthocyacin1 (Ant1). Slant1 was discovered in a T-DNA insertional mutagenesis program and was identified as a MYB transcription factor. Vegetative tissues of Slant1 showed intense purple colour and fruits displayed purple spotting on the epidermis and pericarp. The overexpression of Slant1 upregulated genes encoding enzymes of early and later steps of anthocyanidin biosynthesis as well as genes involved in the glycosylation and transport of anthocyanins into the vacuole [160]. It seems that the original allele from S. chilense ScAnt1 would be more efficient in the production of anthocyanins than the tomato counterpart [161].
The Ant1 paralog gene SlAn2, similar to Petunia anthocyanin2, is found in chromosome 10 and has also been related with the Aft mutant. SlAn2 encodes an R2R3-MYB transcription factor and its overexpression in tomato results in increased anthocyanin accumulation in fruit peel [162]. Additionally, overexpressing fruits display ripening related phenotypes, with enhanced ethylene levels, reduced carotenoid accumulation and faster fruit softening. In fact, the authors found a concomitant accumulation of Rin (Ripening inhibitor) transcripts suggesting that the functions of SlAn2 and Rin may be related. It has been recently studied that between SlAnt1 and SlAn2 only the latter acts as a positive regulator of anthocyanin synthesis in vegetative tissues under high light or low temperature conditions [163].
The Aubergine (Abg) mutant was identified in a cross with Solanum lycopersicoides [164]. This gene also relies in the chromosome 10 and it may be allelic to Aft. The difficulties in the management of S. lycopersicoides introgressions has hindered the development of allelic studies to confirm or discard this relation. In this sense, a paracentric inversion has been identified in the long arm of chromosome 10 of S. lycopersicoides, resulting in the absence of recombination events in this segment [165].
The recessive gene atroviolacea (atv), mapping in chromosome 7, was identified in a segregant population S. cheesmaniae (L. Riley) Fosberg [166]. It has a strongest effect on the accumulation of anthocyanins of vegetative tissues and has a limited effect on the fruit. The atv mutant shows an exaggerated response in the red broad band light, suggesting specificity for the phytochrome phyB1 high irradiance response pathway [167]. Thus, this mutation per se has no important effect on the accumulation of anthocyanins on the fruit. Nonetheless, it has been proved that in the double homozygous mutants Aft Aft atv atv a synergistic effect of both genes arises on the transcription of specific genes of the anthocyanin pathway, resulting in higher anthocyanin contents than in the individual mutants [168]. This effect also applies to the combination Abg with atv. In fact the best combinations to improve anthocyanin content in the fruit include Abg atv atv and Aft Aft atv atv in small fruits, with contents up to 415 and 116 mg 100g-1 fw respectively in the epidermis and subepidermis [36].
Another mutant, anthocyanin free (af), was identified in the fifties as a mutagenesis variant characterized by the lack of anthocyanin production in all plant tissues. Recently, Kang et al. [169] showed that af encodes SlCHI1, but complementation assays demonstrated that SlCHI1 not only complements flavonoid synthesis in the af mutant, but also complements a defect in terpenoid production, suggesting a link between both pathways.
None of these mutants result in the accumulation of anthocyanins in the fruit flesh. Alternatively, tomatoes with purple flesh have been obtained via genetic engineering. In this sense, the transcription Del and Ros1 have been involved in the activation of the flavonoid pathway in tomato [147], as the expression of these factors from Antirrhinum resulted in the accumulation of purple anthocyanins in both peel and flesh. It seems that these factors would stimulate the transcription of the genes encoding PAL, CHI and F3’5’H. This upregulation of the flavonoid pathway and the opening of the anthocyanin gate (F3’5’H) resulted in anthocyanin levels up to 3 mg g−1 fw. Another possible success of the combination of both transcription factors may be related with the upregulation of genes involved in the side chain modification of anthocyanins and genes associated with the transport and accumulation in vacuoles [170]. Later works with the same genes obtained higher levels up 5.2 mg g−1 dry weight (dw), with petunidin-3-(trans-coumaroyl)-rutinoside-5-glucoside and delphinidin-3-(trans-coumaroyl)-rutinoside-5-glucoside representing an 86% of the total anthocyanins [171].
The enzyme F3’5’H would be in fact a key enzyme in restoring the accumulation of anthocyanins in tomato. In fact, the absence of anthocyanins in LC/C1 fruits was attributable primarily to an insufficient expression of F3’5’H, in combination with a strong preference of the tomato dihydroflavonol reductase (DFR) to use dihydromyricetin as a substrate [151].
4.3. Joint Accumulation of Carotenoids and Polyphenols
Following the objective of maximizing the functional value of tomato, several efforts have been made in order to maximize the accumulation of carotenoids and polyphenols in the same material. As stated in the carotenoids section, the use of the high pigment (DDB1:hp-1, hp-1w, DET1: hp-2, hp-2j, hp-2dg) mutants enables an improvement in the accumulation of both types of bioactive compounds. Lines carrying hp-1 apart from increased carotenoid levels can show 13-fold higher levels of quercetin [172]. Long et al. [173] also found higher levels of chlorogenic acid in hp-1-lines (4.3-fold) and similar levels of hydroxycinnamic acids compared to a standard variety. The hp-2dg increased the level of quercetin aglycone 3.2-fold at the red stage, while the differences were not significant for naringenin chalcone [115]. It is not clear the connection of the two pathways, but considering that vitamin C contents are also higher in hp-1 and hp-2 plants [115,174] it could be possible that the expression of these alleles would trigger stress tolerance mechanisms activating the overproduction of antioxidants. Although both pathways seem to be completely independent, surprisingly, the combination of anthocyanin mutants with carotenoid defective mutants (Beta, B, and yellow flesh, r) results in lower total anthocyanin levels [36].
High pigment mutants have also been used in combination with anthocyanin synthesising mutants. Following this strategy, double homozygotes for Aft and hp1 displayed a more than additive effect on the accumulation in anthocyanins and flavonols, with 5-, 19-, and 33-fold increase in the content of petunidin, malvidin and delphinidin respectively [37]. Mes et al. [36] also obtained higher anthocyanin levels combining Aft and hp-1, though the levels obtained were lower than the combination Aft-atv.
Stacking carotenoid and anthocyanin related genes is currently being used in the development of new cultivars with enhanced functional value. As an example, Sestari et al. [175] stacked the genes hp-2, Aft and atv into a commercial cultivar of cherry tomato. Cherry cultivars are selected due to their high ratio surface to volume, as carotenoids and polyphenols tend to accumulate in the external layers of the fruit, and more light is received by the peel increasing anthocyanin synthesis.
5. Conclusions
It is difficult to establish a clear link between a specific component of a complex diet with the prevention of different types of cancer. Especially, when their efficiency may depend on the matrix in which the bioactive compound is present. Consequently, supplementation studies may differ from their natural ingestion in food. Despite these difficulties, carotenoids and polyphenols have proved in different studies to play a role as functional compounds in the prevention of cancer. In this context, despite not outstanding for its nutritional value, the high level of consumption of tomato all year round makes it an important source of bioactive compounds. During the last decades, efforts have been placed in the breeding sector in order to develop new functional tomato varieties with increased levels of carotenoids and polyphenols. These materials would represent an alternative to isolated compounds found in dietary supplements, with the benefits of synergetic effects of these molecules with other phytochemicals presents in the natural matrix. The use of high pigment genes in combination with a restoration of anthocyanin accumulation in the fruit, especially in smaller materials such as cherry varieties, may represent a promising alternative to increment the intake of these bioactive compounds in their natural matrices in the near future. These new materials will obviously be an interesting tool to be combined with other recommendations in order to prevent or even to contribute to delay the progression of cancer.
Acknowledgments
The Author’s thank the editorial office of Cancers for covering the costs to publish in Open Access.
Author Contributions
All the authors contributed to the review of bibliography (especially R.M.) and the writing of the paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Bruhn C.M., Feldman N., Garlitz C., Harwood J., Ivans E., Marshall M., Riley A., Thurber D., Williamson E. Consumer perceptions of quality: Apricots, cantaloupes, peaches, pears, strawberries, and tomatoes. J. Food Qual. 1991;14:187–195. doi: 10.1111/j.1745-4557.1991.tb00060.x. [DOI] [Google Scholar]
- 2.Cappellano K.L. Influencing food choices: Nutrition labeling, health claims, and front-of-the-package labeling. Nutr. Today. 2009;44:269–273. doi: 10.1097/NT.0b013e3181c2637e. [DOI] [Google Scholar]
- 3.Goldman I.L. Molecular breeding of healthy vegetables. EMBO Rep. 2011;12:96–102. doi: 10.1038/embor.2010.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shahidi F., Chandrasekara A., Zhong Y. Bioactive phytochemicals in vegetables. In: Sinha N.K., Hui Y.H., Evranuz E.Ö., Siddiq M., Ahmed J., editors. Handbook of Vegetables and Vegetable Processing. Wiley-Blackwell; Ames, IA, USA: 2011. pp. 125–158. [Google Scholar]
- 5.Kris-Etherton P.M., Hecker K.D., Bonanome A., Coval S.M., Binkoski A.E., Hilpert K.F., Griel A.E., Etherton T.D. Bioactive compounds in foods: their role in the prevention of cardiovascular disease and cancer. Am. J. Med. 2002;113:71–88. doi: 10.1016/S0002-9343(01)00995-0. [DOI] [PubMed] [Google Scholar]
- 6.Trichopoulou A., Lagiou P., Kuper H., Trichopoulos D. Cancer and Mediterranean dietary traditions. Cancer Epidemiol. Biomark. Prev. 2000;9:869–873. [PubMed] [Google Scholar]
- 7.Willett W.C., Sacks F., Trichopoulou A., Drescher G., Ferro-Luzzi A., Helsing E., Trichopoulos D. Mediterranean diet pyramid: a cultural model for healthy eating. Am. J. Clin. Nutr. 1995;61:1402–1406. doi: 10.1093/ajcn/61.6.1402S. [DOI] [PubMed] [Google Scholar]
- 8.Liu R.H. Dietary bioactive compounds and their health implications. J. Food Sci. 2013;78:18–25. doi: 10.1111/1750-3841.12101. [DOI] [PubMed] [Google Scholar]
- 9.Blanck H.M., Gillespie C., Kimmons J.E., Seymour J.D., Serdula M.K. Trends in fruit and vegetable consumption among U.S. men and women, 1994–2005. [(accessed on 1 May 2016)]; Available online: http://www.cdc.gov/pcd/issues/2008/apr/07_0049.htm. [PMC free article] [PubMed]
- 10.Vattem D.A., Ghaedian R., Shetty K. Enhancing health benefits of berries through phenolic antioxidant enrichment: Focus on cranberry. Asia Pac. J. Clin. Nutr. 2005;14:120–130. [PubMed] [Google Scholar]
- 11.Boileau T.W., Liao Z., Kim S., Lemeshow S., Erdman J.W., Clinton S.K. Prostate Carcinogenesis in N-methyl-N-nitrosourea (NMU)-Testosterone-Treated Rats Fed Tomato Powder, Lycopene, or Energy-Restricted Diets. J. Natl. Cancer Inst. 2003;95:1578–1586. doi: 10.1093/jnci/djg081. [DOI] [PubMed] [Google Scholar]
- 12.Wang S., DeGroff V.L., Clinton S.K. Tomato and soy polyphenols reduce insulin-like growth factor-I-stimulated rat prostate cancer cell proliferation and apoptotic resistance in vitro via inhibition of intracellular signaling pathways involving tyrosine kinase. J. Nutr. 2003;133:2367–2376. doi: 10.1093/jn/133.7.2367. [DOI] [PubMed] [Google Scholar]
- 13.Liu R.H. Potential synergy of phytochemicals in cancer prevention: Mechanism of action. J. Nutr. 2004;134:3479–3485. doi: 10.1093/jn/134.12.3479S. [DOI] [PubMed] [Google Scholar]
- 14.Unlu N.Z., Bohn T., Clinton S.K., Schwartz S.J. Carotenoid absorption from salad and salsa by humans is enhanced by the addition of avocado or avocado oil. J. Nutr. 2005;135:431–436. doi: 10.1093/jn/135.3.431. [DOI] [PubMed] [Google Scholar]
- 15.Fielding J.M., Rowley K.G., Cooper P., O’Dea K. Increases in plasma lycopene concentration after consumption of tomatoes cooked with olive oil. Asia Pac. J. Clin. Nutr. 2005;14:131–136. [PubMed] [Google Scholar]
- 16.Kavanaugh C.J., Trumbo P.R., Ellwood K.C. The U.S. food and drug administration’s evidence-based review for qualified health claims: Tomatoes, lycopene, and cancer. J. Natl. Cancer Inst. 2007;99:1074–1085. doi: 10.1093/jnci/djm037. [DOI] [PubMed] [Google Scholar]
- 17.Lin J., Cook N.R., Albert C., Zaharris E., Gaziano J.M., Van Denburgh M., Buring J.E., Manson J.E. Vitamins C and E and beta carotene supplementation and cancer risk: A randomized controlled trial. J. Natl. Cancer Inst. 2009;101:14–23. doi: 10.1093/jnci/djn438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mente A., de Koning L., Shannon H., Anand S. A Systematic Review of the Evidence Supporting a Causal Link Between Dietary Factors and Coronary Heart Disease. Arch. Intern. Med. 2009;169:659–669. doi: 10.1001/archinternmed.2009.38. [DOI] [PubMed] [Google Scholar]
- 19.Mignone L.I., Giovannucci E., Newcomb P.A., Titus-Ernstoff L., Trentham-Dietz A., Hampton J.M., Willett W.C., Egan K.M. Dietary carotenoids and the risk of invasive breast cancer. Int. J. Cancer. 2009;124:2929–2937. doi: 10.1002/ijc.24334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aune D., Chan D.S., Vieira A.R., Rosenblatt D.A., Vieira R., Greenwood D.C., Norat T. Dietary compared with blood concentrations of carotenoids and breast cancer risk : A systematic review and meta-analysis of prospective. Am. J. Clin. Nutr. 2012;2007:356–373. doi: 10.3945/ajcn.112.034165. [DOI] [PubMed] [Google Scholar]
- 21.Chun O.K., Kim D.O., Smith N., Schroeder D., Han J.T., Lee C.Y. Daily consumption of phenolics and total antioxidant capacity from fruit and vegetables in the American diet. J. Sic. Food Agric. 2005;85:1715–1724. doi: 10.1002/jsfa.2176. [DOI] [Google Scholar]
- 22.Leiva-Brondo M., Valcárcel M., Cortés-Olmos C., Roselló S., Cebolla-Cornejo J., Nuez F. Exploring alternative germplasm for the development of stable high vitamin C content in tomato varieties. Sci. Hortic. 2012;133:84–88. doi: 10.1016/j.scienta.2011.10.013. [DOI] [Google Scholar]
- 23.Standfuss J., Terwisscha van Scheltinga A.C., Lamborghini M., Kühlbrandt W. Mechanisms of photoprotection and nonphotochemical quenching in pea light-harvesting complex at 2.5 A resolution. EMBO J. 2005;24:919–928. doi: 10.1038/sj.emboj.7600585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Galpaz N., Galpaz N., Ronen G., Ronen G., Khalfa Z., Khalfa Z., Zamir D., Zamir D., Hirschberg J., Hirschberg J. A Chromoplast-Specific Carotenoid Biosynthesis Pathway Is Revealed by Cloning of the Tomato white-flower Locus. Plant Cell. 2006;18:1–14. doi: 10.1105/tpc.105.039966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Davies J.N., Hobson G.E., McGlasson W.B. The constituents of tomato fruit—the influence of environment, nutrition, and genotype. Crit. Rev. Food Sci. Nutr. 1981;15:205–280. doi: 10.1080/10408398109527317. [DOI] [PubMed] [Google Scholar]
- 26.Martínez-Valverde I., Periago M.J., Provan G., Chesson A. Phenolic compounds, lycopene and antioxidant activity in commercial varieties of tomato (Lycopersicum esculentum) J. Sci. food Agric. 2002;82:323–330. doi: 10.1002/jsfa.1035. [DOI] [Google Scholar]
- 27.Slimestad R., Fossen T., Verheul M.J. The flavonoids of tomatoes. J. Agric. Food Chem. 2008;56:2436–2441. doi: 10.1021/jf073434n. [DOI] [PubMed] [Google Scholar]
- 28.Tiwari U., Cummins E. Factors influencing levels of phytochemicals in selected fruit and vegetables during pre- and post-harvest food processing operations. Food Res. Int. 2013;50:497–506. doi: 10.1016/j.foodres.2011.09.007. [DOI] [Google Scholar]
- 29.Fray R.G., Grierson D. Identification and genetic analysis of normal and mutant phytoene synthase genes of tomato by sequencing, complementation and co-suppression. Plant Mol. Biol. 1993;22:589–602. doi: 10.1007/BF00047400. [DOI] [PubMed] [Google Scholar]
- 30.Qin J., Chao K., Kim M.S. Investigation of Raman chemical imaging for detection of lycopene changes in tomatoes during postharvest ripening. J. Food Eng. 2011;107:277–288. doi: 10.1016/j.jfoodeng.2011.07.021. [DOI] [Google Scholar]
- 31.Shen Y.C., Chen S.L., Wang C.K. Contribution of tomato phenolics to antioxidation and down-regulation of blood lipids. J. Agric. Food Chem. 2007;55:6475–6481. doi: 10.1021/jf070799z. [DOI] [PubMed] [Google Scholar]
- 32.Martí R., Valcárcel M., Herrero-Martínez J.M., Cebolla-Cornejo J., Roselló S. Fast simultaneous determination of prominent polyphenols in vegetables and fruits by reversed phase liquid chromatography using a fused-core column. Food Chem. 2015;169:169–179. doi: 10.1016/j.foodchem.2014.07.151. [DOI] [PubMed] [Google Scholar]
- 33.Muir S.R., Collins G.J., Robinson S., Hughes S., Bovy A., Ric De Vos C.H., van Tunen A.J., Verhoeyen M.E. Overexpression of petunia chalcone isomerase in tomato results in fruit containing increased levels of flavonols. Nat. Biotechnol. 2001;19:470–474. doi: 10.1038/88150. [DOI] [PubMed] [Google Scholar]
- 34.Colliver S., Bovy A., Collins G., Muir S., Robinson S., De Vos C.H., Verhoeyen M.E. Improving the nutritional content of tomatoes through reprogramming their flavonoid biosynthetic pathway. Phytochem. Rev. 2002;1:113–123. doi: 10.1023/A:1015848724102. [DOI] [Google Scholar]
- 35.Stewart A.J., Bozonnet S., Mullen W., Jenkins G.I., Lean M.E., Crozier A. Occurrence of flavonols in tomatoes and tomato-based products. J. Agric. Food Chem. 2000;48:2663–2669. doi: 10.1021/jf000070p. [DOI] [PubMed] [Google Scholar]
- 36.Mes P.J., Boches P., Myers J.R., Durst R. Characterization of tomatoes expressing anthocyanin in the fruit. J. Am. Soc. Hortic. Sci. 2008;133:262–269. [Google Scholar]
- 37.Sapir M., Oren-Shamir M., Ovadia R., Reuveni M., Evenor D., Tadmor Y., Nahon S., Shlomo H., Chen L., Meir A., et al. Molecular aspects of Anthocyanin fruit tomato in relation to high pigment-1. J. Hered. 2008;99:292–303. doi: 10.1093/jhered/esm128. [DOI] [PubMed] [Google Scholar]
- 38.Nicoli M., Anese M., Parpinel M. Influence of processing on the antioxidant properties of fruit and vegetables. Trends Food Sci. Technol. 1999;10:94–100. doi: 10.1016/S0924-2244(99)00023-0. [DOI] [Google Scholar]
- 39.Dewanto V., Wu X., Adom K.K., Liu R.H. Thermal processing enhances the nutritional value of Tomatoes by increasing total antioxidant activity. J. Agric. Food Chem. 2002;50:3010–3014. doi: 10.1021/jf0115589. [DOI] [PubMed] [Google Scholar]
- 40.Crozier A., Lean M.E., McDonald M.S., Black C. Quantitative analysis of the flavonoid content of commercial tomatoes, onions, lettuce, and celery. J. Agric. Food Chem. 1997;45:590–595. doi: 10.1021/jf960339y. [DOI] [Google Scholar]
- 41.Khachik F., Goli M.B., Beecher G.R., Holden J., Lusby W.R., Tenorio M.D., Barrera M.R. Effect of food preparation on qualitative and quantitative distribution of major carotenoid constituents of tomatoes and several green vegetables. J. Agric. Food Chem. 1992;40:390–398. doi: 10.1021/jf00015a006. [DOI] [Google Scholar]
- 42.Giovannucci E. Tomatoes, tomato-based products, lycopene, and cancer: Review of the epidemiologic literature. J. Natl. Cancer Inst. 1999;91:317–331. doi: 10.1093/jnci/91.4.317. [DOI] [PubMed] [Google Scholar]
- 43.Clinton S.K. Lycopene: Chemistry, biology, and implications for human health and disease. Nutr. Rev. 1998;56:35–51. doi: 10.1111/j.1753-4887.1998.tb01691.x. [DOI] [PubMed] [Google Scholar]
- 44.Giovannucci E., Rimm E.B., Liu Y., Stampfer M.J., Willett W.C. A prospective study of tomato products, lycopene, and prostate cancer risk. J. Natl. Cancer Inst. 2002;94:391–398. doi: 10.1093/jnci/94.5.391. [DOI] [PubMed] [Google Scholar]
- 45.Etminan M., Takkouche B., Caamaño-isorna F. The role of tomato products and lycopene in the prevention of prostate cancer: A meta-analysis of observational studies. Cancer Epidemiol. Biomark. Prev. 2004;13:340–345. [PubMed] [Google Scholar]
- 46.Canene-Adams K., Campbell J.K., Zaripheh S., Jeffery E.H., Erdman J.W. The tomato as a functional food. J. Nutr. 2005;134:1226–1230. doi: 10.1093/jn/135.5.1226. [DOI] [PubMed] [Google Scholar]
- 47.Mills P.K., Beeson W.L., Phillips R.L., Fraser G.E. Cohort study of diet, lifestyle, and prostate cancer in Adventist men. Cancer. 1989;64:598–604. doi: 10.1002/1097-0142(19890801)64:3<598::AID-CNCR2820640306>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 48.Wu K., Erdman J.W., Schwartz S.J., Platz E.A., Leitzmann M., Clinton S.K., Degroff V. Plasma and dietary carotenoids, and the risk of prostate cancer: A nested case-control study. Cancer Epidemiol. Biomark. Prev. 2004;13:260–269. doi: 10.1158/1055-9965.EPI-03-0012. [DOI] [PubMed] [Google Scholar]
- 49.Lu Q.Y., Hung J.C., Heber D., Go V.L., Reuter V.E., Cordon-Cardo C., Scher H.I., Marshall J.R., Zhang Z.F. Inverse associations between plasma lycopene and other carotenoids and prostate cancer. Cancer Epidemiol. Biomark. Prev. 2001;10:749–756. [PubMed] [Google Scholar]
- 50.Kristal A.R., Till C., Platz E.A., Song X., King I.B., Neuhouser M.L., Ambrosone C.B., Thompson I.M. Serum lycopene concentration and prostate cancer risk: Results from the prostate cancer prevention trial. Cancer Epidemiol. Biomark. Prev. 2011;20:638–646. doi: 10.1158/1055-9965.EPI-10-1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zu K., Mucci L., Rosner B.A., Clinton S.K., Loda M., Stampfer M.J., Giovannucci E. Dietary lycopene, angiogenesis, and prostate cancer: A prospective study in the prostate-specific antigen era. J. Natl. Cancer Inst. 2014;106:1–10. doi: 10.1093/jnci/djt430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yang C.M., Yen Y.T., Huang C.S., Hu M.L. Growth inhibitory efficacy of lycopene and beta-carotene against androgen-independent prostate tumor cells xenografted in nude mice. Mol. Nutr. Food Res. 2011;55:606–612. doi: 10.1002/mnfr.201000308. [DOI] [PubMed] [Google Scholar]
- 53.Kirsh V.A., Mayne S.T., Peters U., Chatterjee N., Leitzmann M.F., Dixon L.B., Urban D.A., Crawford E.D., Hayes R.B. A prospective study of lycopene and tomato product intake and risk of prostate cancer. Cancer Epidemiol. Biomark. Prev. 2006;15:92–98. doi: 10.1158/1055-9965.EPI-05-0563. [DOI] [PubMed] [Google Scholar]
- 54.AICR Food, Nutrition, Physical Activity, and the Prevention of Cancer: A Global Perspective. [(accessed on 1 May 2016)]. Available online: http://www.aicr.org/assets/docs/pdf/reports/Second_Expert_Report.pdf.
- 55.Kuckuk O., Sarkar F.H., Sakr W., Djuric Z., Pollak M.N., Khachik F., Li Y.W., Banerjee M., Grignon D., Bertram J.S., et al. Phase II randomized clinical trial of lycopene supplementation before radical prostatectomy. Cancer Epidemiol. Biomarkers Prev. 2001;10:861–868. [PubMed] [Google Scholar]
- 56.Ansari M.S., Gupta N.P. A comparison of lycopene and orchidectomy vs. orchidectomy alone in the management of advanced prostate cancer. BJU Int. 2003;92:375–378. doi: 10.1046/j.1464-410X.2003.04370.x. [DOI] [PubMed] [Google Scholar]
- 57.Chen L., Stacewicz-Sapunatzakis M., Duncan C., Sharifi R., Ghosh L., van Breemen R., Ashton D., Bowen P.E. Oxidative DNA damage in prostate cancer patients consuming tomato sauce-based entrees as a whole-food intervention. J. Natl. Cancer Inst. 2001;93:1872–1879. doi: 10.1093/jnci/93.24.1872. [DOI] [PubMed] [Google Scholar]
- 58.Vaishampayan U., Hussain M., Banerjee M., Seren S., Sarkar F.H., Fontana J., Forman J.D., Cher M.L., Powell I., Pontes J.E., et al. Lycopene and soy isoflavones in the treatment of prostate cancer. Nutr. Cancer. 2007;59:1–7. doi: 10.1080/01635580701413934. [DOI] [PubMed] [Google Scholar]
- 59.Mohanty N.K., Saxena S., Singh U.P., Goyal N.K., Arora R.P. Lycopene as a chemopreventive agent in the treatment of high-grade prostate intraepithelial neoplasia. Urol. Oncol. Semin. Orig. Investig. 2005;23:383–385. doi: 10.1016/j.urolonc.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 60.Jatoi A., Burch P., Hillman D., Vanyo J.M., Dakhil S., Nikcevich D., Rowland K., Morton R., Flynn P.J., Young C., et al. A tomato-based, lycopene-containing intervention for androgen-independent prostate cancer: Results of a phase II study from the north central cancer treatment group. Urology. 2007;69:289–294. doi: 10.1016/j.urology.2006.10.019. [DOI] [PubMed] [Google Scholar]
- 61.Clark P.E., Hall M.C., Borden L.S., Miller A.A., Hu J.J., Lee W.R., Stindt D., D’Agostino R., Lovato J., Harmon M., et al. Phase I-II prospective dose-escalating trial of lycopene in patients with biochemical relapse of prostate cancer after definitive local therapy. Urology. 2006;67:1257–1261. doi: 10.1016/j.urology.2005.12.035. [DOI] [PubMed] [Google Scholar]
- 62.Bunker C., McDonald A., Evans R., de la Rosa N., Boumosleh J., Patrick A. A randomized trial of lycopene supplementation in tobago Men with high prostate cancer risk. Nutr. Cancer. 2007;57:130–137. doi: 10.1080/01635580701274046. [DOI] [PubMed] [Google Scholar]
- 63.Neuhouser M.L., Barnett M.J., Kristal A.R., Ambrosone C.B., King I.B., Thornquist M., Goodman G.G. Dietary supplement use and prostate cancer risk in the Carotene and Retinol Efficacy Trial. Cancer Epidemiol. Biomark. Prev. 2009;18:2202–2206. doi: 10.1158/1055-9965.EPI-09-0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ge X., Xing M., Yu L., Shen P. Carotenoid intake and esophageal cancer risk: A meta-analysis. Asian Pacific J. cancer Prev. 2012;14:1911–1918. doi: 10.7314/APJCP.2013.14.3.1911. [DOI] [PubMed] [Google Scholar]
- 65.Ziegler R.G., Mayne S.T., Swanson C.A. Nutrition and lung cancer. Cancer Causes Control. 1996;7:157–177. doi: 10.1007/BF00115646. [DOI] [PubMed] [Google Scholar]
- 66.Heinonen O., Albanes D. The effect of vitamin E and beta carotene on the incidence of lung cancer and other cancers in male smokers. N. Engl. J. Med. 1994;330:1029–1035. doi: 10.1056/NEJM199404143301501. [DOI] [PubMed] [Google Scholar]
- 67.Albanes D., Heinonen O.P., Taylor P.R., Virtamo J., Edwards B.K., Rautalahti M., Hartman A.M., Palmgren J., Freedman L.S., Haapakoski J., et al. Alpha-tocopherol and beta-carotene supplements and lung cancer incidence in the alpha-tocopherol, beta-carotene cancer prevention study: Effects of base-line characteristics and study compliance. J. Natl. Cancer Inst. 1996;88:1560–1570. doi: 10.1093/jnci/88.21.1560. [DOI] [PubMed] [Google Scholar]
- 68.Omenn G.S., Goodman G.E., Thornquist M.D., Balmes J., Cullen M.R., Glass A., Keogh J.P., Meyskens F.L., Valanis B., Williams J.H., et al. Effects of a combination of beta carotene and vitamin a on Lung cancer and cardiovascular disease. N. Engl. J. Med. 1996;334:1150–1155. doi: 10.1056/NEJM199605023341802. [DOI] [PubMed] [Google Scholar]
- 69.Wang X., Russell R. Procarcinogenic and anticarcinogenic effects of beta-carotene. Nutr. Rev. 1999;57:263–272. doi: 10.1111/j.1753-4887.1999.tb01809.x. [DOI] [PubMed] [Google Scholar]
- 70.Wright M.E., Groshong S.D., Husgafvel-Pursiainen K., Genova E., Lucia M.S., Wolff H., Virtamo J., Albanes D. Effects of beta-carotene supplementation on molecular markers of lung carcinogenesis in male smokers. Cancer Prev. Res. 2010;3:745–752. doi: 10.1158/1940-6207.CAPR-09-0107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mondul A.M., Sampson J.N., Moore S.C., Weinstein S.J., Evans A.M., Karoly E.D., Virtamo J., Albanes D. Metabolomic profile of response to supplementation with beta-carotene in the alpha-tocopherol, beta-carotene cancer prevention Study. Am. J. Clin. Nutr. 2013;98:488–493. doi: 10.3945/ajcn.113.062778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tanaka T., Shnimizu M., Moriwaki H. Cancer chemoprevention by carotenoids. Molecules. 2012;17:3202–3242. doi: 10.3390/molecules17033202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hennekens C.H., Buring J.E., Manson J.E., Stampfer M., Rosner B., Cook N.R., Belanger C., LaMotte F., Gaziano J.M., Ridker P.M., et al. Lack of effect of long-term supplementation with beta carotene on the incidence of malignant neoplasms and cardiovascular disease. N. Engl. J. Med. 1996;334:1145–1149. doi: 10.1056/NEJM199605023341801. [DOI] [PubMed] [Google Scholar]
- 74.Eliassen A.H., Hendrickson S.J., Brinton L.A., Buring J.E., Campos H., Dai Q., Dorgan J.F., Franke A.A., Gao Y.T., Goodman M.T., et al. Circulating carotenoids and risk of breast cancer: Pooled analysis of eight prospective studies. J. Natl. Cancer Inst. 2012;104:1905–1916. doi: 10.1093/jnci/djs461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pantavos A., Ruiter R., Feskens E.F., De Keyser C.E., Hofman A., Stricker B.H., Franco O.H., Kiefte-De Jong J.C. Total dietary antioxidant capacity, individual antioxidant intake and breast cancer risk: The Rotterdam study. Int. J. Cancer. 2015;136:2178–2186. doi: 10.1002/ijc.29249. [DOI] [PubMed] [Google Scholar]
- 76.Greenlee H., Kwan M.L., Kushi L.H., Song J., Castillo A., Weltzien E., Quesenberry C.P., Caan B.J. Antioxidant supplement use after breast cancer diagnosis and mortality in the Life after Cancer Epidemiology (LACE) cohort. Cancer. 2012;118:2048–2058. doi: 10.1002/cncr.26526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Blot W.J., Li J.Y., Taylor P.R., Guo W., Dawsey S., Wang G.Q., Yang C.S., Zheng S.F., Gail M., Li G.Y., et al. Nutrition intervention trials in linxian, China: Supplementation with specific vitamin/mineral combinations, cancer incidence, and disease-specific mortality in the general population. J. Natl. Cancer Inst. 1993;85:1483–1491. doi: 10.1093/jnci/85.18.1483. [DOI] [PubMed] [Google Scholar]
- 78.Zhou Y., Wang T., Meng Q., Zhai S. Association of carotenoids with risk of gastric cancer: A meta-analysis. Clin. Nutr. 2015;35:109–116. doi: 10.1016/j.clnu.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 79.Trejo-Solís C., Pedraza-Chaverrí J., Torres-Ramos M., Jiménez-Farfán D., Cruz Salgado A., Serrano-García N., Osorio-Rico L., Sotelo J. Multiple molecular and cellular mechanisms of action of lycopene in cancer inhibition. Evid. Based Complement. Altern. Med. 2013 doi: 10.1155/2013/705121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ramos S. Cancer chemoprevention and chemotherapy: dietary polyphenols and signalling pathways. Mol. Nutr. Food Res. 2008;52:507–526. doi: 10.1002/mnfr.200700326. [DOI] [PubMed] [Google Scholar]
- 81.Korkina L., De Luca C., Kostyuk V., Pastore S. Plant polyphenols and tumors: From mechanisms to therapies, prevention, and protection against toxicity of anti-cancer treatments. Curr. Med. Chem. 2009;16:3943–3965. doi: 10.2174/092986709789352312. [DOI] [PubMed] [Google Scholar]
- 82.Loa J., Chow P., Zhang K. Studies of structure–activity relationship on plant polyphenol-induced suppression of human liver cancer cells. Cancer Chemother. Pharmacol. 2009;63:1007–1016. doi: 10.1007/s00280-008-0802-y. [DOI] [PubMed] [Google Scholar]
- 83.Garcia-Closas R., Gonzalez C.A., Agudo A., Riboli E. Intake of specific carotenoids and flavonoids and the risk of gastric cancer in Spain. Cancer Causes Control. 1999;10:71–75. doi: 10.1023/A:1008867108960. [DOI] [PubMed] [Google Scholar]
- 84.Peterson J., Lagiou P., Samoli E., Lagiou A., Katsouyanni K., La Vecchia C., Dwyer J., Trichopoulos D. Flavonoid intake and breast cancer risk: A case-control study in Greece. Br. J. Cancer. 2003;89:1255–1259. doi: 10.1038/sj.bjc.6601271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bosetti C., Spertini L., Parpinel M., Gnagnarella P., Lagiou P., Negri E., Franceschi S., Montella M., Peterson J., Dwyer J., et al. Flavonoids and breast cancer risk in Italy. Cancer Epidemiol. Biomark. Prev. 2005;14:805–808. doi: 10.1158/1055-9965.EPI-04-0838. [DOI] [PubMed] [Google Scholar]
- 86.Rossi M., Negri E., Talamini R., Bosetti C., Parpinel M., Gnagnarella P., Franceschi S., Dal Maso L., Montella M., Giacosa A., et al. Flavonoids and colorectal cancer in italy. Cancer Epidemiol. Biomark. Prev. 2006;15:1555–1558. doi: 10.1158/1055-9965.EPI-06-0017. [DOI] [PubMed] [Google Scholar]
- 87.Knekt P., Kumpulainen J., Järvinen R., Rissanen H., Heliövaara M., Reunanen A., Hakulinen T., Aromaa A. Flavonoid intake and risk of chronic diseases. Am. J. Clin. Nutr. 2002;76:560–568. doi: 10.1093/ajcn/76.3.560. [DOI] [PubMed] [Google Scholar]
- 88.Cruz–Correa M., Shoskes D., Sanchez P., Zhao R., Hylind L., Wexner D., Giardiello F. Combination treatment with curcumin and quercetin of adenomas in familial adenomatous polyposis. Clin. Gastroenterol. Hepatol. 2006;4:1035–1038. doi: 10.1016/j.cgh.2006.03.020. [DOI] [PubMed] [Google Scholar]
- 89.Neuhouser M.L. Review: Dietary flavonoids and cancer risk: Evidence from human population studies. Nutr. Cancer. 2004;50:1–7. doi: 10.1207/s15327914nc5001_1. [DOI] [PubMed] [Google Scholar]
- 90.Knekt P., Järvinen R., Seppänen R., Hellövaara M., Teppo L., Pukkala E., Aromaa A. Dietary flavonoids and the risk of lung cancer and other malignant neoplasms. Am. J. Epidemiol. 1997;146:223–230. doi: 10.1093/oxfordjournals.aje.a009257. [DOI] [PubMed] [Google Scholar]
- 91.Hirvonen T., Virtamo J., Korhonen P., Albanes D., Pietinen P. Flavonol and flavone intake and the risk of cancer in male smokers (Finland) Cancer Causes Control. 2001;12:789–796. doi: 10.1023/A:1012232008016. [DOI] [PubMed] [Google Scholar]
- 92.Lagiou P., Rossi M., Lagiou A., Tzonou A., La Vecchia C., Trichopoulos D. Flavonoid intake and liver cancer: A case-control study in Greece. Cancer Causes Control. 2008;19:813–818. doi: 10.1007/s10552-008-9144-7. [DOI] [PubMed] [Google Scholar]
- 93.Weng C.J., Yen G.C. Chemopreventive effects of dietary phytochemicals against cancer invasion and metastasis: Phenolic acids, monophenol, polyphenol, and their derivatives. Cancer Treat. Rev. 2012;38:76–87. doi: 10.1016/j.ctrv.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 94.Yang J., Liu C., Ma Y., Weng S., Tang N., Wu S., Ji B.-C., Ma C.-Y., Ko Y.-C., Funayama S., et al. Chlorogenic acid induces apoptotic cell death in U937 leukemia cells through caspase- and mitochondria-dependent pathways. In Vivo. 2012;26:971–978. [PubMed] [Google Scholar]
- 95.Rajendra Prasad N., Karthikeyan A., Karthikeyan S., Venkata Reddy B. Inhibitory effect of caffeic acid on cancer cell proliferation by oxidative mechanism in human HT-1080 fibrosarcoma cell line. Mol. Cell. Biochem. 2011;349:11–19. doi: 10.1007/s11010-010-0655-7. [DOI] [PubMed] [Google Scholar]
- 96.Frydoonfar H.R., McGrath D.R., Spigelman A.D. The variable effect on proliferation of a colon cancer cell line by the citrus fruit flavonoid Naringenin. Color. Dis. 2003;5:149–152. doi: 10.1046/j.1463-1318.2003.00444.x. [DOI] [PubMed] [Google Scholar]
- 97.Harmon A.W., Patel Y.M. Naringenin inhibits glucose uptake in MCF-7 breast cancer cells: A mechanism for impaired cellular proliferation. Breast Cancer Res. Treat. 2004;85:103–110. doi: 10.1023/B:BREA.0000025397.56192.e2. [DOI] [PubMed] [Google Scholar]
- 98.So F., Guthrie N., Chambers A., Moussa M., Carroll K. Inhibition of human breast cancer cell proliferation and delay of mammary tumorigenesis by flavonoids and citrus juices. Nutr. Cancer. 1996;26:167–181. doi: 10.1080/01635589609514473. [DOI] [PubMed] [Google Scholar]
- 99.Stagos D., Amoutzias G.D., Matakos A., Spyrou A., Tsatsakis A.M., Kouretas D. Chemoprevention of liver cancer by plant polyphenols. Food Chem. Toxicol. 2012;50:2155–2170. doi: 10.1016/j.fct.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 100.Gibellini L., Pinti M., Nasi M., Montagna J.P., De Biasi S., Roat E., Bertoncelli L., Cooper E.L., Cossarizza A. Quercetin and cancer chemoprevention. Evid. Based Complement. Altern. Med. 2011 doi: 10.1093/ecam/neq053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Fraser P.D., Enfissi E.M.A., Halket J.M., Truesdale M.R., Yu D., Gerrish C., Bramley P.M. Manipulation of phytoene levels in tomato fruit: Effects on isoprenoids, plastids, and intermediary metabolism. Plant Cell. 2007;19:3194–3211. doi: 10.1105/tpc.106.049817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Pecker I., Chamovitz D., Linden H., Sandmann G., Hirschberg J. A single polypeptide catalyzing the conversion of phytoene to zeta-carotene is transcriptionally regulated during tomato fruit ripening. Proc. Natl. Acad. Sci. USA. 1992;89:4962–4966. doi: 10.1073/pnas.89.11.4962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Isaacson T., Ronen G., Zamir D., Hirschberg J. Cloning of tangerine from tomato reveals a carotenoid isomerase essential for the production of beta-carotene and xanthophylls in plants. Plant Cell. 2002;14:333–342. doi: 10.1105/tpc.010303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Klee H.J., Giovannoni J.J. Genetics and control of tomato fruit ripening and quality attributes. Annu. Rev. Genet. 2011;45:41–59. doi: 10.1146/annurev-genet-110410-132507. [DOI] [PubMed] [Google Scholar]
- 105.Ronen G., Carmel-Goren L., Zamir D., Hirschberg J. An alternative pathway to beta-carotene formation in plant chromoplasts discovered by map-based cloning of beta and old-gold color mutations in tomato. Proc. Natl. Acad. Sci. USA. 2000;97:11102–11107. doi: 10.1073/pnas.190177497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ronen G., Cohen M., Zamir D., Hirschberg J. Regulation of carotenoid biosynthesis during tomato fruit development: expression of the gene for lycopene epsilon cyclase is down- regulated during ripening and is elevated in the mutant Delta. Plant J. 1999;17:341–351. doi: 10.1046/j.1365-313X.1999.00381.x. [DOI] [PubMed] [Google Scholar]
- 107.Zhang Y., Stommel J.R. RAPD and AFLP tagging and mapping of Beta (B) and Beta modifier (MoB), two genes which influence beta-carotene accumulation in fruit of tomato (Lycopersicon esculentum Mill.) Theor. Appl. Genet. 2000;100:368–375. doi: 10.1007/s001220050048. [DOI] [Google Scholar]
- 108.Kasahara H. Contribution of the mevalonate and methylerythritol phosphate pathways to the biosynthesis of gibberellins in Arabidopsis. J. Biol. Chem. 2002;277:45188–45194. doi: 10.1074/jbc.M208659200. [DOI] [PubMed] [Google Scholar]
- 109.Lichtenthaler H.K. The 1-Deoxy-D-Xylulose-5-Phosphate pathway of isoprenoid biosynthesis in plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1999;50:47–65. doi: 10.1146/annurev.arplant.50.1.47. [DOI] [PubMed] [Google Scholar]
- 110.Lois L.M., Rodríguez-Concepción M., Gallego F., Campos N., Boronat A. Carotenoid biosynthesis during tomato fruit development: Regulatory role of 1-deoxy-D-xylulose 5-phosphate synthase. Plant J. 2000;22:503–513. doi: 10.1046/j.1365-313x.2000.00764.x. [DOI] [PubMed] [Google Scholar]
- 111.Galpaz N., Wang Q., Menda N., Zamir D., Hirschberg J. Abscisic acid deficiency in the tomato mutant high-pigment 3 leading to increased plastid number and higher fruit lycopene content. Plant J. 2008;53:717–730. doi: 10.1111/j.1365-313X.2007.03362.x. [DOI] [PubMed] [Google Scholar]
- 112.Cebolla-Cornejo J., Roselló S., Nuez F. Selection of tomato rich in nutritional terpenes. In: Ramawat K., Mérillon J., editors. Natural Products. Springer-Verlag; Berlin, Germany; Heidelberg, Germany: 2013. pp. 2853–2881. [Google Scholar]
- 113.Alba R., Cordonnier-Pratt M.-M., Pratt L.H. Fruit-localized phytochromes regulate lycopene accumulation independently of ethylene production in tomato. Plant Physiol. 2000;123:363–370. doi: 10.1104/pp.123.1.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Liu Y., Roof S., Ye Z., Barry C., Van Tuinent A., Vrebalov J., Bowler C., Giovannoni J. Manipulation of light signal transduction as a means of modifying fruit nutritional quality in tomato. Proc. Natl. Acad. Sci. USA. 2004;101:9897–9902. doi: 10.1073/pnas.0400935101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Bino R.J., De Vos C.H.R., Lieberman M., Hall R.D., Bovy A., Jonker H.H., Tikunov Y., Lommen A., Moco S., Levin I. The light-hyperresponsive high pigment-2dg mutation of tomato: Alterations in the fruit metabolome. New Phytol. 2005;166:427–438. doi: 10.1111/j.1469-8137.2005.01362.x. [DOI] [PubMed] [Google Scholar]
- 116.Mustilli A.C., Fenzi F., Ciliento R., Alfano F., Bowler C. Phenotype of the tomato high pigment-2 mutant is caused by a mutation in the tomato homolog of DEETIOLATED1. Plant Cell. 1999;11:145–157. doi: 10.1105/tpc.11.2.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kendrick R.E., Kerckhoffs L.H., van Tuinen A., Koornneef M. Photomorphogenic mutants of tomato. Plant Cell Environ. 1997;20:746–751. doi: 10.1046/j.1365-3040.1997.d01-109.x. [DOI] [PubMed] [Google Scholar]
- 118.Lavi N., Tadmor Y., Meir A., Bechar A., Oren-Shamir M., Ovadia R., Reuveni M., Nahon S., Shlomo H., Chen L., et al. Characterization of the intense pigment tomato genotype emphasizing targeted fruit metabolites and chloroplast biogenesis. J. Agric. Food Chem. 2009;57:4818–4826. doi: 10.1021/jf900190r. [DOI] [PubMed] [Google Scholar]
- 119.Tomes M.L., Quackenbush F.W. Caro-red, a new provitamin a rich tomato. Econ. Bot. 1958;12:256–260. doi: 10.1007/BF02859771. [DOI] [Google Scholar]
- 120.Tigchelaar C.C., Tomes M.L. “Caro-Rich” Tomato. [(accessed on 1 May 2016)]. Available online: http://hortsci.ashspublications.org/content/by/year.
- 121.Georgiev H., Vulkova-Atchkova Z., Vladimirov B.G., Baralieva D. Breeding determinate tomatoes with higt Beta-carotene content. Gr. Lozar. Nauk. 1981;18:28–31. [Google Scholar]
- 122.Vogel J.T., Tieman D.M., Sims C.A., Odabasi A.Z., Clark D.G., Klee H.J. Carotenoid content impacts flavor acceptability in tomato (Solanum lycopersicum) J. Sci. Food Agric. 2010;90:2233–2240. doi: 10.1002/jsfa.4076. [DOI] [PubMed] [Google Scholar]
- 123.Stigliani A.L., Giorio G., D’Ambrosio C. Characterization of P450 Carotenoid B- And E-hydroxylases of tomato and transcriptional regulation of xanthophyll biosynthesis in root, leaf, petal and fruit. Plant Cell Physiol. 2011;52:851–865. doi: 10.1093/pcp/pcr037. [DOI] [PubMed] [Google Scholar]
- 124.Stommel J.R. Genetic enhancement of tomato fruit nutritive value. In: Razdan M., Matoo A., editors. Genetic Improvement of Solanaceous Crops (Volume 2) CRC Press; Boca Raton, FL, USA: 2007. pp. 193–238. [Google Scholar]
- 125.Rick C.M. High soluble-solids content in large-fruited tomato lines derived from a wild green-fruited species. Hilgardia. 1974;42:493–510. doi: 10.3733/hilg.v42n15p493. [DOI] [Google Scholar]
- 126.Enfissi E.M., Fraser P.D., Lois L.-M., Boronat A., Schuch W., Bramley P.M. Metabolic engineering of the mevalonate and non-mevalonate isopentenyl diphosphate-forming pathways for the production of health-promoting isoprenoids in tomato. Plant Biotechnol. J. 2005;3:17–27. doi: 10.1111/j.1467-7652.2004.00091.x. [DOI] [PubMed] [Google Scholar]
- 127.Fraser P.D., Romer S., Shipton C.A., Mills P.B., Kiano J.W., Misawa N., Drake R.G., Schuch W., Bramley P.M. Evaluation of transgenic tomato plants expressing an additional phytoene synthase in a fruit-specific manner. Proc. Natl. Acad. Sci. USA. 2002;99:1092–1097. doi: 10.1073/pnas.241374598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Fray R.G., Wallace A., Fraser P.D., Valero D., Hedden P., Bramley P.M., Grierson D. Constitutive expression of a fruit phytoene synthase gene in transgenic tomatoes causes dwarfism by redirecting metabolites from the gibberellin pathway. Plant J. 1995;8:693–701. doi: 10.1046/j.1365-313X.1995.08050693.x. [DOI] [Google Scholar]
- 129.Römer S., Fraser P.D., Kiano J.W., Shipton C.A., Misawa N., Schuch W., Bramley P.M. Elevation of the provitamin A content of transgenic tomato plants. Nat. Biotechnol. 2000;18:666–669. doi: 10.1038/76523. [DOI] [PubMed] [Google Scholar]
- 130.Rosati C., Aquilani R., Dharmapuri S., Pallara P., Marusic C., Tavazza R., Bouvier F., Camara B., Giuliano G. Metabolic engineering of beta-carotene and lycopene content in tomato fruit. Plant J. 2000;24:413–419. doi: 10.1046/j.1365-313x.2000.00880.x. [DOI] [PubMed] [Google Scholar]
- 131.D’Ambrosio C., Giorio G., Marino I., Merendino A., Petrozza A., Salfi L., Stigliani A.L., Cellini F. Virtually complete conversion of lycopene into β-carotene in fruits of tomato plants transformed with the tomato lycopene β-cyclase (tlcy-b) cDNA. Plant Sci. 2004;166:207–214. doi: 10.1016/j.plantsci.2003.09.015. [DOI] [Google Scholar]
- 132.Apel W., Bock R. Enhancement of carotenoid biosynthesis in transplastomic tomatoes by induced lycopene-to-provitamin A conversion. Plant Physiol. 2009;151:59–66. doi: 10.1104/pp.109.140533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ma C., Ma B., He J., Hao Q., Lu X., Wang L. Regulation of carotenoid content in tomato by silencing of lycopene β/ε-cyclase genes. Plant Mol. Biol. Rep. 2011;29:117–124. doi: 10.1007/s11105-010-0211-3. [DOI] [Google Scholar]
- 134.Guo F., Zhou W., Zhang J., Xu Q., Deng X. Effect of the citrus lycopene β-Cyclase transgene on carotenoid metabolism in transgenic tomato fruits. PLoS ONE. 2012;7:58. doi: 10.1371/journal.pone.0032221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Corona V., Aracri B., Kosturkova G., Bartley G.E., Pitto L., Giorgetti L., Scolnik P.A., Giuliano G. Regulation of a carotenoid biosynthesis gene promoter during plant development. Plant J. 1996;9:505–512. doi: 10.1046/j.1365-313X.1996.09040505.x. [DOI] [PubMed] [Google Scholar]
- 136.Dalal M., Chinnusamy V., Bansal K.C. Isolation and functional characterization of lycopene beta-cyclase (CYC-B) promoter from Solanum habrochaites. BMC Plant Biol. 2010;10:1–15. doi: 10.1186/1471-2229-10-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Hiwasa-Tanase K., Kuroda H., Hirai T., Aoki K., Takane K., Ezura H. Novel promoters that induce specific transgene expression during the green to ripening stages of tomato fruit development. Plant Cell Rep. 2012;31:1415–1424. doi: 10.1007/s00299-012-1257-5. [DOI] [PubMed] [Google Scholar]
- 138.Davuluri G.R., van Tuinen A., Fraser P.D., Manfredonia A., Newman R., Burgess D., Brummell D.A., King S.R., Palys J., Uhlig J., et al. Fruit-specific RNAi-mediated suppression of DET1 enhances carotenoid and flavonoid content in tomatoes. Nat. Biotechnol. 2005;23:890–895. doi: 10.1038/nbt1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Sun L., Yuan B., Zhang M., Wang L., Cui M., Wang Q., Leng P. Fruit-specific RNAi-mediated suppression of SlNCED1 increases both lycopene and beta-carotene contents in tomato fruit. J. Exp. Bot. 2012;63:3097–3108. doi: 10.1093/jxb/ers026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Sun L., Sun Y., Zhang M., Wang L., Ren J., Cui M., Wang Y., Ji K., Li P., Li Q., et al. Suppression of 9-cis-Epoxycarotenoid dioxygenase, which encodes a key enzyme in abscisic acid biosynthesis, alters fruit texture in transgenic tomato. Plant Physiol. 2012;158:283–298. doi: 10.1104/pp.111.186866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Levin I. Regulating phytonutrient levels in plants-toward modification of plant metabolism for human health. In: Kirakosyan A., Kaufman P.B., editors. Recent Advances in Plant Biotechnology. Springer; New York, NY, USA: 2009. pp. 289–330. [Google Scholar]
- 142.Humphreys J.M., Chapple C. Rewriting the lignin roadmap. Curr. Opin. Plant Biol. 2002;5:224–229. doi: 10.1016/S1369-5266(02)00257-1. [DOI] [PubMed] [Google Scholar]
- 143.Niggeweg R., Michael A.J., Martin C. Engineering plants with increased levels of the antioxidant chlorogenic acid. Nat. Biotechnol. 2004;22:746–754. doi: 10.1038/nbt966. [DOI] [PubMed] [Google Scholar]
- 144.Luo J., Butelli E., Hill L., Parr A., Niggeweg R., Bailey P., Weisshaar B., Martin C. AtMYB12 regulates caffeoyl quinic acid and flavonol synthesis in tomato: Expression in fruit results in very high levels of both types of polyphenol. Plant J. 2008;56:316–326. doi: 10.1111/j.1365-313X.2008.03597.x. [DOI] [PubMed] [Google Scholar]
- 145.Holton T.A., Cornish E.C. Genetics and biochemistry of anthocyanin biosynthesis. Plant Cell. 1995;7:1071–1083. doi: 10.1105/tpc.7.7.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Verhoeyen M.E., Bovy A., Collins G., Muir S., Robinson S., de Vos C.H., Colliver S. Increasing antioxidant levels in tomatoes through modification of the flavonoid biosynthetic pathway. J. Exp. Bot. 2002;53:2099–2106. doi: 10.1093/jxb/erf044. [DOI] [PubMed] [Google Scholar]
- 147.Butelli E., Titta L., Giorgio M., Mock H.P., Matros A., Peterek S., Schijlen E.G., Hall R.D., Bovy A.G., Luo J., et al. Enrichment of tomato fruit with health-promoting anthocyanins by expression of select transcription factors. Nat. Biotechnol. 2008;26:1301–1308. doi: 10.1038/nbt.1506. [DOI] [PubMed] [Google Scholar]
- 148.Yahia E.M., de Jesús Ornelas-Paz J. Chemistry, stability, and biological actions of carotenoids. In: de la Rosa L.A., Alvarez-Parrilla E., González-Aguilar G.A., editors. Fruit and Vegetable Phytochemicals. Wiley-Blackwell; Hoboken, IA, USA: 2010. pp. 177–222. [Google Scholar]
- 149.Sakakibara H., Honda Y., Nakagawa S., Ashida H., Kanazawa K. Simultaneous determination of all polyphenols in vegetables, fruits, and teas. J. Agric. Food Chem. 2003;51:571–581. doi: 10.1021/jf020926l. [DOI] [PubMed] [Google Scholar]
- 150.Zhang Y., Butelli E., Martin C. Engineering anthocyanin biosynthesis in plants. Curr. Opin. Plant Biol. 2014;19:81–90. doi: 10.1016/j.pbi.2014.05.011. [DOI] [PubMed] [Google Scholar]
- 151.Bovy A., de Vos R., Kemper M., Schijlen E., Pertejo M.A., Muir S., Collins G., Robinson S., Verhoeyen M., Hughes S., et al. High-flavonol tomatoes resulting from the heterologous expression of the maize transcription factor genes. Plant Cell. 2002;14:2509–2526. doi: 10.1105/tpc.004218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Ballester A.-R., Molthoff J., de Vos R., Hekkert B.T., Orzaez D., Fernández-Moreno J.-P., Tripodi P., Grandillo S., Martin C., Heldens J., et al. Biochemical and molecular analysis of pink tomatoes: Deregulated expression of the gene encoding transcription factor SlMYB12 leads to pink tomato fruit color. Plant Physiol. 2010;152:71–84. doi: 10.1104/pp.109.147322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Willits M.G., Kramer C.M., Prata R.T., de Luca V., Potter B.G., Steffens J.C., Graser G. Utilization of the genetic resources of wild species to create a nontransgenic high flavonoid tomato. J. Agric. Food Chem. 2005;53:1231–1236. doi: 10.1021/jf049355i. [DOI] [PubMed] [Google Scholar]
- 154.Lindstrom E. Inheritance in tomatoes. Genetics. 1925;10:305–317. doi: 10.1093/genetics/10.4.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Zhang Y., Butelli E., Alseekh S., Tohge T., Rallapalli G., Luo J., Kawar P.G., Hill L., Santino A., Fernie A.R., et al. Multi-level engineering facilitates the production of phenylpropanoid compounds in tomato. Nat. Commun. 2015;6:1–11. doi: 10.1038/ncomms9635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Pandey A., Misra P., Choudhary D., Yadav R., Goel R., Bhambhani S., Sanyal I., Trivedi R., Kumar Trivedi P. AtMYB12 expression in tomato leads to large scale differential modulation in transcriptome and flavonoid content in leaf and fruit tissues. Sci. Rep. 2015 doi: 10.1038/srep12412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Ye J., Hu T., Yang C., Li H., Yang M., Ijaz R., Ye Z., Zhang Y. Transcriptome profiling of tomato fruit development reveals transcription factors associated with ascorbic acid, carotenoid and flavonoid biosynthesis. PLoS ONE. 2015;10:58. doi: 10.1371/journal.pone.0130885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Giuntini D., Lazzeri V., Calvenzani V., Dall’Asta C., Galaverna G., Tonelli C., Petroni K., Ranieri A. Flavonoid profiling and biosynthetic gene expression in flesh and peel of two tomato genotypes grown under UV-B-depleted conditions during ripening. J. Agric. Food Chem. 2008;56:5905–5915. doi: 10.1021/jf8003338. [DOI] [PubMed] [Google Scholar]
- 159.Azari R., Tadmor Y., Meir A., Reuveni M., Evenor D., Nahon S., Shlomo H., Chen L., Levin I. Light signaling genes and their manipulation towards modulation of phytonutrient content in tomato fruits. Biotechnol. Adv. 2010;28:108–118. doi: 10.1016/j.biotechadv.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 160.Mathews H., Clendennen S.K., Caldwell C.G., Liu X.L., Connors K., Matheis N., Schuster D.K., Menasco D.J., Wagoner W., Lightner J., et al. Activation tagging in tomato identifies a transcriptional regulator of anthocyanin biosynthesis, modification, and transport. Plant Cell. 2003;15:1689–1703. doi: 10.1105/tpc.012963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Schreiber G., Reuveni M., Evenor D., Oren-Shamir M., Ovadia R., Sapir-Mir M., Bootbool-Man A., Nahon S., Shlomo H., Chen L., et al. ANTHOCYANIN1 from Solanum chilense is more efficient in accumulating anthocyanin metabolites than its Solanum lycopersicum counterpart in association with the ANTHOCYANIN FRUIT phenotype of tomato. Theor. Appl. Genet. 2012;124:295–307. doi: 10.1007/s00122-011-1705-6. [DOI] [PubMed] [Google Scholar]
- 162.Meng X., Yang D., Li X., Zhao S., Sui N., Meng Q. Physiological changes in fruit ripening caused by overexpression of tomato SlAN2, an R2R3-MYB factor. Plant Physiol. Biochem. 2015;89:24–30. doi: 10.1016/j.plaphy.2015.02.005. [DOI] [PubMed] [Google Scholar]
- 163.Kiferle C., Fantini E., Bassolino L., Povero G., Spelt C., Buti S., Giuliano G., Quattrocchio F., Koes R., Perata P., et al. Tomato R2R3-MYB proteins SlANT1 and SlAN2: Same protein activity, different roles. PLoS ONE. 2015;10:58. doi: 10.1371/journal.pone.0136365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Rick C.M., Cisneros P., Chetelat R.T., DeVerna J.W. Abg—a gene on chromosome 10 for purple fruit derived from S. lycopersicoides. Rep. Tomato Genet. Coop. 1994;44:29–30. [Google Scholar]
- 165.Canady M.A., Ji Y., Chetelat R.T. Homeologous recombination in Solanum lycopersicoides introgression lines of cultivated tomato. Genetics. 2006;174:1775–1788. doi: 10.1534/genetics.106.065144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Rick C.M., Reeves A.F., Zobel R.W. Inheritance and linkage relations of four new mutants. Tomato Genet. Coop. Rep. 1968;18:34–35. [Google Scholar]
- 167.Kerckhoffs L.H., De Groot N.A., van Tuinen A., Schreuder M.E., Nagatani A., Koornneef M., Kendrick R.E. Physiological characterization of exaggerated-photoresponse mutants of tomato. J. Plant Physiol. 1997;150:578–587. doi: 10.1016/S0176-1617(97)80322-7. [DOI] [Google Scholar]
- 168.Povero G., Gonzali S., Bassolino L., Mazzucato A., Perata P. Transcriptional analysis in high-anthocyanin tomatoes reveals synergistic effect of Aft and atv genes. J. Plant Physiol. 2011;168:270–279. doi: 10.1016/j.jplph.2010.07.022. [DOI] [PubMed] [Google Scholar]
- 169.Kang J.-H., Mcroberts J., Shi F., Moreno J.E., Jones A.D., Howe G.A. The flavonoid biosynthetic enzyme chalcone isomerase modulates terpenoid production in glandular trichomes of tomato. Plant Physiol. 2014;164:1161–1174. doi: 10.1104/pp.113.233395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Gonzali S., Mazzucato A., Perata P. Purple as a tomato: Towards high anthocyanin tomatoes. Trends Plant Sci. 2009;14:237–241. doi: 10.1016/j.tplants.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 171.Su X., Xu J., Rhodes D., Shen Y., Song W., Katz B., Tomich J., Wang W. Identification and quantification of anthocyanins in transgenic purple tomato. Food Chem. 2016;202:184–188. doi: 10.1016/j.foodchem.2016.01.128. [DOI] [PubMed] [Google Scholar]
- 172.Yen H.C., Shelton B.A., Howard L.R., Lee S., Vrebalov J., Giovannoni J.J. The tomato high-pigment (hp) locus maps to chromosome 2 and influences plastome copy number and fruit quality. Theor. Appl. Genet. 1997;95:1069–1079. doi: 10.1007/s001220050664. [DOI] [Google Scholar]
- 173.Long M., Millar D.J., Kimura Y., Donovan G., Rees J., Fraser P.D., Bramley P.M., Bolwell G.P. Metabolite profiling of carotenoid and phenolic pathways in mutant and transgenic lines of tomato: identification of a high antioxidant fruit line. Phytochemistry. 2006;67:1750–1757. doi: 10.1016/j.phytochem.2006.02.022. [DOI] [PubMed] [Google Scholar]
- 174.Giuntini D., Graziani G., Lercari B., Fogliano V., Soldatini G.F., Ranieri A. Changes in carotenoid and ascorbic acid contents in fruits of different tomato genotypes related to the depletion of UV-B radiation. J. Agric. Food Chem. 2005;53:3174–3181. doi: 10.1021/jf0401726. [DOI] [PubMed] [Google Scholar]
- 175.Sestari I., Zsögön A., Rehder G.G., Teixeira L. de L., Hassimotto N.M., Purgatto E., Benedito V.A., Pereira-Peres L.E. Near-isogenic lines enhancing ascorbic acid, anthocyanin and carotenoid content in tomato (Solanum lycopersicum L. cv Micro-Tom) as a tool to produce nutrient-rich fruits. Sci. Hortic. 2014;175:111–120. doi: 10.1016/j.scienta.2014.06.010. [DOI] [Google Scholar]