Abstract
Objectives:
To evaluate the role of this polymorphism as a risk factor for breast cancer in Kurdish patients and to investigate the possible association between Arg194Trp x-ray repair cross-complementing group 1 (XRCC1) gene polymorphisms with clinical and histopathological outcomes of patients with breast cancer.
Methods:
A total of 100 breast cancer patients and 200 cancer-free controls in Kurdish population of Kurdistan state admitted to Tohid Hospital, Sanandaj, Kurdistan, Iran between January 2012 and May 2015 were enrolled in this cross-sectional study. Tissue expression of estrogen receptor (ER), progesteron receptor (PR), human epidermal growth factor receptor 2 (Her2/neu), and Ki67 were evaluated by immunohistochemistry (IHC). The Arg194Trp genotypes were determined by polymerase chain reaction- restriction fragment length polymorphism method.
Results:
Our data showed that the risk for breast cancer increased significantly among the Trp variant of XRCC1. Statistically significant association was found between codon 194 polymorphisms and tissue expression of Ki67.
Conclusion:
The Trp allele of codon 194 XRCC1 is a potential risk factor for breast cancer in Kurdish ethnicity. Furthermore, effect of this polymorphism on clinical and histological features of breast cancer was significant.
Breast cancer is the second leading cause of cancer death in women after lung cancer and has the highest incidence rates in women after non-melanoma skin cancer. According to the World Health Organization (WHO) reports, each year over 1.3 million women are diagnosed with breast cancer, and approximately 0.5 million women die of this disease.1 Epidemiological studies show that the incidence of breast cancer is increasing in developing countries. Iran is not exempt from this issue, the breast cancer is the cause of 21.4% of all malignancies and also is the most common cancer in Iranian women.2,3 The incidence of breast cancer increases with age and age-specific incidence rates increases sharply from one in every 230 women between the ages of 30 and 40 to one in 30 between the ages of 60 and 70.4,5 The most common histologic type of invasive breast cancer by far is the invasive (infiltrating) ductal carcinoma (IDC). It comprises approximately 80% of all cases. Invasive lobular carcinoma (ILC) is the second major type (5-10% of all cases) of breast cancer. Finally, medullary carcinoma, already mentioned as a rare subtype of IDC (1%) with a better prognosis.6 Clinical and histological assessment of breast cancer patients for treatment of disease is largely based on clinical and pathologic criteria, including tumor stage, lymph node involvement, histological grade and evaluation of estrogen receptor (ER), progesteron receptor (PR), human epidermal growth factor receptor 2 (HER2)/neu, and Ki67 protein expression by immunohistochemistry (IHC) techniques.7 The x-ray repair cross-complementing group 1 (XRCC1) has an important role in the base excision repair (BER) of single-strand DNA breaks caused by endogenous oxidative species and exogenous carcinogens.8 The XRCC1 gene contains 17 exons and is located on chromosome 19q13.2. This gene encodes a 70-kDa protein consisting of 633 amino acids. Base-excision DNA repair plays an important role in the protection of the genome against chemical carcinogens and ionizing radiations.9,10 These agents induce mutations in DNA and promote malignancies such as leukemia, thyroid, lung, colon, and breast cancers. Altered DNA repair capacity due to gene mutations, or nucleotide substitutions can result in increased susceptibility to these cancers.11,12 X-ray repair cross-complementing group 1 has been shown to have a large number of single-nucleotide polymorphisms (SNPs), several of which are being increasingly studied in cancer epidemiology investigations, in part because of their relative high frequency in the population.13 Among them, Arg194Trp (rs1799782) has been shown to change the neoconservative amino acid, and thus result in alteration of DNA repair capacity.14 The Arg194Trp polymorphism is located in the nuclear antigen-binding region. This linker region separates the Amino terminus (NH2)-terminal domain from the central BRCT1 (BRCA1 C-terminus) domain and interacts with several proteins. Amino acid substitution due to genetic changes might affect its binding efficiency. Arg194Trp SNP, changes the ability of XRCC1 to interact with the other BER enzymes and consequently alter DNA repair capability.9,10,13 The Trp194 variant has been associated with increased BER capacity, whereas the Arg194 allele has been related to significantly higher values of chromosomal breaks. Taken together a protective effect of the 194Trp allele may be suggested.15-17 Several studies have been conducted to evaluate the possible effect of XRCC1 SNPs on breast cancer in different population; however, controversial results have been reported.18,19 Moreover, there is a paucity of information on the association between XRCC1 polymorphisms and histological features in breast cancer patients. Therefore, the present study attempts to analyze the association of the Arg194Trp XRCC1 polymorphisms with breast cancer in a Kurdish population. Furthermore, the possible contribution of Arg194Trp XRCC1 polymorphism on ER, PR, Her2/neu, and Ki67 proteins expression were studied in cancerous patients.
Methods
In this cross-sectional study, all subjects who were admitted to Tohid Hospital, Sanandaj, Kurdistan, Iran, between January 2012 and May 2015 were examined for breast cancer and 100 women were diagnosed as cancerous patients by histopathology of the breast tissue. A total of 200 women with normal mammography result were enrolled in the study as a control group. All patients and control individuals were from Kurdistan, a province in western Iran with a population that is the Kurds. Written informed consent for participation was obtained and the project was approved by the Research Ethics Committee of Kurdistan University of Medical Sciences, Iran. Patients with a history of other organ cancers were excluded from the study. All tumors were graded using the criteria of Scarf Bloom Richardson20 and the clinical staging of patients was evaluated according to TNM staging system for breast cancer.21 Data on morphologic characteristics, grade, and stage of the tumor and adjuvant treatment were obtained from the medical records. The median follow-up was 24 months (range, 6-48 months). The chemotherapy treatment consisted of anthracyclines (doxorubicin and cyclophosphamide) and Paclitaxol.
Tissue preparation
Tissues were fixed in 10% paraformaldehyde, processed routinely and embedded in paraffin. Sections (2 µm) were mounted on super frost slides. Hematoxylin and eosin staining was used for histological evaluation under light microscope. Sequential sections were used for ER, PR, Ki67 and Her2/neu staining.
Immunohistochemistry
The immunostains were performed on an automated stainer (XT; Ventana Systems, Phoenix, AZ, Arizona). The primary antibody incubation time for all assays was 32 minutes after antigen retrieval in Tris based buffer (60 minutes at 95-100°C). The protein expression assessment was performed by scoring based on the percentage of stained cells and the intensity of nuclear stain, according to the method described previously.22-24
Deoxyribonucleic acid extraction.25
Whole blood was collected in ethylenediaminetetraacetic acid (EDTA) for DNA analyses. Genomic DNA was extracted from whole blood using DNA extraction kit (DNPTM, CinnaGen Inc, Tehran, Iran) according to the manufacturer’s instructions. Blood samples were incubated with lysis buffer, and then DNA selectively precipitated. The insoluble DNA has washed and desalted by wash buffer and it was stored at -20°C pending simultaneous analysis.
Polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) analysis.26,27
The Arg194Trp (C27157T) XRCC1 SNP was identified using PCR restriction fragment length polymorphism. The PCR reaction was performed in a final volume of 25 µL using PCR Master mix kit (CinnaGen Inc, Tehran, Iran), 10 pmol of each primer with final concentration of 400 nM, and 100 ng DNA. Two primers were used to amplify a fragment of 491bp of exon 6-XRCC1 gene. The XRCC1-Exon 6 forward primer was 5¢-GCCCCGTCCCAGGTA-3¢, and XRCC1-Exon 6 reverse primer was 5¢-AGTGAAAGGGTCTTGGGGCT-3¢. The PCR conditions was: 5 min at 95°C (initial denaturation), followed by 45 cycles of 95°C for 45 seconds (denaturation), 58°C for 45 seconds (annealing) and 72°C for 45s using an Eppendorf Mastercycler (Eppendorf AG, Hamburg, Germany). In each PCR run, samples with no DNA template were used as negative controls. Amplified DNA fragments (491 bp) were cut by restriction enzyme HpaII (Jena Bioscience, Germany) for 30 min at 37°C. The genotypes were identified by electrophoresis of DNA fragments generated after digestion (2 bands: 198 and 293 bp for Arg/Arg genotype (27157 CC), one band: 491 bp for Trp/Trp genotype (27157 TT) and three bands: 491bp, 198 bp and 293 bp for heterozygous (27157 CT) genotype).
Statistical analysis
Data was analyzed by SAS 9.2 and one-sample Kolmogorov-Smirnov test was applied to determine normal distribution of data. Results were presented as Mean±SD and independent samples T-test used to compare mean differences. One-way analysis of variance followed by Post Hoc, Tukey, and Dunnett tests was used to analyze differences between groups and the p-value <0.05 was considered as significant. For variables with abnormal distribution equivalent non-parametric tests, Kruskal-Wallis and Chi-Square were performed.
Results
Clinical characteristics of patients with invasive breast cancer are shown in Table 1. Eighty-eight patients had IDC and 12 had ILC. Histological grading was evaluated for all patients. Of 100 breast cancer patients, 12 cases were low grade, 60 cases were intermediate grade and 28 cases were high grade. Modalities of treatment were surgery (60 patients), chemotherapy regimen (88 patients), and radiotherapy (59 patients), as needed.
Table 1.
Clinical outcomes of breast cancer patients.

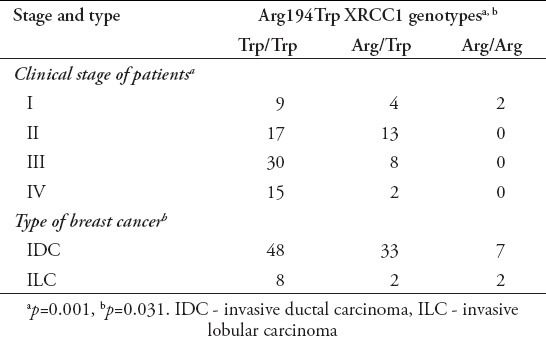
A total of 100 women with breast cancer (age: 47.13±8.4 years) and 200 healthy subjects (age: 46.8±7.3 years) evaluated for Arg194Trp XRCC1 gene polymorphism. Figure 1 shows the results of XRCC1 Arg194Trp SNP genotyping in studied groups. Moreover, according to our study, Trp allele of XRCC1 Arg194Trp polymorphism was associated with the IDC (p<0.05). Furthermore, there was a significant correlation between Trp allele of XRCC1 Arg194Trp polymorphism and clinical staging of breast cancer patients (p<0.05) (Table 2).
Figure 1.
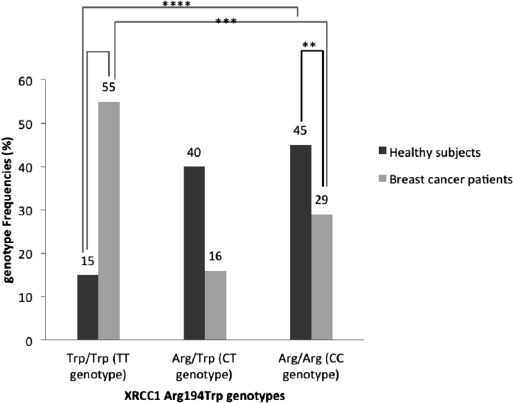
Prevalence of Arg194Trp x-ray repair cross-complementing group 1 (XRCC1) genotypes in studied subjects. The prevalence of Arg194Trp XRCC1 gene polymorphism was statistically significant (p<0.05), *between the Trp/Trp (TT genotype) in patients and controls, **between Arg/Arg (CC genotype) in patients and controls, ***between Trp/Trp (TT genotype) and Arg/Arg (CC genotype) in patients, ****between Trp/Trp (TT genotype) and Arg/Arg (CC genotype) in controls
Table 2.
Correlation of Arg194 x-ray repair cross-complementing group 1 (XRCC1) genotype with stage and type of breast cancer.
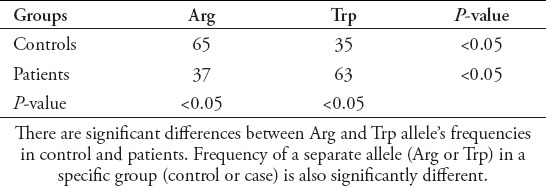
Genotype distribution for controls (p=0.23), but not for patients (p<0.0001) were in agreement with the Hardy-Weinberg equilibrium. The Trp/Trp genotype was detected more frequently in breast cancer patients than in controls and the difference was significant (62% versus 2%, p<0.05). The rate of Trp and Arg alleles of XRCC1 gene in the patients and control groups were 63 and 35 % for Trp allele, and 37 and 65 % for Arg allele, respectively (Table 3). Furthermore, the most frequent genotype of Arg194Trp SNP of XRCC1 gene in patients was the Trp/Trp (55%) followed by Arg/Arg (29%), and Arg/Trp (16 %), while the frequencies of these genotypes in healthy subjects were 15% for Trp/Trp, 45% for Arg/Arg, and 40% for Arg/Trp. The Trp allele of Arg194Trp XRCC1 gene was associated with breast cancer risk (odds ratio [OR]: 3.1622, 95% confident intervals: 1.7747-5.6344, Z statistic: 3.9006, p=0.0001).
Table 3.
X-ray repair cross-complementing group 1 alleles frequency in studied groups.
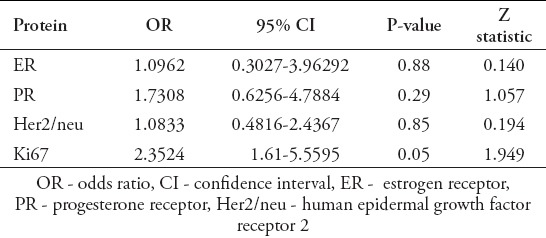
In the second part of our study, we assessed the correlation between ER, PR, Her2/neu and Ki67 protein expression and the XRCC1 Arg194Trp genotypes. In regards to Trp allele of XRCC1 Arg194Trp genotypes, the calculated OR for ER protein expression was 1.0962 and PR was 1.7308. The corresponding OR for Ki67 was 2.3524 and Her2/neu was 1.0833. According to the results, Trp genotype of codon 194 of XRCC1 gene has statistically significant association with Ki67 protein expression in breast tumors. However, our results showed that there is not any significant difference between XRCC1 Arg194Trp polymorphism and ER, PR and Her2/neu proteins expression (Table 4).
Table 4.
Association of Trp genotype of Arg194Trp SNP of x-ray repair cross-complementing group 1 (XRCC1)with protein expression in breast tumors.
Discussion
In the present study, for the first time we determined the association of XRCC1 Arg194Trp polymorphism and risk of breast cancer and with clinical outcomes of breast cancer in a Kurdish population. There are too many studies, which have been conducted in regards to the role of Arg194Trp of XRCC1 gene polymorphism and the risk of breast cancer.28,29 In line with previous studies,30,31 our results demonstrated the potential role of Trp variant of codon 194 XRCC1 gene as a potential risk factor of breast cancer. There are some studies in regards to Arg194 XRCC1 role in breast cancer. For example, in a Polish population Trp194 XRCC1 allele has associated with breast cancer.32 In addition, Smith et al,33 reported a weak association of the Trp194 allele with a risk of breast cancer occurrence in white women. Furthermore, some studies did not find any correlation between polymorphism of codon 194 XRCC1 and risk of breast cancer.34 Taking together, these findings reveal that there is not an overall agreement to the effect of Arg194Trp XRCC1 polymorphism on breast cancer. It has been found that the distribution of Arg194Trp polymorphism is significantly influenced by ethnicity.13 The frequency of the Trp allele of XRCC1 Arg194Trp was higher in Asian populations than in African and Caucasian populations. African and Caucasian populations showed higher Arg allele frequencies.13 In our study, the frequency of Trp allele was higher in patients group, this genotype had a great correlation with risk of breast cancer.
Codon 194 XRCC1 was located on the highly conserved region. This region is a protein interacting domain and has an important role in base excision DNA repair mechanism. Therefore, amino acid substitution in this region could greatly alter the XRCC1 capability to interact with other DNA repair protein components of BER complex.32 However, laboratory experiments have failed to reach an agreement on the functional effects of the Arg194Trp polymorphism. Previous studies35 which have been conducted by the mutagen sensitivity assay suggest that individuals with the Trp194 allele exhibited a significantly higher frequency of chromatid exchanges than did those with the Arg194 allele. It has been showed that patients with the Arg194Arg genotype have less tolerance to genotoxic agents, such as lymphocyte exposure to bleomycin and benzo [a] pyrene-diol-epoxide, than patients with the194Trp.14 Furthermore, it has been observed that patients with the Arg194Arg genotype exhibited significantly higher levels of chromosomal breaks than those with the Trp194 allele.36 Our results showed no statistical association between the Arg194Trp XRCC1 gene polymorphism and ER, PR or Her2/neu proteins expression in breast tumor tissues. However, our data suggested a potential association between Trp194 variant XRCC1 gene and Ki67 protein expression. Dufloth et al37 evaluated the association between the XRCC1, XPD, XRCC3, and RAD51 gene polymorphisms and ER, or PR expression. However, similar to our results, they could not find any significant association. The Ki67 protein is a cellular marker of cell proliferation and currently included in panel test of histological evaluation of breast cancer.38 The direct correlation between the mutation of cell cycle regulatory genes, such as P53, and Ki67 protein expression39 and the association of XRCC1 polymorphism with P53 gene mutations40 have been separately studied. In the present study, we show the direct relationship between Trp allele of codon 194 XRCC1 and Ki67 protein expression in breast tumor tissues. Although we did not evaluate the P53 in this study, but it has been proved that gene mutations of P53 potentially diminish the tumor inhibitory effect of P53 protein and Arg194Trp polymorphism of XRCC1 increase the mutations of P53 gene. The P53 mutations result in higher cell proliferation and increasing Ki67 protein expression in tumor tissues. Finally, our results show that there is an association between the stage and type of breast cancer and Arg194Trp XRCC1 genotypes.
Study limitation
We did not study the other important SNPs in XRCC1 gene. Hence, future studies with different population for evaluating the relationship between other XRCC1 gene polymorphisms and breast cancer are suggested.
In conclusion, we show the high frequency of Trp194 allele of XRCC1 gene in Kurdish patients with breast cancer and Trp194 allele of XRCC1 could be proposed as a potential risk factor in Kurdish patients with breast cancer. Moreover, despite no correlation between Trp194 allele with ER, PR and Her2/neu, it has a direct association with Ki67 protein expression in breast cancer tissues. Further studies with greater population should be carried out to ascertain the relation between XRCC1 genetic polymorphisms, and Ki67 protein.
Footnotes
Statistics.
Excerpts from the Uniform Requirements for Manuscripts Submitted to Biomedical Journals updated November 2003.
Available from www.icmje.org
Describe statistical methods with enough detail to enable a knowledgeable reader with access to the original data to verify the reported results. When possible, quantify findings and present them with appropriate indicators of measurement error or uncertainty (such as confidence intervals). Avoid relying solely on statistical hypothesis testing, such as the use of P values, which fails to convey important information about effect size. References for the design of the study and statistical methods should be to standard works when possible (with pages stated). Define statistical terms, abbreviations, and most symbols. Specify the computer software used.
References
- 1.Benson JR, Jatoi I. The global breast cancer burden. Future Oncol. 2012;8:697–702. doi: 10.2217/fon.12.61. [DOI] [PubMed] [Google Scholar]
- 2.Mousavi SM1, Montazeri A, Mohagheghi MA, Jarrahi AM, Harirchi I, Najafi M, et al. Breast cancer in Iran: an epidemiological review. Breast J. 2007;13:383–391. doi: 10.1111/j.1524-4741.2007.00446.x. [DOI] [PubMed] [Google Scholar]
- 3.Jafari-Koshki T, Schmid VJ, Mahaki B. Trends of breast cancer incidence in Iran during 2004-2008: a Bayesian space-time model. Asian Pac J Cancer Prev. 2014;15:1557–1561. doi: 10.7314/apjcp.2014.15.4.1557. [DOI] [PubMed] [Google Scholar]
- 4.Reyna C, Lee MC. Breast cancer in young women: special considerations in multidisciplinary care. J Multidiscip Healthc. 2014;7:419–429. doi: 10.2147/JMDH.S49994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Toriola AT, Colditz GA. Trends in breast cancer incidence and mortality in the United States: implications for prevention. Breast Cancer Res Treat. 2013;138:665–673. doi: 10.1007/s10549-013-2500-7. [DOI] [PubMed] [Google Scholar]
- 6.Gannon LM, Cotter MB, Quinn CM. The classification of invasive carcinoma of the breast. Expert Rev Anticancer Ther. 2013;13:941–954. doi: 10.1586/14737140.2013.820577. [DOI] [PubMed] [Google Scholar]
- 7.Rakha EA, Ellis IO. Modern classification of breast cancer: should we stick with morphology or convert to molecular profile characteristics. Adv Anat Pathol. 2011;18:255–267. doi: 10.1097/PAP.0b013e318220f5d1. [DOI] [PubMed] [Google Scholar]
- 8.Cleator S, Heller W, Coombes RC. Triple-negative breast cancer: therapeutic options. Lancet Oncol. 2007;8:235–244. doi: 10.1016/S1470-2045(07)70074-8. [DOI] [PubMed] [Google Scholar]
- 9.Dai L, Wang K, Zhang J, Lv Q, Wu X, Wang Y. XRCC1 gene polymorphisms and esophageal squamous cell carcinoma risk in Chinese population: A meta-analysis of case-control studies. Int J Cancer. 2009;125:1102–11029. doi: 10.1002/ijc.24446. [DOI] [PubMed] [Google Scholar]
- 10.Yu H, Fu C, Wang J, Xue H, Xu B. Interaction between XRCC1 polymorphisms and intake of long-term stored rice in the risk of esophageal squamous cell carcinoma: a case-control study. Biomed Environ Sci. 2011;24:268–274. doi: 10.3967/0895-3988.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 11.Hu Z, Ma H, Chen F, Wei Q, Shen H. XRCC1 polymorphisms and cancer risk: a meta-analysis of 38 case-control studies. Cancer Epidemiol Biomarkers Prev. 2005;14:1810–1818. doi: 10.1158/1055-9965.EPI-04-0793. [DOI] [PubMed] [Google Scholar]
- 12.Vodicka P, Stetina R, Polakova V, Tulupova E, Naccarati A, Vodickova L, et al. Association of DNA repair polymorphisms with DNA repair functional outcomes in healthy human subjects. Carcinogenesis. 2007;28:657–664. doi: 10.1093/carcin/bgl187. [DOI] [PubMed] [Google Scholar]
- 13.Takeshita H, Fujihara J, Yasuda T, Kimura-Kataoka K. Worldwide distribution of four snps in x-ray and repair and cross-complementing group 1 (XRCC1) Clin Transl Sci. 2014;8:347–350. doi: 10.1111/cts.12237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang Y, Spitz MR, Zhu Y, Dong Q, Shete S, Wu X. From genotype to phenotype: correlating XRCC1 polymorphisms with mutagen sensitivity. DNA Repair (Amst) 2003;2:901–908. doi: 10.1016/s1568-7864(03)00085-5. [DOI] [PubMed] [Google Scholar]
- 15.Metsola K, Kataja V, Sillanpää P, Siivola P, Heikinheimo L, Eskelinen M, et al. XRCC1 and XPD genetic polymorphisms, smoking and breast cancer risk in a Finnish case-control study. Breast Cancer Res. 2005;7:R987–R997. doi: 10.1186/bcr1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pachkowski BF, Winkel S, Kubota Y, Swenberg JA, Millikan RC, Nakamura J. XRCC1 genotype and breast cancer: functional studies and epidemiologic data show interactions between XRCC1 codon 280 His and smoking. Cancer Res. 2006;66:2860–2868. doi: 10.1158/0008-5472.CAN-05-3388. [DOI] [PubMed] [Google Scholar]
- 17.Patel AV, Calle EE, Pavluck AL, Feigelson HS, Thun MJ, Rodriguez C. A prospective study of XRCC1 (X-ray cross-complementing group 1) polymorphisms and breast cancer risk. Breast Cancer Res. 2005;7:R1168–R1173. doi: 10.1186/bcr1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Macías-Gómez NM, Peralta-Leal V, Meza-Espinoza JP, Gutiérrez-Angulo M, Durán-González J, Ramírez-González JM, et al. Polymorphisms of the XRCC1 gene and breast cancer risk in the Mexican population. Fam Cancer. 2015;14:349–354. doi: 10.1007/s10689-015-9787-y. [DOI] [PubMed] [Google Scholar]
- 19.Saadat M, Kohan L, Omidvari S. Genetic polymorphisms of XRCC1 (codon 399) and susceptibility to breast cancer in Iranian women, a case-control study. Breast Cancer Res Treat. 2008;111:549–553. doi: 10.1007/s10549-007-9811-5. [DOI] [PubMed] [Google Scholar]
- 20.Elston CW, Ellis IO. Pathological prognostic factors in breast cancer I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology. 2002;41:154–161. [PubMed] [Google Scholar]
- 21.Edge S, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A, editors. AJCC cancer staging manual. 7th ed. New York (NY): Springer-Verlag; 2010. [Google Scholar]
- 22.Chambers JT, Carcangiu ML, Voynick IM, Schwartz PE. Immunohistochemical evaluation of estrogen and progesterone receptor content in 183 patients with endometrial carcinoma. Part II: Correlation between biochemical and immunohistochemical methods and survival. Am J Clin Pathol. 1990;94:255–260. doi: 10.1093/ajcp/94.3.255. [DOI] [PubMed] [Google Scholar]
- 23.Carcangiu ML, Chambers JT, Voynick IM, Pirro M, Schwartz PE. Immunohistochemical evaluation of estrogen and progesterone receptor content in 183 patients with endometrial carcinoma. Part I: Clinical and histologic correlations. Am J Clin Pathol. 1990;94:247–254. doi: 10.1093/ajcp/94.3.247. [DOI] [PubMed] [Google Scholar]
- 24.Ahmadi A, Poorfathollah AA, Aghaiipour M, Rezaei M, Nikoo-ghoftar M, Abdi M, et al. Diagnostic value of CD117 in differential diagnosis of acute leukemias. Tumor Biology. 2014;35:6763–6768. doi: 10.1007/s13277-014-1899-8. [DOI] [PubMed] [Google Scholar]
- 25.Maroofi F, Amini S, Roshani D, Ghaderi B, Abdi M. Different frequencies and effects of ABCB1 T3435C polymorphism on clinical and laboratory features of B cell chronic lymphocytic leukemia in Kurdish patients. Tumor Biology. 2015;36:2863–2868. doi: 10.1007/s13277-014-2914-9. [DOI] [PubMed] [Google Scholar]
- 26.Salimizand H, Amini S, Abdi M, Ghaderi B, Azadi N-A. Concurrent effects of ABCB1, C3435T, ABCG2, C421A and XRCC1 Arg194Trp genetic polymorphisms with risk of cancer, clinical output, and response to treatment with imatinib mesylate in patients with chronic myeloid leukemia. Tumor Biology. 2016;37:791–798. doi: 10.1007/s13277-015-3874-4. [DOI] [PubMed] [Google Scholar]
- 27.Ghafouri H, Ghaderi B, Amini S, Nikkhoo B, Abdi M, Hoseini A. Association of ABCB1 and ABCG2 single nucleotide polymorphisms with clinical findings and response to chemotherapy treatments in Kurdish patients with breast cancer. Tumour Biol. 2015 Dec;:1–6. doi: 10.1007/s13277-015-4679-1. [DOI] [PubMed] [Google Scholar]
- 28.Bu T, Liu L, Sun Y, Zhao L, Peng Y, Zhou S, et al. XRCC1 Arg399Gln polymorphism confers risk of breast cancer in American population: a meta-analysis of 10846 cases and 11723 controls. PLoS One. 2014;9:86086. doi: 10.1371/journal.pone.0086086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tengstrom M, Mannermaa A, Kosma VM, Hirvonen A, Kataja V. XRCC1 rs25487 polymorphism predicts the survival of patients after postoperative radiotherapy and adjuvant chemotherapy for breast cancer. Anticancer Res. 2014;34:3031–3037. [PubMed] [Google Scholar]
- 30.Feng YZ, Liu YL, He XF, Wei W, Shen XL, Xie DL. Association between the XRCC1 Arg194Trp polymorphism and risk of cancer: evidence from 201 case-control studies. Tumour Biol. 2014;35:10677–97. doi: 10.1007/s13277-014-2326-x. [DOI] [PubMed] [Google Scholar]
- 31.Ginsberg G, Angle K, Guyton K, Sonawane B. Polymorphism in the DNA repair enzyme XRCC1: utility of current database and implications for human health risk assessment. Mutat Res. 2011;727:1–15. doi: 10.1016/j.mrrev.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 32.Przybylowska-Sygut K, Stanczyk M, Kusinska R, Kordek R, Majsterek I. Association of the Arg194Trp and the Arg399Gln polymorphisms of the XRCC1 gene with risk occurrence and the response to adjuvant therapy among Polish women with breast cancer. Clin Breast Cancer. 2013;13:61–68. doi: 10.1016/j.clbc.2012.09.019. [DOI] [PubMed] [Google Scholar]
- 33.Smith TR, Miller MS, Lohman K, et al. Polymorphisms of XRCC1 and XRCC3 genes and susceptibility to breast cancer. Cancer Lett. 2003;190:183–190. doi: 10.1016/s0304-3835(02)00595-5. [DOI] [PubMed] [Google Scholar]
- 34.Sliwinski T, Krupa R, Wisniewska-Jarosinska M, Lech J, Morawiec Z, Chojnacki J, et al. No association between the Arg194Trp and Arg399Gln polymorphisms of the XRCC1 gene and colorectal cancer risk and progression in a Polish population. Exp Oncol. 2008;30:253–254. [PubMed] [Google Scholar]
- 35.Au WW, Salama SA, Sierra-Torres CH. Functional characterization of polymorphisms in DNA repair genes using cytogenetic challenge assays. Environ Health Perspect. 2003;111:1843–1850. doi: 10.1289/ehp.6632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tuimala J, Szekely G, Wikman H, Järventaus H, Hirvonen A, Gundy S, et al. Genetic polymorphisms of DNA repair and xenobiotic-metabolizing enzymes: effects on levels of sister chromatid exchanges and chromosomal aberrations. Mutat Res. 2004;554:319–333. doi: 10.1016/j.mrfmmm.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 37.Dufloth RM, Arruda A, Heinrich JK, Schmitt F, Zeferino LC. The investigation of DNA repair polymorphisms with histopathological characteristics and hormone receptors in a group of Brazilian women with breast cancer. Genet Mol Res. 2008;7:574–582. doi: 10.4238/vol7-3gmr376. [DOI] [PubMed] [Google Scholar]
- 38.Scholzen T, Gerdes J. The Ki-67 protein: from the known and the unknown. J Cell Physiol. 2000;182:311–322. doi: 10.1002/(SICI)1097-4652(200003)182:3<311::AID-JCP1>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 39.Gissi DB, Gabusi A, Servidio D, Cervellati F, Montebugnoli L. Predictive role of p53 protein as a single marker or associated with ki67 antigen in oral leukoplakia: A retrospective longitudinal study. Open Dent J. 2015;9:41–45. doi: 10.2174/1874210601509010041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hsieh LL, Chien HT, Chen IH, Liao CT, Wang HM, Jung SM, et al. The XRCC1 399Gln polymorphism and the frequency of p53 mutations in Taiwanese oral squamous cell carcinomas. Cancer Epidemiol Biomarkers Prev. 2003;12:439–443. [PubMed] [Google Scholar]


