Abstract
Objectives:
To assess the significance of thyroid autoimmune screening in alopecia areata (AA) patients in Saudi population, and to determine whether there is a difference in thyroid autoimmune susceptibility between mild and severe AA.
Methods:
In a prospective case-control study, we included 50 alopecia totalis (AT) and alopecia universalis (AU) patients, 50 age- and gender-matched patients with localized AA, and 50 age- and gender- matched healthy subjects between March 2015 and August 2015. Patients with AA were consecutively recruited from the hair disorders out-patient clinic of King Khalid University Hospital, Riyadh, Kingdom of Saudi Arabia.
Results:
Thyroid autoantibodies (TAAs) were positive in AT/AU (40%), mild AA (14%), and healthy subjects (4%). The frequency of TAAs was significantly higher in patients with AT/AU than in mild AA (p=0.001) and healthy controls (p<0.001). The frequency of thyroid peroxidase antibody (TPO-Abs) was significantly higher in patients with AT/AU than in mild AA and healthy controls (p<0.001 for both). The frequency of TG-Abs was significantly higher in patients with AT/AU (p=0.003) and mild AA (p=0.043) than in healthy controls. Serum TSH level was significantly higher in AT/AU patients than in mild AA patients (p=0.006) and healthy controls (p=0.005).
Conclusion:
Severe subtype of AA is associated with a high risk of autoimmune thyroid disease. This highlights the significance of screening for thyroid abnormalities and TAAs in patients with AT/AU.
Alopecia areata (AA) is the most frequent cause of inflammation-induced hair loss, with a reported incidence of 0.1-0.2% and a lifetime risk of 1.7%. Alopecia areata is usually manifested as patchy hair loss in oval-shaped areas, most commonly on the scalp. Sometimes, AA can progress into severe forms named alopecia totalis (AT), which involves the whole scalp hair and alopecia universalis (AU), which involves the whole body hair.1 Currently available evidence suggests that AA is a T-cell mediated organ-specific auto-immune disease with genetic predisposition and environmental trigger.1 Alopecia areata is associated with an increased overall risk of autoimmune disorders including vitiligo, psoriasis, celiac disease, lupus erythematosus, and diabetes mellitus, as well as chronic inflammatory diseases including atopy.2,4 The reported prevalence of thyroid diseases among AA patients is ranging between 0% and 28%.1,4-6 There is marked inconsistency of findings among the published data. Until now, there is no well-designed controlled study confirming that thyroid autoimmunity (TAI) is pathogenic, or related to severity of hair loss in AA. However, AA is believed to be associated with thyroid autoantibodies (TAAs) as an autoimmune phenomenon.1 The purpose of the present study was to assess the significance of thyroid autoimmune screening in AA patients in Saudi population, and to determine whether there is a difference in thyroid autoimmune susceptibility between mild and severe AA.
Methods
In a prospective case-control study, we included 50 patients presenting with severe AA, 50 age- and gender- matched mild AA patients, and age- and gender- matched control group of 50 healthy subjects. Patients with AA were consecutively recruited from the hair disorders out-patient clinic of King Khalid University Hospital, Riyadh, Saudi Arabia between March 2015 and August 2015. Diagnosis of AA was made based on clinical ground. Patients were included in the severe AA group if they were having AT and AU; and in the mild AA group if having <3 alopecic patches with a widest diameter of <3 cm.7 Patients were excluded if having other forms of AA, or on any thyroid related medications. All patients were subjected to thorough history taking and cutaneous examination. They were also screened for thyroid dysfunction by means of serum thyroid stimulating hormone (TSH), free thyroxine (FT4), and for the presence of TAAs by mean of thyroid peroxidase autoantibodies (TPO-Abs) and thyroglobulin autoantibodies (TG-Abs). This study was approved by the Institutional Review Board, College of Medicine, King Saud University, Riyadh, Saudi Arabia. This study was performed in accordance with the ethical standards laid down in the Declaration of Helsinki. All volunteers provided written informed consent and were free to withdraw from the study at any time.
Serum assay
Serum TSH and FT4 were measured using electrochemiluminescence immunoassay (Cobas e411 immunoassay analyzer, Roche Diagnostics, Mannheim, Germany). The reference values were 0.25-5.0 µIU/ml for TSH, and 10.3-25.8 pmol/L for FT4. Patients were diagnosed to have overt hypothyroidism when TSH was >5.0 µIU/ml and FT4 <10.3 pmol/L, while subclinical hypothyroidism when TSH>5.0 µIU/ml with normal FT4. Patients were diagnosed to have overt hyperthyroidism when TSH was <0.25 µIU/ml and FT4 >25.8 pmol/L; while subclinical hyperthyroidism when TSH was <0.25 µIU/ml with normal FT4. Thyroglobulin autoantibodies and TPO-Abs were measured by antibody agglutination test (Serodia-ATG and Serodia-AMC, Fujirebio Inc., Tokyo, Japan). Sera were considered negative for TG-Abs and TPO-Abs when agglutination did not occur at a dilution of 1:100. Patients were diagnosed with TAI when titer of TG-Abs, or TPO-Abs were positive.
Statistical analysis
All statistical analyses were performed using IBM SPSS Statistics, version 22 (IBM Corp, Armonk, NY, USA). Descriptive statistics for quantitative variables were presented as mean ± standard deviation. The significance of differences was assessed using an independent t test for all variables between 2 groups. Analysis of variance with Post-Hoc test was used to determine significance between groups. Statistical significance was set at p<0.05.
Results
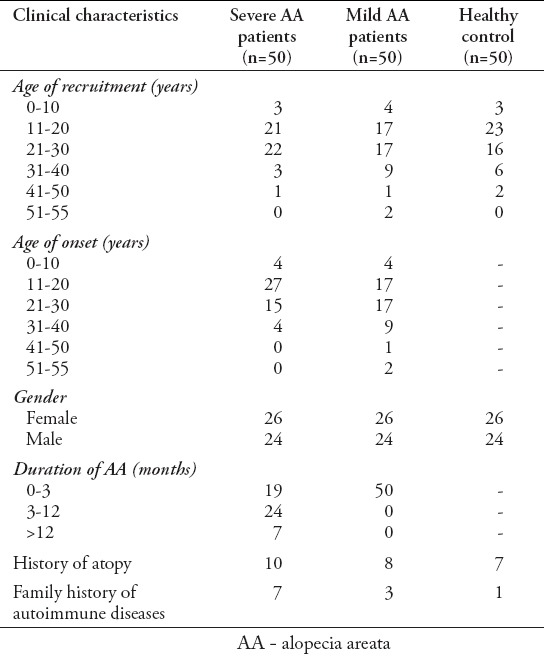
Fifty severe AA patients (26 women, 24 men) aged between 10 and 43 years (mean age 21.5±7.7 years), 50 mild AA patients (26 women, 24 men) aged between 6 and 54 years (mean age 23.9±10.4 years), and 50 healthy subjects (26 women, 24 men) aged between 8 and 47 years (mean age 22.4±7.5 years) participated in this study (Table 1).
Table 1.
Clinical characteristics of 50 patients with severe AA, 50 age- and gender- matched mild AA patients, and age- and gender- matched control group of 50 healthy subjects.
Thyroid autoimmunity
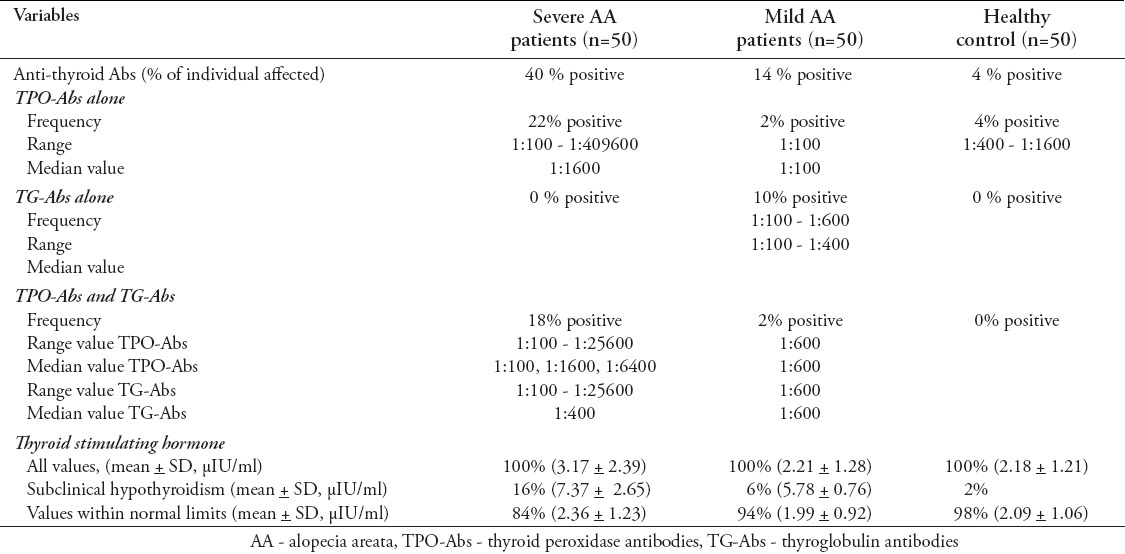
Thyroid autoantibodies were positive in 40% of severe AA, 14% of mild AA, and 4% of healthy subjects (Table 2). The frequency of TAAs was significantly higher in patients with AA (27%) than in healthy controls (4%) (p<0.001). There was also a significant difference in frequency of TAAs between patients with severe AA (40%), patients with mild AA (14%), and healthy controls (4%) (p<0.001). The frequency of TAAs was significantly higher in patients with severe AA than in mild AA (p=0.001) and healthy controls (p<0.001). However, no significant difference in frequency of TAAs was noted between mild AA patients and healthy controls (p=0.177). Among AA patients with TAAs, the presence of TPO-Abs seems to be slightly predominant (Table 2). The frequency of TPO-Abs was significantly higher in patients with AA (22%) than in healthy controls (4%) (p=0.002). There was a significant difference in frequency of TPO-Abs between patients with severe AA (40%), patients with mild AA (4%) and healthy controls (4%) (p<0.001). The frequency of TPO-Abs was significantly higher in patients with severe AA than in mild AA and healthy controls (p<0.001 for both). However, there was no significant difference in frequency of TPO-Abs was noted between mild AA patients and healthy controls (p=1). The frequency of TG-Abs was significantly higher in patients with AA (15%) than in healthy controls (0%) (p=0.002). There was a significant difference in frequency of TG-Abs between patients with severe AA (18%), patients with mild AA (2%), and healthy controls (0%) (p=0.009). The frequency of TG-Abs was significantly higher in patients with severe AA and mild AA (p=0.003) than in healthy controls (p=0.043). However, no significant difference in frequency of TG-Abs was noted between severe AA patients and mild AA patients (p=0.308).
Table 2.
Comparison of anti-thyroid antibodies and thyroid function between severe AA patients, mild AA patients and healthy controls.
Thyroid function
Table 2 shows thyroid function test of the study subjects. Serum TSH was significantly higher in AA patients (2.69±1.97 µIU/ml) than in healthy controls (2.18±1.21 µIU/ml) (p=0.047). There was a significant difference in serum TSH level between severe AA patients, mild AA patients, and healthy controls (p=0.006). Serum TSH level was significantly higher in severe AA patients than in mild AA patients and healthy controls (p=0.006, and p=0.005). However, no significant difference in serum TSH level was noted between mild AA patients and healthy controls (p=0.916). Serum FT4 levels were comparable in all groups with no significant difference between healthy controls and AA patients (p=0.24); nor between healthy controls, mild AA patients, and severe AA patients (p=0.775).
Discussion
This study proves that the frequency of TAAs and serum TSH level were significantly higher in patients with AT and AU than in localized AA patients. The overall TAA positivity was 40% (18% were positive for both TG-Abs and TPO-Abs; and 22% were positive for TPO-Abs only) in patients with AT/AU. This study also confirmed the existence of differences in serum TSH level and TAAs frequency between AA patients and healthy controls. However, mild AA patients were comparable to healthy controls in terms of TSH level and TPO-Abs frequency. Autoimmune thyroid disease (ATD) is one of the most common autoimmune diseases, affecting 2-5% of the general population.8 The development of TPO-Abs, TG-Abs, and anti-TSH antibodies is the main feature of ATD.9 Thyroid peroxidase autoantibodies was defined as the most sensitive marker of TAI.10 Thyroid peroxidase autoantibodiesplay a key role in thyroid hormone synthesis.8-10 The presence of TPO-Abs increases the risk of developing (sub) clinical hypothyroidism and also associated with hypo- or hyperthyroidism, which is not the case for TG-Abs.10 Thyroid peroxidase autoantibodies are present in 90% of patients with Hashimoto’s thyroiditis.10 In a large epidemiological survey performed in individuals without thyroid disease, or any treatment that could have influenced thyroid function, TPO-Abs was detected in 4.4%, TG-Abs was detected in 3.4%, and both TPO-Abs and TG-Abs were detected in 6.9% of the subjects. Thyroid stimulating hormone and the prevalence of TAAs were greater in females and increase with age.11 Similar findings were reported in women of reproductive age.10-12 It is unknown why people develop TAAs, nor why not all individuals with TAI develop clinical thyroid disease. It is estimated that approximately 70% of the susceptibility to develop TAAs is due to genetic factors. Recently, Medici et al8 identified 5 genetic loci associated with TPO-Abs, 3 of which were also associated with clinical thyroid disease. Although several studies have examined the association between TAI and AA risk, no complete consensus exists about the prevalence and risk of thyroid disease in AA. In previous studies,5,6,13-15 it was found that there is no association between ATD and AA risk. Those studies did not recommend the routine use of thyroid tests in AA patients, unless signs, or symptoms suggestive of thyroid disease are present. In contrast, an association between thyroid diseases and AA has been reported by other studies.1,16-21 The explanation for this inconsistency of findings may lie in variations in studies design and methodology including, but not limited to patient self-reported data, small population size, homogeneous populations, or disease heterogeneity in term of degree of disease activity and disease extent. Differences with respect to severity of AA have been well documented in the literature.1 Many studies were reported that individuals with AT/AU have longer duration and younger age of onset of disease than those with patchy AA.22-25 Few studies16,17,25 evaluated TAI in relation to severity of AA and they found no correlation between TAI and the severity of AA. However, our data shows that serum TSH level and frequency of TAAs are significantly higher in patients with severe AA than in mild AA patients. Bakry et al26 also found that levels of TPO-Abs and TG-Abs are significantly higher in severe AA compared with mild and moderate cases. In addition, Seirafi et al27 found that abnormal T3, T4, and TSH are significantly higher in AT/AU compared with healthy individuals. Another study28 used the National AA Registry database found that individuals with self-reported history of an autoimmune disease, or hypothyroidism were associated with an increased risk of AA, and this was consistent for both the severe subtype of AA (AT/AU) and the localized subtype (AA persistent). In addition, history of hypothyroidism, or an autoimmune disease were more common in patients with AU than localized persistent subtype of AA.28 Furthermore, Goh et al22 supported a general theory of 2 clinically distinct subtypes of AA; the patchy AA and the more severe AT/AU. The severe AT/AU subtype is more often associated with thyroid disease, atopic dermatitis, and extensive family history of AA. This association might be helpful in explaining the pathogenesis and genetic basis of the more severe clinical subtype of AA.22 This is supported by the association reports of several immune-related genes with the susceptibility to severe forms of AA including DRB1*0401 (DR4), DQB1*0301 (DQ7), HLA-DRB5, MICA, PTPN22, interleukin-1ß single-nucleotide polymorphisms C-511T, IL1RN, IL2RA, TNF/LTA locus, and TRAF1/C5.29-32 We planned to investigated thyroid autoimmunity status among different AA subtypes according to the onset age, but, unfortunately, few of our study population illnesses started in childhood. Based on the onset age in adolescence (10 years of age and above) only 8 patients were classified in an early-onset group and the other 92 patients in a late-onset group. The standard treatment of TAI is levothyroxine (LT4). Administration of low dose levothyroxine leads to TSH suppression and reduction of the exaggerated inflammatory response induced by TSH.33 Since TSH has been shown to induce direct T cell, B cell, and dendritic cell activation in addition to the release of proinflammatory cytokines, which trigger an inflammatory state in the thyroid gland and other tissues including the skin.33 In addition, treating patients with LT4 can decrease the levels of TPO-Ab and TG-Ab, while patients without LT4 substitution had a gradual increase of TPO-Ab and TG-Ab levels, as well as a tendency to gradual increase of TSH levels overtime leading to thyroid dysfunction.34-36 Another effective complementary treatment for TAI is the trace element Selenium. A recent systematic review found that Selenium supplementation is associated with a significant decrease in TPO-Ab titers at 6 and 12 months; meanwhile, the TG-Ab titers can be dropped at 12 months.37
In conclusion, the present study indicates a high risk of ATD in severe subtype of AA. Thyroid autoantibodies were found in a high percentage of AT/AU patients. These antibodies seem to dysregulate TSH secretion and the inflammatory state could mediate AT/AU clinical manifestation. This indicates the necessity of screening for thyroid abnormalities and TAAs in patients with AT/AU, which is of benefit for early diagnosis and treatment.
Footnotes
Clinical Practice Guidelines.
Clinical Practice Guidelines must include a short abstract. There should be an Introduction section addressing the objective in producing the guideline, what the guideline is about and who will benefit from the guideline. It should describe the population, conditions, health care setting and clinical management/diagnostic test. Authors should adequately describe the methods used to collect and analyze evidence, recommendations and validation. If it is adapted, authors should include the source, how, and why it is adapted? The guidelines should include not more than 50 references, 2-4 illustrations/tables, and an algorithm.
References
- 1.Alkhalifah A, Alsantali A, Wang E, McElwee KJ, Shapiro J. Alopecia areata update: part I. Clinical picture, histopathology, and pathogenesis. J Am Acad Dermatol. 2010;62:177–188. doi: 10.1016/j.jaad.2009.10.032. [DOI] [PubMed] [Google Scholar]
- 2.Gilhar A, Etzioni A, Paus R. Alopecia areata. N Engl J Med. 2012;366:1515–1525. doi: 10.1056/NEJMra1103442. [DOI] [PubMed] [Google Scholar]
- 3.Bertolini M, Gilhar A, Paus R. Alopecia areata as a model for T cell-dependent autoimmune diseases. Exp Dermatol. 2012;21:477–479. doi: 10.1111/j.1600-0625.2011.01427.x. [DOI] [PubMed] [Google Scholar]
- 4.Huang KP, Mullangi S, Guo Y, Qureshi AA. Autoimmune, atopic, and mental health comorbid conditions associated with alopecia areata in the United States. JAMA Dermatol. 2013;149:789–794. doi: 10.1001/jamadermatol.2013.3049. [DOI] [PubMed] [Google Scholar]
- 5.Puavilai S, Puavilai G, Charuwichitratana S, Sakuntabhai A, Sriprachya-Anunt S. Prevalence of thyroid diseases in patients with alopecia areata. Int J Dermatol. 1994;33:632–633. doi: 10.1111/j.1365-4362.1994.tb02921.x. [DOI] [PubMed] [Google Scholar]
- 6.Rahnama Z, Farajzadeh S, Mohamamdi S, Masoudi MA. Prevalence of thyroid disorders in patients with alopecia areata. J Pak Assoc Derma. 2014;24:246–250. [Google Scholar]
- 7.Kavak A, Baykal C, Ozarmağan G, Akar U. HLA in alopecia areata. Int J Dermatol. 2000;39:589–592. doi: 10.1046/j.1365-4362.2000.00921.x. [DOI] [PubMed] [Google Scholar]
- 8.Medici M, Porcu E, Pistis G, Teumer A, Brown SJ, Jensen RA, et al. Identification of novel genetic Loci associated with thyroid peroxidase antibodies and clinical thyroid disease. PLoS Genet. 2014;10:e1004123. doi: 10.1371/journal.pgen.1004123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khan FA, Al-Jameil N, Khan MF, Al-Rashid M, Tabassum H. Thyroid dysfunction: an autoimmune aspect. Int J Clin Exp Med. 2015;8:6677–6681. [PMC free article] [PubMed] [Google Scholar]
- 10.Unuane D, Velkeniers B, Anckaert E, Schiettecatte J, Tournaye H, Haentjens P, et al. Thyroglobulin autoantibodies: is there any added value in the detection of thyroid auto-immunity in women consulting for fertility treatment? Thyroid. 2013;23:1022–1028. doi: 10.1089/thy.2012.0562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hollowell JG, Staehling NW, Flanders WD, Hannon WH, Gunter EW, Spencer CA, et al. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III) J Clin Endocrinol Metab. 2002;87:489–499. doi: 10.1210/jcem.87.2.8182. [DOI] [PubMed] [Google Scholar]
- 12.Strieder TG, Prummel MF, Tijssen JG, Endert E, Wiersinga WM. Risk factors for and prevalence of thyroid disorders in a cross-sectional study among healthy female relatives of patients with autoimmune thyroid disease. Clin Endocrinol (Oxf) 2003;59:396–401. doi: 10.1046/j.1365-2265.2003.01862.x. [DOI] [PubMed] [Google Scholar]
- 13.Al-Khawajah M. Alopecia areata and associated diseases in Saudi patients. Ann Saudi Med. 1991;11:651–654. doi: 10.5144/0256-4947.1991.651. [DOI] [PubMed] [Google Scholar]
- 14.Serarslan G, Savaş N, Yenin JZ. Is atopy and autoimmunity more prevalent in patients with alopecia areata?A comparative study. J Eur Acad Dermatol Venereol. 2012;26:720–723. doi: 10.1111/j.1468-3083.2011.04152.x. [DOI] [PubMed] [Google Scholar]
- 15.Cunliffe WJ, Hall R, Stevenson CJ, Weightman D. Alopecia areata, thyroid disease and autoimmunity. Br J Dermatol. 1969;81:877–881. doi: 10.1111/j.1365-2133.1969.tb15967.x. [DOI] [PubMed] [Google Scholar]
- 16.Kasumagić-Halilović E. Thyroid autoimmunity in patients with alopecia areata. Acta Dermatovenerol Croat. 2008;16:123–125. [PubMed] [Google Scholar]
- 17.Seyrafi H, Akhiani M, Abbasi H, Mirpour S, Gholamrezanezhad A. Evaluation of the profile of alopecia areata and the prevalence of thyroid function test abnormalities and serum autoantibodies in Iranian patients. BMC Dermatol. 2005;5:11. doi: 10.1186/1471-5945-5-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baars MP, Greebe RJ, Pop VJ. High prevalence of thyroid peroxidase antibodies in patients with alopecia areata. J Eur Acad Dermatol Venereol. 2013;27:137–139. doi: 10.1111/j.1468-3083.2011.04420.x. [DOI] [PubMed] [Google Scholar]
- 19.Kasumagić-Halilović E, Prohić A, Ovčina-Kurtović N, Čavaljuga S. Structural and functional abnormalities of the thyroid gland in patients with alopecia areata. Medicinski zurnal. 2010;16:52–54. [Google Scholar]
- 20.Chu SY, Chen YJ, Tseng WC, Lin MW, Chen TJ, Hwang CY, et al. Comorbidity profiles among patients with alopecia areata: the importance of onset age, a nationwide population-based study. J Am Acad Dermatol. 2011;65:949–956. doi: 10.1016/j.jaad.2010.08.032. [DOI] [PubMed] [Google Scholar]
- 21.Noso S, Park C, Babaya N, Hiromine Y, Harada T, Ito H, et al. Organ specificity in auto-immune diseases: thyroid and islet autoimmunity in alopecia areata. J Clin Endocrinol Metab. 2015;100:1976–1983. doi: 10.1210/jc.2014-3985. [DOI] [PubMed] [Google Scholar]
- 22.Goh C, Finkel M, Christos PJ, Sinha AA. Profile of 513 patients with alopecia areata: associations of disease subtypes with atopy, autoimmune disease and positive family histo-ry. J Eur Acad Dermatol Venereol. 2006;20:1055–1060. doi: 10.1111/j.1468-3083.2006.01676.x. [DOI] [PubMed] [Google Scholar]
- 23.Tan E, Tay YK, Goh CL, Chin Giam Y. The pattern and profile of alopecia areata in Singapore-a study of 219 Asians. Int J Dermatol. 2002;41:748–753. doi: 10.1046/j.1365-4362.2002.01357.x. [DOI] [PubMed] [Google Scholar]
- 24.Green J, Sinclair RD. Genetics of alopecia areata. Australas J Dermatol. 2000;41:213–218. doi: 10.1046/j.1440-0960.2000.00439.x. [DOI] [PubMed] [Google Scholar]
- 25.Sharma VK, Dawn G, Kumar B. Profile of alopecia areata in northern India. Int J Dermatol. 1996;35:22–27. doi: 10.1111/j.1365-4362.1996.tb01610.x. [DOI] [PubMed] [Google Scholar]
- 26.Bakry OA, Basha MA, El Shafiee MK, Shehata WA. Thyroid disorders associated with alopecia areata in egyptian patients. Indian J Dermatol. 2014;59:49–55. doi: 10.4103/0019-5154.123494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seirafi H, Ehsani A, Hosseini M, Samavati B, Gholamali F, Noormohammadpour P. Comparison of thyroid function tests in alopecia totalis and universalis with control group. Tehran Univ Med J. 2013;71:238–243. [Google Scholar]
- 28.Barahmani N, Schabath MB, Duvic M. National Alopecia Areata Registry.History of atopy or autoimmunity increases risk of alopecia areata. J Am Acad Dermatol. 2009;61:581–591. doi: 10.1016/j.jaad.2009.04.031. [DOI] [PubMed] [Google Scholar]
- 29.Colombe BW, Lou CD, Price VH. The genetic basis of alopecia areata: HLA associations with patchy alopecia areata versus alopecia totalis and alopecia universalis. J Investig Dermatol Symp Proc. 1999;4:216–219. doi: 10.1038/sj.jidsp.5640214. [DOI] [PubMed] [Google Scholar]
- 30.Lee S, Paik SH, Kim HJ, Ryu HH, Cha S, Jo SJ, et al. Exomic sequencing of immune-related genes reveals novel candidate variants associated with alopecia universalis. PLoS One. 2013;8:e53613. doi: 10.1371/journal.pone.0053613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alfadhli S, Nanda A. Genetic analysis of interleukin-1 receptor antagonist and interleukin-1ßsingle-nucleotide polymorphisms C-511T and C+3953T in alopecia areata: susceptibility and severity association. Clin Exp Med. 2014;14:197–202. doi: 10.1007/s10238-013-0228-7. [DOI] [PubMed] [Google Scholar]
- 32.Redler S, Albert F, Brockschmidt FF, Herold C, Hanneken S, Eigelshoven S, et al. Investigation of selected cytokine genes suggests that IL2RA and the TNF/LTA locus are risk factors for severe alopecia areata. Br J Dermatol. 2012;167:1360–1365. doi: 10.1111/bjd.12004. [DOI] [PubMed] [Google Scholar]
- 33.Bumbacea RS, Popa LG, Orzan OA, Voiculescu VM, Giurcaneanu C. Clinical and therapeutic implications of the association between chronic urticaria and autoimmune thyroiditis. Acta Endocrinologica. 2014;10:595–604. [Google Scholar]
- 34.Padberg S, Heller K, Usadel KH, Schumm Draeger PM. One-year prophylactic treatment of euthyroid Hashimoto’s thyroiditis patients with levothyroxine: is there a benefit? Thyroid. 2001;11:249–255. doi: 10.1089/105072501750159651. [DOI] [PubMed] [Google Scholar]
- 35.Schmidt M, Voell M, Rahlff I, Dietlein M, Kobe C, Faust M, et al. Long-term follow-up of antithyroid peroxidase antibodies in patients with chronic autoimmune thyroiditis (Hashimoto’s thyroiditis) treated with levothyroxine. Thyroid. 2008;18:755–760. doi: 10.1089/thy.2008.0008. [DOI] [PubMed] [Google Scholar]
- 36.Aksoy DY, Kerimoglu U, Okur H, Canpinar H, Karaağaoğlu E, Yetgin S, et al. Effects of prophylactic thyroid hormone replacement in euthyroid Hashimoto’s thyroiditis. Endocr J. 2005;52:337–343. doi: 10.1507/endocrj.52.337. [DOI] [PubMed] [Google Scholar]
- 37.Fan Y, Xu S, Zhang H, Cao W, Wang K, Chen G, et al. Selenium supplementation for autoimmune thyroiditis: a systematic review and meta-analysis. Int J Endocrinol. 2014;2014:904573. doi: 10.1155/2014/904573. [DOI] [PMC free article] [PubMed] [Google Scholar]


