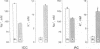Abstract
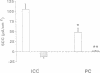
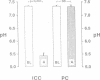
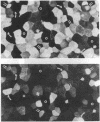
The renal collecting duct is a heterogenous epithelium consisting of intercalated cells (ICC) and principal cells (PC). The origin of this cellular heterogeneity is not clear. To test the hypothesis that the two cell types might originate from one another, pure populations of ICC (beta subtype) and PC were isolated by fluorescence-activated cell sorting and grown on permeable supports. After the monolayers reached confluence, the expression of ICC- and PC-specific functions and antigens was monitored. Cultures of sorted beta-ICC, in addition to expressing ICC-specific functions (such as an electrogenic H+ secretion) and antigens, progressively acquired PC functions (amiloride-sensitive Na+ transport and K+ secretion). On day 6, cultures of sorted beta-ICC exhibited a lumen-negative transepithelial potential difference of 83 +/- 4 mV and a short circuit current of 107 +/- 15 microA/cm2 and created a lumen-to-bath K+ concentration ratio of approximately 10. The percentage of cells staining with two PC-specific antibodies was 53% and 65%. On the other hand, cultures of sorted PC failed to acquire ICC-specific functions while maintaining PC characteristics. To rule out preferential proliferation of a few contaminating PC as an explanation of these results, we have generated a continuous collecting duct cell line (M-1) originating from mice transgenic for the early region of simian virus 40. Cell lines cloned from M-1 cells exhibit both PC and ICC functions and show mutually exclusive heterogenous expression of PC and ICC antigens, demonstrating a common origin of the two cell types. These data indicate that while beta-ICC in culture can give rise to both alpha-ICC and PC, PC cannot convert to ICC, which raises the possibility that beta-ICC is the stem cell in the renal collecting duct. Differentiation of ICC to PC may explain the cellular heterogeneity in the cortical collecting duct.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bastani B., Purcell H., Hemken P., Trigg D., Gluck S. Expression and distribution of renal vacuolar proton-translocating adenosine triphosphatase in response to chronic acid and alkali loads in the rat. J Clin Invest. 1991 Jul;88(1):126–136. doi: 10.1172/JCI115268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D., Hirsch S., Gluck S. An H+-ATPase in opposite plasma membrane domains in kidney epithelial cell subpopulations. Nature. 1988 Feb 18;331(6157):622–624. doi: 10.1038/331622a0. [DOI] [PubMed] [Google Scholar]
- Campbell S. M., Taha M. M., Medina D., Rosen J. M. A clonal derivative of mammary epithelial cell line COMMA-D retains stem cell characteristics of unique morphological and functional heterogeneity. Exp Cell Res. 1988 Jul;177(1):109–121. doi: 10.1016/0014-4827(88)90029-8. [DOI] [PubMed] [Google Scholar]
- Fejes-Tóth G., Náray-Fejes-Tóth A. Differentiated transport functions in primary cultures of rabbit collecting ducts. Am J Physiol. 1987 Dec;253(6 Pt 2):F1302–F1307. doi: 10.1152/ajprenal.1987.253.6.F1302. [DOI] [PubMed] [Google Scholar]
- Fejes-Tóth G., Náray-Fejes-Tóth A. Isolated principal and intercalated cells: hormone responsiveness and Na+-K+-ATPase activity. Am J Physiol. 1989 Apr;256(4 Pt 2):F742–F750. doi: 10.1152/ajprenal.1989.256.4.F742. [DOI] [PubMed] [Google Scholar]
- Hayashi M., Schuster V. L., Stokes J. B. Absence of transepithelial anion exchange by rabbit OMCD: evidence against reversal of cell polarity. Am J Physiol. 1988 Aug;255(2 Pt 2):F220–F228. doi: 10.1152/ajprenal.1988.255.2.F220. [DOI] [PubMed] [Google Scholar]
- Jakobsson B., Bohman S. O., Sundelin B., Aperia A. Mitotic response to high protein intake in different renal cell types in weanling rats. Kidney Int. 1988 Mar;33(3):662–666. doi: 10.1038/ki.1988.50. [DOI] [PubMed] [Google Scholar]
- Kirk K. L., Buku A., Eggena P. Cell specificity of vasopressin binding in renal collecting duct: computer-enhanced imaging of a fluorescent hormone analog. Proc Natl Acad Sci U S A. 1987 Aug;84(16):6000–6004. doi: 10.1073/pnas.84.16.6000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moberly J. B., Fanestil D. D. Heterogeneity of expression of apical membrane determinants in A6 epithelial cells. Am J Physiol. 1987 Jul;253(1 Pt 1):C37–C44. doi: 10.1152/ajpcell.1987.253.1.C37. [DOI] [PubMed] [Google Scholar]
- Muto S., Giebisch G., Sansom S. Effects of adrenalectomy on CCD: evidence for differential response of two cell types. Am J Physiol. 1987 Oct;253(4 Pt 2):F742–F752. doi: 10.1152/ajprenal.1987.253.4.F742. [DOI] [PubMed] [Google Scholar]
- Náray-Fejes-Tóth A., Fejes-Tóth G. Glucocorticoid receptors mediate mineralocorticoid-like effects in cultured collecting duct cells. Am J Physiol. 1990 Oct;259(4 Pt 2):F672–F678. doi: 10.1152/ajprenal.1990.259.4.F672. [DOI] [PubMed] [Google Scholar]
- Ostedgaard L. S., Jennings M. L., Karniski L. P., Schuster V. L. A 45-kDa protein antigenically related to band 3 is selectively expressed in kidney mitochondria. Proc Natl Acad Sci U S A. 1991 Feb 1;88(3):981–985. doi: 10.1073/pnas.88.3.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuster V. L., Fejes-Tóth G., Naray-Fejes-Tóth A., Gluck S. Colocalization of H(+)-ATPase and band 3 anion exchanger in rabbit collecting duct intercalated cells. Am J Physiol. 1991 Apr;260(4 Pt 2):F506–F517. doi: 10.1152/ajprenal.1991.260.4.F506. [DOI] [PubMed] [Google Scholar]
- Schuster V. L., Stokes J. B. Chloride transport by the cortical and outer medullary collecting duct. Am J Physiol. 1987 Aug;253(2 Pt 2):F203–F212. doi: 10.1152/ajprenal.1987.253.2.F203. [DOI] [PubMed] [Google Scholar]
- Schwartz G. J., Barasch J., Al-Awqati Q. Plasticity of functional epithelial polarity. 1985 Nov 28-Dec 4Nature. 318(6044):368–371. doi: 10.1038/318368a0. [DOI] [PubMed] [Google Scholar]
- Steinmetz P. R. Cellular organization of urinary acidification. Am J Physiol. 1986 Aug;251(2 Pt 2):F173–F187. doi: 10.1152/ajprenal.1986.251.2.F173. [DOI] [PubMed] [Google Scholar]
- Stoos B. A., Náray-Fejes-Tóth A., Carretero O. A., Ito S., Fejes-Tóth G. Characterization of a mouse cortical collecting duct cell line. Kidney Int. 1991 Jun;39(6):1168–1175. doi: 10.1038/ki.1991.148. [DOI] [PubMed] [Google Scholar]