Abstract
Histone post-translational modification patterns represent epigenetic states of genomic genes and denote the state of their transcription, past history, and future potential in gene expression. Genome-wide chromatin modification patterns reported from various laboratories are assembled in the ENCODE database, providing a fertile ground for understanding epigenetic regulation of any genes of interest across many cell types. The IRF family genes critically control innate immunity as they direct expression and activities of interferons. While these genes have similar structural and functional traits, their chromatin landscapes and epigenetic features have not been systematically evaluated. Here, by mining ENCODE database using an imputational approach, we summarize chromatin modification patterns for 6 of 9 IRF genes and show characteristic features that connote their epigenetic states. BRD4 is a BET bromodomain protein that “reads and translates” epigenetic marks into transcription. We review recent findings that BRD4 controls constitutive and signal-dependent transcription of many genes, including IRF genes. BRD4 dynamically binds to various genomic genes with a spatial and temporal specificity. Of particular importance, BRD4 is shown to critically regulate IRF-dependent anti-pathogen protection, inflammatory responses triggered by NF-κB, and the growth and spread of many cancers. The advent of small molecule inhibitors that disrupt binding of BET bromdomain to acetylated histone marks has opened new therapeutic possibilities for cancer and inflammatory diseases.
Introduction
Post-translational modification of core histones including acetylation and methylation, occurring on the lysine residues, and other modifications represent epigenetic marks that denote the states of gene expression, lineage derivation, and transcriptional and replicative potential (Dawson and Kouzarides 2012; Rando 2012). Widely accepted signatures are histone H3 lysine 4 trimethylation (H3K4me3) associated with active transcription and initiation, H3K36me3 and H3K79me2 indicating active elongation. Additional marks are H3K9me3 denoting transcription repression and heterochromatin, H3K27me3 signifying polycomb-dependent repression (Vire and others 2006). Acetylation of H3 and H4 indicates open chromatin and active gene expression (Wang and others 2008). More recently, enhancers, including super-enhancers are shown to be marked by H3K4me1and H3K27ac (Hnisz and others 2013; Loven and others 2013). Besides classical acetylation and methylation marks, new types of histone modification are reported, adding further understanding of epigenetic landscapes (Huang and others 2014).
DNA methylation is another mechanism by which genes are epigenetically marked (Esteller 2007). DNA methylation almost always signifies transcriptional repression, involved in developmental and cell type specific gene silencing and promoting growth of some cancers by silencing tumor suppressor genes.
IRF family proteins globally control interferon (IFN) activities, as they direct transcription of type I and type III IFN genes and numerous IFN-stimulated genes (ISGs) (Tamura and others 2008). Thus, they exert a decisive influence on innate immune responses. The role of this family in innate immunity is further reinforced by IRF4 and IRF8, which have additional ability to control transcription of genes specific for the innate and adaptive immune responses, besides IFNs. Although, the 9 IRF genes share structural and functional similarity, are dispersed in different chromosomes, and have distinct expression profiles, presumably influenced by the surrounding chromatin environment.
Chromatin Landscape of the Human IRF Genes
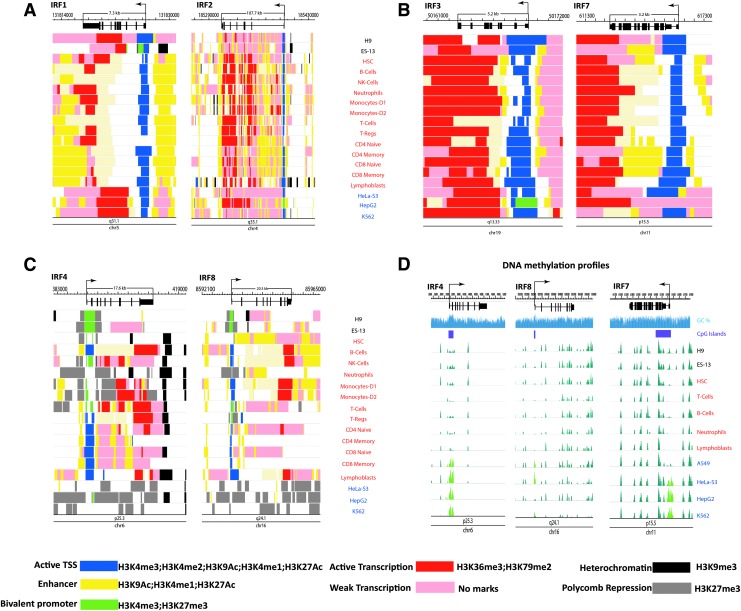
Genome-wide histone tail modifications are now available on public databases, including the ENCODE program at the NIH Roadmap Epigenomics Mapping Consortium (www.roadmapepigenomics.org). In this work, we examined epigenetic states of 6 human IRF genes by mining genome-wide histone modification data reported for various human cells that are deposited in the ENCODE database, and results summarized in Fig. 1A–C. Our data consist of imputed tracks from 12 histone marks (H3K4me1, H3K4me2, H3K4me3, H3K9ac, H3K27ac, H4K20me1, H3K79me2, H3K36me3, H3K9me3, H3K27me3, H2A.Z) from a collection of 127 reference epigenomes and a total of 25 epigenetic states. DNA methylation data were obtained from roadmap of epigenomes project hosted at NIH Roadmap Epigenomics Mapping Consortium (http://egg2.wustl.edu/roadmap/data/byDataType/dnamethylation/RRBS/) Data were visualized using the Washington University Epigenome Browser tool (http://epigenomegateway.wustl.edu/browser/). Although we examined 127 epigenomes, data summarized here are from15 “normal” and/or fresh cells and 3 representative cancer cell lines. Our data reveal that the 6 IRF genes we chose to study can be divided into 3 groups that exhibit distinct chromatin landscapes, (A) IRF1 and IRF2, (B) IRF3 and IRF7, and (C) IRF4 and IRF8.
FIG. 1.
The epigenomic chromatin landscape of the IRF genes. A color-coded illustration of chromatin-state annotation was deduced from histone modification patterns on various human cells deposited in the ENCODE database. Specific histone tail modifications and the corresponding epigenetic and transcriptional states are shown in the bottom. Visualization of the data was performed in the Washington University Epigenome Browser (http://epigenomegateway.wustl.edu/browser/). (A–C) Human IRF genes exhibiting similar chromatin landscapes in various cell types (center) are grouped: A (IRF1 and IRF2), B (IRF3 and IRF7), and C (IRF4 and IRF8). (D) DNA methylation signatures of the human IRF genes. The peaks represent the fractional methylation signals obtained by the reduced representation bisulfite sequencing for indicated cell types. The CpG islands at the promoter region are shown as a purple box.
IRF1 and IRF2
IRF1 and IRF2, the founding IRF members, have the smallest C-terminal regulatory domain in the family. These IRFs exhibited an open chromatin configuration throughout the gene in all cell types presented here, from totipotent embryonic stem cells (ESC), pluripotent hematopoietic stem cells (HSCs) to differentiated immune cells, including T, B lymphocytes and all myeloid lineage cells and established cancer cell lines. Both IRF1 and IRF2 had the marks indicated for active transcription initiation from the TSS and the marks indicating positive transcription in the gene body. The 2 genes also possessed enhancer marks both upstream and downstream regions. Enhancer marks were also present in the gene body, which may contribute to the intragenic control of transcription (Kowalczyk and others 2012). These features are consistent with the expression of the 2 genes in many cell types. Although IRF1 and IRF2 are reported to act in an antagonistic manner (activation vs. repression of target genes, anti-oncogenic vs. oncogenic property), no chromatin marks suggestive of their functional dichotomy were evident.
IRF3 and IRF7
IRF3 and IRF7 displayed similar open chromatin landscape in all cell spectrums. The 2 IRFs showed extended TSS associated active chromatin marks in the 5′ coding regions, along with prominent active transcription signatures in the 3′ coding regions. These features were not seen in IRF1 and IRF2, although both groups are constitutively expressed in a wide variety of cells. The strong active transcription marks were noted in the region beyond the transcription end site both in IRF3 and IRF7, an intriguing, yet mysterious feature. This feature may be reinforced by the adjacent genes that may be active. IRF3 and IRF7 are both expressed in an latent state before stimulation, but activated to induce type I IFN genes upon pathogen recognition receptor signaling. Both genes are known to produce multiple isoforms. The histone modification patterns distinct from IRF1 and IRF2 revealed here may reflect these properties.
IRF4 and IRF8
IRF4 and IRF8 are structurally more similar to each other than other IRF members, and are probable paralogues that co-evolved from a common ancestor. Unlike other IRF genes, IRF4 and IRF8 are expressed in an immune cell specific manner, and contribute to the development and activity of lymphocytes and myeloid cells, a role unrelated to IFNs (Tamura and others 2008). Consistent with cell restricted expression, the promoter regions of IRF4 and IRF8 showed the H3K27me3 mark that indicate polycomb mediated repression in most cell lineages except in immune cell system where active marks were more dominant. Further, IRF4 had active marks (H3K4me3, H3K36me3, H3K76me2) in B cells and some T cells, but repressive and/or bivalent signatures in NK, myeloid cells and some T cells. Whereas, IRF8 had positive marks in monocytes, B cells, CD4/8 T, but had repressive marks in neutrophils and T cells. Bivalent marks were evident in 2 ES cells for both IRF4 and IRF8. However, in HSCs, IRF4 had a bivalent mark, while IRF8 showed active TSS. In the 3 established cell lines, IRF4 and IRF8 both showed repressive marks throughout the genes, unlike other IRFs. In ES cells, active chromatin marks are largely absent in both genes, although IRF8 showed curious enhancer marks in HSCs and immune cells throughout the gene, whereas IRF4 was devoid of this signature in HSCs. These features suggest the possibility that IRF4 and IRF8 are differentially regulated in early stem cells.
DNA Methylation Landscape of IRF Genes in Cancer and Normal Cells
DNA methylation takes place in the CpG islands of genomic DNA, that are for the most part located near the promoter regions, and results in gene silencing (Esteller 2007; Rodriguez-Paredes and Esteller 2011). DNA methylation is mediated by DNA methyltransferases (DNMTs) and reversed by TET DNA demethylases (Shen and others 2014). Aberrant DNA methylation has been noted in various cancers, for example, in tumor suppressor genes (eg, BRACA1), demethylation of proto-oncogenes (eg, IGF2). An alteration has been noted for DNMT3a in AML (Ley and others 2010). Moreover, aberrant DNA methylation has been reported in some cancers for several IRF genes. For example, methylation of IRF1, IRF4 and IRF7 is reported for various gastric cancers (Jee and others 2009; Yamashita and others 2010; Mitchell and others 2014). Abnormal IRF4 methylation has also been found in pediatric B cell ALL (Musialik and others 2015). Hypermethylaion of IRF6 is found in squamous cell carcinoma (Botti and others 2011). DNA methylation has also been reported for IRF8 in renal cell carcinoma, colorectal cancer, melanoma and other cancers (Yang and others 2007; Lee and others 2008; Tshuikina and others 2008). DNA methylation-controlled activity of IRF8 has been recently described for differentiation of the osteoclasts (Nishikawa and others 2015).
Figure 1D summarizes the methylation state of IRF4, IRF7 and IRF8 genes for the same set of cells as above. Methylated sites were present over the CpG islands of these IRFs, located near the promoter and other intragenic regions even outside the gene body. Interestingly, the overall methylation patterns were similar across different cell types, including IRF4 and IRF8, whose expression vary among these cells. The lack of cell type difference, contrasts histone modification patterns, where notable differences were evident that corresponded to cell type dependent expression, which may suggest that DNA methylation does not contributes to cell type specific expression of IRF 4 and IRF8. However, DNA methylation patterns of established cancer cell lines (HeLa, HepG2, K562) were markedly different from those of normal cells for all 3 IRFs, showing prominent hypermethylation in the CpG islands. It is possible that these IRFs are downregulated in these established cells resulting in transcriptional repression, which may potentially be linked to their cancer status. Other IRF genes (IRF2, IRF3, IRF5, IRF9) were also found to have similar DNA methylation patterns across the cells, except IRF1 which displayed increased methylation in ESC and HSC stem cells (not shown).
BRD4 Translates Chromatin Signatures into Gene Expression
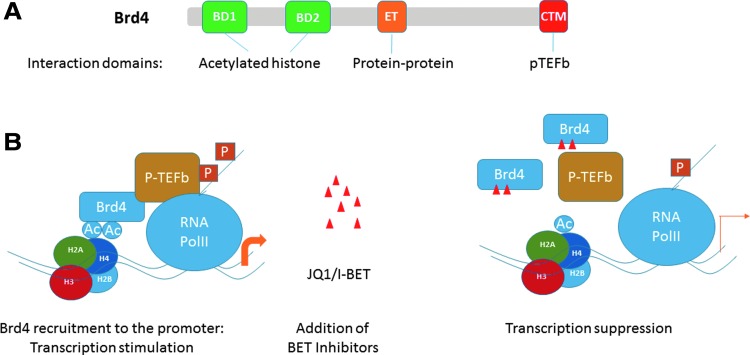
Histone modifications only provide epigenetic marks, but do not directly alter gene activity. There are proteins specialized in reading histone marks and translating into transcription. Proteins of the BET bromodomain family are a noted example. The family consists of BRD2, BRD3, BRD4 and BRDT, have 2 conserved bromodomains and the extra-terminal (ET) domain (Fig. 2A). The former 3 proteins are expressed ubiquitously in most cell types, while BRDT expression is limited to testis. Through the bromodomains, they bind acetylated histone tails of various core histones H3 and H4, displaying considerable lysine residue specificity (Dey and others 2000, 2003; Wu and Chiang 2007).
FIG. 2.
Bromodomain protein BRD4. (A) Schematic structure: BRD4 are nuclear protein of ∼200 kDa, and it is abundantly expressed in most if not all cell types. BD1 and BD2 represent the bromodomains, composed of 4 αhelices through which they bind acetylated lysine on core histones, showing considerable specificity. The extra-terminal (ET) domain offers a protein–protein interaction surface. The extreme C-terminal motif (CTM) confers binding of core P-TEFb (cyclin T1 and CDK9) that phosphorylates Pol II C-terminal domain to start transcription elongation. BRD4 also has an independent kinase activity that contributes to enhanced transcription (Devaiah and others 2012). (B) Inhibition of transcription by small molecule inhibitors (JQ1, I-BET). JQ1 and I-BET reversibly inhibits binding of acetylated histone tails to BET bromdomains. This in turn interferes with constitutive and induced recruitment of BRD4 to the TSS, gene body and enhancers where histones are acetylated or induced to be acetylated. This blocks transcription initiation. The inhibitors also hamper BRD4-dependent P-TEFb recruitment, leading to inhibition of transcription elongation.
Because of the abundant expression and its known role in transcription, BRD4 has been most extensively studied among other members. We have been studying BRD4 and showed that BRD4 recruits P-TEFb, a transcription elongation factor, through its C-terminal motif to activates expression of many genes (Jang and others 2005). BRD4 tightly binds to chromosomes during mitosis and affects gene expression in the newly divided cells (Dey and others 2009). BRD4 binds to a large array of constitutively expressed and signal-induced genes in normal and cancer cells (Dey and others 2009; Hargreaves and others 2009; Zhang and others 2012). IRF genes are also shown to be occupied by BRD4 at the promoters and the enhancers and are regulated by BRD4 in macrophages, T cells and cancer cells (Nicodeme and others 2010; Zhang and others 2012; Pelish and others 2015).
As a reader/translator of the epigenetic marks, BRD4 plays a critical role in innate immune responses and inflammation. For example, IFN stimulation causes recruitment of BRD4 and then P-TEFb to many ISGs, a process essential for ISG transcription induction (Patel and others 2013; Sarai and others 2013). BRD4 recruitment is dependent on the initial binding of ISGF3 complex (STAT1/2/IRF9) and ensuing histone acetylation. Similarly, stimulation of macrophages with LPS triggers BRD4 recruitment to many proinflammatory genes activated by NF-κB, which is also caused by induced histone acetylation in inflammatory genes (Hargreaves and others 2009)
Development of small molecule inhibitors that disrupt the interaction between acetyl-histones and the BET bromodomains has opened many new avenues of research, and offers therapeutic possibilities for some diseases (Filippakopoulos and others 2010; Nicodeme and others 2010) (Fig. 2B). The small molecule inhibitors, representatives being JQ1 and I-BET are shown to have the remarkable capacity to inhibit growth of various cancers, including acute myeloid leukemia, myeloma, diffuse large B cell lymphoma and various solid tumors, in many cases prolonging host survival in mouse models (Filippakopoulos and others 2010; Zuber and others 2011; Loven and others 2013; Ceribelli and others 2014; Shi and others 2014; Pelish and others 2015). These studies led to the view that BRD4 targets MYC, the growth promoting oncogene to enhance cancer cell growth, and that the inhibitors preferentially attack BRD4. These studies further showed that high MYC expression is bolstered by the super-enhancer in the MYC gene that forms a stable DNA loop, to which BRD4 is recruited and functions along with other chromatin binding factors such as Mediators and BAF complexes (Hnisz and others 2013; Loven and others 2013; Pelish and others 2015). Super-enhancers are reported for other BRD4 targets, including IRF4 and IRF8 (Loven and others 2013; Pelish and others 2015). Super-enhancer-bound BRD4 are reported to be more susceptible to JQ1 and I-BET than BRD4 bound to other regions, leading to preferential attenuation of MYC activity and potent anti-oncogenic effect.
BET inhibitors are also shown to be efficacious in regulating inflammation and related diseases by preferentially attacking BRD4. In LPS stimulated bone marrow derived macrophages, BET inhibitors suppress induction of a subset of inflammatory genes, including Il1b, Il6, Ccl2, Ccl12, Cxcl9 and Ifnb1, although the inhibitors downregulated only <25% of all LPS induced genes (Nicodeme and others 2010; Belkina and others 2013). The inhibition was largely attributed to that of BRD4, and to some degree BRD2, causing inhibition of Pol II elongation. Although not all inflammatory genes were downregulated, injection of mice with the inhibitor provided full protection from lethal endotoxin shock caused by LPS, Salmonella, and CLP (Nicodeme and others 2010). Further, BET inhibitors are shown to reduce development of inflammatory Th17 cells in vivo and in vitro. In this case also, the inhibitors downregulated only a subset of Th17-polarized genes (Bandukwala and others 2012; Mele and others 2013). Nevertheless, IL-17A was one of BRD4's targets, and the inhibitor prevented BRD4 recruitment to the Il17 promoter. Consistent with this selective effect, BET inhibitors significantly ameliorated a mouse model of neuro inflammatory disease, experimental allergic encephalomyelitis without strongly limiting Th1 cells (Bandukwala and others 2012; Mele and others 2013). BET inhibitors are also shown to inhibit TNFα-induced inflammatory changes in endothelial cells. These inflammatory genes are, for the most part, targets of NF-κB and shown to form BRD4-containing super-enhancers upon stimulation, and this was preferentially inhibited by JQ1 (Brown and others 2014). Extending to in vivo JQ1 markedly diminished plaque formation and atherogenesis caused by endothelial inflammation in Ldl−/− mice (Brown and others 2014). These reports highlight therapeutic potentials of BET inhibitors for cancers and inflammatory diseases.
However, it is important to note that BET inhibitors likely have a negative side, since they hamper protective innate immunity in both cancer and inflammatory diseases. Consistent with this concern, these inhibitors compromise IFN mediated transcription pathways that are important for protection against infectious pathogens and cancer immune surveillance (Patel and others 2013; Wienerroither and others 2014; Gubin and others 2015).
Conclusions and Perspectives
Chromatin marks created by histone tail modifications are the basis of epigenetic regulation. Genome-wide epigenome information reveals that IRF genes carry characteristic chromatin marks that signify and predict their expression and the activity controlling innate immune responses. Identification of chromatin readers that recognize specific histone modification capable of regulating transcription provides mechanistic understanding of epigenetic regulation. In addition to BET inhibitors discussed above, pharmacological agents that aim at other epigenetic marks are being developed, with a notable example of a H3K27 methylase inhibitor, shown to inhibit pediatric glioma growth (Hashizume and others 2014). These efforts are likely to have substantive impacts on prophylactic and therapeutic strategies to deal with chronic infectious diseases, inflammatory disorders, and some types of cancers.
Acknowledgments
The authors thank colleagues in the Ozato Lab and DDB for discussion and critical reading of the article. This work was supported by the intramural program of the NICHD, National Institutes of Health (ZIA HD001310-28).
Author Disclosure Statement
No competing financial interests exist.
References
- Bandukwala HS, Gagnon J, Togher S, Greenbaum JA, Lamperti ED, Parr NJ, Molesworth AMH, Smithers N, Lee K, Witherington J, Tough DF, Prinjha RK, Peters B, Rao A. 2012. Selective inhibition of CD4+ T-cell cytokine production and autoimmunity by BET protein and c-Myc inhibitors. Proc Natl Acad Sci U S A 109(36):14532–14537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belkina AC, Nikolajczyk BS, Denis GV. 2013. BET protein function is required for inflammation: Brd2 genetic disruption and BET inhibitor JQ1 impair mouse macrophage inflammatory responses. J Immunol 190(7):3670–3678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botti E, Spallone G, Moretti F, Marinari B, Pinetti V, Galanti S, De Meo PD, De Nicola F, Ganci F, Castrignano T, Pesole G, Chimenti S, Guerrini L, Fanciulli M, Blandino G, Karin M, Costanzo A. 2011. Developmental factor IRF6 exhibits tumor suppressor activity in squamous cell carcinomas. Proc Natl Acad Sci U S A 108(33):13710–13715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown Jonathan D, Lin Charles Y, Duan Q, Griffin G, Federation AJ, Paranal Ronald M, Bair S, Newton G, Lichtman AH, Kung AL, Yang T, Wang H, Luscinskas Francis W, Croce KJ, Bradner James E, Plutzky J. 2014. NF-κB directs dynamic super enhancer formation in inflammation and atherogenesis. Mol Cell 56(2):219–231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceribelli M, Kelly PN, Shaffer AL, Wright GW, Xiao W, Yang Y, Mathews Griner LA, Guha R, Shinn P, Keller JM, Liu D, Patel PR, Ferrer M, Joshi S, Nerle S, Sandy P, Normant E, Thomas CJ, Staudt LM. 2014. Blockade of oncogenic IkappaB kinase activity in diffuse large B-cell lymphoma by bromodomain and extraterminal domain protein inhibitors. Proc Natl Acad Sci U S A 111(31):11365–11370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson MA, Kouzarides T. 2012. Cancer epigenetics: from mechanism to therapy. Cell 150(1):12–27 [DOI] [PubMed] [Google Scholar]
- Devaiah BN, Lewis BA, Cherman N, Hewitt MC, Albrecht BK, Robey PG, Ozato K, Sims RJ, 3rd, Singer DS. 2012. BRD4 is an atypical kinase that phosphorylates serine2 of the RNA polymerase II carboxy-terminal domain. Proc Natl Acad Sci U S A 109(18):6927–6932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dey A, Chitsaz F, Abbasi A, Misteli T, Ozato K. 2003. The double bromodomain protein Brd4 binds to acetylated chromatin during interphase and mitosis. Proc Natl Acad Sci U S A 100(15):8758–8763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dey A, Ellenberg J, Farina A, Coleman AE, Maruyama T, Sciortino S, Lippincott-Schwartz J, Ozato K. 2000. A bromodomain protein, MCAP, associates with mitotic chromosomes and affects G2-to-M transition. Mol Cell Biol 20(17):6537–6549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dey A, Nishiyama A, Karpova T, McNally J, Ozato K. 2009. Brd4 marks select genes on mitotic chromatin and directs postmitotic transcription. Mol Biol Cell 20(23):4899–4909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esteller M. 2007. Cancer epigenomics: DNA methylomes and histone-modification maps. Nat Rev Genet 8(4):286–298 [DOI] [PubMed] [Google Scholar]
- Filippakopoulos P, Qi J, Picaud S, Shen Y, Smith WB, Fedorov O, Morse EM, Keates T, Hickman TT, Felletar I, Philpott M, Munro S, McKeown MR, Wang Y, Christie AL, West N, Cameron MJ, Schwartz B, Heightman TD, La Thangue N, French CA, Wiest O, Kung AL, Knapp S, Bradner JE. 2010. Selective inhibition of BET bromodomains. Nature 468(7327):1067–1073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubin MM, Artyomov MN, Mardis ER, Schreiber RD. 2015. Tumor neoantigens: building a framework for personalized cancer immunotherapy. J Clin Invest 125(9):3413–3421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hargreaves DC, Horng T, Medzhitov R. 2009. Control of inducible gene expression by signal-dependent transcriptional elongation. Cell 138(1):129–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashizume R, Andor N, Ihara Y, Lerner R, Gan H, Chen X, Fang D, Huang X, Tom MW, Ngo V, Solomon D, Mueller S, Paris PL, Zhang Z, Petritsch C, Gupta N, Waldman TA, James CD. 2014. Pharmacologic inhibition of histone demethylation as a therapy for pediatric brainstem glioma. Nat Med 20(12):1394–1396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hnisz D, Abraham BJ, Lee TI, Lau A, Saint-Andre V, Sigova AA, Hoke HA, Young RA. 2013. Super-enhancers in the control of cell identity and disease. Cell 155(4):934–947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H, Sabari BR, Garcia BA, Allis CD, Zhao Y. 2014. SnapShot: histone modifications. Cell 159(2):458–458.e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang MK, Mochizuki K, Zhou M, Jeong H-S, Brady JN, Ozato K. 2005. The bromodomain protein Brd4 is a positive regulatory component of P-TEFb and stimulates RNA polymerase II-dependent transcription. Mol Cell 19(4):523–534 [DOI] [PubMed] [Google Scholar]
- Jee CD, Kim MA, Jung EJ, Kim J, Kim WH. 2009. Identification of genes epigenetically silenced by CpG methylation in human gastric carcinoma. Eur J Cancer 45(7):1282–1293 [DOI] [PubMed] [Google Scholar]
- Kowalczyk MS, Hughes JR, Garrick D, Lynch MD, Sharpe JA, Sloane-Stanley JA, McGowan SJ, De Gobbi M, Hosseini M, Vernimmen D, Brown JM, Gray NE, Collavin L, Gibbons RJ, Flint J, Taylor S, Buckle VJ, Milne TA, Wood WG, Higgs DR. 2012. Intragenic enhancers act as alternative promoters. Mol Cell 45(4):447–458 [DOI] [PubMed] [Google Scholar]
- Lee KY, Geng H, Ng KM, Yu J, van Hasselt A, Cao Y, Zeng YX, Wong AH, Wang X, Ying J, Srivastava G, Lung ML, Wang LD, Kwok TT, Levi BZ, Chan AT, Sung JJ, Tao Q. 2008. Epigenetic disruption of interferon-gamma response through silencing the tumor suppressor interferon regulatory factor 8 in nasopharyngeal, esophageal and multiple other carcinomas. Oncogene 27(39):5267–5276 [DOI] [PubMed] [Google Scholar]
- Ley TJ, Ding L, Walter MJ, McLellan MD, Lamprecht T, Larson DE, Kandoth C, Payton JE, Baty J, Welch J, Harris CC, Lichti CF, Townsend RR, Fulton RS, Dooling DJ, Koboldt DC, Schmidt H, Zhang Q, Osborne JR, Lin L, O'Laughlin M, McMichael JF, Delehaunty KD, McGrath SD, Fulton LA, Magrini VJ, Vickery TL, Hundal J, Cook LL, Conyers JJ, Swift GW, Reed JP, Alldredge PA, Wylie T, Walker J, Kalicki J, Watson MA, Heath S, Shannon WD, Varghese N, Nagarajan R, Westervelt P, Tomasson MH, Link DC, Graubert TA, DiPersio JF, Mardis ER, Wilson RK. 2010. DNMT3A mutations in acute myeloid leukemia. N Engl J Med 363(25):2424–2433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loven J, Hoke HA, Lin CY, Lau A, Orlando DA, Vakoc CR, Bradner JE, Lee TI, Young RA. 2013. Selective inhibition of tumor oncogenes by disruption of super-enhancers. Cell 153(2):320–334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mele DA, Salmeron A, Ghosh S, Huang H-R, Bryant BM, Lora JM. 2013. BET bromodomain inhibition suppresses TH17-mediated pathology. J Exp Med 210(11):2181–2190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell SM, Ross JP, Drew HR, Ho T, Brown GS, Saunders NF, Duesing KR, Buckley MJ, Dunne R, Beetson I, Rand KN, McEvoy A, Thomas ML, Baker RT, Wattchow DA, Young GP, Lockett TJ, Pedersen SK, Lapointe LC, Molloy PL. 2014. A panel of genes methylated with high frequency in colorectal cancer. BMC Cancer 14:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musialik E, Bujko M, Kober P, Wypych A, Gawle-Krawczyk K, Matysiak M, Siedlecki JA. 2015. Promoter methylation and expression levels of selected hematopoietic genes in pediatric B-cell acute lymphoblastic leukemia. Blood Res 50(1):26–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicodeme E, Jeffrey KL, Schaefer U, Beinke S, Dewell S, Chung C-w, Chandwani R, Marazzi I, Wilson P, Coste H, White J, Kirilovsky J, Rice CM, Lora JM, Prinjha RK, Lee K, Tarakhovsky A. 2010. Suppression of inflammation by a synthetic histone mimic. Nature 468(7327):1119–1123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishikawa K, Iwamoto Y, Kobayashi Y, Katsuoka F, Kawaguchi S, Tsujita T, Nakamura T, Kato S, Yamamoto M, Takayanagi H, Ishii M. 2015. DNA methyltransferase 3a regulates osteoclast differentiation by coupling to an S-adenosylmethionine-producing metabolic pathway. Nat Med 21(3):281–287 [DOI] [PubMed] [Google Scholar]
- Patel MC, Debrosse M, Smith M, Dey A, Huynh W, Sarai N, Heightman TD, Tamura T, Ozato K. 2013. BRD4 coordinates recruitment of pause release factor P-TEFb and the pausing complex NELF/DSIF to regulate transcription elongation of interferon-stimulated genes. Mol Cell Biol 33(12):2497–2507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelish HE, Liau BB, Nitulescu II, Tangpeerachaikul A, Poss ZC, Da Silva DH, Caruso BT, Arefolov A, Fadeyi O, Christie AL, Du K, Banka D, Schneider EV, Jestel A, Zou G, Si C, Ebmeier CC, Bronson RT, Krivtsov AV, Myers AG, Kohl NE, Kung AL, Armstrong SA, Lemieux ME, Taatjes DJ, Shair MD. 2015. Mediator kinase inhibition further activates super-enhancer-associated genes in AML. Nature 526(7572):273–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rando OJ. 2012. Combinatorial complexity in chromatin structure and function: revisiting the histone code. Curr Opin Genet Dev 22(2):148–155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Paredes M, Esteller M. 2011. Cancer epigenetics reaches mainstream oncology. Nat Med 17(3):330–339 [DOI] [PubMed] [Google Scholar]
- Sarai N, Nimura K, Tamura T, Kanno T, Patel MC, Heightman TD, Ura K, Ozato K. 2013. WHSC1 links transcription elongation to HIRA-mediated histone H3.3 deposition. EMBO J 32(17):2392–2406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen L, Song CX, He C, Zhang Y. 2014. Mechanism and function of oxidative reversal of DNA and RNA methylation. Annu Rev Biochem 83:585–614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi J, Wang Y, Zeng L, Wu Y, Deng J, Zhang Q, Lin Y, Li J, Kang T, Tao M, Rusinova E, Zhang G, Wang C, Zhu H, Yao J, Zeng YX, Evers BM, Zhou MM, Zhou BP. 2014. Disrupting the interaction of BRD4 with diacetylated Twist suppresses tumorigenesis in basal-like breast cancer. Cancer Cell 25(2):210–225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura T, Yanai H, Savitsky D, Taniguchi T. 2008. The IRF family transcription factors in immunity and oncogenesis. Annu Rev Immunol 26:535–584 [DOI] [PubMed] [Google Scholar]
- Tshuikina M, Jernberg-Wiklund H, Nilsson K, Oberg F. 2008. Epigenetic silencing of the interferon regulatory factor ICSBP/IRF8 in human multiple myeloma. Exp Hematol 36(12):1673–1681 [DOI] [PubMed] [Google Scholar]
- Vire E, Brenner C, Deplus R, Blanchon L, Fraga M, Didelot C, Morey L, Van Eynde A, Bernard D, Vanderwinden JM, Bollen M, Esteller M, Di Croce L, de Launoit Y, Fuks F. 2006. The Polycomb group protein EZH2 directly controls DNA methylation. Nature 439(7078):871–874 [DOI] [PubMed] [Google Scholar]
- Wang Z, Zang C, Rosenfeld JA, Schones DE, Barski A, Cuddapah S, Cui K, Roh TY, Peng W, Zhang MQ, Zhao K. 2008. Combinatorial patterns of histone acetylations and methylations in the human genome. Nat Genet 40(7):897–903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wienerroither S, Rauch I, Rosebrock F, Jamieson AM, Bradner J, Muhar M, Zuber J, Müller M, Decker T. 2014. Regulation of NO synthesis, local inflammation, and innate immunity to pathogens by BET family proteins. Mol Cell Biol 34(3):415–427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu SY, Chiang CM. 2007. The double bromodomain-containing chromatin adaptor Brd4 and transcriptional regulation. J Biol Chem 282(18):13141–13145 [DOI] [PubMed] [Google Scholar]
- Yamashita M, Toyota M, Suzuki H, Nojima M, Yamamoto E, Kamimae S, Watanabe Y, Kai M, Akashi H, Maruyama R, Sasaki Y, Yamano H, Sugai T, Shinomura Y, Imai K, Tokino T, Itoh F. 2010. DNA methylation of interferon regulatory factors in gastric cancer and noncancerous gastric mucosae. Cancer Sci 101(7):1708–1716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang D, Thangaraju M, Greeneltch K, Browning DD, Schoenlein PV, Tamura T, Ozato K, Ganapathy V, Abrams SI, Liu K. 2007. Repression of IFN regulatory factor 8 by DNA methylation is a molecular determinant of apoptotic resistance and metastatic phenotype in metastatic tumor cells. Cancer Res 67(7):3301–3309 [DOI] [PubMed] [Google Scholar]
- Zhang W, Prakash C, Sum C, Gong Y, Li Y, Kwok JJ, Thiessen N, Pettersson S, Jones SJ, Knapp S, Yang H, Chin KC. 2012. Bromodomain-containing protein 4 (BRD4) regulates RNA polymerase II serine 2 phosphorylation in human CD4+ T cells. J Biol Chem 287(51):43137–43155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuber J, Shi J, Wang E, Rappaport AR, Herrmann H, Sison EA, Magoon D, Qi J, Blatt K, Wunderlich M, Taylor MJ, Johns C, Chicas A, Mulloy JC, Kogan SC, Brown P, Valent P, Bradner JE, Lowe SW, Vakoc CR. 2011. RNAi screen identifies Brd4 as a therapeutic target in acute myeloid leukaemia. Nature 478(7370):524–528 [DOI] [PMC free article] [PubMed] [Google Scholar]