Abstract
Platelet factor 4 (PF4) has been recently shown to inhibit infection by a broad range of human immunodeficiency virus type 1 (HIV-1) isolates in vitro. We found that the inhibitory effects of PF4 are limited to a defined concentration range where PF4 exists largely in a monomeric state. Under these conditions, PF4 bound the HIV-1 envelope protein and inhibited HIV-1 attachment to the cell surface. However, as concentrations increased to the point where PF4 exists largely in tetrameric or higher-order forms, viral infection in vitro was enhanced. Enhancement could be inhibited by mutations in PF4 that shift the oligomeric equilibrium toward the monomeric state, or by using soluble glycosaminoglycans (GAGs) to which tetrameric PF4 avidly binds. We conclude that at physiologically relevant concentrations, oligomeric PF4 enhances infection by HIV-1 by interacting with the viral envelope protein as well as cell surface GAGs, enhancing virus attachment to the cell surface. This effect was not specific to HIV-1, as enhancement was seen with some but not all other viruses tested. The biphasic effects of PF4 on HIV-1 infection suggest that native PF4 will not be a useful antiviral agent and that PF4 could contribute to the hematologic abnormalities commonly seen in HIV-infected individuals by enhancing virus infection in the bone marrow.
Introduction
Human immunodeficiency virus type 1 (HIV-1) entry into target cells results from sequential interactions between the HIV-1 envelope glycoprotein (Env) with the cellular receptor CD4 and a coreceptor, either CCR5 or CXCR4.1–6 The efficiency of this process can be regulated in vivo by cytokines and chemokines that bind to the viral coreceptors or that influence coreceptor expression levels.7–10 A variety of cell types secrete cytokines or chemokines that can modulate HIV infection, including activated platelets that have been shown to possess anti HIV-1 properties in vitro.7–9,11,12
During vascular injury, activated platelets release a number of mediators from their α-granules, including connective tissue-activating peptide III (CTAP-III/CXCL7), RANTES (CCL5), and Platelet-factor 4 (PF4/CXCL4).13,14 PF4 is a cationic α-chemokine that functions primarily to promote coagulation by moderating the effects of heparin-like molecules.15,16 PF4 is present in nanomolar and micromolar concentrations within plasma (0.5–3 nM) and serum (0.4–1.9 μM), respectively.17–20 PF4 has been shown to be chemotactic for immune cells by acting through interactions with a splice variant of the G-protein-coupled receptor CXCR3B and cell surface proteoglycans.21–23 Recently, PF4 has been described to possess potent and broadly active antiviral activity against HIV-1 in vitro at concentrations less than 0.5 mM,12,24 although the mechanism and in vivo relevance of these results are uncertain.
In this study, we find that the previously reported inhibitory effects of PF4 are limited to a narrow concentration range where PF4 exists predominantly as a monomer.25 Under these conditions, PF4 binds directly to Env and inhibits virus infection by preventing its attachment to the cell surface. At physiologic concentrations, where PF4 exists largely as a tetramer, it enhanced infection several fold above untreated controls. This biphasic activity of PF4 was not restricted to the HIV-1 Env, as we observed similar results with HIV-1 pseudoviruses bearing the glycoproteins of murine leukemia virus (HIV-1MLV), simian immunodeficiency viruses (HIV-1SIVmac316 and HIV-1SIVsmmE660), and vesicular stomatitis virus (HIV-1VSV-G). However, PF4 did not antagonize or enhance the entry of pseudoviruses bearing the glycoprotein of influenza (HIV-1H5N1). We further demonstrated that PF4 carries out its dual activity during viral infection by modulating viral attachment to the cell. Finally, we provide evidence that oligomeric PF4 directly interacts with cellular glycosaminoglycans (GAGs) as well as the HIV-1 envelope glycoprotein gp120, perhaps serving as a bridge between cell surface GAGs and the viral envelope glycoprotein, thus enhancing virus attachment and infection at high PF4 concentrations. This could play a role in vivo, as PF4 produced in the bone marrow could impact virus infection of stem cells and other progenitors, contributing to the hematologic abnormalities commonly associated with HIV/AIDS.
Materials and Methods
Cell culture
293T17 and HeLa-derived JC53 cells were maintained in Dulbecco's modified Eagle medium (DMEM) with 10% (vol/vol) fetal bovine serum (FBS)—D10F media. Multinuclear activation of galactosidase indicator cells stably expressing human CD4 and CCR5 (MAGI-CCR5) was obtained from the National Institutes of Health AIDS Research and Reference Reagent Program and maintained in DMEM supplemented with 10% FBS and 1 mg/ml puromycin. SupT1-CCR5 and Jurkat-CCR5 immortalized cell lines were maintained in Roswell Park Memorial Institute (RPMI) medium containing 10% FBS (R10F media). Primary human CD4+ T cells were obtained from the Human Immunology Core of the University of Pennsylvania's Center for AIDS Research.
Virus production and normalization
HIV-1 Env pseudoviruses were produced by calcium phosphate cotransfection of 6 μg of pcDNA3.1+ containing the desired Env clone with 10 μg of HIV-1 core (pNL43-ΔEnv-vpr+ -luc+ or pNL43-ΔEnv-vpr+ -eGFP) into 293T17 cells. At 72 h post-transfection, the pseudovirus-containing supernatant was harvested and filtered through a 0.45-μm-pore-size filter and stored at −80°C. Influenza (H5N1) pseudoviruses were produced by calcium phosphate cotransfection of 400 ng of pCMV8/R containing H5 (VRC 7705) and 100 ng of pCMV8/R containing N1 (VRC 7708) with 10 μg of HIV-1 core (pNL43-ΔEnv-vpr+ -luc+) into 293T17 cells. HEPES buffer (1:100; Invitrogen) was added to the media to maintain basic pH and minimize acid-induced HA triggering. At 48 h post-transfection, the pseudovirus-containing supernatant was harvested as described for HIV-1 Env pseudoviruses. Green fluorescent protein (GFP) pseudoviruses were concentrated ∼100-fold by ultracentrifugation at 113,000×g for 2 h at 4°C through a 20% sucrose cushion. Pelleted pseudovirus was then resuspended in phosphate-buffered saline (PBS) and stored at −80°C.
Virus inhibition assay
MAGI-CCR5 cells were treated with varying concentrations of PF4 or media only before infection with the indicated luciferase reporter pseudovirus or full-length infectious molecular clone. After addition of pseudovirus, plates were spinoculated at 1,200×g for 2 h at 4°C and then incubated at 37°C. Viral inoculum was replaced with fresh complete media (supplemented with PF4 for replication competent infections) after 4 h. Inhibition assays were also conducted with the SupT1-CCR5 and Jurkat-CCR5 cell lines as described above. For single-cycle infections using luciferase-encoding pseudovirus, cells were lysed with Brite-Glo (Promega) at 72 h postinfection and relative light units (RLUs) were measured. For spreading infections of replication-competent infectious virus, HIV-1 replication was assessed by measuring p24 Gag protein in cell-free culture supernatants taken between days 3 and 9 postinfection using a commercial enzyme immunoassay (AlphaLISA; PerkinElmer). All inhibition assays were done in at least duplicate in each of at least three independent experiments.
Viral attachment assay
Human CD4+ T cells (106 cells per condition) were stimulated for 3–5 days with anti-CD3/anti-CD28 beads (Invitrogen) and 20 U/ml recombinant interleukin-2 (IL-2, Aldesleukin; Prometheus Laboratories) in R10F media. Cells were pretreated with 200 μl of 200 nM or 4 μM PF4WT with or without 10 μg/ml soluble heparan sulfate proteoglycan (HSP), 4 μM PF4K50E, 15 μM maraviroc (MVC), 15 μM plerixafor (AMD3100), 40 μg/ml DEAE-dextran, or serum-free PBS at room temperature for 30 min. Cells were subsequently exposed to 200 μl of undiluted HIV-1R3A (2.3 μg p24/ml) and incubated at 37°C for 4 h in the absence or presence of each drug treatment. To determine background signal level, 2 × 106 untreated cells were infected and incubated at 4°C for 4 h. After incubation, cells were washed twice in PBS to remove unbound virus. Cells were then split into two aliquots: one aliquot was treated with 50 μl of prewarmed 0.05% trypsin-EDTA at 37°C for 10 min, followed by trypsin inactivation with 5 ml cold R10F media. The other aliquot was left trypsin untreated in R10F media. Both trypsin-treated and -untreated cells were washed thrice with cold PBS, then the cell pellets lysed with 100 μl of 0.5% (wt/vol) Triton X-100. Cell-associated p24 was measured using the p24 AlphaLISA (PerkinElmer). The final p24 concentration was calculated by subtracting the concentration of the trypsin-treated cells incubated at 4°C from the p24 signal measured in each test sample.
Primary human CD4+ T-cell infections
Primary human CD4+ T cells (106 cells per condition) were stimulated with anti-CD3/anti-CD28 magnetic beads (Invitrogen) and 20 U/ml recombinant IL-2 in R10F media. Three days poststimulation, cells were transferred to 24-well plates and incubated for 30 min with no PF4, 200 nM PF4, or 4 μM PF4. Viral input was normalized by reverse transcriptase (RT) activity as determined by a colorimetric assay (Roche). Approximately 3 ng RT of replication-competent HIV-1CH077 was used to infect cells in duplicate in a total volume of 250 μl. Plates were incubated at 37°C, and media were replaced every 48 h with fresh IL-2 containing R10F with PF4. HIV-1 replication was assessed by measuring the p24 Gag protein in cell-free culture supernatants collected 6 days postinfection. Assays were done in duplicate with each of at least three independent donors.
Generation and purification of PF4 in S2 cells
cDNA encoding human PF4 was cloned into the plasmid pMT/BiP/V5-His A (Invitrogen) for expression. PF4 expression was induced by adding copper sulfate (0.5 mM) to S2 cells. The induced S2 cells were then incubated in serum-free medium Insect-Xpress (Lonza) for 3–5 days; supernatants were collected and the media filtered through a 0.22-μm filter. PF4WT was purified from the media on a heparin HiTrap column on an ATKA Prime (GE Healthcare) at 4°C in 10 mM Tris, 1 mM EDTA, pH 8.0 buffer. Media were loaded in buffer containing 0.5 M NaCl, and PF4 was eluted at 1.8 M NaCl using a linear gradient. Fractions containing purified PF4 as detected by silver staining of 12% NuPAGE Bis-Tris gels (Invitrogen) were pooled, concentrated, and the buffer was exchanged into 50 mM HEPES, 0.5 M NaCl, pH ∼7.2 using an Amicon Ultra centrifugal filter (3K NMWL; Millipore). Protein was quantified using a bicinchoninic acid assay (Pierce Chemical). PF4K50E and PF4E28R/K50E were purified as PF4WT, with the following modifications: the column buffer system used was 50 mM MES, 1 mM EDTA, and pH 6.5. Media were loaded in buffer containing 0.3 M NaCl, and the proteins were eluted at 1.3 M NaCl using a linear gradient. Commercially available PF4 isolated from human platelets (Calbiochem) and commercially available recombinant PF4 (R&D Systems) were tested along with laboratory-generated recombinant PF4.
Enzyme-linked immunosorbent assay
Chemokines were immobilized on 96-well plates (Immulon 4HBX; Thermo Scientific) in phosphate-buffered saline without calcium and magnesium (PBS-/-) (Life Technologies) overnight at room temperature. Wells were washed thrice with 250 μl PBS containing 0.1% Tween-20 (PBST), followed by blocking with 200 μl of 1% BSA in PBS (BPBS) at room temperature for 1 h. Wells were then washed thrice with PBST. In binding experiments, 50 μl of purified gp120 was added to chemokine-coated or control wells and allowed to react for 30 min at room temperature. Wells were then washed thrice. To detect bound gp120, 50 μl of a gp120-specific polyclonal rabbit serum (1170) created in our laboratory (1:1,250 dilution in BPBS) was added to wells and reacted at room temperature for 30 min. The wells were washed thrice before the addition of 50 μl HRP-conjugated secondary goat anti-rabbit antibody (1:2,500 dilution in BPBS; Cell Signaling Technology). After 30-min reaction at room temperature, the wells were washed six times. To visualize color, 100 μl of the tetramethylbenzidine (TMB) substrate solution (R&D Systems) was added to the wells. The OD450 was measured using an MRX Revelation microplate reader (Dynex Technologies) immediately after the addition of 50 μl 2 N sulfuric acid stop solution (R&D Systems).
Antibody inhibition assays
PF4 was preincubated in the absence or presence of 2× excess RTO or KKO anti-hPF4 antibodies at room temperature for 25 min. The antibody-PF4 mixture was then added to MAGI-CCR5 cells before infection with the indicated luciferase reporter pseudovirus. After addition of pseudovirus, plates were spinoculated at 1,200×g for 2 h at 4°C and then incubated at 37°C. Infection inoculum was replaced with fresh complete media after 2 h. Cells were then lysed with Brite-Glo (Promega) at 72 h postinfection, and RLUs were measured.
Statistical analysis
Infection values obtained with or without PF4 treatment were compared using t-tests. p-Values of less than 0.05 were considered significant. Data were analyzed with Prism 5.0 software.
Ethics statement
These studies were approved by the University of Pennsylvania's Institutional Review Board. All human cells used in this study were from normal healthy donors who provided written informed consent.
Results
PF4 exhibits a biphasic effect on HIV-1 entry
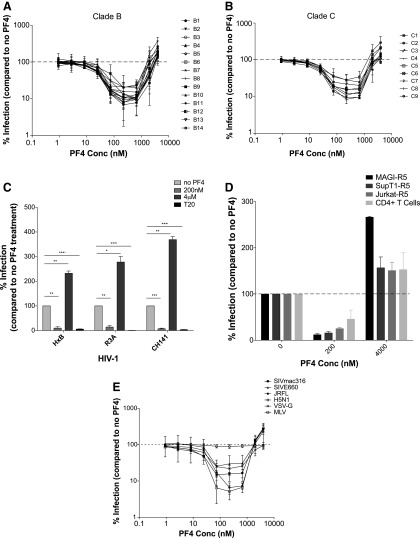
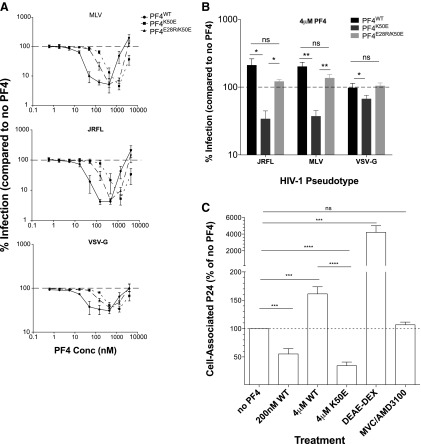
Initial studies evaluating the effect of recombinant human PF4 on HIV-1 infection in vitro demonstrated that PF4 possesses broad antiviral activity against multiple HIV-1 genetic clades, irrespective of coreceptor tropism.12,24 We utilized an independent panel of previously described26,2714 clade B and 9 clade C HIV-1 Envs (Table 1) to further explore the antiviral properties of this chemokine. Of the 23 Envs tested, 18 were CCR5 (R5)-tropic, 1 was CXCR4 (X4)-tropic, and 4 were R5/X4 (dual)-tropic, and 12 of the 23 Envs were derived from transmitted/founder (T/F) viruses.27–33 Multinuclear activation of galactosidase indicator cells endogenously expressing human CXCR4 and stably expressing human CD4 and CCR5 (MAGI-R5 cells)34 was pretreated with increasing concentrations of recombinant PF4 for 30 min, and subsequently infected with HIV-1 Env pseudoviruses. PF4 inhibited infection by all HIV-1 Env pseudoviruses tested, with maximal inhibition occurring at ∼200 nM added protein (mean% inhibition = 85 ± 6; p < 0.0001). T/F Envs were inhibited as efficiently as Envs derived from other viruses. However, when PF4 was added at concentrations above 200 nM, infection was inhibited less efficiently and was enhanced above (mean% infection = 174 ± 67; p < 0.0001) that of untreated controls at the highest concentration (4 μM) of PF4 tested (Fig. 1A, B).
Table 1.
Summary of T/F and Chronic HIV-1 Env Clones
| Env type | Clade | Numerical designation | Env clone designation | Coreceptor tropism | References | Accession number |
|---|---|---|---|---|---|---|
| T/F | B | 1 | REJO.D12.1972 | R5 | 26 | EU576707 |
| B | 2 | WEAUd15.410.5017 | R5/X4 | 26 | EU289202 | |
| B | 3 | 700010058.A4.4375 | R5 | 26 | EU576440 | |
| B | 4 | 700010077.SA2.6559 | R5/X4 | 26 | EU578999.2 | |
| B | 5 | TT35P.11H8.2874 | R5 | 26 | EU577329 | |
| B | 6 | 1006-11.C3.1601 | R5 | 26 | EU575025 | |
| B | 7 | 1056-10.TA11.1826 | R5 | 26 | EU575305 | |
| B | 8 | 1058-11.B11.1550 | R5/X4 | 26 | EU289187 | |
| C | 1 | 2833264_3G11 | R5 | 31 | HQ595757 | |
| C | 2 | 1245045_3C7 | R5 | 31 | HQ595742 | |
| C | 3 | ZM246F.F | R5 | 27–29 | n/a | |
| C | 4 | ZM247Fv2.fs | R5 | 27–29 | n/a | |
| Chronic | B | 9 | CRPE.B28.4072 | R5/X4 | 26 | EU578065.1 |
| B | 10 | JOTO.TA1.2247 | X4 | 26 | EU578181.1 | |
| B | 11 | OLLA.A14.1923 | R5 | 26 | EU578231 | |
| B | 12 | SAMI.A8.1863 | R5 | 26 | EU578272 | |
| B | 13 | SHKE.A26.4112 | R5 | 26 | EU578453 | |
| B | 14 | 1632-a6 | R5 | 30 | HQ216883 | |
| C | 5 | 704010330.G5h | R5 | 31 | JQ777128 | |
| C | 6 | 702010141_w12_e80.F | R5 | 31 | JQ779320 | |
| C | 7 | 704010461.A7h | R5 | 31 | JQ777137 | |
| C | 8 | 4403.A18 | R5 | 31 | HM070677 | |
| C | 9 | 4403.bmL.B6 | R5 | 31 | HM070754 |
HIV-1, human immunodeficiency virus type 1; HSX, heterosexual exposure; MSM, men who have sex with men; SPD, serial plasma donor; T/F, transmitted/founder.
FIG. 1.
PF4 exhibits biphasic activity on viral entry. (A) Fourteen clade B and (B) nine clade C HIV envelope pseudotypes were tested on MAGI-R5 cells in the absence or presence of increasing concentrations of PF4. PF4 inhibited infection of all Env pseudoviruses tested at concentrations below 200 nM. However, at concentrations above 2 μM, infection of all viruses was enhanced twofold to fivefold by PF4. (C) The dual activity of PF4 was recapitulated with CCR5- and CXCR4-using replication competent HIV-1. (D) PF4 exhibited biphasic activity against a replication competent, dual-tropic primary HIV-1 isolate (CH077) on multiple cell lines. (E) The effect of PF4 was not specific to HIV-1 envelope as the dual activity was evident to varying degrees with HIV-1 pseudoviruses bearing the glycoprotein of SIVmac316, SIVE660, VSV, and MLV. HIV-1 pseudotypes bearing the influenza glycoprotein H5N1 infection were unaffected by the presence of PF4. All experiments were done in at least triplicate in each of at least three independent experiments. Error bars represent standard deviations. ns, p > 0.05; *p < 0.05; **p < 0.005; ***p < 0.0001. HIV-1, human immunodeficiency virus type 1; PF4, Platelet-factor 4.
For our studies, we tested commercially available PF4 isolated from human platelets, commercially available recombinant PF4, as well as laboratory-produced recombinant PF4. We found that both recombinant forms of PF4 impacted HIV-1 infection identically over a broad concentration range, while the concentration needed for native PF4 to maximally inhibit (24 nM) and then enhance (200 nM) HIV-1 infection was ∼1-log less, perhaps reflecting inefficient refolding of the recombinant proteins. Nonetheless, as all three forms of PF4 exhibited similar biphasic effects on HIV-1 infection, we proceeded with the laboratory-developed recombinant PF4 for subsequent experiments.
The MAGI assay was also performed utilizing replication competent R5-, X4-, and dual-tropic HIV-1 (CH141, HxB, and R3A, respectively). As was observed with the pseudoviruses, 200 nM PF4 maximally inhibited infection of the clade C primary isolate HIV-1CH141 (mean% inhibition = 93 ± 4; p = 0.0007), the clade B primary isolate HIV-1R3A (mean% inhibition = 86, ± 10; p = 0.0044), and the laboratory-adapted strain HIV-1HxB (mean% inhibition = 90 ± 9; p = 0.0034). In contrast, at 4 μM, PF4 enhanced infection of all three viruses by twofold to threefold (Fig. 1C). A saturating concentration of the membrane fusion inhibitor enfuvirtide (T20) was used as a negative control in these experiments, inhibiting infection of all three viruses by 94%–99% of untreated control.
PF4 activity is evident on multiple cell types against a variety of viruses
To evaluate whether the activity of PF4 was specific to the MAGI-R5 cells, CD4+ T-cell lines stably expressing CCR5 (SupT1-R5 and Jurkat-R5) and primary human CD4+ T cells were infected with either HIV-1 pseudoviruses or replication-competent HIV-1 in the absence or presence of PF4. The biphasic activity of PF4 was observed in all cell types with all viruses tested (Fig. 1D). These results confirm the findings of previous studies12,24 that infection by R5- and X4-tropic HIV-1 strains is reduced in the presence of PF4. However, the inhibitory effects of PF4 were limited to a relatively narrow concentration range, above which viral infection in vitro was consistently enhanced.
To assess the specificity of anti-viral PF4 activity, we examined the ability of HIV-1 pseudotyped viruses bearing the envelope glycoproteins from SIVsmE660, SIVmac316, MLV, influenza (H5N1), or VSV to infect MAGI-R5 cells in the absence or presence of increasing concentrations of PF4. Infection by HIV-1SIVmac316 and HIV-1MLV was robustly inhibited by PF4 at ∼200 nM (mean% inhibition = 84 ± 1; p < 0.0001 and 95 ± 2; p = 0.0002, respectively), but significantly enhanced (mean% infection = 265 ± 71; p < 0.0212 and 286 ± 65; p = 0.0015, respectively) by 4 μM PF4. Infection by HIV-1VSV-G and HIV-1SIVsmmE660 was inhibited to a lesser degree (mean% inhibition = 78 ± 5; p = 0.0013 and 70 ± 20; p = 0.0256, respectively) with inhibition being lost as PF4 concentrations were increased, while HIV-1H5N1 infection was unaffected by PF4 (mean% inhibition = 4 ± 8; p = 0.2483) (Fig. 1E). Given that these pseudoviruses share a common HIV-1 core (NL4.3), yet were inhibited to varying degrees by PF4, we conclude that both the inhibitory as well as the viral enhancement effects of PF4 occur at the level of entry and are not restricted to HIV-1.
PF4 modulates viral entry by acting on viral attachment
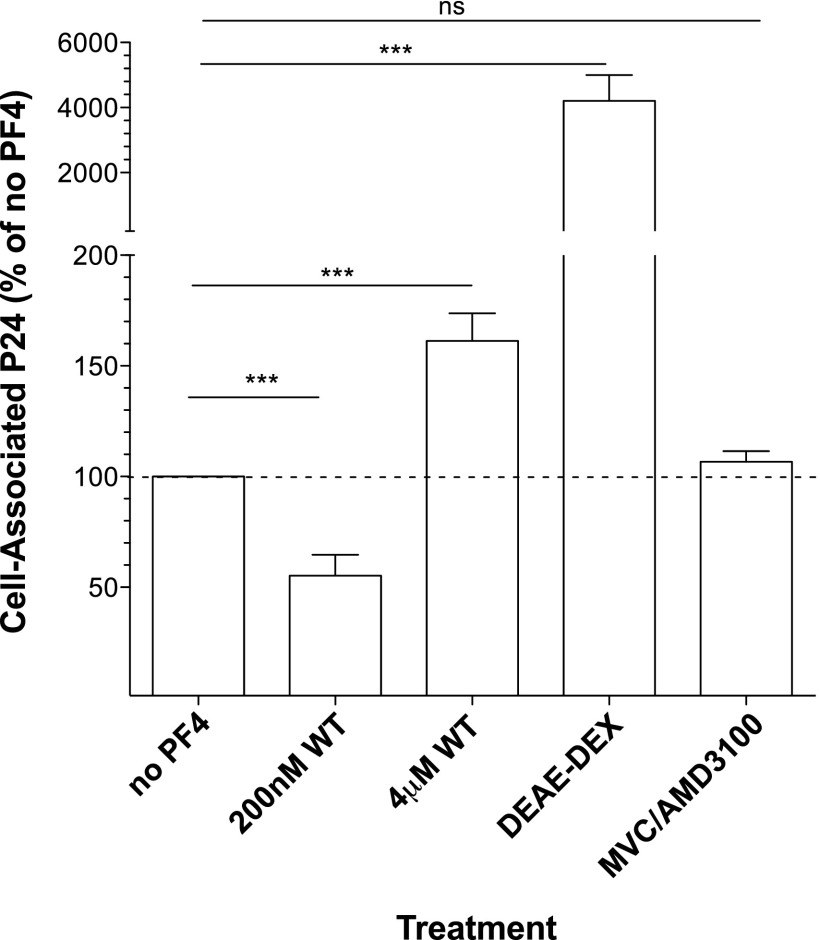
To explore the mechanism of action by which PF4 modulates HIV-1 infection, we first performed time of addition experiments and found that, as previously reported,24 PF4 most strongly inhibited HIV-1 when added before or simultaneously with the virus, consistent with it impacting HIV-1 entry (data not shown). To determine if PF4 impacts the first step of virus infection—binding of virions to the cell surface—we examined the ability of low and high concentrations of the chemokine to interfere with viral attachment. For this purpose, primary human CD4+ T cells were exposed to HIV-1R3A for 4 h at 37°C in the presence or absence of PF4. Cells were then split into two aliquots; one aliquot was treated with trypsin to remove attached viral particles remaining on the cell surface, while the other aliquot was left untreated. Attachment was measured by quantifying total versus trypsin-resistant cell-associated HIV-1 p24 Gag protein. When added at 200 nM, PF4 inhibited virus infection and also significantly reduced viral attachment to cells by 55% ± 9% compared to the no treatment control (p = 0.0008) (Fig. 2). In contrast, the infection-enhancing concentration of PF4 (4 μM) increased viral attachment to primary cells by 61% ± 12% relative to the no treatment control (p = 0.0006). The polycation DEAE-dextran was used as a positive control and enhanced viral attachment 42-fold above the untreated control (p = 0.0004). The coreceptor small molecule antagonists MVC and AMD-3100, which block viral engagement of CCR5 and CXCR4, respectively, had no significant effect on viral attachment as expected (Fig. 2). These data suggest that low levels of PF4 inhibit viral infection by decreasing viral adsorption to cells, while higher concentrations of the chemokine enhance viral attachment and thus increase infection.
FIG. 2.
PF4 modulates viral attachment to cells. Replication competent HIV-1 R3A binding to the cell surface was assessed in the absence or presence of PF4. The inhibitory concentration of PF4 (200 nM) significantly decreased viral attachment to cells compared to the untreated control (% cell-associated p24 = 55.2 ± 9.4; p = 0.0008). Contrastingly, the enhancing concentration of PF4 (4 μM) significantly increased viral binding to cells compared to control (% cell-associated p24 = 161.3 ± 12.5; p = 0.0006). The positive control DEAE-dextran increased viral attachment ∼42-fold above control (% cell-associated p24 = 4,207.0 ± 790.7; p = 0.0004). The small molecule coreceptor antagonists maraviroc (MVC) and AMD3100 served as negative controls and did not impact viral attachment as they work downstream of early attachment events. All experiments were done in duplicate with three donors in at least three independent experiments. Error bars represent standard error.
PF4 interacts with cellular GAGs
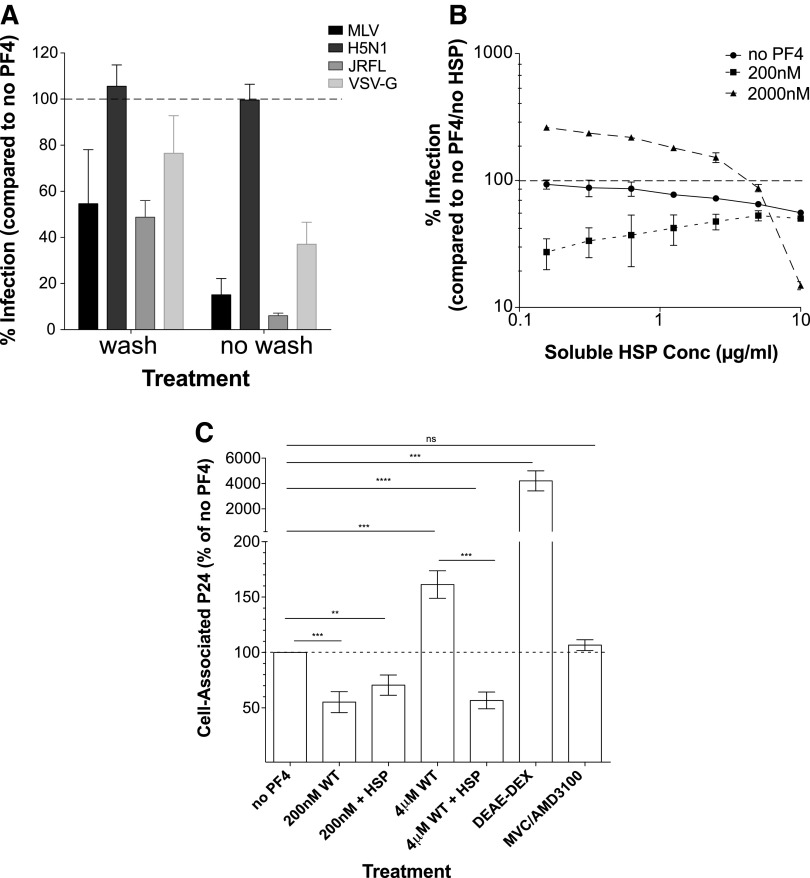
PF4 could potentially impact virus attachment by interacting with cell surface components, with the viral glycoprotein or with both. There is considerable evidence that many chemokines interact with cell surface GAGs, which in turn facilitate attachment of many viruses to cells.35–37 To determine if PF4 may interact with cell surface molecules in a manner that impacts virus attachment, we treated MAGI-R5 cells with an inhibitory concentration of PF4 (200 nM) for 30 min, then either washed the cells with PBS (wash) or left unwashed (no wash), and subsequently infected with HIV-1 pseudovirus. We hypothesized that if PF4's mechanism of action entailed interactions with the viral glycoprotein, then prebinding to the cell surface followed by washing should have little impact on virus infection. If, however, PF4 binds to a cell surface molecule that participates in virus attachment, then washing off prebound PF4 should reduce the chemokine's effect on virus infection depending on the affinity of the interaction. Prebinding PF4 to the cell surface followed by extensive washing decreased subsequent infection by HIV-1MLV, HIV-1JRFL, and HIV-1VSV-G by 45% ± 12% (p = 0.016), 51% ± 7% (p = 0.007), and 24% ± 16% (p = 0.13), respectively (Fig. 3A), consistent with PF4 interacting with a cell surface molecule(s) to antagonize viral infection. As expected, prebinding and then washing PF4 from cells had no effect on HIV-1H5N1 infection (mean% infection = 106 ± 9; p = 0.4080) (Fig. 3A). While these results do not exclude the possibility of unwashed PF4 interacting with the viral glycoprotein to inhibit infection, they do suggest that PF4 activity is significantly dependent upon its interaction with cell surface molecules.
FIG. 3.
PF4 interacts with cell surface GAGs. (A) To address whether PF4 interacts with a cell surface molecule to modulate infection, cells were pretreated with 200 nM PF4 and either washed five times with PBS or left unwashed, followed by infection with HIV-1 pseudoviruses bearing the glycoprotein of MLV, H5N1, JRFL, or VSV. Washing off PF4 before infection reduced entry by approximately twofold. Infection was inhibited at least threefold when PF4 was left on cells. The negative control HIV-1H5N1 infection was unaffected by either treatment. (B) Effect of cell surface GAGs on PF4 activity. Infection of HIV-1JRFL was measured in the presence of 0, 200, or 2,000 nM PF4 with increasing concentrations of soluble HSP. 10 μg/ml HSP significantly diminished the effect of 2,000 nM PF4 on viral infection, impacting infection ∼18-fold. HSP had a twofold effect on infection in the presence of low concentrations of PF4 and in the absence of PF4. (C) Addition of soluble HSP to the low concentration of PF4 did not significantly impact its effect on viral binding. However, adding HSP to the high concentration of PF4 significantly decreased cell-associated virus to levels comparable to 200 nM PF4. All experiments were done in at least triplicate in each of at least three independent experiments. Error bars represent standard deviations. GAGs, glycosaminoglycans; HSP, heparan sulfate proteoglycan; PBS, phosphate-buffered saline.
Since it is well established that PF4 binds to negatively charged GAGs such as HSPs and chondroitin sulfate proteoglycans (CSP),38–41 we performed competition assays using soluble HSP. MAGI-R5 cells were infected with HIV-1 pseudoviruses in the presence or absence of increasing concentrations of soluble HSP and no low (200 nM) or high (2 μM) concentrations of PF4. As the concentration of soluble HSP was increased, the ability of the low concentration of PF4 to inhibit infection waned, eventually reaching the same level as no PF4. In addition, high amounts of HSP ablated the capacity of the high concentration of PF4 to enhance infection (HIV-1JRFL shown in Fig. 3B). As previously reported, in the absence of PF4, exogenous HSP inhibited HIV-1 infection in a concentration-dependent manner.42 Similar results were observed with soluble CSP (data not shown). The fact that soluble HSP reduced the ability of PF4 to inhibit virus infection and entirely ablated the ability of high concentrations of PF4 to enhance virus infection could be linked to virus binding—at inhibitory concentrations of PF4, the presence of 10 μg/ml soluble HSP slightly increased virus binding (mean% cell-associated p24 200 nM vs. 200 nM + HSP = 55 ± 9 vs. 70 ± 9; p = 0.3), while at enhancing concentrations of PF4, the presence of HSP strongly reduced virus binding to the cell surface (mean% cell-associated p24 = 57 ± 7; p < 0.0001) (Fig. 3C). These data are consistent with PF4 being able to bind to GAGs, particularly at high PF4 concentrations, with this in turn being linked to enhanced virus binding to the cell surface.
HIV-1 gp120 binds specifically to PF4
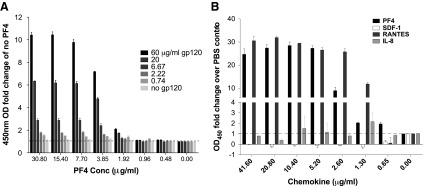
The ability of PF4 to bind to GAGs does not, by itself, explain how it can enhance virus infection at high concentrations. Therefore, we hypothesized that at high concentrations, PF4 might function as an electrostatic bridge between virions and cell surface GAGs to modulate attachment. If this is true, not only should PF4 interact with cell surface GAGs but it should also interact with the virus. To test this, we assessed the ability of purified HIV-1 envelope glycoprotein gp120 to bind to polystyrene-immobilized PF4 by the enzyme-linked immunosorbent assay (ELISA). Graded amounts of PF4 were immobilized overnight before incubation with varying concentrations of purified HIV-1JRFL gp120 for 30 min. After washing, bound gp120 was detected using a rabbit polyclonal serum.43 We found that gp120 bound to PF4 in a concentration-dependent manner (Fig. 4A). Likewise, gp120 also bound to RANTES in a concentration-dependent manner, consistent with previous work.44 In contrast, gp120 failed to bind to immobilized IL-8 or SDF-1 using the same assay conditions (Fig. 4B). From these results, we conclude that the bimolecular interaction observed between gp120 and PF4/RANTES is direct and has some degree of specificity.
FIG. 4.
HIV-1 gp120 binds specifically to immobilized PF4. (A) Graded amounts of PF4 were immobilized overnight before incubating with varying concentrations of purified HIV-1 JRFL gp120. JRFL gp120 bound to PF4 in a concentration-dependent manner. (B) To assess selectivity of gp120 binding, we tested the ability of other chemokines of similar size and charge to PF4 to bind gp120. RANTES was used as a positive control and bound JRFL gp120. However, gp120 did not bind IL-8 or SDF-1. All experiments were done in at least triplicate in each of at least three independent experiments. Error bars represent standard deviations. IL-8, interleukin-8; SDF-1, stromal cell-derived factor 1.
PF4 oligomerization state correlates with its effects on virus infection
PF4 in solution exists in a dynamic state; at low concentrations it is largely monomeric, while at high concentrations it forms tetramers.15,25,45,46 Chemical crosslinking was used to confirm this equilibrium using the recombinant PF4 used in our studies.47 Although this equilibrium exists in solution in vitro, under physiologic conditions it has been hypothesized that PF4 exists primarily as a tetramer and avidly binds to heparin and GAGs to form the ultralarge antigenic complexes noted in the clinical disorder of heparin-induced thrombocytopenia (HIT).15,25,45,47–49 Mutations that decrease the formation of PF4 tetramers have been shown to reduce the formation of these large complexes. Rauova et al. disrupted the ionic interactions between recombinant PF4 dimers by substituting Lys at position 50 with Glu to create PF4K50E, which shifts the equilibrium of PF4 to favor dimers and monomers.47 In addition, a double mutant was generated in which the Glu28 and Lys50 in PF4 were replaced with Arg and Glu (PF4E28R/K50E) to reinstate the ionic interactions between dimers, significantly restoring the ability to form tetramers at high concentrations.47 Given this concentration-dependent oligomerization of PF4 and its two opposing effects on viral entry, we reasoned that inhibition might be linked to the presence of monomers that bind directly to Env and prevent it from interacting with the cell surface, whereas PF4 tetramers may function to enhance viral infection by forming a bridge between cell surface GAGs and Env, overriding the inhibitory effect and thereby enhancing virus attachment.
To explore the effect of oligomeric state on PF4 activity, MAGI-R5 cells were infected with HIV-1 pseudoviruses in the absence or presence of increasing concentrations of recombinant PF4WT, PF4K50E, or PF4E28R/K50E. For all viruses tested, PF4K50E exhibited an ∼1-log increase in IC50 compared to PF4WT, while the double mutant PF4E28R/K50E partially rescued this loss in potency (Fig. 5A). As previously observed, HIV-1JRFL and HIV-1MLV infection was enhanced approximately twofold of untreated control (mean% infection = 210 ± 51 and 200 ± 32, respectively) at the highest concentration (4 μM) of PF4WT tested. Relative to PF4WT, 4 μM of the monomer-favoring PF4K50E did not enhance, but rather inhibited HIV-1JRFL and HIV-1MLV infection (mean% inhibition = 66 ± 11; p = 0.028 and 63% ± 8%; p = 0.008, respectively), while the complementary mutant PF4E28R/K50E restored the enhancing activity (mean% infection = 121 ± 9; p = 0.45 and 136% ± 18%; p = 0.16, respectively), although not to the levels of PF4WT (Fig. 5A, 5B). Compared to the untreated control, HIV-1VSV-G infection was inhibited by 4 μM PF4K50E (mean% inhibition = 33 ± 9; p = 0.0154), but not significantly impacted by 4 μM PF4WT or PF4E28R/K50E (mean% infection = 97 ± 16; p = 0.89 and 104 ± 11; p = 0.19, respectively) (Fig. 5A, B). In addition, we tested the ability of PF4K50E to modulate viral attachment as we had previously done with low and high concentrations of PF4WT. As observed with the low concentration of PF4WT, the monomer-favoring PF4K50E significantly decreased viral attachment at 4 μM compared to untreated controls (% cell-associated p24 = 35 ± 6; p < 0.0001) (Fig. 5C). From these data, we conclude that the antiviral property of PF4 occurs under conditions where tetramers are not prevalent, while the presence of tetramers is associated with enhancement of viral infection.
FIG. 5.
PF4 oligomeric state correlates with its biphasic activity on viral infection. (A) MAGI-R5 cells were infected with HIV-1 pseudoviruses bearing the glycoprotein of MLV (top), JRFL (middle), and VSV (bottom) in the absence or increasing presence of PF4WT, PF4K50E, or PF4E28R/K50E. (B) Virus entry was measured at high (4 μM) concentrations of PF4WT, PF4K50E, or PF4E28R/K50E. PF4WT enhanced infection of HIV-1JRFL and HIV-1MLV approximately twofold. PF4K50E significantly inhibited HIV-1JRFL, HIV-1MLV, and HIV-1VSV-G entry. PF4E28R/K50E restored the enhancement observed with PF4WT. PF4WT and PF4E28R/K50E did not significantly impact HIV-1VSV-G entry. (C) High concentrations of the monomer-favoring mutant PF4K50E significantly reduced viral binding to cells. All experiments were done in at least triplicate in each of at least three independent experiments. Error bars represent standard deviations. ns, p > 0.05; *p < 0.05; **p < 0.005; ***p < 0.0001.
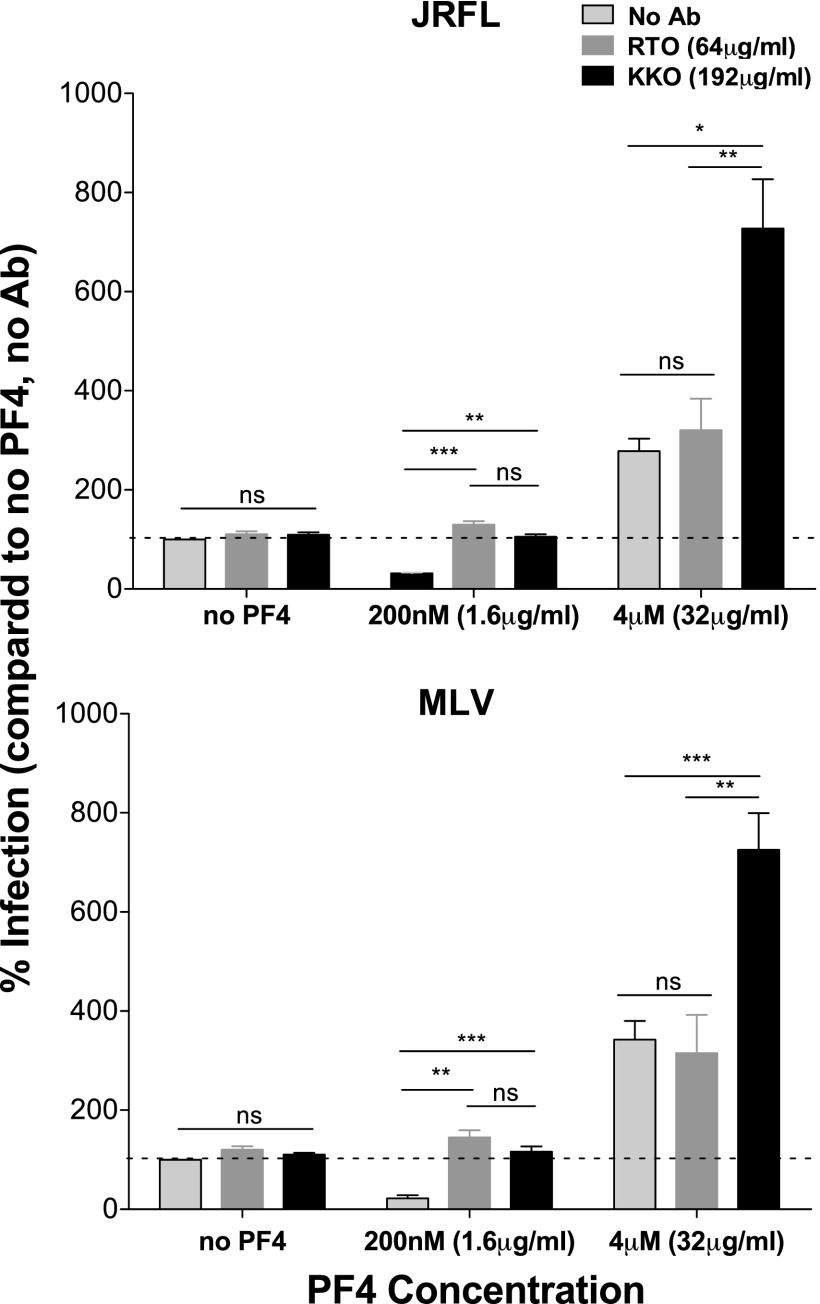
Antibodies that recognize distinct oligomeric forms of PF4 were used to further probe the role of oligomeric state on PF4 activity during viral infection. The monoclonal anti-hPF4 antibody RTO selectively binds to PF4 monomers, while the HIT-like monoclonal antibody KKO induces the oligomerization of PF4 tetramers and preferentially recognizes large complexes that comprise PF4 tetramers.50,51 If monomers and dimers are responsible for the inhibitory effect of PF4 on virus infection, then both RTO and KKO should ablate the inhibitory effect of 200 nM PF4, which in fact we observed (Fig. 6). However, at 4 μM PF4, where preformed tetramers predominate, the antibodies should differ in their effects—RTO should not impact PF4 activity since it preferentially binds monomers, while KKO may further enhance infectivity by inducing even greater PF4 oligomerization, which enhances attachment of virus to the cell surface. We found this to be the case, with KKO further enhancing infectivity of both HIV-1JRFL and HIV-1MLV pseudotypes in the presence of 4 μM PF4 (Fig. 6). These results further support the hypothesis that oligomeric forms of PF4 enhance viral infection.
FIG. 6.
Antibodies recognizing distinct oligomeric forms of PF4 impact chemokine activity during infection. 0, 200 nM, or 4 μM PF4 was preincubated in the absence or presence of excess RTO or KKO anti-hPF4 antibodies. The mixture was then added to MAGI-R5 cells, followed by HIV-1JRFL (top) or HIV-1MLV (bottom) infection. For both pseudoviruses tested, RTO and KKO did not significantly impact viral infection in the absence of PF4. However, both antibodies prevented 200 nM PF4-mediated viral inhibition. At 4 μM PF4, RTO did not impact viral enhancement compared to no antibody control. KKO further enhanced viral infection in the presence of 4 μM PF4. All experiments were done in at least triplicate in each of at least three independent experiments. Error bars represent standard deviations. ns, p > 0.05; *p < 0.05; **p < 0.005; ***p < 0.0001.
Discussion
Increased platelet activation, thrombocytopenia, and thrombosis are complications associated with HIV-1 infection.49,52–55 Several studies describe an antiviral role for chemokines released from activated platelets, including RANTES and PF4.9,12,24 RANTES can modulate virus infection through diverse mechanisms that are dependent upon its concentration. At low concentrations, RANTES can inhibit HIV infection by blocking its interaction with the viral coreceptor CCR5.9,44,56–61 At high and likely supraphysiological concentrations, RANTES forms higher order oligomers that bind to the viral Env protein as well as cell surface GAGs, enhancing virus attachment and infection, at least in vitro.44 RANTES can also modulate virus infection by transducing signals through CCR5 that over time render cells more permissive for virus replication.62 Likewise, we find that PF4 can both inhibit and enhance HIV-1 infection in a concentration-dependent manner, with enhancement being observed at PF4 concentrations likely to be found in proximity to cell surfaces.
Recent studies that examined the impact of PF4 on HIV-1 infection suggested that the in vitro inhibitory effects of PF4 could be exploited therapeutically.12,24 Enhancement of infection by PF4 was not reported in these studies, although concentrations greater than 650 nM were not tested. However, a previous study by Schwartzkopff et al. showed that higher concentrations of PF4 (4 μM) actually enhanced HIV-1 infection in macrophages.63 The availability of recombinant PF4, PF4 mutations that impact its oligomeric properties, and oligomeric state-specific anti-PF4 antibodies allowed us to more fully explore this chemokine's biphasic effects on HIV-1 infection. We confirmed earlier findings that at low concentrations, PF4 inhibits HIV-1 infection by ∼1-log and linked this inhibition to the ability of PF4 to bind directly to the gp120 subunit of the viral Env protein and to decrease binding of virions to the cell surface. However, PF4 also inhibited infection by several other viruses that bear little similarity to HIV-1 and inhibited all of the genetically diverse HIV-1 strains that we tested, indicting that it has broad antiviral potency. From this we conclude that while PF4 can engage the viral Env protein, it likely does so in a relatively nonspecific manner, increasing the likelihood of off-target effects, thus potentially limiting its therapeutic use. Furthermore, the fact that PF4 inhibited infection of all HIV-1 strains we tested in vitro suggests that it has not applied sufficient selective pressure in vivo to drive the development of widespread resistance.
PF4 exists in dynamic equilibrium in solution, where monomers assemble into tetramers through dimer intermediates in a concentration-dependent manner.15,25,45,46 However, it is likely that under physiological conditions, PF4 exists predominantly as a tetramer complexed with GAGs.15,25,45,48 The biphasic effect of PF4 on HIV-1 infection can be explained by its tendency to oligomerize at physiologically relevant concentrations and its ability to bind to cell surface GAGs in a manner analogous to RANTES.15,48 As PF4 concentrations increased, with a concomitant shift toward tetrameric and higher-order complexes, the inhibitory capacity of PF4 waned and viral infection was ultimately enhanced. This finding was observed for all HIV-1 strains tested as well as virions bearing the SIV and MLV glycoproteins. Collectively, these data suggest that in vivo, where PF4 likely exists primarily as a GAG-associated tetramer, the inhibitory effects of the monomeric chemokine are less likely to predominate.
The mechanism by which PF4 enhanced virus infection at high concentrations was again at the level of virus binding. Like most chemokines, oligomeric PF4 binds with high affinity to polyanionic GAGs such as heparin and HSPs.64 Since PF4 can also bind to the viral Env protein, we hypothesized that PF4 oligomers can function as an electrostatic bridge between virions and cell surface GAGs. In support of this hypothesis, we found that high concentrations of PF4 enhanced virus binding and that the addition of soluble heparan and CSP significantly mitigated the enhancing, but not the inhibitory, effects of PF4 by decreasing viral attachment to cells. This is not entirely surprising, as PF4 tetramers have been shown to exhibit higher affinity for GAGs due to their favorable quaternary structure, which exposes a ring of basic amino acids.64,65 In addition, by promoting oligomerization, the HIT-like antibody KKO likely induced the formation of ultralarge complexes of PF4 tetramers that further potentiated the basal enhancement effect of 4 μM PF4 in vitro.
While RANTES and PF4 can both inhibit viral infection at low concentrations, the mechanisms are different: RANTES inhibits virus infection in a highly specific manner by interacting with the viral coreceptor, while PF4 inhibits HIV-1 infection by binding to the viral Env protein. In contrast, both chemokines can enhance virus infection at high concentrations by forming higher ordered complexes and enhancing virus binding to cells through interactions with cell surface GAGs. However, while the concentrations at which RANTES enhances HIV-1 infection in vitro are unlikely to be found in vivo, it is likely that physiological concentrations of PF4 can enhance virus infection. These findings underscore the importance of examining the full spectrum of relevant concentrations when assessing the impact of chemokines on virus infection, given their ability to form oligomers that in turn influence their interactions with cell surface molecules. In addition, it is possible that the enhancing effect of PF4 on virus infection could impact HIV-1 pathogenesis. PF4 is produced by megakaryocytes in the bone marrow leading to intramedullary release66 and regulates hematopoietic stem cell cycle activity.67 The presence of PF4 in the bone marrow could enhance HIV-1 infection of stem cells and other progenitors, contributing to the well-documented hematopoietic abnormalities that are common in HIV-infected individuals.
Acknowledgments
The authors thank Beatrice Hahn for providing HIV-1 viral stocks. They thank Gary Nabel and the Vaccine Research Center (VRC) of the National Institute of Allergy and Infectious Diseases for providing influenza plasmids. They also thank Chuka Didigu, Shilpa Iyer, Paul Bates, and Craig Wilen for helpful comments and technical advice. In addition, they thank the University of Pennsylvania's Center for Aids Research (CFAR) human immunology and flow cytometry core facilities for reagents and equipment. This work was supported by the National Institutes of Health (NIH) grant P01HL110860-01. The content in this publication is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. Z.F.P. is the recipient of a National Science Foundation Graduate Research Fellowship (fellow ID: 2012140562).
Authorship
Contribution: Z.F.P., A.H.R., A.M.R., F-H.L., L.R., D.B.C., M.P., B.S.S., and R.W.D. Conceived and designed the experiments: Z.F.P., A.H.R., B.S.S., M.P., and R.W.D. Performed the experiments: Z.F.P., A.H.R., A.M.R., and F-H.L. Analyzed the data: Z.F.P. and A.H.R. Contributed reagents/materials/analysis tools: L.R., A.H.R., B.S.S., and M.P. Wrote the manuscript: Z.F.P. and R.W.D.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Feng Y, Broder CC, Kennedy PE, Berger EA: HIV-1 entry cofactor: Functional cDNA cloning of a seven-transmembrane, G protein-coupled receptor. Science 1996;272:872–877 [DOI] [PubMed] [Google Scholar]
- 2.Dragic T, Litwin V, Allaway GP, et al. : HIV-1 entry into CD4+ cells is mediated by the chemokine receptor CC-CKR-5. Nature 1996;381:667–673 [DOI] [PubMed] [Google Scholar]
- 3.Doranz B: A dual-tropic primary HIV-1 isolate that uses fusin and the beta-chemokine receptors CKR-5, CKR-3, and CKR-2b as fusion cofactors. Cell 1996;85:1149–1158 [DOI] [PubMed] [Google Scholar]
- 4.Deng H, Liu R, Ellmeier W, et al. : Identification of a major co-receptor for primary isolates of HIV-1. Nature 1996;381:661–666 [DOI] [PubMed] [Google Scholar]
- 5.Choe H, Farzan M, Sun Y, et al. : The beta-chemokine receptors CCR3 and CCR5 facilitate infection by primary HIV-1 isolates. Cell 1996;85:1135–1148 [DOI] [PubMed] [Google Scholar]
- 6.Alkhatib G, Combadiere C, Broder CC, et al. : CC CKR5: A RANTES, MIP-1alpha, MIP-1beta receptor as a fusion cofactor for macrophage-tropic HIV-1. Science 1996;272:1955–1958 [DOI] [PubMed] [Google Scholar]
- 7.Walker CM, Levy JA: A diffusible lymphokine produced by CD8+ T lymphocytes suppresses HIV replication. Immunology 1989;66:628–630 [PMC free article] [PubMed] [Google Scholar]
- 8.Brinchmann JE, Gaudernack G, Vartdal F: CD8+ T cells inhibit HIV replication in naturally infected CD4+ T cells. Evidence for a soluble inhibitor. J Immunol 1990;144:2961–2966 [PubMed] [Google Scholar]
- 9.Cocchi F, DeVico AL, Garzino-Demo A, Arya SK, Gallo RC, Lusso P: Identification of RANTES, MIP-1 alpha, and MIP-1 beta as the major HIV-suppressive factors produced by CD8+ T cells. Science 1995;270:1811–1815 [DOI] [PubMed] [Google Scholar]
- 10.DeVico AL, Gallo RC: Control of HIV-1 infection by soluble factors of the immune response. Nat Rev Microbiol 2004;2:401–413 [DOI] [PubMed] [Google Scholar]
- 11.Walker CM, Moody DJ, Stites DP, Levy JA: CD8+ T lymphocyte control of HIV replication in cultured CD4+ cells varies among infected individuals. Cell Immunol 1989;119:470–475 [DOI] [PubMed] [Google Scholar]
- 12.Solomon Tsegaye T, Gnirß K, Rahe-Meyer N, et al. : Platelet activation suppresses HIV-1 infection of T cells. Retrovirology 2013;10:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brandt E, Ludwig A, Petersen F, Flad HD: Platelet-derived CXC chemokines: Old players in new games. Immunol Rev 2000;177:204–216 [DOI] [PubMed] [Google Scholar]
- 14.Hundelshausen von P, Petersen F, Brandt E: Platelet-derived chemokines in vascular biology. Thromb Haemost 2007;97:704–713 [DOI] [PubMed] [Google Scholar]
- 15.Barber AJ, Käser-Glanzmann R, Jakábová M, Lüscher EF: Characterization of a chondroitin 4-sulfate proteoglycan carrier for heparin neutralizing activity (platelet factor 4) released from human blood platelets. Biochim Biophys Acta 1972;286:312–329 [PubMed] [Google Scholar]
- 16.Slungaard A: Platelet factor 4: A chemokine enigma. Int J Biochem Cell Biol 2005;37:1162–1167 [DOI] [PubMed] [Google Scholar]
- 17.Files JC, Malpass TW, Yee EK, Ritchie JL, Harker LA: Studies of human plate alpha-granule release in vivo. Blood 1981;58:607–618 [PubMed] [Google Scholar]
- 18.Brandt E, Petersen F, Ludwig A, Ehlert JE, Bock L, Flad HD: The beta-thromboglobulins and platelet factor 4: Blood platelet-derived CXC chemokines with divergent roles in early neutrophil regulation. J Leukoc Biol 2000;67:471–478 [DOI] [PubMed] [Google Scholar]
- 19.Kaplan KL, Owen J: Plasma levels of beta-thromboglobulin and platelet factor 4 as indices of platelet activation in vivo. Blood 1981;57:199–202 [PubMed] [Google Scholar]
- 20.Chesterman CN, McGready JR, Doyle DJ, Morgan FJ: Plasma levels of platelet factor 4 measured by radioimmunoassay. Br J Haematol 1978;40:489–500 [DOI] [PubMed] [Google Scholar]
- 21.Lasagni L, Francalanci M, Annunziato F, et al. : An alternatively spliced variant of CXCR3 mediates the inhibition of endothelial cell growth induced by IP-10, Mig, and I-TAC, and acts as functional receptor for platelet factor 4. J Exp Med 2003;197:1537–1549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mueller A, Meiser A, McDonagh EM, et al. : CXCL4-induced migration of activated T lymphocytes is mediated by the chemokine receptor CXCR3. J Leukoc Biol 2008;83:875–882 [DOI] [PubMed] [Google Scholar]
- 23.Korniejewska A, McKnight AJ, Johnson Z, Watson ML, Ward SG: Expression and agonist responsiveness of CXCR3 variants in human T lymphocytes. Immunology 2011;132:503–515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Auerbach DJ, Lin Y, Miao H, et al. : Identification of the platelet-derived chemokine CXCL4/PF-4 as a broad-spectrum HIV-1 inhibitor. Proc Natl Acad Sci U S A 2012;109:9569–9574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mayo KH, Chen MJ: Human platelet factor 4 monomer-dimer-tetramer equilibria investigated by proton NMR spectroscopy. Biochemistry 1989;28:9469–9478 [DOI] [PubMed] [Google Scholar]
- 26.Wilen CB, Parrish NF, Pfaff JM, et al. : Phenotypic and immunologic comparison of clade B transmitted/founder and chronic HIV-1 envelope glycoproteins. J Virol 2011;85:8514–8527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Parrish NF, Wilen CB, Banks LB, et al. : Transmitted/founder and chronic subtype C HIV-1 use CD4 and CCR5 receptors with equal efficiency and are not inhibited by blocking the integrin α4β7. PLoS Pathog 2012;8:e1002686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Keele BF, Giorgi EE, Salazar-Gonzalez JF, et al. : Identification and characterization of transmitted and early founder virus envelopes in primary HIV-1 infection. Proc Natl Acad Sci U S A 2008;105:7552–7557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Salazar-Gonzalez JF, Bailes E, Pham KT, et al. : Deciphering human immunodeficiency virus type 1 transmission and early envelope diversification by single-genome amplification and sequencing. J Virol 2008;82:3952–3970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Salazar-Gonzalez JF, Salazar MG, Keele BF, et al. : Genetic identity, biological phenotype, and evolutionary pathways of transmitted/founder viruses in acute and early HIV-1 infection. J Exp Med 2009;206:1273–1289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jiang C, Parrish NF, Wilen CB, et al. : Primary infection by a human immunodeficiency virus with atypical coreceptor tropism. J Virol 2011;85:10669–10681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gnanakaran S, Bhattacharya T, Daniels M, et al. : Recurrent signature patterns in HIV-1 B clade envelope glycoproteins associated with either early or chronic infections. PLoS Pathog 2011;7:e1002209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kirchherr JL, Hamilton J, Lu X, et al. : Identification of amino acid substitutions associated with neutralization phenotype in the human immunodeficiency virus type-1 subtype C gp120. Virology 2011;409:163–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Charneau P, Mirambeau G, Roux P, Paulous S, Buc H, Clavel F: HIV-1 reverse transcription. A termination step at the center of the genome. J Mol Biol 1994;241:651–662 [DOI] [PubMed] [Google Scholar]
- 35.Handel TM, Johnson Z, Crown SE, Lau EK, Proudfoot AE: Regulation of protein function by glycosaminoglycans—As exemplified by chemokines. Annu Rev Biochem 2005;74:385–410 [DOI] [PubMed] [Google Scholar]
- 36.Proudfoot AEI, Handel TM, Johnson Z, et al. : Glycosaminoglycan binding and oligomerization are essential for the in vivo activity of certain chemokines. Proc Natl Acad Sci U S A 2003;100:1885–1890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Johnson Z, Proudfoot AE, Handel TM: Interaction of chemokines and glycosaminoglycans: A new twist in the regulation of chemokine function with opportunities for therapeutic intervention. Cytokine Growth Factor Rev 2005;16:625–636 [DOI] [PubMed] [Google Scholar]
- 38.Petersen F, Brandt E, Lindahl U, Spillmann D: Characterization of a neutrophil cell surface glycosaminoglycan that mediates binding of platelet factor 4. J Biol Chem 1999;274:12376–12382 [DOI] [PubMed] [Google Scholar]
- 39.Sachais BS, Higazi AA-R, Cines DB, Poncz M, Kowalska MA: Interactions of platelet factor 4 with the vessel wall. Semin Thromb Hemost 2004;30:351–358 [DOI] [PubMed] [Google Scholar]
- 40.Loscalzo J, Melnick B, Handin RI: The interaction of platelet factor four and glycosaminoglycans. Arch Biochem Biophys 1985;240:446–455 [DOI] [PubMed] [Google Scholar]
- 41.Kowalska MA, Rauova L, Poncz M: Role of the platelet chemokine platelet factor 4 (PF4) in hemostasis and thrombosis. Thromb Res 2010;125:292–296 [DOI] [PubMed] [Google Scholar]
- 42.Patel M, Yanagishita M, Roderiquez G, et al. : Cell-surface heparan sulfate proteoglycan mediates HIV-1 infection of T-cell lines. AIDS Res Hum Retroviruses 2009;9:167–174 [DOI] [PubMed] [Google Scholar]
- 43.Agrawal-Gamse C, Luallen RJ, Liu B, et al. : Yeast-elicited cross-reactive antibodies to HIV Env glycans efficiently neutralize virions expressing exclusively high-mannose N-linked glycans. J Virol 2011;85:470–480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Trkola A, Gordon C, Matthews J, et al. : The CC-chemokine RANTES increases the attachment of human immunodeficiency virus type 1 to target cells via glycosaminoglycans and also activates a signal transduction pathway that enhances viral infectivity. J Virol 1999;73:6370–6379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Moore S, Pepper DS, Cash JD: Platelet antiheparin activity. The isolation and characterisation of platelet factor 4 released from thrombin-aggregated washed human platelets and its dissociation into subunits and the isolation of membrane-bound antiheparin activity. Biochim Biophys Acta 1975;379:370–384 [PubMed] [Google Scholar]
- 46.Sachais BS, Rux AH, Cines DB, et al. : Rational design and characterization of platelet factor 4 antagonists for the study of heparin-induced thrombocytopenia. Blood 2012;119:5955–5962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rauova L, Poncz M, McKenzie SE, et al. : Ultralarge complexes of PF4 and heparin are central to the pathogenesis of heparin-induced thrombocytopenia. Blood 2005;105:131–138 [DOI] [PubMed] [Google Scholar]
- 48.Huang SS, Huang JS, Deuel TF: Proteoglycan carrier of human platelet factor 4. Isolation and characterization. J Biol Chem 1982;257:11546–11550 [PubMed] [Google Scholar]
- 49.Amiral J, Bridey F, Dreyfus M, et al. : Platelet factor 4 complexed to heparin is the target for antibodies generated in heparin-induced thrombocytopenia. Thromb Haemost 1992;68:95–96 [PubMed] [Google Scholar]
- 50.Arepally GM, Kamei S, Park KS, et al. : Characterization of a murine monoclonal antibody that mimics heparin-induced thrombocytopenia antibodies. Blood 2000;95:1533–1540 [PubMed] [Google Scholar]
- 51.Sachais BS, Litvinov RI, Yarovoi SV, et al. : Dynamic antibody-binding properties in the pathogenesis of HIT. Blood 2012;120:1137–1142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Holme PA, Müller F, Solum NO, Brosstad F, Frøland SS, Aukrust P: Enhanced activation of platelets with abnormal release of RANTES in human immunodeficiency virus type 1 infection. FASEB J 1998;12:79–90 [DOI] [PubMed] [Google Scholar]
- 53.Servais J, Nkoghe D, Schmit JC, et al. : HIV-associated hematologic disorders are correlated with plasma viral load and improve under highly active antiretroviral therapy. J Acquir Immune Defic Syndr 2001;28:221–225 [DOI] [PubMed] [Google Scholar]
- 54.Corrales-Medina VF, Simkins J, Chirinos JA, et al. : Increased levels of platelet microparticles in HIV-infected patients with good response to highly active antiretroviral therapy. J Acquir Immune Defic Syndr 2010;54:217–218 [DOI] [PubMed] [Google Scholar]
- 55.Metcalf Pate KA, Pate KM, Mankowski JL, Mankowski JL. HIV and SIV associated thrombocytopenia: An expanding role for platelets in the pathogenesis of HIV. Drug Discov Today Dis Mech 2011;8:e25–e32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Arenzana-Seisdedos F, Virelizier JL, Rousset D, et al. : HIV blocked by chemokine antagonist. Nature 1996;383:400. [DOI] [PubMed] [Google Scholar]
- 57.Burns JM, Gallo RC, DeVico AL, Lewis GK: A new monoclonal antibody, mAb 4A12, identifies a role for the glycosaminoglycan (GAG) binding domain of RANTES in the antiviral effect against HIV-1 and intracellular Ca2+ signaling. J Exp Med 1998;188:1917–1927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mack M, Luckow B, Nelson PJ, et al. : Aminooxypentane-RANTES induces CCR5 internalization but inhibits recycling: A novel inhibitory mechanism of HIV infectivity. J Exp Med 1998;187:1215–1224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Oravecz T, Pall M, Norcross MA: Beta-chemokine inhibition of monocytotropic HIV-1 infection. Interference with a postbinding fusion step. J Immunol 1996;157:1329–1332 [PubMed] [Google Scholar]
- 60.Simmons G, Clapham PR, Picard L, et al. : Potent inhibition of HIV-1 infectivity in macrophages and lymphocytes by a novel CCR5 antagonist. Science 1997;276:276–279 [DOI] [PubMed] [Google Scholar]
- 61.Trkola A, Paxton WA, Monard SP, et al. : Genetic subtype-independent inhibition of human immunodeficiency virus type 1 replication by CC and CXC chemokines. J Virol 1998;72:396–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gordon CJ, Muesing MA, Proudfoot AE, Power CA, Moore JP, Trkola A: Enhancement of human immunodeficiency virus type 1 infection by the CC-chemokine RANTES is independent of the mechanism of virus-cell fusion. J Virol 1999;73:684–694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Schwartzkopff F, Grimm TA, Lankford CSR, et al. : Platelet factor 4 (CXCL4) facilitates human macrophage infection with HIV-1 and potentiates virus replication. Innate Immun 2009;15:368–379 [DOI] [PubMed] [Google Scholar]
- 64.Stringer SE, Gallagher JT: Specific binding of the chemokine platelet factor 4 to heparan sulfate. J Biol Chem 1997;272:20508–20514 [DOI] [PubMed] [Google Scholar]
- 65.Zhang X, Chen L, Bancroft DP, Lai CK, Maione TE: Crystal structure of recombinant human platelet factor 4. Biochemistry 1994;33:8361–8366 [DOI] [PubMed] [Google Scholar]
- 66.Lambert M, Meng R, Xiao L, et al. : Intramedullary megakaryocytes internalize released platelet factor 4 and store it in alpha granules. J Thromb Haemost 2015;13(10):1888–1899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bruns I, Lucas D, Pinho S, et al. : Megakaryocytes regulate hematopoietic stem cell quiescence through CXCL4 secretion. Nat Med 2014;20:1315–1320 [DOI] [PMC free article] [PubMed] [Google Scholar]