Abstract
Background: Relatives with single positive islet autoantibodies have a much lower risk of progression to diabetes than those with multiple autoantibodies.
Materials and Methods: TrialNet subjects positive for single autoantibody to insulin (mIAA) (n = 50) or single autoantibody to glutamic acid decarboxylase (GADA) (n = 50) were analyzed using new electrochemiluminescence (ECL) assays (ECL-IAA and ECL-GADA, respectively) at their initial visit and longitudinally over time. Affinity assays were performed on a subset of single autoantibody-positive subjects at initial and most recent visits.
Results: After a mean follow-up of 5.3 years, 20 subjects developed type 1 diabetes. Among either single GADA or single mIAA subjects, those who were positive in the ECL assay showed higher affinity at the initial visit, and affinity results stayed consistent over time. No converting events from low to high or high to low affinity were seen over time.
Conclusions: Confirmed positivity for ECL is associated with high affinity and can help staging of risk for type 1 diabetes in single autoantibody-positive subjects.
Introduction
Screening for risk of type 1 diabetes uses autoantibody (Ab) radioimmunoassays for specific islet autoantigens, including Abs to insulin (mIAA),1 glutamic acid decarboxylase (GADA),2 islet antigen-2 (ICA512),3 and most recently zinc transporter 8.4,5 Individuals having a single Ab positivity are at low risk for progression to diabetes, whereas individuals expressing two or more Abs have a risk of 80% of developing diabetes in 15 years.6 Prevention trials are currently available for subjects with multiple Ab positivity, but no prevention trials are offered for single Ab-positive subjects. Cumulative risk of development of diabetes has been associated with young age at seroconversion, positivity for multiple Abs, high Ab levels, persistent positivity for mIAA, and high Ab affinity.7–12 Children with persistent single high-affinity mIAA or GADA have been shown to be more likely to progress to type 1 diabetes than children with single low-affinity Abs.11,12
Recently, our center developed new electrochemiluminescence (ECL) assays for insulin (ECL-IAA) and GADA (ECL-GADA).13 Both ECL-IAA and ECL-GADA have been shown to be more disease specific in the Diabetes Autoimmunity Study in the Young (DAISY) and the TrialNet Pathway to Prevention studies.14,15 Most children with two or more radioimmunoassay- positive Abs were found to be positive by ECL assays, whereas only some children with a single radioimmunoassay-positive Ab were confirmed by ECL-IAA and ECL-GADA assays, respectively.14,15 The goal of this study was to evaluate ECL assays and Ab affinity over time in TrialNet single Ab-positive subjects.
Research Design and Methods
Subjects
The TrialNet Pathway to Prevention Study screens relatives of type 1 diabetes patients for the presence of islet Abs and offers close monitoring and/or prevention trials.16 In total, 100 single Ab subjects confirmed positive on two consecutive visits were selected from the TrialNet Pathway to Prevention Study (50 single mIAA and 50 single GADA subjects) and were analyzed for ECL-IAA and ECL-GADA at their initial visit and longitudinally over time (yearly samples). Subjects were selected to be confirmed single Ab positive on two consecutive visits with no additional Ab positivity on follow-up. All subjects provided written informed consent, and the study was approved by the ethical boards of all participating institutions.
Radioimmunoassays and ECL assays
The radioimmunoassays for mIAA and GADA used in the present study were all performed in the Barbara Davis Center laboratory as previously described.17,18 The radioimmunoassay cutoffs were set at the 99th percentile of 500 normal control samples for GADA and 106 controls for mIAA, respectively. Based on the report by the Islet Autoantibody Standardization Program Committee,19 sensitivities and specificities were 52% and 100%, respectively, for mIAA and 82% and 99%, respectively, for GADA.
ECL-IAA and ECL-GADA assays were measured longitudinally on all subjects at yearly intervals at the Barbara Davis Center laboratory as previously described.13,14 In brief, serum samples were mixed with both sulfo-tag and biotin-labeled antigen proteins (either proinsulin or glutamic acid decarboxylase 65 [GAD65]) for overnight incubation at 4°C. The antigen–antibody complexes with biotin were captured by a streptavidin-coated plate, and the sulfo-tag gave the signals with ECL. The results were expressed as an index against internal standard positive controls of either insulin or GAD65 monoclonal antibody. The ECL assay cutoff indexes of 0.006 for mIAA or 0.023 for GADA were set at the 99th percentile over 100 healthy controls, and the ECL interassay coefficiencies of variation were 4.8% (n = 20) for mIAA and 8.8% (n = 10) for GADA, respectively. Sensitivities and specificities for the ECL assays were 60% and 98%, respectively, for mIAA and 78% and 96%, respectively, for GADA among patients with newly diagnosed type 1 diabetes.19
GADA and mIAA affinity assays
Affinity measures the strength of interaction between an antibody and an antigen. It is defined by the same basic thermodynamic principles that govern any reversible biomolecular interaction. High-affinity antibodies will bind a greater amount of antigen at a lower concentration of antigen than low-affinity antibodies during the same period of time. For affinity testing, 20 subjects were selected from each group with consistent ECL status at initial and last visit (i.e., 20 single GADA-positive subjects who were confirmed ECL-GADA positive at initial and last visits [n = 10] or confirmed ECL-GADA negative at initial and last visits [n = 10]). For single mIAA-positive subjects, the same selection criteria were used for 20 single mIAA-positive subjects with 10 confirmed ECL-IAA positive and 10 confirmed ECL-IAA negative; however, only seven single mIAA subjects were confirmed positive by ECL-IAA at initial and last visits and therefore included in the affinity analysis.
The GADA and IAA affinity analyses were performed using the standard GADA and mIAA radioimmunoassays with a serial absorption of unlabeled antigen protein as previously published.13,14 Each serum was assessed in seven or eight separated wells, one without competition and six or seven with competition by adding unlabeled GAD65 or insulin in six different concentrations (5.7 × 10–4–4.6 × 10–9 M), respectively, into the serum incubation mixture to compete with radiolabeled GAD65 or insulin. For competition, the serum samples were mixed with radiolabeled and unlabeled GAD65 or insulin, respectively, at the same time, and then incubated for overnight at 4°C. The complexes of antibody–antigen were precipitated with protein A/G Sepharose® (GE Healthcare, Little Chalfont, United Kingdom), and radioactive signals were counted. The signal inhibitions of 50% by unlabeled GAD65 or insulin were calculated and compared for relative affinity of antibodies.
Statistics
Statistical analyses were performed using SAS software (version 9.2; SAS Institute, Cary, NC). Categorical variables were analyzed using Fisher's exact test. Continuous variables were tested using the t test for difference in means or the Wilcoxon rank-sum test for difference in medians. Because of different Ab cutoff values and change in the type of assay over time, both ECL and radioimmunoassays Ab levels were converted to SD units away from threshold (“Z scores”) for analyses. All subjects were selected to be confirmed positive for either mIAA or GADA, but many of the samples were negative in ECL assays, leading to mean values (Table 1) that can be negative for ECL assays. Both initial and mean levels of Abs over time were calculated. A P value of <0.05 was considered statistically significant.
Table 1.
Characteristics of TrialNet Subjects with Single Autoantibody to Glutamic Acid Decarboxylase or Single Autoantibody to Insulin
| Single Ab by radioimmunoassay | No diabetes | Developed diabetes | P value |
|---|---|---|---|
| GADA (n = 50) | |||
| n | 35 | 15 | |
| Age at initial visit (years) | 6.6 ± 2.5 | 23.5 ± 15.1 | <0.0001 |
| Follow up duration (years) | 6.3 ± 1.8 | 2.2 ± 2.3 | <0.0001 |
| Gender male, [n (%)] | 21 (60%) | 6 (40%) | 0.23 |
| Ethnicity NHW [n (%)] | 26 (74%) | 11 (73%) | 1.0 |
| Initial GADA | 0.01 ± 0.18 | 0.43 ± 0.28 | <0.0001 |
| Initial ECL-GADA | −0.09 ± 0.03 | 0.19 ± 0.33 | 0.001 |
| Mean GADA | 0.0 ± 0.12 | 0.33 ± 0.26 | <0.0001 |
| Mean ECL-GADA | −0.07 ± 0.07 | 0.17 ± 0.29 | 0.001 |
| HLA-DR3/4*0302 [n (%)]a | 4 (13%) | 4 (33%) | 0.18 |
| HLA-DR2*0602 [n (%)]a | 4 (13%) | 1 (8%) | 1.0 |
| mIAA (n = 50) | |||
| n | 45 | 5 | |
| Age at initial visit (years) | 7.3 ± 3.1 | 8.1 ± 4.4 | 0.56 |
| Follow-up duration (years) | 6.0 ± 2.3 | 2.1 ± 2.5 | 0.007 |
| Gender male [n (%)] | 21 (47%) | 4 (80%) | 0.35 |
| Ethnicity NHW [n (%)] | 37 (82%) | 4 (80%) | 1.0 |
| Initial mIAA | 0.08 ± 0.22 | 0.04 ± 0.08 | 0.85 |
| Initial ECL-IAA | −0.04 ± 0.10 | 0.15 ± 0.19 | 0.06 |
| Mean mIAA | 0.02 ± 0.18 | 0.03 ± 0.12 | 0.65 |
| Mean ECL-IAA | −0.02 ± 0.15 | 0.16 ± 0.20 | 0.06 |
| HLA-DR3/4*0302 [n (%)]a | 3 (7%) | 2 (40%) | 0.08 |
| HLA-DR2*0602 [n (%)]a | 5 (12%) | 1 (20%) | 0.51 |
Data are mean ± SD values unless specified otherwise. Autoantibody (Ab) levels were converted to SD units away from threshold (Z scores).
Missing human leukocyte antigen (HLA) genotyping in a few subjects (six of 50 single Ab to glutamic acid decarboxylase [GADA] and three of 50 single Ab to insulin [mIAA]).
ECL, electrochemiluminescence; NHW, non-Hispanic white.
Results
After a median follow-up of 5.3 (± 2.6) years, 20 of these subjects developed type 1 diabetes, including 15 single GADA and five single mIAA. Among the 50 single mIAA-positive subjects, in total 32 (64%) were positive at least once by ECL-IAA, whereas 34 (68%) of the 50 single GADA-positive subjects were positive at least once by ECL-GADA. Characteristics of these single Ab-positive subjects are described in Table 1. Follow-up duration was significantly shorter among subjects who developed diabetes compared with those who stayed diabetes free (2.2 vs. 6.3 years [P < 0.0001] and 2.1 vs. 6.0 years [P = 0.007] for single GADA and single mIAA, respectively). Age at initial visit was similar for single mIAA subjects (8.1 vs. 7.3 years; P = 0.56) but significantly older for those single GADA subjects who developed diabetes (23.5 vs. 6.6 years; P < 0.0001).
Single GADA subjects who developed diabetes compared with those who stayed diabetes free had higher initial GADA levels (ECL-GADA, respectively, 0.19 vs. −0.09 [P = 0.001]; GADA, respectively, 0.43 vs. 0.01[P < 0.0001]) as well as higher mean GADA levels over time (ECL-GADA, respectively, 0.17 vs. −0.07 [P = 0.001]; GADA, respectively, 0.33 vs. 0.0 [P < 0.0001]). Among single mIAA subjects, similar results were found for ECL-IAA, with higher levels in those who developed diabetes compared with those who stayed diabetes free (initial ECL-IAA, respectively, 0.15 vs. −0.04 [P = 0.06]; mean ECL-IAA, respectively, 0.16 vs. −0.02 [P = 0.06]), but no differences were found for mIAA levels (initial mIAA, respectively, 0.04 vs. 0.08 [P = 0.85]; mean mIAA, respectively, 0.03 vs. 0.02 [P = 0.65]).
There were no statistically significant differences in human leukocyte antigen genotypes among the subjects who progressed to diabetes versus those who did not progress.
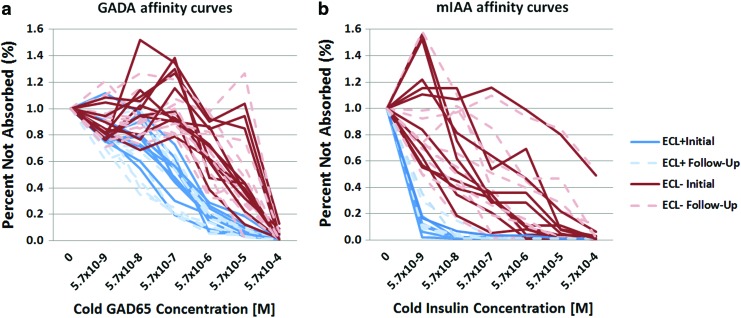
Affinity assays were performed on a subset of single Ab-positive subjects (17 for mIAA and 20 for GADA) to assess whether affinity stays consistent over time. Among these single GADA subjects, half (n = 10) were positive by ECL-GADA assay. The results of the competition assay are illustrated in Figure 1a. GADA not detectable by ECL assay required a 10–1,000-fold higher concentration of unlabeled GAD65 protein for 50% inhibition of binding of GADA to labeled GAD65 protein for samples negative by ECL-GADA than for samples positive by ECL-GADA. Affinity results were found to be consistent between initial and follow-up visits.
FIG. 1.
(a) Longitudinal affinity results for single autoantibody to glutamic acid decarboxylase (GADA)-positive samples according to electrochemiluminescence (ECL)-GADA status: GADA from ECL-negative subjects (red or black lines), compared with ECL-positive subjects (blue or gray lines), required higher concentrations of glutamic acid decarboxylase 65 (GAD65) for 50% maximal inhibition. Results are expressed as percentages of signal not absorbed. The solid line indicates the initial sample; the dotted line indicates the follow-up sample. (b) Longitudinal affinity results for single autoantibody to insulin (mIAA)-positive samples according to ECL-IAA status: insulin autoantibody from ECL-negative subjects (red or black lines), compared with ECL-positive subjects (blue or gray lines), required higher concentrations of insulin for 50% maximal inhibition. Results are expressed as percentages of signal not absorbed. The solid line indicates the initial sample; the dotted line indicates the follow-up sample. (Color graphics available at www.liebertonline.com/dia)
Among the 17 single mIAA subjects, seven were positive by ECL-IAA, whereas 10 were negative. The results of the competition assay were similar to those found for GADA (Fig. 1b). At 50% inhibition, those ECL-positive were significantly different from those ECL-negative. Samples that were positive for ECL-IAA showed higher affinity already at the initial visit, and affinity results stayed consistent over time.
Among subjects who developed diabetes with available affinity results (n = 14), all except one were ECL-positive and had high affinity for GADA or mIAA. One subject with a low level of GADA who developed diabetes was ECL-GADA negative and had low affinity results. No converting events from low to high or high to low affinity were seen over time.
Discussion
In this study, single Ab-positive subjects were evaluated using ECL assays and affinity assays over time. Previous preliminary findings from the DAISY study showed that multiple Ab-positive subjects (typically positive also for ECL) have higher-affinity antibodies than single Ab-positive subjects.13 This is the first study to show that single Ab-positive (mIAA or GADA) subjects confirmed positive for ECL have higher-affinity Ab compared with single Ab-positive TrialNet subjects negative for ECL and that higher affinity is already present at the initial visit in these single Ab-positive subjects and stays consistent over time.
Our findings suggest that ECL measurements may have important practical implications regarding staging of diabetes risk. Subjects who were confirmed positive by ECL assay showed higher affinity already at the initial visit, and no converting affinity events were seen over time, implicating that high affinity of these ECL assays can help define risk for diabetes early on. Different risk scores for type 1 diabetes have been proposed, including number and levels of Ab as well as metabolic markers.9,10,20,21 Currently, prevention trials are only available for relatives of patients with diabetes who have two or more Abs. However, single Ab-positive subjects confirmed positive for ECL may qualify for enrollment into prevention trials as their risk for diabetes is much higher than those single Ab-positive subjects negative for ECL.15 On the other hand, subjects found to be negative by ECL assays may benefit from less intensive monitoring in these longitudinal prospective studies.22
The higher affinity of these Abs detected by the ECL assays may be explained by several factors. The ECL assay is a bivalent assay where Abs to insulin in serum link to both the sulfo-tagged proinsulin and the biotinylated proinsulin, thereby potentially increasing specificity.13 In addition, ECL assays are designed to capture all immunoglobulins, whereas only immunoglobulin Gs are captured by radioimmunoassays. Further evaluation is needed to confirm these hypotheses, which could have important implications for understanding the pathophysiology of type 1 diabetes.
Only single mIAA and single GADA subjects were analyzed in this study. Islet antigen-2 and zinc transporter 8 antibodies are only rarely present as a single antibody.23 Although subjects were selected randomly from the monitoring phase of TrialNet, duration of follow-up and age at initial visit were significantly different between subjects who developed diabetes and those who stayed diabetes free. One possible explanation is that most young subjects who develop diabetes have multiple Abs, whereas older subjects who develop diabetes may be positive for GADA only. The number of subjects analyzed in this study was small, and further evaluation of ECL assays in prospective studies such as TrialNet and The Environmental Determinants of Diabetes in the Young (TEDDY) is warranted.
In conclusion, among single Ab-positive subjects, those confirmed positive by ECL assays show high affinity at initial visit, and the affinity stays consistent over time. Positivity for ECL at initial visit can help staging of risk for type 1 diabetes in single Ab-positive subjects.
Supplementary Material
Contributor Information
Collaborators: and the TrialNet Study Group
Acknowledgments
This research was supported by grant DK32083 from the National Institutes of Health, grants 17-2013-535 and 47-2013-581 from the JDRF, and grant 1-14-CD-17 from the American Diabetes Association. The Type 1 Diabetes TrialNet Pathway to Prevention Study Group is a clinical trials network funded by the National Institutes of Health through the National Institute of Diabetes and Digestive and Kidney Diseases, the National Institute of Allergy and Infectious Diseases, and The Eunice Kennedy Shriver National Institute of Child Health and Human Development, through the cooperative agreements U01 DK061010, U01 DK061034, U01 DK061042, U01 DK061058, U01 DK085465, U01 DK085453, U01 DK085461, U01 DK085463, U01 DK085466, U01 DK085499, U01 DK085504, U01 DK085505, U01 DK085509, U01 DK103180, U01-DK103153, U01-DK085476, and U01-DK103266 and the JDRF.
Author Disclosure Statement
No competing financial interests exist.
A.K.S. designed the study, wrote the manuscript, and contributed to the discussion. A.F., D.M., Z.Z., and F.D. researched data. J.S., P.G., and M.J.R. contributed to the discussion and reviewed/edited the manuscript. L.Y. designed the study, contributed to the discussion, and reviewed/edited the manuscript. A.K.S. takes full responsibility for the contents of the article.
References
- 1.Greenbaum C, Palmer JP, Kuglin B, et al. : Insulin autoantibodies measured by radioimmunoassay methodology are more related to insulin-dependent diabetes mellitus than those measured by enzyme-linked immunosorbent assay: results of the Fourth International Workshop on the Standardization of Insulin Autoantibody Measurement. J Clin Endocrinol Metab 1992;74:1040–1044 [DOI] [PubMed] [Google Scholar]
- 2.Baekkeskov S, Aanstoot H-J, Christgau S, et al. : Identification of the 64K autoantigen in insulin-dependent diabetes as the GABA-synthesizing enzyme glutamic acid decarboxylase. Nature 1990;347:151–156. Erratum in Nature 1990;347:782. [DOI] [PubMed] [Google Scholar]
- 3.Gianani R, Rabin DU, Verge CF, et al. : ICA512 autoantibody radioassay. Diabetes 1995;44:1340–1344 [DOI] [PubMed] [Google Scholar]
- 4.Yu L, Cuthbertson DD, Maclaren N, et al. : Expression of GAD65 and islet cell antibody (ICA512) autoantibodies among cytoplasmic ICA+ relatives is associated with eligibility for the Diabetes Prevention Trial-Type 1. Diabetes 2001;50:1735–1740 [DOI] [PubMed] [Google Scholar]
- 5.Schlosser M, Mueller PW, Torn C, et al. : Diabetes Antibody Standardization Program: evaluation of assays for insulin autoantibodies. Diabetologia 2010;53:2611–2620 [DOI] [PubMed] [Google Scholar]
- 6.Ziegler AG, Rewers M, Simell O, et al. : Seroconversion to multiple islet autoantibodies and risk of progression to diabetes in children. JAMA 2013;309:2473–2479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Orban T, Sosenko JM, Cuthbertson D, et al. : Pancreatic islet autoantibodies as predictors of type 1 diabetes in the Diabetes Prevention Trial-Type 1. Diabetes Care 2009;32:2269–2274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Siljander HT, Simell S, Hekkala A, et al. : Predictive characteristics of diabetes-associated autoantibodies among children with HLA-conferred disease susceptibility in the general population. Diabetes 2009;58:2835–2842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Steck AK, Johnson K, Barriga KJ, et al. : Age of islet autoantibody appearance and mean levels of insulin, but not GAD or IA-2 autoantibodies, predict age of diagnosis of type 1 diabetes: Diabetes Autoimmunity Study in the Young. Diabetes Care 2011;34:1397–1399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Steck AK, Vehik K, Bonifacio E, et al. : Predictors of progression from the appearance of islet autoantibodies to early childhood diabetes: The Environmental Determinants of Diabetes in the Young (TEDDY). Diabetes Care 2015;38:808–813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bender C, Schlosser M, Christen U, et al. : GAD autoantibody affinity in schoolchildren from the general population. Diabetologia 2014;57:1911–1918 [DOI] [PubMed] [Google Scholar]
- 12.Giannopoulou EZ, Winkler C, Chmiel R, et al. : Islet autoantibody phenotypes and incidence in children at increased risk for type 1 diabetes. Diabetologia 2015;58:2317–2323 [DOI] [PubMed] [Google Scholar]
- 13.Yu L, Miao D, Scrimgeour L, et al. : Distinguishing persistent insulin autoantibodies with differential risk: nonradioactive bivalent proinsulin/insulin autoantibody assay. Diabetes 2012;61:179–186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miao D, Guyer KM, Dong F, et al. : GAD65 autoantibodies detected by electrochemiluminescence assay identify high risk for type 1 diabetes. Diabetes 2013;62:4174–4178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miao D, Steck AK, Zhang L, et al. : Electrochemiluminescence assays for insulin and glutamic acid decarboxylase autoantibodies improve prediction of type 1 diabetes risk. Diabetes Technol Ther 2015;17:119–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mahon JL, Sosenko JM, Rafkin-Mervis L, et al. : The TrialNet Natural History Study of the Development of Type 1 Diabetes: objectives, design, and initial results. Pediatr Diabetes 2009;10:97–104 [DOI] [PubMed] [Google Scholar]
- 17.Yu L, Robles DT, Abiru N, et al. : Early expression of anti-insulin autoantibodies of humans and the NOD mouse: evidence for early determination of subsequent diabetes. Proc Natl Acad Sci U S A 2000;97:1701–1706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bonifacio E, Yu L, Williams AK, et al. : Harmonization of glutamic acid decarboxylase and islet antigen-2 autoantibody assays for National Institute of Diabetes and Digestive and Kidney Diseases Consortia. J Clin Endocrinol Metab 2010;95:3360–3367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Islet Autoantibody Standardization Program Certification of 2015 Workshop Participation (May 8, 2015) [Pittman D, Winter WE, Schlosser M, Williams A, Lampascona V, Achenbach P.], 2015
- 20.Sosenko JM, Skyler JS, Palmer JP, et al. : The prediction of type 1 diabetes by multiple autoantibody levels and their incorporation into an autoantibody risk score in relatives of type 1 diabetic patients. Diabetes Care 2013;36:2615–2620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sosenko JM, Skyler JS, Mahon J, et al. : The application of the diabetes prevention trial-type 1 risk score for identifying a preclinical state of type 1 diabetes. Diabetes Care 2012;35:1552–1555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vehik K, Beam CA, Mahon JL, et al. : Development of autoantibodies in the TrialNet Natural History Study. Diabetes Care 2011;34:1897–1901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wenzlau JM, Juhl K, Yu L, et al. : The cation efflux transporter ZnT8 (Slc30A8) is a major autoantigen in human type 1 diabetes. Proc Natl Acad Sci U S A 2007;104:17040–17045 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.