Abstract
The Amplatzer Vascular Plug (AVP) is a cylindrical plug made of self-expanding nitinol wire mesh with precise delivery control, which can be used for a variety of vascular pathologies. An AVP is an ideal vascular occlusion device particularly in high-flow vessels, where there is high risk of migration and systemic embolization with traditional occlusion devices. We performed 28 embolizations using the AVP from 2009 to 2014 and achieved complete occlusion without complications.
Keywords: Amplatzer vascular plug, embolization device, vascular embolization
Introduction
In the 1990s, Kurt Amplatz developed a novel vascular “plug” which allows for minimally invasive closure of atrial septal defects.[1] In 2005, the same idea was used in the development and subsequent Food and Drug Administration (FDA) approval of vascular plugs for peripheral arterial and venous occlusion. Traditional options for vascular occlusion included mechanical occlusion devices such as coils or detachable balloons, liquid embolic agents, particulate embolic agents, and sclerosing agents. Precise control of the location of embolization is often not possible with these agents. Also, the majority of traditional embolic agents cannot be retrieved once deployed or injected into the circulation.[2] The vascular plug solves many of these issues, while providing low recanalization rates. FDA premarket notification mentions the advantages of significantly shorter occlusion times, improved deployment precision without device migration, and significantly decreased recanalization rate at 2 months after the procedure with the Amplatzer Vascular Plug (AVP) compared to coil embolization.[3] An AVP also can be recaptured and repositioned, and has the potential to occlude large blood vessels which would normally require a number of coils or a combination of embolic agents.[4,5] This pictorial essay will discuss the use of AVP (St Jude Medical, Inc, St Paul, MN, USA) for extracardiac indications at our institution and review the published literature.
Device Description and Deployment Technique
AVP is a cylindrical plug made of self-expanding nitinol wire mesh, with sizes ranging from 4 to 16 mm in 2 mm increments. It is deployed through sheaths ranging from 3 to 8 French. The first generation has a single-lobe design for rapid occlusion in short vessel landing zones. The second generation is multi-layered, created for enhanced conformability to the variable vessel landing zones. The fourth generation has recently been approved by the FDA and can be deployed through a microcatheter.[6,7] There are radiopaque bands at each end of the plug. The proximal end has a stainless steel microscrew that keeps the plug attached to the stainless steel delivery cable. The delivery cable with the plug attached comes preloaded within an introducer sheath. The device can be then introduced into the delivery catheter. The plug can be withdrawn and repositioned through the catheter before detachment. The plug is detached by rotating the delivery cable in a counterclockwise direction, which unscrews the cable from the vascular plug. The manufacturer recommends oversizing the AVP by 30-50% to ensure proper fit within the vessel, limiting the potential complication of device migration.[6] Stasis is achieved by thrombus formation within and around the plug.
Our Experience
All instances of the use of an AVP in different clinical scenarios in this series were in accordance with the FDA approval as an arterial or venous occlusion device. The retrospective analysis was approved by our institutional review board (IRB).
In our experience, eight attending vascular and interventional radiologists were involved in 28 embolizations using the AVP from 2009 to 2014 [Table 1]. An AVP with diameter 30-50% greater than the diameter of the target vessel was chosen in all cases. Patients’ age ranged from 13 to 70 years (average 48 years). Thirteen patients were male and 15 were female. The right common femoral artery was accessed in 17 patients, the right common femoral vein in 3 patients, the right internal jugular vein in 1 patient, the right subclavian vein and brachiocephalic vein junction in 1 patient, and the arteriovenous fistula directly in 6 patients [Table 2]. Of the 17 arterial punctures, 13 were closed with an Angioseal closure device. The remaining three arterial punctures and the venous punctures were closed with manual compression. Two of the six arteriovenous fistula accesses were closed with purse string suture technique. The AVP embolization resulted in complete distal vascular stasis in all patients, and we experienced no minor or major complications.
Table 1.
Number of patients undergoing amplatzer vascular plug embolization for various indications
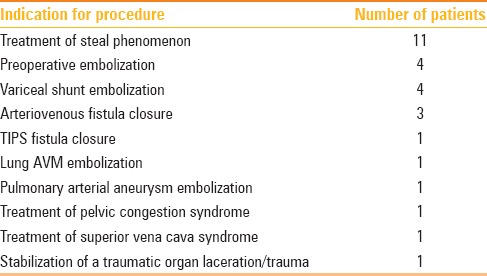
Table 2.
Frequency of vascular access site for amplatzer vascular plug embolization procedures

Arteriovenous Fistula Closure
The most common indication for deployment of an AVP in our patient population was the treatment of vascular steal. Peripheral vascular steal syndromes can be debilitating phenomena and cause significant morbidity including disabling edema and ischemic symptoms such as pain or ulceration.[8,9]
One patient with an arteriovenous fistula for hemodialysis access had arm pain from insufficient outflow from the upper extremities that resolved completely after closure of the left axillary artery to left axillary vein fistula [Figure 1]. Another patient had right hand ischemia that clinically improved after closure of the right brachiocephalic fistula [Figure 2]. We were able to deploy a single AVP and eliminate the steal phenomenon with immediate and lasting results at follow-up of 7 months [Figures 1 and 2]. In 2009, Bui et al. treated vascular steal related to upper extremity hemodialysis arteriovenous fistulae in five patients with AVPs and also documented immediate improvement in distal arterial flow with resolution of clinical symptoms in all patients.[10]
Figure 1 (A and B).
12 mm × 9 mm AVP used to occlude a left axillary artery to left axillary vein dialysis graft to successfully treat symptomatic vascular steal and superior vena cava syndrome. (A) Pre-deployment shows accessed arterial limb of the graft (dotted arrow) and (B) post-deployment of the AVP (arrow)
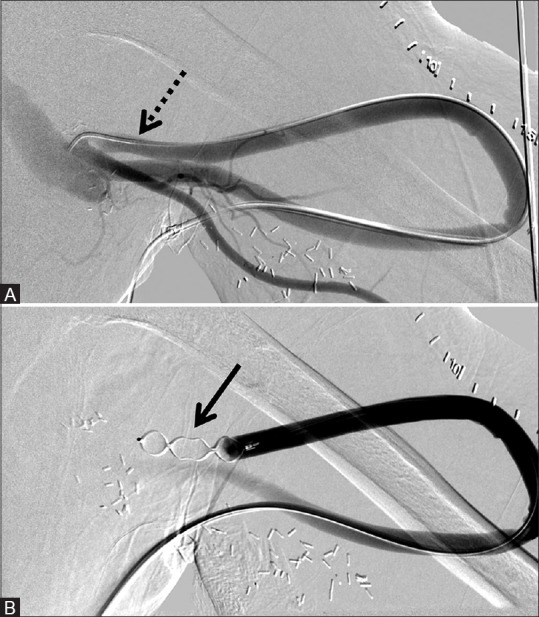
Figure 2 (A and B).
Two 12 mm × 9 mm AVPs used to embolize the right brachiocephalic fistula, improving circulation through the superior vena cava and right hand ischemic symptoms. (A) Pre-deployment shows accessed venous limb of the fistula (dotted arrow) and (B) post-deployment of the AVP (arrow)
AVPs are useful in the treatment of vascular shunts, particularly those with high flow or large feeding vessels, where the risk of distal embolization of coils is substantial.[11,12,13,14,15,16,17] There were five cases of occlusion of arteriovenous fistula venous outflow tract collateral veins, including one brachiocephalic and two radiocephalic fistulas. In all cases, the collateral veins were successfully occluded with the AVP device, increasing the venous outflow from the fistula for hemodialysis (not shown).
In 2007, Recto et al. reported a patient who sustained two transient ischemic attacks after intravenous saline flushes flowed through a fistula between the superior vena cava and the left upper pulmonary vein. The authors prevented further neurologic events by embolizing the vascular shunt with an AVP.[18]
In the lower extremities, there are reported cases of an iatrogenic arteriovenous fistula and a pseudoaneurysm that were successfully closed with an AVP.[19,20]
Splenic Artery Embolization
Steal phenomenon may also compromise blood flow to the transplanted liver. The splenic artery may steal blood from hepatic arteries which may already be compromised due to anastomotic stricture. Controlled embolization of the proximal splenic artery with preservation of collateral circulation to the spleen will maintain splenic perfusion while effectively shunting blood to the liver, which is evident by improved liver function tests and decrease in ascites volume in these cases. There were eight patients who underwent splenic embolization to increase hepatic artery flow following liver transplant [Figure 3].
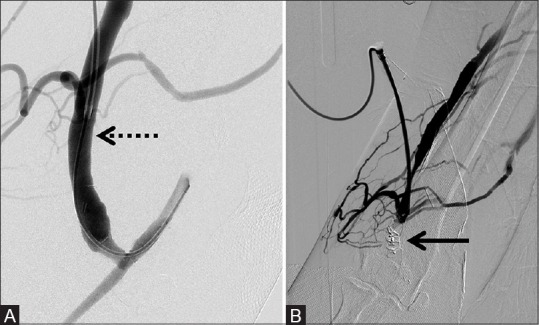
Figure 3 (A and B).
8 mm × 7 mm AVP used to successfully prevent splenic artery steal and increase hepatic artery flow in a hepatic transplant patient. (A) Pre-deployment reveals relatively prominent splenic perfusion (dotted arrow) with relatively diminutive hepatic perfusion (dotted double arrow) and (B) post-deployment of the AVP (arrow) with absence of flow in the splenic artery and relatively increased hepatic perfusion (double arrow)
In 2011, Zhu et al. reported a series of 23 cases of splenic artery steal after liver transplant, where the AVP was compared with coils, and they successfully used the AVP as their primary embolic agent in 10 cases.[21] In 2010, Maurer et al. also showed improved hepatic perfusion, decreased transaminase levels, decreased ascites, and improved platelet counts after embolization of the splenic artery with one or two AVPs for splenic artery steal in 13 patients.[22]
Another patient underwent splenic artery embolization to reduce portal venous pressures due to mesenteric vein thrombosis [Figure 4]. Two patients underwent splenic artery embolization to reduce portal pressures in order to treat variceal bleeding, with subsequent resolution of the melena and stabilization of the hemoglobin/hematocrit levels (not shown). We also utilized an AVP in the splenic artery of another patient and the coronary vein in a different patient to reduce portal pressures. Post-embolization, both patients had resolution of gastrointestinal bleeding. One patient underwent coronary vein embolization concomitantly with transjugular intrahepatic portosystemic shunt (TIPS) revision due to fundal variceal shunting and demonstrated complete occlusion of the coronary vein with improved flow through the portal vein post-embolization [Figure 5]. Our findings are compatible with those of Pattynama et al., who reported the use of AVPs in portal vein collaterals to decrease the risk of subsequent re-bleeding in eight patients.[23]
Figure 4 (A and B).
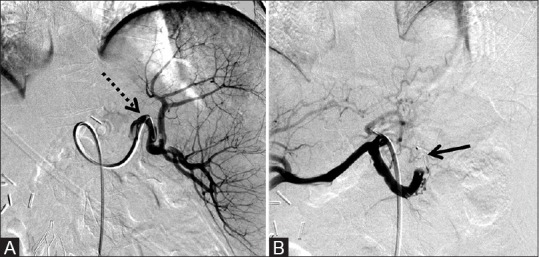
8 mm × 7 mm AVP used to embolize the splenic artery to successfully reduce portal venous pressure in a patient with mesenteric vein thrombosis and upper gastrointestinal bleeding. (A) Pre-deployment shows accessed splenic artery (dotted arrow) and (B) post-deployment of the AVP (arrow) with absence of flow in the distal splenic artery with a few collateral vessels
Figure 5 (A and B).
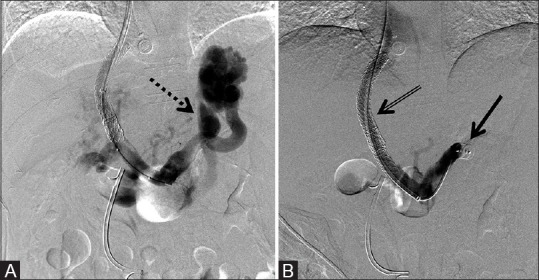
12 mm × 9 mm AVP used to embolize the coronary vein concomitantly with TIPS revision due to gastric fundal varices, with resultant improved portal flows post-embolization. (A) Pre-deployment shows prominent porto-systemic shunting through the coronary vein (dotted arrow) and (B) post-deployment of the AVP (arrow) occluding the coronary vein with improved flow through the TIPS shunt (double arrow)
Transjugular Intrahepatic Portosystemic Shunt
In our series, one patient underwent TIPS and was later identified as having a fistula between the right posterior segmental branch of the hepatic artery and the hepatic vein. A branch of the right hepatic artery was selectively embolized with an AVP, sparing the remaining right lobe arterial supply and the cystic artery [Figure 6].
Figure 6 (A and B).
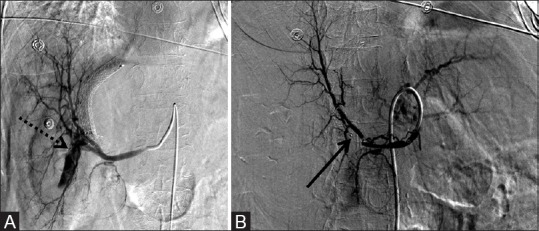
6 mm × 6 mm AVP used to successfully occlude a right posterior branch hepatic artery to right hepatic vein fistula in a TIPS patient. (A) Pre-deployment celiac angiogram reveals the large hematoma with flow in the TIPS shunt corresponding to the fistula (dotted arrow) and (B) post-deployment of the AVP (arrow) with resolution of the fistula
Also, TIPS may be closed using an AVP if hepatic encephalopathy severely impairs mental functioning.[11,23,24,25] There have been reports of non-cirrhotic portal-systemic shunts with encephalopathy that were occluded with an AVP, leading to normalization of blood ammonia levels.[26,27]
Preoperative Embolization
Four patients underwent preoperative embolization of various vessels to reduce intraoperative bleeding. The left renal artery was embolized using two AVPs in one patient with renal cell carcinoma [Figure 7]. Two patients required jump graft embolization of donor superior mesenteric artery for rejected small bowel transplants prior to enterectomy [Figure 8]. One patient with portal hypertension and thrombocytopenia underwent splenic artery embolization prior to splenectomy (not shown).
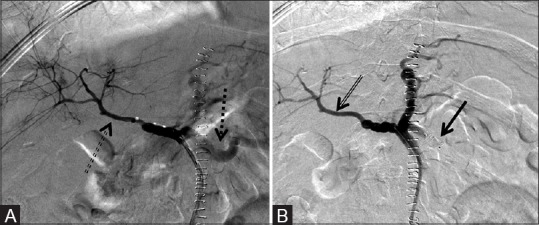
Figure 7 (A and B).
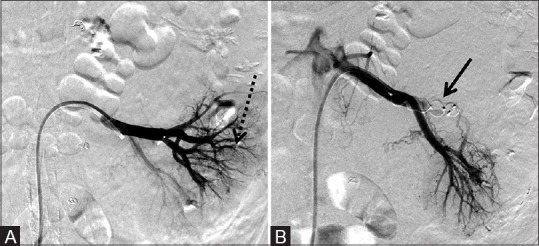
One 8 mm × 7 mm AVP and one 12 mm × 9 mm AVP used to successfully preoperatively embolize the left renal artery to reduce intraoperative blood loss during resection of a renal cell carcinoma. (A) Pre-deployment and (B) post-deployment of the AVP (arrow) revealing at least 70% decrease in vascularity of the mass
Figure 8 (A and B).
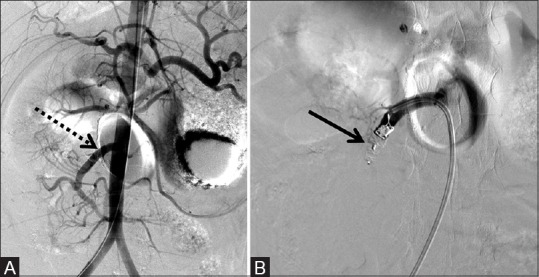
One 6 mm × 6 mm AVP and one 8 mm × 7 mm AVP used to successfully embolize the SMA jump graft of the donor SMA for rejected small bowel transplant prior to enterectomy and transplant revision. (A) Pre-deployment shows the patent jump graft (dotted arrow) and (B) post-deployment of the AVP (arrow). SMA, Superior mesenteric artery
Portal vein embolization prior to hemi-hepatectomy has also been performed successfully with an AVP. Mordasini et al. in 2010 and Ringe et al. in 2007 embolized the portal vein in a total of five patients prior to right hepatectomy, without complications.[25,28] In addition to decreased intraoperative blood loss, preoperative unilateral portal vein embolization also promotes segmental hypertrophy of the liver. Libicher and colleagues occluded the right portal vein using an AVP and microparticles in 10 patients and noted a 27% increase in the volume of the left lobe of the liver after 4 weeks without evidence of recanalization or migration.[29] Only one patient in the literature sustained hepatic infarction within 12 h after preoperative portal vein embolization. However, post-procedural CT scan revealed the AVP to protrude into the main portal vein causing extensive portal thrombosis. The same authors report partial recanalization of the second-order occluded portal vein in one patient with an AVP, but post-procedural CT scan revealed the AVP to completely cover the anterior portal vein branch but only partially cover the posterior portal vein branch. The authors concluded that portal vein embolization with the AVP is a safe and effective technique to induce hypertrophy of the future liver remnant.[30]
Pulmonary Arterial Aneurysm and Arteriovenous Malformation Embolization
There were two cases using an AVP in pulmonary vasculature. AVP deployment into the segmental branch of the right upper lobe pulmonary artery successfully occluded the arteriovenous malformation in one patient after failed coil embolization [Figure 9]. Another patient underwent successful occlusion of the left lower lobar pulmonary arterial aneurysm with a single AVP [Figure 10].
Figure 9 (A and B).
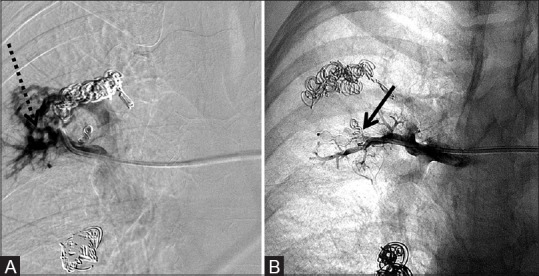
6 mm × 6 mm AVP deployed into the right upper lobe pulmonary artery main feeding trunk to occlude a pulmonary arteriovenous malformation after failure of attempted coil embolization twice with 3 mm × 3 cm microcoils. (A) Pre-deployment shows persistent vascular malformation (dotted arrow) and (B) post-deployment of the AVP (arrow) with lack of filling of the malformation
Figure 10 (A and B).
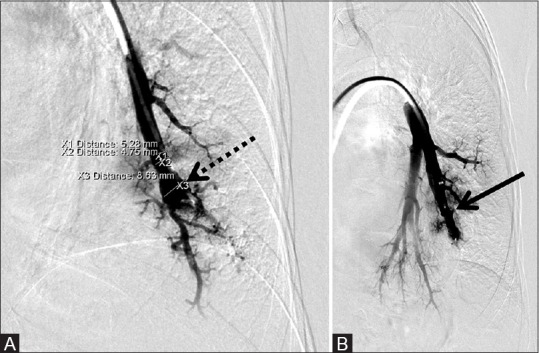
8 mm × 7 mm AVP used to successfully occlude a pulmonary artery aneurysm. (A) Pre-deployment shows the aneurysm (dotted arrow) and (B) post-deployment of the AVP (arrow) occluding the aneurysm
Pulmonary arteriovenous malformations (PAVMs) may occur in isolation or associated with Osler–Weber–Rendu syndrome or hereditary hemorrhagic telangiectasia. The goal of treating PAVMs is to prevent cerebral thromboembolism, pulmonary hemorrhage, and brain abscess, and to increase blood oxygenation by eliminating the right-to-left intrapulmonary shunt.[31,32] Historically, nonoperative treatment of PAVMs included embolization with coils or detachable balloons, and has been extensively described. However, a significant limitation of coil or balloon therapy is the potential migration of these devices into the systemic circulation. The risk is higher in patients whose PAVMs are fed by large arteries with short courses.[11,31,33,34,35,36] AVPs are particularly well suited to treat such PAVMs, as they can be deployed precisely and can be repositioned if necessary with little risk of systemic embolization.[11,24,32,37,38,39] Our patient with multiple PAVMs failed attempted coil embolization twice with 3 mm × 3 cm microcoils due to persistent arterial feeding branches, but showed immediate occlusion of the PAVM after deployment of an AVP into the main feeding trunk. In 2010, Hart et al. successfully embolized 120 of 161 PAVMs with a single AVP without any complications.[40] While the majority of studies describe PAVMs in the adult population, the AVP has also been described to successfully occlude a PAVM in a pediatric patient.[41]
Treatment of Pelvic Congestion Syndrome
There was one case of pelvic congestion syndrome which was treated with bilateral gonadal vein occlusion. Two AVPs were used per vessel, one proximal and one distal. Sodium tetradecy sulfate (STS) foam was also used in the deep parauterine and ovarian veins for complete venous stasis. Follow-up venogram at 18 months demonstrated resolution of the pelvic varices and no significant reflux of contrast into the gonadal veins [Figure 11].
Figure 11 (A-C).
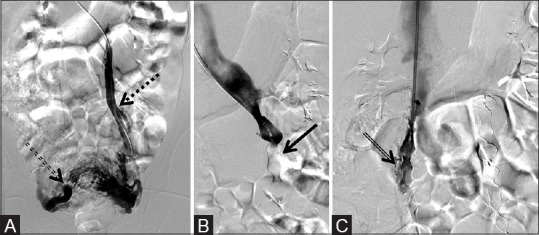
One 16 mm × 12 mm AVP and one 18 mm × 14 mm AVP in the left gonadal vein and one 14 mm × 10 mm AVP and one 16 mm × 12 mm AVP in the right gonadal vein used to embolize symptomatic pelvic varices. (A) Pre-deployment reveals dilation of the left gonadal vein (dotted arrow) with reflux into pelvic varicosities (double dotted arrow), (B) post-deployment of the AVP (arrow) on the left, and (C) post-deployment of the AVP (double arrow) on the right
Trauma
Proximal splenic arterial embolization is indicated prior to or in lieu of splenectomy and has traditionally been performed using coils. The AVP may be deployed rapidly and precisely, making it quite useful for emergent procedures. Hemorrhage was controlled using an AVP in a patient with an ilioenteric fistula and in another patient with high-flow traumatic hepatic arteriovenous fistula.[42,43] We also emergently treated a patient with subcapsular splenic traumatic hematoma by splenic artery occlusion with an AVP [Figure 12].
Figure 12 (A and B).
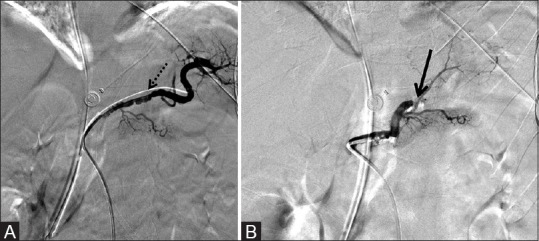
6 mm × 6 mm AVP used to successfully occlude the splenic artery in a trauma patient while preserving collateral blood supply to the spleen, avoiding splenectomy. (A) Pre-deployment reveals no active extravasation and vasospasm of the splenic artery (dotted arrow) which resolved after slow instillation of 500 mcg nitroglycerine and (B) post-deployment of the AVP (arrow) with lack of flow in the splenic artery and preservation of collateral perfusion
Complications and Difficulties
Complications arising from embolization with an AVP are uncommon in the literature, and our data demonstrate no complications in 28 embolizations. Reported complications include device migration, which is rare given the self-expanding device design that exerts a radially directed force on the vessel wall to maintain its position.[7,14] Only one study described migration of an AVP into the abdominal aorta, for which conservative management was elected.[44] Vessel recanalization is rare, complicating approximately 0.4% of AVP deployments in the literature.[7] In 2014, Guneyli et al. experienced recanalization in one patient out of their series of 41 AVP placements over an average follow-up period of 4.7 months.[45] In 2006, Hoit et al. deployed the AVP as a buttress against which they packed coils, improving vascular occlusion and preventing coil migration.[46]
Use of the first through third generations of the AVP in tortuous vessels is difficult and a 5-French sheath is required. In 2011, Zhu et al. observed that only 5 of 22 patients presenting for splenic artery embolization had splenic arteries that were sufficiently non-tortuous to allow deployment of an AVP.[21] The newest generation in the AVP family, the AVP 4, overcomes this limitation as it can be deployed through a 0.038 inch microcatheter. While the AVP 4 can be deployed via a microcatheter, its use is limited to vessels no larger than 6 mm in diameter.[21,25,47]
Advantages
The main advantages of utilization of the AVP over traditional coils described in the literature include reduction in procedure times, reduction in materials used, ability to reposition the plug for precise deployment, reduction in radiation times, and reduction in procedure costs.[2,3,5,6,48] In 2009, Pech et al. analyzed their experience with gastroduodenal artery embolization with the AVP 2 or platinum fiber coils and found a 29% reduction in materials’ cost per patient, decreasing from $1298 for platinum fiber coils to $898 for the AVP 2. Their procedure time decreased by an average of 13.3 min, their embolization time decreased by 10.5 min, and their fluoroscopy time decreased by an average of 5.8 min with the AVP 2. They used an average of six coils per patient, but only one AVP in each gastroduodenal artery.[48] In 2008, Pellerin et al. experienced similar results for internal iliac artery embolization with AVP or coils prior to endovascular aortic repair. All patients experienced complete arterial occlusion; however, only a single AVP was required, compared to an average of seven coils, for a 72% cost saving in the materials used.[49]
Conclusion
The AVP has a wide array of potential indications given its utility in high-flow vessels that would otherwise have a high risk of migration and possible systemic embolization with traditional embolic agents. The AVP can be precisely positioned and can be retrieved and redeployed if necessary. Few studies have documented complications such as recanalization, incomplete embolization, and migration, although these have been rare.[23] We experienced complete occlusion without complications in 28 embolizations using an AVP. Further data are needed to determine the long-term efficacy of AVP occlusion; however, current reports are optimistic for its future in vascular and interventional radiology.
Financial support and sponsorship
Nil.
Conflicts of interest
The authors declare that they have no conflicts of interest or relevant grant information.
References
- 1.Masura J, Gavora P, Formanek A, Hijazi ZM. Transcatheter closure of secundum atrial septal defects using the new self-centering amplatzer septal occluder: Initial human experience. Cathet Cardiovasc Diagn. 1997;42:388–93. doi: 10.1002/(sici)1097-0304(199712)42:4<388::aid-ccd7>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 2.Bilbao JI, Martínez-Cuesta A, Urtasun F, Cosín O. Complications of embolization. Semin Intervent Radiol. 2006;23:126–42. doi: 10.1055/s-2006-941443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stromberg P. Amplatzer Vascular Plug and Delivery System. US Food and Drug Administration Premarket Notification Rockville, MD. 2003. [Last accessed on 2014 Nov 20]. Available from: http://www.accessdatafdagov/cdrh_docs/pdf3/k031810pdf .
- 4.Vascular Plug Family. [Last accessed on 2014 Nov 20]. Available from: http://www.amplatzercom/products/VascularPlugFamily/tabid/813/Defaultaspx .
- 5.Idowu O, Barodawala F, Nemeth A, Trerotola SO. Dual use of an amplatzer device in the transcatheter embolization of a large high-flow renal arteriovenous fistula. J Vasc Interv Radiol. 2007;18:671–6. doi: 10.1016/j.jvir.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 6.Gandhi RT, Quintana D. Advancing the standard of care in peripheral embolization. Endovasc Today. 2014;4:1–4. [Google Scholar]
- 7.Wang W, Li H, Tam MD, Zhou D, Wang DX, Spain J. The amplatzer vascular plug: A review of the device and its clinical applications. Cardiovasc Intervent Radiol. 2012;35:725–40. doi: 10.1007/s00270-012-0387-z. [DOI] [PubMed] [Google Scholar]
- 8.Ozyer U, Harman A, Aytekin C, Boyvat F, Karakayali F. Application of the AMPLATZER vascular plug in endovascular occlusion of dialysis accesses. Cardiovasc Intervent Radiol. 2009;32:967–73. doi: 10.1007/s00270-009-9574-y. [DOI] [PubMed] [Google Scholar]
- 9.Powell S, Narlawar R, Odetoyinbo T, Littler P, Oweis D, Sharma A, et al. Early experience with the amplatzer vascular plug II for occlusive purposes in arteriovenous hemodialysis access. Cardiovasc Intervent Radiol. 2010;33:150–6. doi: 10.1007/s00270-009-9755-8. [DOI] [PubMed] [Google Scholar]
- 10.Bui JT, Gaba RC, Knuttinen MG, West DL, Owens CA. Amplatzer vascular plug for arteriovenous hemodialysis access occlusion: Initial experience. J Vasc Access. 2009;10:5–10. doi: 10.1177/112972980901000102. [DOI] [PubMed] [Google Scholar]
- 11.Ferro C, Petrocelli F, Rossi UG, Bovio G, Dahmane M, Seitun S. Vascular percutaneous transcatheter embolisation with a new device: Amplatzer vascular plug. Radiol Med. 2007;112:239–51. doi: 10.1007/s11547-007-0138-4. [DOI] [PubMed] [Google Scholar]
- 12.Owens CA, Bui JT, West DL, Sepahdari A. Use of the amplatzer vascular plug as a coil constrainer during endovascular occlusion of a dialysis shunt. Cardiovasc Intervent Radiol. 2007;30:754–6. doi: 10.1007/s00270-007-9065-y. [DOI] [PubMed] [Google Scholar]
- 13.Campbell JE, Davis C, Defade BP, Tierney JP, Stone PA. Use of an amplatzer vascular plug for transcatheter embolization of a renal arteriovenous fistula. Vascular. 2009;17:40–3. doi: 10.2310/6670.2008.00071. [DOI] [PubMed] [Google Scholar]
- 14.Tuite DJ, Kessel DO, Nicholson AA, Patel JV, McPherson SJ, Shaw DR. Initial clinical experience using the amplatzer vascular plug. Cardiovasc Intervent Radiol. 2007;30:650–4. doi: 10.1007/s00270-007-9044-3. [DOI] [PubMed] [Google Scholar]
- 15.Brountzos EN, Ptohis N, Grammenou-Pomoni M, Panagiotou I, Kelekis D, Gouliamos A, et al. High-flow renal arteriovenous fistula treated with the amplatzer vascular plug: Implementation of an arterial and venous approach. Cardiovasc Intervent Radiol. 2009;32:543–7. doi: 10.1007/s00270-008-9383-8. [DOI] [PubMed] [Google Scholar]
- 16.Peynircioglu B, Cil B. Amplatzer stuffing technique in the treatment of an iatrogenic mesenteric arteriovenous fistula. Cardiovasc Intervent Radiol. 2009;32:1247–51. doi: 10.1007/s00270-009-9614-7. [DOI] [PubMed] [Google Scholar]
- 17.Ferro C, Rossi UG, Bovio G, Petrocelli F, Seitun S. The amplatzer vascular plug 4: Preliminary experience. Cardiovasc Intervent Radiol. 2010;33:844–8. doi: 10.1007/s00270-009-9749-6. [DOI] [PubMed] [Google Scholar]
- 18.Recto MR, Sadlo H, Sobczyk WL. Rare case of persistent left superior vena cava to left upper pulmonary vein: Pathway for paradoxical embolization and development of transient ischemic attack and subsequent occlusion with an amplatzer vascular plug. J Invasive Cardiol. 2007;19:E313–6. [PubMed] [Google Scholar]
- 19.Chang DH, Liebig T, Bangard C. Closure of an iatrogenic arteriovenous fistula and an pseudoaneurysm of the lower leg with the amplatzer vascular plug. Rofo. 2011;183:394–6. doi: 10.1055/s-0029-1245968. [DOI] [PubMed] [Google Scholar]
- 20.Park YN, Kim JH, Choi ST, Byun SS, Kang JM, Kim HS, et al. Endovascular therapy of a traumatic chronic popliteal arteriovenous fistula using an AMPLATZER vascular plug. J Vasc Interv Radiol. 2010;21:1779–82. doi: 10.1016/j.jvir.2010.07.023. [DOI] [PubMed] [Google Scholar]
- 21.Zhu X, Tam MD, Pierce G, McLennan G, Sands MJ, Lieber MS, et al. Utility of the amplatzer vascular plug in splenic artery embolization: A comparison study with conventional coil technique. Cardiovasc Intervent Radiol. 2011;34:522–31. doi: 10.1007/s00270-010-9957-0. [DOI] [PubMed] [Google Scholar]
- 22.Maurer MH, Mogl MT, Podrabsky P, Denecke T, Grieser C, Fröling V, et al. Splenic artery syndrome after orthotopic liver transplantation: Treatment with the amplatzer vascular plug. Cardiovasc Intervent Radiol. 2011;34:1208–13. doi: 10.1007/s00270-010-0083-9. [DOI] [PubMed] [Google Scholar]
- 23.Pattynama PM, Wils A, van der Linden E, van Dijk LC. Embolization with the amplatzer vascular plug in TIPS patients. Cardiovasc Intervent Radiol. 2007;30:1218–21. doi: 10.1007/s00270-007-9089-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cil B, Peynircioglu B, Canyigit M, Geyik S, Ciftçi T. Peripheral vascular applications of the amplatzer vascular plug. Diagn Interv Radiol. 2008;14:35–9. [PubMed] [Google Scholar]
- 25.Mordasini P, Szucs-Farkas Z, Do DD, Gralla J, Kettenbach J, Hoppe H. Use of a latest-generation vascular plug for peripheral vascular embolization with use of a diagnostic catheter: Preliminary clinical experience. J Vasc Interv Radiol. 2010;21:1185–90. doi: 10.1016/j.jvir.2010.03.013. [DOI] [PubMed] [Google Scholar]
- 26.Lee SA, Lee YS, Lee KS, Jeon GS. Congenital intrahepatic portosystemic venous shunt and liver mass in a child patient: Successful endovascular treatment with an amplatzer vascular plug (AVP) Korean J Radiol. 2010;11:583–6. doi: 10.3348/kjr.2010.11.5.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Park SW, Kang HS, Kim YJ, Lee MW, Roh HG. Successful occlusion of spontaneous portosystemic shunts leading to encephalopathy in a non-cirrhotic patient by using the amplatzer vascular plug. Acta Radiol. 2007;48:1077–81. doi: 10.1080/02841850701545847. [DOI] [PubMed] [Google Scholar]
- 28.Ringe KI, Weidemann J, Rosenthal H, Keberle M, Chavan A, Baus S, et al. Transhepatic preoperative portal vein embolization using the amplatzer vascular plug: Report of four cases. Cardiovasc Intervent Radiol. 2007;30:1245–7. doi: 10.1007/s00270-007-9158-7. [DOI] [PubMed] [Google Scholar]
- 29.Libicher M, Herbrik M, Stippel D, Poggenborg J, Bovenschulte H, Schwabe H. Portal vein embolization using the amplatzer vascular plug II: Preliminary results. Rofo. 2010;182:501–6. doi: 10.1055/s-0028-1110019. [DOI] [PubMed] [Google Scholar]
- 30.Yoo H, Ko GY, Gwon DI, Kim JH, Yoon HK, Sung KB, et al. Preoperative portal vein embolization using an amplatzer vascular plug. Eur Radiol. 2009;19:1054–61. doi: 10.1007/s00330-008-1240-2. [DOI] [PubMed] [Google Scholar]
- 31.Conti V, Fiorucci F, Serpilli M, Patrizi A, Paone G, Neri P, et al. Osler-Rendu-Weber syndrome: Congenital arteriovenous intrapulmonary fistula treated using a percutaneous amplatzer plug. Eur Rev Med Pharmacol Sci. 2008;12:213–6. [PubMed] [Google Scholar]
- 32.Santos CS, Norte A, Ferreira I, Almeida P, Segorbe Luís A, Loureiro M, et al. Hereditary hemorrhagic telangiectasia and pulmonary arteriovenous malformations - embolization with amplatzer vascular plug. Rev Port Pneumol. 2009;15:331–7. [PubMed] [Google Scholar]
- 33.Andersen PE, Kjeldsen AD. Occlusion of pulmonary arteriovenous malformations by use of vascular plug. Acta Radiol. 2007;48:496–9. doi: 10.1080/02841850701280866. [DOI] [PubMed] [Google Scholar]
- 34.Baldi S, Rostagno RD, Zander T, Rabellino M, Maynar M. Occlusion of a pulmonary arteriovenous fistula with an amplatzer vascular plug. Arch Bronconeumol. 2007;43:239–41. doi: 10.1016/s1579-2129(07)60057-3. [DOI] [PubMed] [Google Scholar]
- 35.Cil B, Canyigit M, Ozkan OS, Pamuk GA, Dogan R. Bilateral multiple pulmonary arteriovenous malformations: Endovascular treatment with the amplatzer vascular plug. J Vasc Interv Radiol. 2006;17:141–5. doi: 10.1097/01.rvi.0000186954.74462.ce. [DOI] [PubMed] [Google Scholar]
- 36.Kessler J, Trerotola SO. Use of the amplatzer vascular plug for embolization of a large retroperitoneal shunt during transjugular intrahepatic portosystemic shunt creation for gastric variceal bleeding. J Vasc Interv Radiol. 2006;17:135–40. doi: 10.1097/01.rvi.0000186958.59457.10. [DOI] [PubMed] [Google Scholar]
- 37.Abdel Aal AK, Hamed MF, Biosca RF, Saddekni S, Raghuram K. Occlusion time for amplatzer vascular plug in the management of pulmonary arteriovenous malformations. AJR Am J Roentgenol. 2009;192:793–9. doi: 10.2214/AJR.08.1534. [DOI] [PubMed] [Google Scholar]
- 38.Ferro C, Rossi UG, Bovio G, Seitun S, Rossi GA. Percutaneous transcatheter embolization of a large pulmonary arteriovenous fistula with an Amplatzer vascular plug. Cardiovasc Intervent Radiol. 2007;30:328–31. doi: 10.1007/s00270-005-0348-x. [DOI] [PubMed] [Google Scholar]
- 39.Rossi M, Rebonato A, Greco L, Stefanini G, Citone M, Speranza A, et al. A new device for vascular embolization: Report on case of two pulmonary arteriovenous fistulas embolization using the amplatzer vascular plug. Cardiovasc Intervent Radiol. 2006;29:902–6. doi: 10.1007/s00270-005-0160-7. [DOI] [PubMed] [Google Scholar]
- 40.Hart JL, Aldin Z, Braude P, Shovlin CL, Jackson J. Embolization of pulmonary arteriovenous malformations using the amplatzer vascular plug: Successful treatment of 69 consecutive patients. Eur Radiol. 2010;20:2663–70. doi: 10.1007/s00330-010-1851-2. [DOI] [PubMed] [Google Scholar]
- 41.Farra H, Balzer DT. Transcatheter occlusion of a large pulmonary arteriovenous malformation using the amplatzer vascular plug. Pediatr Cardiol. 2005;26:683–5. doi: 10.1007/s00246-004-0857-4. [DOI] [PubMed] [Google Scholar]
- 42.Hicks TD, Kedora JC, Shutze WP. Treatment of an ilioenteric fistula with an amplatzer vascular plug. J Vasc Surg. 2011;54:1495–7. doi: 10.1016/j.jvs.2011.05.012. [DOI] [PubMed] [Google Scholar]
- 43.Koc O, Cil BE, Peynircioglu B, Emlik D, Ozbek O. Complementary use of NBCA with the amplatzer vascular plug for embolization of a high-flow traumatic hepatic arteriovenous fistula. Cardiovasc Intervent Radiol. 2009;32:1105–7. doi: 10.1007/s00270-009-9505-y. [DOI] [PubMed] [Google Scholar]
- 44.Maleux G, Rega F, Heye S, Troost E, Budts W. Asymptomatic migration of a first-generation AMPLATZER vascular plug into the abdominal aorta: Conservative management may be an option. J Vasc Interv Radiol. 2011;22:569–70. doi: 10.1016/j.jvir.2010.11.033. [DOI] [PubMed] [Google Scholar]
- 45.Güneyli S, Çinar C, Bozkaya H, Parildar M, Oran I. Applications of the amplatzer vascular plug to various vascular lesions. Diagn Interv Radiol. 2014;20:155–9. doi: 10.5152/dir.2013.13139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hoit DA, Schirmer CM, Malek AM. Use of the amplatzer vascular plug as an anchoring scaffold for coil-mediated parent vessel occlusion: Technical case report. Neurosurgery. 2006;59(1 Suppl 1):ONSE171–2. doi: 10.1227/01.NEU.0000219856.66842.EF. [DOI] [PubMed] [Google Scholar]
- 47.Widlus DM, Moeslein FM, Richard HM., 3rd Evaluation of the amplatzer vascular plug for proximal splenic artery embolization. J Vasc Interv Radiol. 2008;19:652–6. doi: 10.1016/j.jvir.2007.11.025. [DOI] [PubMed] [Google Scholar]
- 48.Pech M, Kraetsch A, Wieners G, Redlich U, Gaffke G, Ricke J, et al. Embolization of the gastroduodenal artery before selective internal radiotherapy: A prospectively randomized trial comparing platinum-fibered microcoils with the amplatzer vascular plug II. Cardiovasc Intervent Radiol. 2009;32:455–61. doi: 10.1007/s00270-008-9498-y. [DOI] [PubMed] [Google Scholar]
- 49.Pellerin O, Caruba T, Kandounakis Y, Novelli L, Pineau J, Prognon P, et al. Embolization of the internal iliac artery: Cost-effectiveness of two different techniques. Cardiovasc Intervent Radiol. 2008;31:1088–93. doi: 10.1007/s00270-008-9374-9. [DOI] [PubMed] [Google Scholar]


