Abstract
Reduced brain-derived neurotrophic factor (BDNF) signaling is considered as a pathogenic event in early Alzheimer’s disease (AD), but the influence of apathy and apolipoprotein E epsilon-4 allele (APOE4) on serum BDNF values was not previously investigated in AD. We evaluated serum BDNF levels in AD, amnestic mild cognitive impairment (MCI) and control subjects. Baseline BDNF levels were similar in AD, MCI and controls. AD patients having apathy showed lower BDNF values than patients without apathy (p<0.05). After correction for the influence of apathy, APOE4 carriers showed lower BDNF levels (p<0.01) and MMSE scores (p<0.01) than non-APOE4 carriers in the subgroup of AD females, but not in males. Significant (p<0.05) positive correlations between BDNF values and MMSE scores were only observed in subgroups of AD males and of AD patients without apathy. These results are showing the association of apathy and APOE4 with reduced serum BDNF levels in AD, and are suggesting that BDNF reductions might contribute to the worse cognitive performance exhibited by AD apathetic patients and female APOE4 carriers.
Keywords: Alzheimer’s disease, mild cognitive impairment, elderly control subjects, brain-derived neurotrophic factor, apathy, apolipoprotein E epsilon-4 allele, serum
1. Introduction
A down regulation of brain-derived neurotrophic factor (BDNF) signaling seems to be an early event in the pathogenesis of Alzheimer’s disease (AD) [1, 2]. Decreased levels of BNDF and its precursor protein (proBDNF) in the hippocampus and cortex, a reduced expression of the high-affinity BDNF receptor tyrosine kinase B (trkB) in cortical areas and in basal forebrain cholinergic neurons, and low BDNF concentrations in the cerebrospinal fluid (CSF) have been reported in AD and mild cognitive impairment (MCI) [1, 3–6].
BDNF seems to be essential for neural plasticity and memory, may reduce the amyloidogenic cleavage of the amyloid precursor protein and prevent apoptotic neuronal death through activation of its specific trkB receptors, and was found to protect against neurodegeneration in various animal models of AD [7, 8]. Therefore, a reduction of brain BDNF may contribute to synaptic and neuronal damage, amyloid deposition, and cognitive deficits characteristic of AD. In fact, decreases of BDNF, proBDNF or trkB in cortical areas or in cholinergic neurons of the basal forebrain were found to correlate with accumulation of neuritic plaques and synaptophysin loss [4], and with scores of cognitive impairment [3, 6] in AD patients. A positive association between low CSF BDNF concentrations and poor memory performance was also reported in non-demented subjects [5].
Alterations in circulating BDNF have been described in AD and MCI, although with controversial results. Several reports showed low BDNF levels [9–12], but other studies found unchanged [13–15] or even elevated [16] concentrations of BDNF in the serum/plasma of AD and MCI patients compared to controls. Investigations about the influence of serum BDNF on cognitive functions in AD and MCI are also inconclusive. Whereas some authors found that serum BDNF levels were reduced in AD patients with a fast cognitive decline [17] and correlated positively with episodic memory scores in MCI patients [12], other investigators reported an association of increased BDNF with poorer memory performance in AD [14] and a lack of association of serum BDNF with the progression of cognitive impairment or with the conversion to AD in MCI subjects [9].
AD is frequently associated with depression, and depressive symptoms constitute a significant risk factor for AD [18]. On the other hand, dysphoria/depression symptoms show an important overlapping with apathy, the more prevalent neuropsychiatric symptom in AD [19]. According to recent publications, some BDNF gene polymorphisms increase the risk of AD-related depression and are associated with the response to antidepressant treatment in AD patients [20–22]. Investigations on the relationship between circulating BDNF and depression indicate that BDNF levels are reduced in major depression, and tend to normalize after treatment with selective serotonin reuptake inhibitors (SSRIs) and during clinical remission [23]. However, there are no previous studies evaluating the influence of dysphoria/depression and apathy on serum BDNF levels in AD patients.
In the present study we investigated the influence of apathy on serum BDNF in AD patients. Associations of BDNF values with apolipoprotein E epsilon-4 allele (APOE4) status (present or absent), disease severity and dysphoria/depression in AD were also investigated. In addition, we evaluated group differences in circulating BDNF levels and its relationships with age, sex and cognitive performance in elderly controls, in subjects with amnestic MCI and in AD patients.
2. Methods
2.1. Subjects
The study sample included 362 Caucasian subjects evaluated at three institutions specialized on cognitive disorders from A Coruña, Granada and Málaga (Spain). It consisted of 252 patients with AD (196 women; mean age: 74.99±7.35 years), 48 amnestic MCI patients (35 women; mean age: 73.46±7.57 years) and sixty two healthy cognitive controls (43 women; mean age: 68.44±7.20 years). AD patients met DSM-IV criteria [24] and NINCDS-ADRDA criteria for probable AD [25]. Amnesic MCI patients were selected according to Petersen criteria revised [26]. Subjects having any other significant neurological or psychiatric disease, active allergies, unstable medical conditions or clinically significant laboratory abnormalities were not included in the study. Patients and controls were not taking systemic corticosteroids, anti-parkinsonian agents, narcotics or cholinesterase inhibitors for at least one month prior to blood sampling. Patients showing clinically significant depression in the medical evaluation and/or scores higher than fifteen in the 17-item subscale of the Hamilton Depression Scale [27] were not included in the study. Although the general level of physical activity was not quantified in the present study, none of the participants reported to be on specific exercise programs. The study was conducted according to Good Clinical Practice guidelines and written informed consent was obtained from all participants.
2.2. Measurement of serum BDNF levels and APOE genotyping
A butterfly-21 INT (Venisystems, Abbott Ireland Ltd., Sligo, Ireland) was inserted into the antecubital vein and baseline blood samples were taken during the morning using evacuated blood collecting tubes (Venojet, Terumo Europe N.V., Leuven, Belgium). Then, serum samples were extracted and stored at −40 °C until assays. Serum BDNF levels were determined by using a solid phase enzyme-linked immunosorbent assay (ELISA) kit specific for the quantitative determination of both natural and recombinant human BDNF in cell culture supernates, serum and plasma (R&D Systems, Inc., Minneapolis, MN, USA) provided by Vitro SA (Spain). The minimum detection limit of the assay was <62.5 pg/ml. The intra- and inter-assay coefficients of variation were <10%.
Apolipoprotein E (APOE) polymorphisms were identified in all participants according to the method previously described [28].
2.3. Clinical and neuropsychological evaluations
Cognitive performance was evaluated in all participants by using the Mini Mental State Examination (MMSE) [29] and the ADAS-cog+ [30], a 14-item extended version of the AD Assessment Scale-cognitive subscale with an increased sensitivity to detect cognitive changes in milder patients. AD severity was assessed by using the Clinical Interview Based Impression of Severity with Caregiver Input (CIBIS+) [31]. CIBIS+ scores in all controls and MCI cases included in this study were 1 and 2, respectively. The presence of a major depressive episode was ruled out through clinical interview and evaluation with the 17-item subscale of the Hamilton Depression Scale. Scores of apathy/indifference (apathy) and depression/dysphoria (dysphoria) as evaluated with the Neuropsychiatric Inventory (NPI) [32] were obtained for 250 AD patients. Apathy in the NPI is rated according to the frequency (1 to 4, occasionally to very frequently) and severity (1 to 3, mild to severe) of alterations in eight sub-items assessing the lack of spontaneity, initiative, emotional response, participation in activities, motivation and plans, social interaction, interests, and care about doing new things. Total apathy and dysphoria scores (frequency × severity) range from 0 to 12 points. The maximum sum score for the NPI-12 items is 144.
2.4. Statistical Analysis
Data are presented as means plus/minus standard deviations (X±SD). BDNF data showed a normal distribution as evaluated with the Kolmogorov-Smirnov test in each of the three diagnostic groups. Group comparisons were done by Chi-Square and ANOVA analyses as appropriate. The influences of gender (female, male) and APOE4 status (present, absent) on BDNF levels in the whole population and in each diagnostic group (Controls, MCI and AD) were evaluated by ANCOVA using age and APOE4 or gender, respectively, as covariates. In the AD group, BDNF levels were further analyzed by MANCOVA with age as a covariate, and gender, APOE4, apathy (present, absent) and dysphoria (present, absent) as between-subjects factors. Since this analysis revealed a significant effect of apathy and a significant interaction of gender and APOE4, additional data analyses were conducted for apathy-related subgroups and for female AD patients with and without APOE4. The influence of apathy on BDNF levels, cognitive performance measures (MMSE and ADAS-cog+ scores) and NPI scores in AD patients was analyzed by ANCOVA/MANCOVA, including age, disease severity (CIBIS+ score), dysphoria and SSRIs treatment (yes, no) as covariates or between-subjects factors when appropriate. The effects of APOE4 on BDNF levels and MMSE scores in female AD patients were also analyzed by ANCOVA/MANCOVA using age, CIBIS+, apathy, dysphoria and SSRIs treatment as covariates or between-subjects factors. The potential relationships between BDNF levels and the scores of cognitive performance (MMSE and ADAS-cog+), apathy, dysphoria and total NPI were analyzed by using a stepwise linear regression method including BDNF levels, CIBIC+ scores, age, sex and APOE4 as independent variables, and the psychometric parameter of interest as the dependent variable. Probability values lower than 0.05 were considered statistically significant.
3. Results
Since average age was lower in the whole group of control subjects than in MCI and AD groups, comparisons were also done with the subgroup of elderly controls (EC) aged 66 or more years (table 1). Sex distribution was similar in control, MCI and AD populations, representing females more than seventy percent of the cases (table 1). APOE epsilon-4 allele (APOE4) was significantly overrepresented in AD (47.2%; allelic frequency: 27.6%) and MCI patients (41.7%; allelic frequency: 28.1%) as compared to controls (12.9%; allelic frequency: 7.3%).
Table 1.
Baseline serum BDNF levels and clinical characteristics in controls, MCI patients and AD patients.
| Controls | MCI (N=48) |
AD (n=252) |
Analysis | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| AC (n=62) | EC (n=38) | ||||||||||
| N (%) | N (%) | N (%) | N (%) | X2 | df | P | |||||
| Female gender | 43 (69.4) | 30 (78.9) | 35 (72.9) | 196 (77.8) | 2.15 0.61 |
2 2 |
ns ns |
||||
| APOE ε4 allele | 8 (12.9) | 5 (13.2) | 20 (41.7) | 119 (47.2) | 24.33 15.69 |
2 2 |
<0.001 <0.001 |
||||
| Apathy | 167 (66.8) | -- | -- | -- | |||||||
| Dysphoria | 135 (54.0) | -- | -- | -- | |||||||
| SSRIs treatment | 88 (35.2) | -- | -- | -- | |||||||
| Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | F | df | P | |||||
| Age (years) | 68.44±7.20 | 72.74±5.69 | 73.46±7.57† | 74.99±7.35† | 19.77 2.21 |
2, 359 2, 335 |
<0.001 ns |
||||
| BDNF (ng/ml) | 15.08±10.79 | 16.73±11.83 | 14.33±9.12 | 15.16±9.48 | 0.14 0.67 |
2, 359 2, 335 |
ns ns |
||||
| MMSE (score) | 27.94±1.56 | 27.68±1.69 | 24.83±1.34†** | 17.46±4.12‡ | 264.93 186.29 |
2, 359 2, 335 |
<0.001 <0.001 |
||||
| ADAS-cog+ (score) | 14.84±4.40 | 15.88±4.45 | 24.20±4.87†* | 39.90±15.16‡ | 107.89 71.86 |
2, 359 2, 335 |
<0.001 <0.001 |
||||
| NPI (score) | 13.47±10.30 | -- | -- | -- | |||||||
| Apathy (score) | 2.90±2.87 | -- | -- | -- | |||||||
| Dysphoria (score) | 1.62±2.23 | -- | -- | -- | |||||||
Control subjects: AC (All controls: 56–86 years); EC (elderly controls: 66–86 years).
MCI: Mild Cognitive Impairment. AD: Alzheimer’s disease.
p<0.01 versus AC;
p<0.05 &
p<0.01 versus EC;
p<0.01 versus AC, EC & MCI (Tukey test).
Chi-Square and ANOVA analyses done with AC and EC are presented in upper and lower lines, respectively.
Baseline serum BDNF levels were similar in AD, MCI and controls (table 1), and showed no age-related variations in any of the diagnostic groups. Overall, in the whole study sample BDNF levels were lower in males than in females (12.94±9.45 ng/ml versus 15.69±9.63 ng/ml) after corrections for age and APOE4 status [F(3,358)=2.28; p=0.016]. Gender-related differences (males versus females) in circulating BDNF concentrations were significant in the MCI group (8.94±5.00 ng/ml versus 16.33±9.53 ng/ml; p=0.004), but not in control subjects (11.90±8.46 ng/ml versus 16.48±11.47 ng/ml; p=0.186) nor in AD patients (14.23±10.32 ng/ml versus 15.40±9.24 ng/ml; p=0.296). When corrected for age and sex, APOE4 carriers showed lower BDNF levels than non-APOE4 carriers in the subgroup of AD patients (13.87±9.31 ng/ml versus 16.27±9.51 ng/ml; p=0.037), but not in the other diagnostic groups.
In the AD group, NPI scores of apathy and dysphoria were present in 66.8% and 54% of the cases, respectively; and 35.2% of the patients received treatment with SSRIs (table 1). Analysis of BDNF levels by MANCOVA using age as a covariate, and sex, APOE4 status, apathy and dysphoria as between-subjects factors, demonstrated significant effects for apathy [F(16,233)=7.24; p=0.008], as well as for the interaction of sex and APOE4 [F(16,233)=4.46; p=0.036].
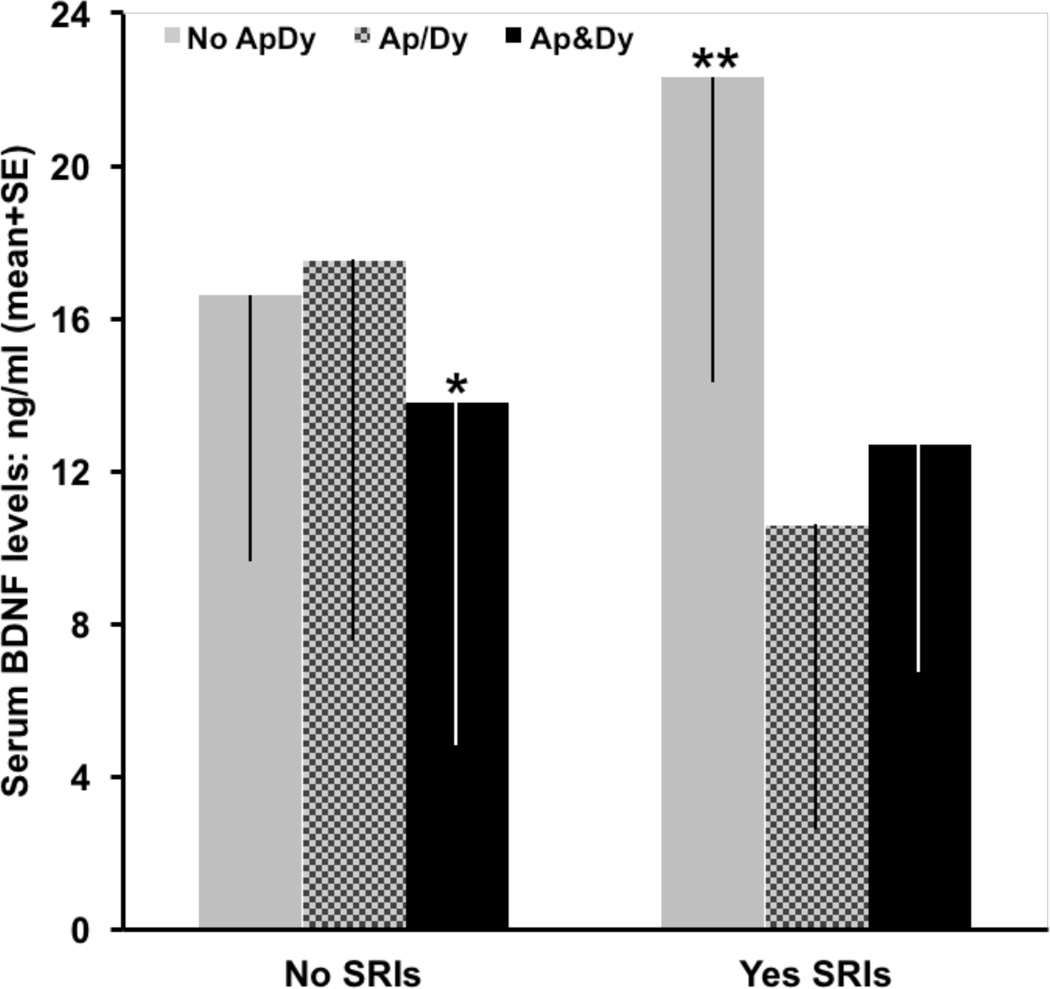
Gender distribution, frequency of APOE4 and age were similar in AD patients with and without apathy (table 2); whereas SSRIs treatment was more frequent, and dysphoria was more prevalent and severe in the apathy subgroup (table 2). Apathy was significantly associated to reduced BDNF levels, and to increases in CIBIS+ scores (AD severity), ADAS-cog+ scores (cognitive impairment) and NPI scores (behavioural disturbances) (table 2). AD patients having apathy showed a 20% reduction in serum BDNF values as compared to patients without apathy (p=0.043) after corrections for the influence of CIBIS+, dysphoria and SSRIs treatment (table 2). A significant effect of the interaction of apathy, dysphoria and SSRIs treatment on BDNF levels was also revealed by the MANCOVA analysis [F(31,218)=7.85; p=0.006]. Subsequent subgroup analyses showed that apathy and dysphoria interacted to reduce circulating BDNF in patients without SSRIs treatment [F(13,148)=3.38; p=0.037] (figure 1), and that treatment with SSRIs was associated to a significant increase of BDNF levels in AD patients without apathy and dysphoria as compared to those patients having apathy, dysphoria or both [F(12,75)=7.29; p=0.001] (figure 1). The significant effects of apathy on measures of cognitive impairment and behavioural disturbances (table 2) were independent of the significant influence of CIBIS+ on ADAS-cog+ [F(6,243)=211.56; p=0.000) and on NPI scores [F(12,237)=5.55; p=0.004], and of the significant contribution of dysphoria to NPI scores [F(12,237)=14.88; p=0.000].
Table 2.
Serum BDNF levels and clinical characteristics in AD patients without and with apathy.
| AD patients | Analysis | ||||||
|---|---|---|---|---|---|---|---|
| No Apathy (n=83) | Apathy (n=167) | ||||||
| N (%) | N (%) | X2 | df | P | |||
| Femalegender | 66 (79.5) | 128 (76.6) | 0.26 | 1 | ns | ||
| APOE ε4 allele | 38 (45.8) | 80 (47.9) | 0.75 | 1 | ns | ||
| Dysphoria | 37 (44.6) | 98 (58.7) | 4.44 | 1 | <0.05 | ||
| SSRIs treatment | 21 (25.3) | 67 (40.1) | 5.34 | 1 | <0.05 | ||
| CIBIS+: 3 (mild) 4 (moderate) 5 (moderately severe) |
38 (45.8) 33 (39.8) 12 (14.5) |
33 (19.8) 91 (54.5) 43 (25.7) |
18.86 | 2 | <0.001 | ||
| Mean ± SD | Mean ± SD | F | df | P | |||
| Age (years) | 75.31±7.10 | 74.83±7.53 | 0.24 | 1, 248 | Ns | ||
| MMSE (score) | 18.89±4.22 | 16.78±3.91 | 15.37 | 1, 248 | <0.001 | ||
| Apathy (score) | 0.00±0.00 | 4.34±2.46 | 258.23 | 1, 248 | <0.001 | ||
| Dysphoria (score) | 1.02±1.67 | 1.92±2.41 | 9.30 | 1, 248 | <0.01 | ||
| BDNF (ng/ml)a | 17.02±9.55 | 14.15±9.22 | 10.09 | 4, 245 | <0.05 | ||
| ADAS-cog+ (score)b |
33.93±13.97 | 42.90±14.89 | 7.75 | 6, 243 | <0.01 | ||
| NPI (score)c | 8.39±8.25 | 15.99±10.31 | 18.74 | 12, 237 | <0.001 | ||
ANCOVA using CIBIS+, dysphoria and SSRIs treatment as covariates, and apathy as between-subjects factor.
MANCOVA using age as a covariate, and apathy and CIBIS+ as between-subjects factors. Independent effect of CIBIS+ [F(6,243)=211.56; p<0.001].
MANCOVA using age as a covariate, and apathy, dysphoria and CIBIS+ as between-subjects factors. Independent effects of CIBIS+ [F(12,237)=5.55; p<0.01] and dysphoria [F(12,237)=14.88; p<0.001].
Figure 1.
Serum BDNF levels in AD patients without (No) and with (Yes) SSRIs treatment as a function of the presence or not of apathy and/or dysphoria. No ApDy: patients showing no apathy or dysphoria. Ap/Dy: Patients having apathy or dysphoria. Ap&Dy: Patients showing both apathy and dysphoria.
MANCOVA analyses using age and CIBIS+ as covariates, and apathy-dysphoria, sex and APOE4 as between-subjects factors. In patients without SSRI treatment, the presence of both apathy and dysphoria was associated to a significant reduction of serum BDNF [F(13,148)=3.38; *p<0.05]. In the group of patients treated with SSRIs, serum BDNF levels were higher in patients without apathy or dysphoria than in to those patients having apathy, dysphoria or both [F(12,75)=7.29; **p<0.01].
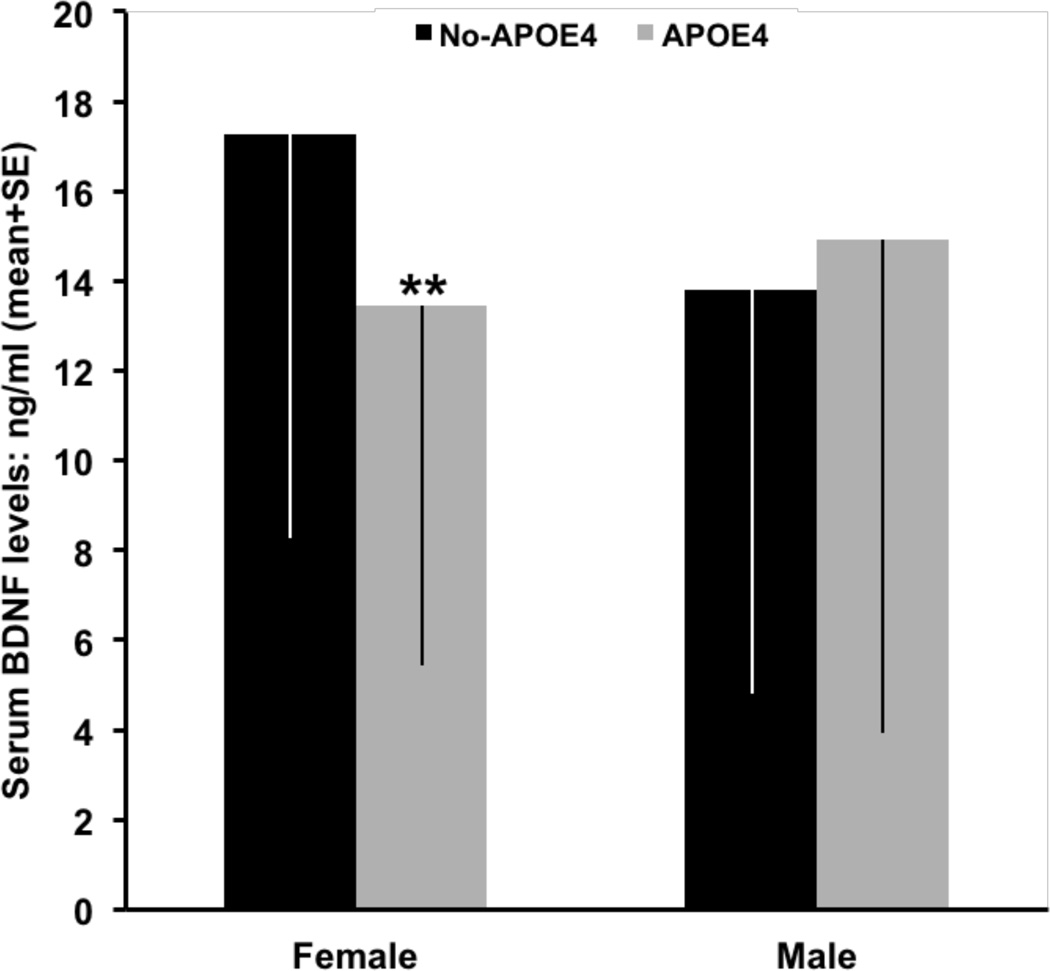
Serum BDNF levels were lower by 25% in APOE4 than in non-APOE4 carriers in the subgroup of female AD patients (table 3, figure 2), but not in males (figure 2). These APOE-related differences in BDNF values were even more marked in female AD patients without treatment with SSRIs (18.74±9.35 ng/ml versus 14.05±8.70 ng/ml; p=0.009) and were not influenced by apathy and dysphoria factors. MMSE scores were also significantly lower in APOE4 carriers than in non-carriers (table 3), and independently of the significant effects of CIBIS+ on cognitive performance. As depicted in table 3, all the other evaluated parameters were similar in the two APOE-related subgroups of female AD patients.
Table 3.
Serum BDNF levels and clinical characteristics in female AD patients without and with the APOE E4 allele.
| Female AD patients | Analysis | ||||||
|---|---|---|---|---|---|---|---|
| APOE No-E4 (n=98) | APOE E4 (n=98) | ||||||
| N (%) | N (%) | X2 | df | P | |||
| Apathy | 62 (63.9) | 66 (68.0) | 0.37 | 1 | ns | ||
| Dysphoria | 54 (55.7) | 55 (56.7) | 0.02 | 1 | ns | ||
| SRIs treatment | 39 (39.8) | 30 (30.6) | 1.81 | 1 | ns | ||
| CIBIS+: 3 (mild) 4 (moderate) 5 (moderately severe) |
28 (28.9) 44 (45.4) 25 (25.8) |
21 (21.6) 55 (56.7) 21 (21.6) |
2.57 | 2 | ns | ||
| Mean ± SD | Mean ± SD | F | df | P | |||
| Age (years) | 75.14±7.86 | 75.42±6.28 | 0.07 | 1, 192 | ns | ||
| Apathy (score) | 2.85±3.03 | 2.84±2.76 | 0.00 | 1, 192 | ns | ||
| Dysphoria (score) | 1.77±2.26 | 1.73±2.46 | 0.02 | 1, 192 | ns | ||
| ADAS-cog+ (score) |
40.40±16.43 | 40.85±14.15 | 0.04 | 1, 192 | ns | ||
| NPI (score) | 15.02±11.60 | 13.02±9.92 | 1.67 | 1, 192 | ns | ||
| BDNF (ng/ml)a | 17.28±9.52 | 13.44±8.37 | 9.58 | 6, 187 | <0.01 | ||
| MMSE (score)b | 18.11±4.26 | 16.30±3.58 | 18.87 | 9, 184 | <0.001 | ||
AD: Alzheimer’s disease.
ANCOVA, using age, CIBIS+, apathy, dysphoria and SSRIs treatment as covariates and APOE4 as between-subjects factor.
MANCOVA, using age, apathy, dysphoria and SSRIs treatment as covariates, and APOE4 and CIBIS+ as between-subjects factors. Also significant effects of CIBIS+ [F(9,184)=179.01; p<0.001) without significant interaction with APOE4.
Figure 2.
APOE4-related levels of serum BDNF in female and male AD patients. After corrections for age, CIBIS+, apathy, dysphoria and SSRIs treatment, APOE4 was associated to a significant (**p<0.01) reduction in serum BDNF levels in females, but not in males.
Linear regression analysis showed only mild but significant positive associations between BDNF levels and MMSE scores in the subgroup of male AD patients (B=0.08, SE=0.03, t=2.28, p=0.026), and in the subgroup of AD patients without apathy (B=0.06, SE=0.03, t =2.12, p=0.037).
4. Discussion
Baseline serum BDNF levels showed no significant group differences among controls, MCI and AD patients in the present study (Table 1). Our results are in agreement with recent findings of unchanged serum BDNF levels in AD and MCI cases compared to controls [13–15], but are in contrast with other studies reporting reduced [9–12] or increased [16] circulating levels of BDNF in AD and MCI patients. Discrepancies among studies might be owing to differences of the evaluated populations in factors influencing BDNF levels other than the diagnosis. According to results of our study, females had higher BDNF values than males, achieving statistically significant differences in MCI cases, and APOE4 carriers showed significantly lower BDNF levels than non-APOE4 carriers in the group of AD patients. Therefore, variations among study populations in gender distribution and/or in the proportion of APOE4 carriers might account, at least in part, for the variability of group differences reported by investigations on serum BDNF levels in AD, MCI and controls. Increased plasma and CSF BDNF levels in females compared to males were also observed by others in non-demented subjects [5, 33, 34]; but there are no previous studies assessing the influence of the APOE4 status on serum BDNF concentrations in AD patients. On the other hand, we found no significant influences of age or AD severity on BDNF levels. Although age-related decreases of BDNF concentrations were reported for normal adults and for institutionalized elderly subjects with depressive disorder [5, 35], most investigations failed to show any correlations of BDNF levels with age or disease severity in AD [10, 13, 16].
Results of the present investigation also indicated that the presence of apathy symptoms was associated to a significant reduction of serum BDNF levels in AD patients (table 2), with independency of the potential effects of gender, APOE4, dysphoria, and SSRIs treatment on BDNF concentrations. The lack of previous studies assessing the impact of apathy on circulating BDNF don’t allows the contrast of our results. In an attempt to delineate the specific influence of apathy on serum BDNF levels in AD, no patients with clinically significant depression were included in our study because most publications reported decreased peripheral levels of BDNF in patients with major depression or dysthymic disorder [23, 35]. Even though, mild dysphoria symptoms suggestive of subclinical depression were present in half of our AD patients and, as expected, these symptoms were more marked and prevalent in the subgroup of patients showing NPI scores for apathy. Also considering that antidepressant treatment with SSRIs was found to induce an upregulation or normalization of serum BDNF in depressed patients [23, 36], we analyzed BDNF levels according to the presence of apathy, dysphoria or both in AD patients without SSRIs treatment and in those receiving SSRIs. These analyses revealed a significant interaction of dysphoria and apathy to reduce serum BDNF in AD patients without SSRIs treatment, and a significant increase of BDNF levels in SSRIs-treated patients showing neither apathy nor dysphoria symptoms as compared to treated patients having apathy, dysphoria or both (figure 1). Our findings are consistent with results of recent studies demonstrating reduced plasma BDNF in community-dwelling and institutionalized elderly people with depressive symptoms or subclinical depression [35, 37], and with publications confirming the value of an increase in plasma BDNF as a predictive marker of the positive response to anti-depressants [36].
Low serum BDNF might constitute a biomarker for AD-related apathy, and probably depression, but the specific mechanisms underlying such an association between BDNF and apathy are unknown. Recent genetic studies found variants of the BDNF gene Val66Met polymorphism to be associated with an increased risk for AD-associated depression [20–22], with a significant influence on executive dysfunction in patients with mild AD [38], and with the response to the SSRI paroxetine in AD-related depression [22]. In our study, apathetic AD patients showed elevated scores of dysphoria/depression, cognitive impairment, disease severity (table 2) and executive dysfunction (data not shown) remembering alterations aforementioned in relationship with the BDNF Val66Met polymorphism. If genetic variants of this or other BDNF polymorphisms contribute to the association of apathy with low BDNF levels in AD is a possibility to be further explored.
A significant reduction of BDNF levels was found for APOE4 carriers compared to non-carriers in the subgroup of female AD patients, but not in males (figure 2). This APOE4-related BDNF reduction was independent of the effects of apathy and dysphoria on serum BDNF, and was associated to a significant decrease in MMSE scores (table 3). The influence of the APOE4 status on circulating BNDF was not evaluated before in AD, but the association of APOE4 with low MMSE scores in AD patients was already observed by other authors [39]. Associations of low serum BDNF and reduced MMSE scores have also been found in previous dementia studies showing that BDNF levels correlate significantly with MMSE scores and with the rate of cognitive decline as assessed with the MMSE [11, 17]. A reduced brain expression of BDNF and its receptors was also shown to be associated with cognitive impairment measured by the MMSE in AD and MCI [3, 6]. Although mechanisms underlying the associations of APOE4 with low BDNF and MMSE scores in AD females are to be determined, our results show a significant interaction of gender and APOE4 in influencing circulating BDNF and MMSE performance. The lack of APOE4-related differences observed for these parameters in AD males must be interpreted with caution owing to the small representation of male patients. In line with our findings, there are previous studies showing that lower plasma BDNF was associated with steeper brain volume loss and with cognitive decline in female but not male elderly subjects [33, 34], and that the Val66Met BDNF gene polymorphism has a sexually dimorphic effect on the susceptibility to late-onset AD, the Met66 allele conferring AD susceptibility to women but not to men [40]. On the other hand, interactions of BDNF Val66Met polymorphism with gender and with APOE4 have been reported to be significant determinants, respectively, of serum BDNF levels in patients with depression [41] and of the progression of cognitive impairment and brain atrophy in preclinical AD [9, 42]. An overrepresentation of the BDNF-270 C allele among APOE4 carriers has also been reported in AD patients [4]. Associations of APOE and BDNF polymorphisms might, therefore, account to some extent for the APOE4-related differences in BDNF and MMSE we found in female AD patients.
The significant positive correlations between BDNF and MMSE scores observed in the small subgroups of AD patients without apathy and of AD males, as well as the associations of reduced serum BDNF levels with lower MMSE scores in female APOE4 carriers and with a worse ADAScog+ performance in apathetic patients, are suggesting that reductions in serum BDNF might contribute to cognitive decline in AD. These results, however, must be interpreted with caution because the correlations were very week and the associations were restricted to some subgroups of patients and cognitive measures (MMSE in female APOE4 carriers and ADAScog+ in apathetic patients). Although several studies found that serum BDNF levels correlate positively with MMSE scores and with a lower rate of cognitive decline in AD patients [11, 17], as well as with memory performance in MCI patients [12], other authors showed no correlations of BDNF with global cognitive measures [10, 13] or even negative associations between BDNF levels and memory scores in AD [14].
Main results of the present study indicate that serum BDNF levels are significantly reduced in AD patients having apathy symptoms as compared to those patients without apathy, and in female AD cases carrying the APOE4 allele compared to non-carriers. Further studies are warranted to confirm these findings because there are no previous reports on the influence of apathy and APOE4 on circulating BDNF in AD. Future investigations must overcome limitations of our study related to small sample sizes, particularly the low number of AD males, and should address the evaluation of the potential interaction of apathy and dysphoria-depression to reduce BDNF levels in a clinical sample of AD patients not excluding depression or prominent neuropsychiatric symptoms, as well as the determination of the influence of BDNF polymorphisms on the associations of apathy and APOE4 with low BDNF levels in AD.
Acknowledgments
This study was supported by Research Grants from the Fundación Antidemencia Al-Andalus (Córdoba, Spain), and from Ever NeuroPharma (Unterach, Austria). AA was also supported by the Sixth Framework Programme of the European Union (LSHB-CT-2006-037702).
Footnotes
Disclosure statement
The authors declare no conflicts of interest.
References
- 1.Mufson EJ, Counts SE, Fahnestock M, Ginsberg SD. Cholinotrophic molecular substrates of mild cognitive impairment in the elderly. Curr Alzheimer Res. 2007;4:340–350. doi: 10.2174/156720507781788855. [DOI] [PubMed] [Google Scholar]
- 2.Ye X, Tai W, Zhang D. The early events of Alzheimer's disease pathology: from mitochondrial dysfunction to BDNF axonal transport deficits. Neurobiol Aging. 2012;33:1122e.1–1122e.10. doi: 10.1016/j.neurobiolaging.2011.11.004. [DOI] [PubMed] [Google Scholar]
- 3.Ginsberg SD, Wuu J, Counts SE, Mufson EJ. Down regulation of trk but not p75NTR gene expression in single cholinergic basal forebrain neurons mark the progression of Alzheimer´s disease. J Neurochem. 2006;97:475–487. doi: 10.1111/j.1471-4159.2006.03764.x. [DOI] [PubMed] [Google Scholar]
- 4.Lee J, Fukumoto H, Orne J, Klucken J, Raju S, Vanderburg CR, Irizarry MC, Hyman BT, Ingelsson M. Decreased levels of BDNF protein in Alzheimer temporal cortex are independent of BDNF polymorphisms. Exp Neurol. 2005;194:91–96. doi: 10.1016/j.expneurol.2005.01.026. [DOI] [PubMed] [Google Scholar]
- 5.Li G, Peskind ER, Millard SP, Chi P, Sokal I, Yu CE, Bekris LM, Raskind MA, Galasko DR, Montine TJ. Cerebrospinal fluid concentration of brain-derived neurotrophic factor and cognitive function in non-demented subjects. PLoS One. 2009;4:e5424. doi: 10.1371/journal.pone.0005424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peng S, Wuu J, Mufson EJ, Fahnestock M. Precursor form of brain-derived neurotrophic factor and mature brain-derived neurotrophic factor are decreased in the pre-clinical stages of Alzheimer's disease. J Neurochem. 2005;93:1412–1421. doi: 10.1111/j.1471-4159.2005.03135.x. [DOI] [PubMed] [Google Scholar]
- 7.Nagahara AH, Merrill DA, Coppola G, Tsukada S, Schroeder BE, Shaked GM, Wang L, Blesch A, Kim A, Conner JM, Rockenstein E, Chao MV, Koo EH, Geschwind D, Masliah E, Chiba AA, Tuszynski MH. Neuroprotective effects of brain-derived neurotrophic factor in rodent and primate models of Alzheimer’s disease. Nat Med. 2009;15:331–337. doi: 10.1038/nm.1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nikolaev A, McLaughlin T, O'Leary DD, Tessier-Lavigne M. APP binds DR6 to trigger axon pruning and neuron death via distinct caspases. Nature. 2009;457:981–989. doi: 10.1038/nature07767. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 9.Forlenza OV, Diniz BS, Teixeira AL, Ojopi EB, Talib LL, Mendonça VA, Izzo G, Gattaz WF. Effect of brain-derived neurotrophic factor Val66Met polymorphism and serum levels on the progression of mild cognitive impairment. World J Biol Psychiatry. 2010;11:774–780. doi: 10.3109/15622971003797241. [DOI] [PubMed] [Google Scholar]
- 10.Laske C, Stransky E, Leyhe T, Eschweiler GW, Maetzler W, Wittorf A, Soekadar S, Richartz E, Koehler N, Bartels M, Buchkremer G, Schott K. BDNF serum and CSF concentrations in Alzheimer's disease, normal pressure hydrocephalus and healthy controls. J Psychiatr Res. 2007;41:387–394. doi: 10.1016/j.jpsychires.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 11.Lee JG, Shin BS, You YS, Kim JE, Yoon SW, Jeon DW, Baek JH, Park SW, Kim YH. Decreased serum brain-derived neurotrophic factor levels in elderly korean with dementia. Psychiatry Investig. 2009;6:299–305. doi: 10.4306/pi.2009.6.4.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yu H, Zhang Z, Shi Y, Bai F, Xie C, Qian Y, Yuan Y, Deng L. Association study of the decreased serum BDNF concentrations in amnestic mild cognitive impairment and the Val66Met polymorphism in Chinese Han. J Clin Psychiatry. 2008;69:1104–1111. doi: 10.4088/jcp.v69n0710. [DOI] [PubMed] [Google Scholar]
- 13.O'Bryant SE, Hobson V, Hall JR, Waring SC, Chan W, Massman P, Lacritz L, Cullum CM, Diaz-Arrastia R. Texas Alzheimer's Research Consortium. Brain-derived neurotrophic factor levels in Alzheimer's disease. J Alzheimers Dis. 2009;17:337–341. doi: 10.3233/JAD-2009-1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O'Bryant SE, Hobson VL, Hall JR, Barber RC, Zhang S, Johnson L, Diaz-Arrastia R. Texas Alzheimer's Research Consortium. Serum brain-derived neurotrophic factor levels are specifically associated with memory performance among Alzheimer's disease cases. Dement Geriatr Cogn Disord. 2011;31:31–36. doi: 10.1159/000321980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Woolley JD, Strobl EV, Shelly WB, Miller BL, Mellon SH, Rankin KP. BDNF serum concentrations show no relationship with diagnostic group or medication status in neurodegenerative disease. Curr Alzheimer Res. 2012;9:815–821. doi: 10.2174/156720512802455395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Angelucci F, Spalletta G, di Iulio F, Ciaramella A, Salani F, Colantoni L, Varsi AE, Gianni W, Sancesario G, Caltagirone C, Bossù P. Alzheimer's disease (AD) and Mild Cognitive Impairment (MCI) patients are characterized by increased BDNF serum levels. Curr Alzheimer Res. 2010;7:15–20. doi: 10.2174/156720510790274473. [DOI] [PubMed] [Google Scholar]
- 17.Laske C, Stellos K, Hoffmann N, Stransky E, Straten G, Eschweiler GW, Leyhe T. Higher BDNF serum levels predict slower cognitive decline in Alzheimer's disease patients. Int J Neuropsychopharmacol. 2011;14:399–404. doi: 10.1017/S1461145710001008. [DOI] [PubMed] [Google Scholar]
- 18.Grünblatt E, Zehetmayer S, Bartl J, Löffler C, Wichart I, Rainer MK, Jungwirth S, Bauer P, Danielczyk W, Tragl KH, Riederer P, Fischer P. Genetic risk factors and markers for Alzheimer's disease and/or depression in the VITA study. J Psychiatr Res. 2009;43:298–308. doi: 10.1016/j.jpsychires.2008.05.008. [DOI] [PubMed] [Google Scholar]
- 19.Starkstein SE, Ingram L, Garau ML, Mizrahi R. On the overlap between apathy and depression in dementia. J Neurol Neurosurg Psychiatry. 2005;76:1070–1074. doi: 10.1136/jnnp.2004.052795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arlt S, Demiralay C, Tharun B, Geisel O, Storm N, Eichenlaub M, Lehmbeck JT, Wiedemann K, Leuenberger B, Jahn H. Genetic Risk Factors for Depression in Alzheimer´s Disease Patients. Curr Alzheimer Res. 2013;10:72–81. [PubMed] [Google Scholar]
- 21.Borroni B, Archetti S, Costanzi C, Grassi M, Ferrari M, Radeghieri A, Caimi L, Caltagirone C, Di Luca M, Padovani A ITINAD Working Group. Role of BDNF Val66Met functional polymorphism in Alzheimer's disease-related depression. Neurobiol Aging. 2009;30:1406–1412. doi: 10.1016/j.neurobiolaging.2007.11.023. [DOI] [PubMed] [Google Scholar]
- 22.Zhang L, Fang Y, Zeng Z, Lian Y, Wei J, Zhu H, Jia Y, Zhao X, Xu Y. BDNF Gene Polymorphisms are Associated with Alzheimer's Disease-Related Depression and Antidepressant Response. J Alzheimers Dis. 2011;26:523–530. doi: 10.3233/JAD-2011-110113. [DOI] [PubMed] [Google Scholar]
- 23.Molendijk ML, Bus BA, Spinhoven P, Penninx BW, Kenis G, Prickaerts J, Voshaar RC, Elzinga BM. Serum levels of brain-derived neurotrophic factor in major depressive disorder: state-trait issues, clinical features and pharmacological treatment. Mol Psychiatry. 2011;16:1088–1095. doi: 10.1038/mp.2010.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th. Washington DC: American Psychiatric Association; 1994. [Google Scholar]
- 25.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: Report of NINCDS-ADRDA Work Group. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 26.Artero S, Petersen R, Touchon J, Ritchie K. Revised criteria for mild cognitive impairment: validation within a longitudinal population study. Dement Geriatr Cogn Disord. 2006;22:465–470. doi: 10.1159/000096287. [DOI] [PubMed] [Google Scholar]
- 27.Hamilton M. Development of a rating scale for primary depressive illness. Br J Soc Clin Psychol. 1967;6:278–296. doi: 10.1111/j.2044-8260.1967.tb00530.x. [DOI] [PubMed] [Google Scholar]
- 28.Alvarez A, Cacabelos R, Sampedro C, Garcia-Fantini M, Aleixandre M. Serum TNF-alpha levels are increased and correlate negatively with free IGF-I in Alzheimer disease. Neurobiol Aging. 2007;18:533–536. doi: 10.1016/j.neurobiolaging.2006.02.012. [DOI] [PubMed] [Google Scholar]
- 29.Folstein MF, Folstein SE, McHugh PR. "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician". J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 30.Mohs RC, Knopman D, Petersen RC, Ferris SH, Ernesto C, Grundman M, Sano M, Bieliauskas L, Geldmacher D, Clark C, Thal LJ. Development of cognitive instruments for use in clinical trials of antidementia drugs: additions to the Alzheimer's Disease Assessment Scale that broaden its scope. The Alzheimer's Disease Cooperative Study. Alzheimer Dis Assoc Disord. 1997;11:S13–S21. [PubMed] [Google Scholar]
- 31.Knopman DS, Knapp MJ, Gracon SI, Davis CS. The Clinician Interview-Based Impression (CIBI): a clinician’s global change rating scale in Alzheimer’s disease. Neurology. 1994;44:2315–2321. doi: 10.1212/wnl.44.12.2315. [DOI] [PubMed] [Google Scholar]
- 32.Cummings JL, Mega M, Gray K, Rosenberg-Thompson S, Carusi DA, Gornbein J. The Neuropsychiatric Inventory: Comprehensive Assessment of Pychopathology in Dementia. Neurology. 1994;44:2308–2314. doi: 10.1212/wnl.44.12.2308. [DOI] [PubMed] [Google Scholar]
- 33.Driscoll I, Martin B, An Y, Maudsley S, Ferrucci L, Mattson MP, Resnick SM. Plasma BDNF is associated with age-related white matter atrophy but not with cognitive function in older, non-demented adults. PLoS One. 2012;7:e35217. doi: 10.1371/journal.pone.0035217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Komulainen P, Pedersen M, Hänninen T, Bruunsgaard H, Lakka TA, Kivipelto M, Hassinen M, Rauramaa TH, Pedersen BK, Rauramaa R. BDNF is a novel marker of cognitive function in ageing women: the DR's EXTRA Study. Neurobiol Learn Mem. 2008;90:596–603. doi: 10.1016/j.nlm.2008.07.014. [DOI] [PubMed] [Google Scholar]
- 35.Chu CL, Liang CK, Chou MY, Lin YT, Pan CC, Lu T, Chen LK, Chow PC. Decreased plasma brain-derived neurotrophic factor levels in institutionalized elderly with depressive disorder. J Am Med Dir Assoc. 2012;13:434–437. doi: 10.1016/j.jamda.2011.08.006. [DOI] [PubMed] [Google Scholar]
- 36.Dreimüller N, Schlicht KF, Wagner S, Peetz D, Borysenko L, Hiemke C, Lieb K, Tadić A. Early reactions of brain-derived neurotrophic factor in plasma (pBDNF) and outcome to acute antidepressant treatment in patients with Major Depression. Neuropharmacology. 2012;62:264–269. doi: 10.1016/j.neuropharm.2011.07.017. [DOI] [PubMed] [Google Scholar]
- 37.Bus BA, Tendolkar I, Franke B, de Graaf J, Heijer MD, Buitelaar JK, Oude Voshaar RC. Serum brain-derived neurotrophic factor: determinants and relationship with depressive symptoms in a community population of middle-aged and elderly people. World J Biol Psychiatry. 2012;13:39–47. doi: 10.3109/15622975.2010.545187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nagata T, Shinagawa S, Nukariya K, Yamada H, Nakayama K. Association between BDNF polymorphism (Val66Met) and executive function in patients with amnestic mild cognitive impairment or mild Alzheimer disease. Dement Geriatr Cogn Disord. 2012;33:266–272. doi: 10.1159/000339358. [DOI] [PubMed] [Google Scholar]
- 39.Tschanz JT, Corcoran CD, Schwartz S, Treiber K, Green RC, Norton MC, Mielke MM, Piercy K, Steinberg M, Rabins PV, Leoutsakos JM, Welsh-Bohmer KA, Breitner JC, Lyketsos CG. Progression of cognitive, functional, and neuropsychiatric symptom domains in a population cohort with Alzheimer dementia: the Cache County Dementia Progression study. Am J Geriatr Psychiatry. 2011;19:532–342. doi: 10.1097/JGP.0b013e3181faec23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fukumoto N, Fujii T, Combarros O, Kamboh MI, Tsai SJ, Matsushita S, Nacmias B, Comings DE, Arboleda H, Ingelsson M, Hyman BT, Akatsu H, Grupe A, Nishimura AL, Zatz M, Mattila KM, Rinne J, Goto YI, Asada T, Nakamura S, Kunugi H. Sexually dimorphic effect of the Val66Met polymorphism of BDNF on susceptibility to Alzheimer's disease: New data and meta-analysis. Am J Med Genet B Neuropsychiatr Genet. 2009;153B:235–242. doi: 10.1002/ajmg.b.30986. [DOI] [PubMed] [Google Scholar]
- 41.Elfving B, Buttenschøn HN, Foldager L, Poulsen PH, Andersen JH, Grynderup MB, Hansen ÅM, Kolstad HA, Kaerlev L, Mikkelsen S, Thomsen JF, Børglum AD, Wegener G, Mors O. Depression, the Val66Met polymorphism, age, and gender influence the serum BDNF level. J Psychiatr Res. 2012;46:1118–1125. doi: 10.1016/j.jpsychires.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 42.Hashimoto R, Hirata Y, Asada T, Yamashita F, Nemoto K, Mori T, Moriguchi Y, Kunugi H, Arima K, Ohnishi T. Effect of the brain-derived neurotrophic factor and the apolipoprotein E polymorphisms on disease progression in preclinical Alzheimer's disease. Genes Brain Behav. 2009;8:43–52. doi: 10.1111/j.1601-183X.2008.00440.x. [DOI] [PubMed] [Google Scholar]